Abstract
Objectives
To determine the prevalence and distribution of sleep-disordered breathing and associated correlates in a large cohort of older men using several standardized definitions.
Design
Cross-sectional analyses.
Setting
Six U.S. communities.
Participants
Polysomnography was performed on 2,911 participants of the Outcomes of Sleep Disorders in Older Men Sleep Study (mean age ± standard deviation 76.38 ± 5.53; body mass index 27.17 ± 3.8 kg/m2).
Measurements
Three outcomes were assessed: sleep-disordered breathing (respiratory disturbance index ≥15), obstructive apnea (obstructive apnea index ≥5), and central apnea (central apnea index ≥5).
Results
The prevalence of moderate–severe sleep-disordered breathing was estimated to be 21.4% to 26.4%. Multivariable logistic regression models demonstrated that age (adjusted odds ratio (AOR) per 5-year increase = 1.24, 95% confidence interval (CI) = 1.15–1.34), obesity (AOR = 2.54, 95% CI = 2.09–3.09), Asian versus Caucasian race (AOR = 2.14, 95% CI = 1.33–3.45), snoring (AOR = 2.01, 95% CI = 1.62–2.49), sleepiness (AOR = 1.41, 95% CI = 1.11–1.79), hypertension (AOR = 1.26, 95% CI = 1.06–1.50), cardiovascular disease (AOR = 1.24, 95% CI = 1.19–1.29), and heart failure (AOR = 1.81, 1.31–2.51) were independently associated with sleep-disordered breathing; snoring (AOR = 2.10, 95% CI = 1.67–2.70), age (AOR per 5-year increase = 1.27, 95% CI = 1.18–1.38), obesity (AOR = 1.48, 95% CI = 1.21–1.82), and heart failure (AOR = 1.60, 95% CI = 1.15–2.24) were associated with obstructive apnea; and age (AOR = 1.33, 1.17–1.50) and heart failure (AOR = 1.88, 95% CI = 1.17–3.04) were associated with central apnea.
Conclusion
Regardless of definition, a high prevalence of sleep disorders is observed in community-dwelling older men. Qualitatively similar associations were observed between sleep disorders and snoring, obesity, and comorbidities, as reported for middle aged populations. Asian race was associated with sleep-disordered breathing.
Keywords: sleep apnea, geriatrics, cohort study
Repetitive partial or complete upper airway obstruction resulting in sleep fragmentation and nocturnal chronic intermittent hypoxia associated with a myriad of pathophysiological effects characterize sleep-disordered breathing (SDB). These effects are believed to contribute to adverse cardiovascular health outcomes and to negatively influence quality of life. Population differences in SDB include a higher prevalence in older individuals and men. Whereas the prevalence of SDB in middle-aged adults has been reported to range from 2% to 4%;1 in older people, prevalence ranges from 6% to 70% in samples derived from referral-based and population-based sources.2–6 SDB prevalence ranges appear to be higher in older men than in older women.2 Longitudinal data suggest that the highest rates of SDB progression occur in older men, thereby highlighting the potential vulnerability of older men to SDB.7–9 The sex-specific disparity of SDB occurrence is likely attributable to a variety of factors, including hormonal influences and sex-related differences in upper airway anatomy and body fat deposition.
In older people, it has been intensely debated whether SDB represents a similar condition as in middle-aged adults.10 Support for the hypothesis that it does not is derived from studies that have reported a different constellation of associated risk factors, symptoms, and apnea characteristics in older samples than in middle-aged adults. Specifically, the reported snoring prevalence has tended to be lower in the elderly population,11 possibly because older individuals, especially those who live alone, may systematically underreport snoring. Because snoring reflects airway turbulence, which is a function of the flow characteristics in the airway, differences in snoring across populations could also reflect population differences in airway anatomy and physiology. In addition, underlying comorbidities, survival bias, competing risks, or differences in pathophysiology may influence the measurable consequences of SDB. Some studies of older populations report weaker associations between SDB and cardiovascular disease (CVD) in older than in middle-aged populations.12,13–15 Support for SDB as a unique disorder in this older cohort is derived from knowledge that aging is associated with greater comorbidity and that cardiovascular comorbidities are associated with the central type of apneic respiratory events. Some of these results have been published in the form of an abstract.16
Rigorously collected data from a large, prospective, multicenter, population-based cohort of older men (Outcomes of Sleep Disorders in Older Men Study, or the Osteoporotic Fractures in Men (MrOS) Sleep Study) were analyzed to address the hypothesis that SDB in elderly men is associated with a similar set of risk factors and comorbidities as has been reported in middle-aged populations. The MrOS Sleep Study cohort provides a unique opportunity to investigate sleep disorders in the largest collection of sleep data in elderly men. Definitions of SDB were similar to those used in the Sleep Heart Health Study (SHHS) and the Wisconsin Cohort Study to facilitate comparisons across populations. A secondary goal was to address potential differences in the prevalence estimates and risk relationships according to different definitions of SDB, including reclassifying SDB based on distinguishing obstructive from central events and variation of the amplitude criterion for hypopneas.
Methods
Subjects and Study Design
The MrOS Study, the parent cohort for the MrOS Sleep Study, enrolled 5,995 community-dwelling men aged 65 and older during the baseline examination between 2000 and 2002. To be eligible for the study, men had to be able to walk without assistance and not have had a bilateral hip replacement. Participants were recruited at six clinical centers (Birmingham, AL; Minneapolis, MN; Palo Alto, CA; Monongahela Valley near Pittsburgh, PA; Portland, OR; and San Diego, CA). The study design and methods of recruitment and demographics of the initial cohort have been previously published.17,18 The MrOS Sleep Study visit occurred on average 3.4 ± 0.5 years (range 1.9–4.9) after the baseline examination, between December 2003 and March 2005. Ethics approval was obtained from the institutional review board at each site and the Coordinating Center and Reading Center. Written informed consent for participation in the MrOS Sleep Study was obtained for all individuals. The MrOS Sleep Study is an ancillary study with a target recruitment number of 3,000 men from the parent MrOS Study. Of the 5,995 MrOS participants, 3,135 participated in the MrOS Sleep Study, with 2,860 participants in the main cohort who did not participate in the sleep study visit (1,995 were unwilling to participate, 421 did not undergo screening because recruitment goals were met, 270 died before the sleep study visit, 150 were ineligible because they were receiving therapy for sleep apnea (continuous positive airway pressure or oxygen) and 24 were terminated before the sleep study visit). The 1,995 MrOS Study participants unwilling to participate were older by 1 year (74.01 ± 5.88 vs 73.05 ± 5.55, P < .001) compared to the 3,135 MrOS Sleep Study participants and not significantly different with respect to body mass index (BMI; 27.20 ± 3.72 kg/m2 vs 27.38 ± 3.72 kg/m2, P = .10).
Data Collection
In-home sleep studies using unattended polysomnography (Safiro, Compumedics, Inc., Melbourne, Australia) were performed. The recording montage consisted of C3/A2 and C4/A1 electroencephalograms, bilateral electrooculograms, a bipolar submental electromyogram, thoracic and abdominal respiratory inductance plethysmography, airflow (using nasal-oral thermocouple and nasal pressure cannula), finger pulse oximetry, electrocardiogram, body position (mercury switch sensor), and bilateral leg movements (piezoelectric sensors). Trained certified staff members performed home visits for setup of the sleep study units. After sensors were placed and calibrated, signal quality and impedance were checked, and sensors were repositioned as needed to improve signal quality, replacing electrodes if impedances were greater than 5 kΩ, using approaches similar to those in the SHHS.19 After studies were downloaded, they were transferred to the Case Reading Center (Cleveland, OH) for centralized scoring by a trained technician. Polysomnography data quality was excellent, with a failure rate of less than 4% and more than 70% of studies graded as being of excellent or outstanding quality. Quality codes for signals and studies were graded using previously described approaches, which included coding the duration of artifact-free data per channel and overall study quality (reflecting the combination of grades for each channel).19 Snoring was assessed according to self-report. Information about daytime sleepiness was assessed according to self-report using the Epworth Sleepiness Scale (ESS).20 The standard ESS cutpoint of 10 was used to define excessive daytime sleepiness.21
Assessment of SDB
The severity of SDB was determined according to the Respiratory Disturbance Index (RDI; number of apneas and hypopneas per hour of sleep). Apneas were identified if the amplitude of the airflow was flat or nearly flat for more than 10 seconds. Obstructive apneas were scored if persistence of effort on abdominal or thoracic inductance plethysmography was noted, and central apneas were scored if there was no evident effort on either the abdominal and thoracic plethysmography bands. Hypopneas were scored using SHHS criteria (requiring a “discernible” (>30%) reduction in amplitude of respiratory effort or airflow) and, in secondary analyses, using the American Academy of Sleep Medicine (AASM) criteria22 (≥50% reduction in amplitude of signals), considered according to the following hierarchy: summed inductance plethysmography channel, abdominal or thoracic inductance plethysmography, nasal pressure, or thermistor. For the current study, only respiratory events (apneas and hypopneas) associated with a 4% oxygen desaturation were included, and the SHHS definition was used for primary analyses. Using similar approaches, between- and within-scorer reliability for various derivations of the RDI in the same scoring group has been excellent, with intraclass correlation coefficients ranging from 0.76 to 0.99.23
Other Measures
All covariate data were collected at the time of the sleep study visit. All participants completed questionnaire data, which included questions about medical history, smoking, and alcohol intake. Race was based on self-report and categorized as Caucasian, African American, Asian, or Hispanic/other. BMI was calculated as weight (kg)/height (m2), and obesity was defined as a BMI greater than 30 kg/ m2.24,25 During the home or clinic visits, body weight was measured using a standard balance beam scale and height using a wall-mounted Harpenden stadiometer (Holtain, UK). Neck and waist circumference were also measured using standard methods.26 Snoring was assessed according to self-report. The MacArthur Subjective Status Scale (range 1–10) was used to assess perceived social status, with higher scores representing higher perceived social status.27
Statistical Analysis
The primary correlates (risk factor, comorbidities, or symptoms) of sleep disorders considered were subject characteristics (age (per 5-year increase), race (referent race: Caucasian), obesity (BMI > 30 kg/m2, per the World Health Organization classification),24,25 neck circumference (per 5-cm increase), waist circumference (per 5-cm increase), socioeconomic status, current tobacco smoking and alcohol use), self-reported doctor-diagnosed diseases (hypertension, diabetes mellitus, CVD (angina pectoris, myocardial infarction, coronary percutaneous intervention, coronary artery bypass graft surgery, vascular surgery, or pacemaker placement), heart failure, and stroke), symptoms (snoring and subjective sleepiness defined as an ESS score >1020), and study site. Exploratory analyses assessed the association between each correlate and RDI, categorized as less than 5, 5 to less than 15, 15 to less than 30, and 30 or more, using ordinal logistic regression models. Because results yielded similar findings as logistic regression models using the dichotomous variable of RDI of 15 or greater versus less than 15, the latter results are presented. The three primary outcomes in this analysis were SDB (RDI ≥15), obstructive apnea (OA, obstructive apnea index ≥5), and central apnea (CA, central apnea index ≥5). Separate logistic regression models were constructed to describe the relative strength of each covariate with each outcome; unadjusted and age-, obesity-, and race-adjusted odds ratios with 95% confidence intervals are presented. Analyses were performed using SAS statistical software (version 9.1, SAS Institute, Inc., Cary, NC).
Results
Subject Characteristics
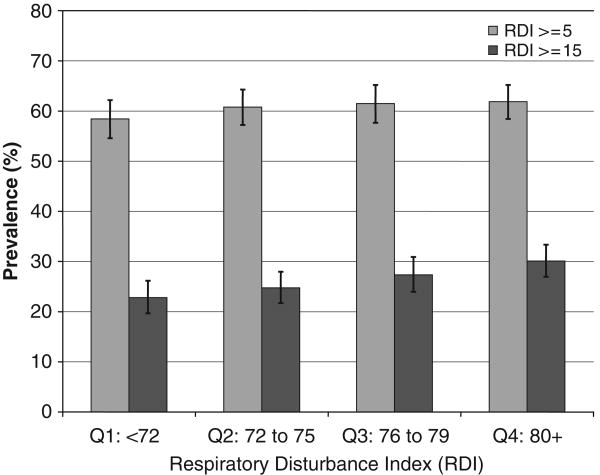
Of the 3,135 participants who attended the sleep visit, data from 2,911 (96.2%) had adequate polysomnography signal quality for at least 4 hours and constitute the current analysis. The sample was elderly, predominantly Caucasian, and had a wide distribution of BMI (Table 1). A statistically significant P-value for trend (P = .005) was observed with SDB defined as RDI of 15 or greater and increasing age quartile, which conversely was not observed with SDB defined as RDI of 5 or greater and increasing age quartile (P = .68) (Figure 1).
Table 1. Subject Characteristics According to Sleep-Disordered Breathing (SDB).
| Characteristic | Overall (N = 2,911) |
SDB (RDI > 15) (n = 768) |
Non-SDB (RDI <15) (n = 2,143) |
P-Value |
|---|---|---|---|---|
| Subject characteristic | ||||
| Age, mean ± SD | 76.4 ± 5.5 | 76.9 ± 5.5 | 76.2 ± 5.6 | .003 |
| Race, % | ||||
| Caucasian | 90.7 | 89.3 | 91.2 | .22 |
| African American | 3.4 | 3.5 | 3.4 | |
| Asian | 2.9 | 3.7 | 2.6 | |
| Other | 3.0 | 3.5 | 2.8 | |
| Body mass index (kg/m2) | ||||
| Mean ± SD | 27.2 ± 3.8 | 28.5 ± 4.2 | 26.7 ± 3.6 | <.001 |
| >30 kg/m2, % | 20.3 | 31.5 | 16.3 | <.001 |
| Neck circumference, cm, mean ± SD | 39.5 ± 3.0 | 40.2 ± 2.9 | 39.2 ± 2.9 | <.001 |
| Waist circumference, cm, mean ± SD | 99.7 ± 11.2 | 102.9 ± 11.4 | 98.5 ± 10.9 | <.001 |
| Hip circumference, cm, mean ± SD | 102.9 ± 8.6 | 105.0 ± 9.0 | 102.2 ± 8.3 | <.001 |
| Waist–hip circumference ratio, mean ± SD | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | <.001 |
| Symptoms | ||||
| Current snoring ≥3–5 times per week (vs < 3 times per week) | 29.9 | 41.2 | 25.6 | <.001 |
| Epworth sleepiness scale (ESS) (range 0–24), mean ± SD | 6.2 ± 3.7 | 6.6 ± 4.0 | 6.0 ± 3.6 | <.001 |
| Excessive daytime sleepiness (ESS > 10), % | 13.1 | 16.7 | 11.8 | <.001 |
| Loud snoring (bed partner report vs no snoring) | 57.5 | 63.9 | 55.2 | <.001 |
| Witnessed apnea (bed partner report vs no witnessed apneas) | 27.5 | 36.6 | 24.3 | <.001 |
| Comorbidities | ||||
| Diabetes mellitus, % | 13.3 | 16.8 | 12.0 | <.001 |
| Hypertension, % | 49.9 | 57.3 | 47.3 | <.001 |
| Cardiovascular disease, % | 33.3 | 37.4 | 31.9 | .005 |
| Heart failure, % | 6.0 | 9.2 | 4.8 | <.001 |
| Stroke, % | 3.8 | 3.5 | 3.9 | .61 |
| Alcoholic drinks per week, % | ||||
| 0 | 34.5 | 35.1 | 34.3 | .54 |
| <1 | 12.4 | 12.0 | 12.5 | |
| 1–2 | 12.7 | 13.6 | 12.3 | |
| 3–5 | 15.5 | 16.6 | 15.2 | |
| 6–13 | 19.5 | 18.1 | 20.0 | |
| ≥14 | 5.4 | 4.6 | 5.7 | |
| Current cigarette smoker (vs other), % | 2.0 | 1.3 | 2.2 | .13 |
| Number of cigarettes smoked per day, mean ± SD | 0.3 ± 2.3 | 0.2 ± 2.0 | 0.3 ± 2.4 | .13 |
RDI = Respiratory Disturbance Index; SD = standard deviation.
Figure 1.
Sleep-disordered breathing (SDB) prevalence estimates with 95% confidence intervals are illustrated based upon respiratory disturbance index (RDI) cutoffs of 5 (P-value for trend = .68) and 15 (P-value for trend = .005) according to age quartile.
Prevalence and Correlates of SDB
In the sample, 26.4% of participants were classified with SDB as defined by a RDI of 15 or greater and with use of the SHHS definitions for hypopnea. With this cutoff, SDB prevalence increased with increasing age, from 22.8% (95% confidence interval (CI) = 19.7–26.2%) for those younger than 72 to 30.1% (95% CI = 26.9–33.3%) for those aged 80 and older (Figure 1), and increasing BMI, from 16.5% (95% CI = 13.9–19.4%) for those with BMI of less than 24.6 to 38.5% (95% CI = 34.9–42.1%) for those with BMI of 29.4 or greater (Figure 2) (P-values for trend < .001). In contrast, prevalence of SDB defined using a lower cutoff (RDI ≥5) was higher (58.4–61.9%) and did not vary according to age category (Figure 1), although it did vary according to increasing BMI (Figure 2). (All subsequent primary analyses are based on an RDI cutoff of 15.)
Figure 2.
Sleep-disordered breathing (SDB) prevalence estimates with 95% confidence intervals are illustrated based upon respiratory disturbance index (RDI) cutoffs of 5 (P-value for trend < .001) and 15 (P-value for trend < .001) according to body mass index (BMI) quartile.
In unadjusted analyses, SDB was significantly associated with the following symptoms and additional demographic and anthropometric factors: snoring, excessive daytime sleepiness, obesity status, and neck and waist circumference (Table 2). SDB was also significantly associated with the following medical diagnoses in unadjusted models: hypertension, diabetes mellitus, CVD, and heart failure. After adjusting for race and obesity, SDB was significantly associated with age (odds ratio (OR) = 1.24, 95% CI = 1.15–1.34), and after adjusting for age and obesity, SDB was significantly associated with Asian race (OR = 2.2495% CI = 1.37–3.66). BMI and Asian race were negatively correlated (Spearman correlation coefficient = −0.13, P< .001); therefore, once the effects of obesity were adjusted for, Asian race was a stronger risk factor. After adjusting for age, race, and obesity, SDB remained associated with snoring (OR = 2.04, 95% CI = 1.66–2.50), excessive daytime sleepiness (OR = 1.50, 95% CI = 1.19–1.89), neck circumference (OR = 2.19, 95% CI = 1.88–2.56), waist circumference (per 5 cm; OR = 1.24, 95% CI = 1.19–1.29), hypertension (OR = 1.26, 95% CI = 1.06–1.50), CVD (OR = 1.24, 95% CI = 1.03–1.48), and heart failure (OR = 1.81, 95% CI = 1.31–2.51).
Table 2. Logistic Regression with Sleep-Disordered Breathing (SDB) (Respiratory Disturbance Index > 15) as Outcome.
| Characteristic | Odds Ratio (95% Confidence Interval) | |
|---|---|---|
| Unadjusted* | Adjusted† | |
| Symptom | ||
| Snoring (>3–5 times/week) | 2.04 (1.66–2.50)‡ | 2.01 (1.62–2.49)‡ |
| Excessive daytime somnolence (Epworth Sleepiness Scale score >10) | 1.50 (1.19–1.89)‡ | 1.41 (1.11–1.79)‡ |
| Subject characteristic | ||
| Age (per 5-year increase) | 1.12 (1.04–1.20)‡ | 1.24 (1.15–1.34)‡ |
| Race (reference: Caucasian) | ||
| African American | 1.07 (0.68–1.68) | 1.05 (0.66–1.68) |
| Asian | 1.42 (0.90–2.26) | 2.14 (1.33–3.45)‡ |
| Hispanic | 1.28 (0.81–2.04) | 1.38 (0.85–2.22) |
| Obesity (BMI >30 kg/m2) | 2.36 (1.95–2.86)‡ | 2.54 (2.09–3.09)‡ |
| Neck circumference (per 5-cm increase) | 1.96 (1.69–2.27)‡ | 2.19 (1.88–2.56)‡ |
| Waist circumference (per 5-cm increase) | 1.21 (1.16–1.25)‡ | 1.24 (1.19–1.29)‡ |
| Socioeconomic status (range 1–10, per 1-unit increase) | 0.95 (0.91–1.00) | 0.95 (0.90–1.00) |
| Current smoker | 0.60 (0.30–1.17) | 0.63 (0.31–1.29) |
| Alcohol use (per category increase) | 0.98 (0.93–1.03) | 1.01 (0.96–1.06) |
| Comorbid factors | ||
| Hypertension | 1.50 (1.27–1.77)‡ | 1.26 (1.06–1.50)‡ |
| Diabetes mellitus | 1.47 (1.17–1.86)‡ | 1.18 (0.93–1.51) |
| Cardiovascular disease | 1.28 (1.08–1.52)‡ | 1.24 (1.03–1.48)‡ |
| Heart failure | 2.02 (1.47–2.76)‡ | 1.81 (1.31–2.51)‡ |
| Stroke | 0.89 (0.57–1.39) | 0.86 (0.55–1.37) |
Unadjusted univariate models presented.
Models adjusted for age, race, and body mass index (BMI) except age, race, and obesity measures, which were adjusted for respective other subject characteristics.
Statistically significant.
Prevalence and Correlates of Obstructive Apnea and Central Apnea
OA (defined as an OA index (OAI) ≥5) was observed in 35.1% of the sample. In contrast, CA (defined as a CA index (CAI) ≥5) was observed in only 7.5% of participants. Although SDB includes apneas and hypopneas in its characterization; the definition of OA and CA include only the respective apneic events.
Analyses evaluating OA as the outcome revealed similar correlates as SDB. In unadjusted analyses, OA was associated with snoring (OR = 1.98, 95% CI = 1.61–2.44), age (per 5-year increase, OR = 1.22, 95% CI = 1.14–1.32), obesity (OR = 1.37, 95% CI = 1.12–1.67), neck circumference (OR = 1.40, 95% CI = 1.21–1.63), waist circumference (OR = 1.07, 95% CI = 1.03–1.11), diabetes mellitus (OR = 1.28, 95% CI = 1.01–1.62), and heart failure (OR = 1.60, 95% CI = 1.15–2.24). These associations persisted in models adjusted for age, race, and obesity except that the association with diabetes mellitus was no longer statistically significant (OR = 1.20, 95% CI = 0.94–1.53). Sensitivity analyses performed including only participants with an OAI of 5 or greater without a concomitant CAI of 5 or greater corresponded with a reduction in sample size from 2,911 to 2,624. These sensitivity analyses in which all individuals with a CAI of 5 or greater were excluded revealed generally similar associations, as do analyses including all individuals with a CAI of 5 or greater, although, a significant association with congestive heart failure was not noted with a reduction in OR point estimate (1.88 to 1.25) and less precision shown by an increase in 95% CI (1.17–3.04 to 0.49–3.17).
In contrast, in unadjusted and age-, race-, and obesity-adjusted models, CAwas associated only with age and heart failure (Table 3). Sensitivity analyses performed including only participants with a CAI of 5 or greater without a concomitant OAI of 5 or greater revealed a significant association with age, although a significant association with congestive heart failure was not noted, which is likely due to limited power to detect the association. (OR point estimate was reduced from 1.47 to 1.29, and confidence interval increased from 1.05–1.87 to 0.90–2.06.)
Table 3. Logistic Regression with Central Apnea (Central Apnea Index >5) as Outcome.
| Characteristic | Odds Ratio (95% Confidence Interval) | |
|---|---|---|
| Unadjusted* | Adjusted† | |
| Symptom | ||
| Snoring (>3–5 times/week) | 0.91 (0.63–1.32) | 1.03 (0.70–1.51) |
| Excessive daytime somnolence (Epworth Sleepiness Scale score >10) | 1.44 (0.99–2.10) | 1.46 (1.00–2.14) |
| Subject characteristic | ||
| Age (per 5-year increase) | 1.33 (1.17–1.50)‡ | 1.34 (1.18–1.51)‡ |
| Race (reference: Caucasian) | ||
| African American | 0.70 (0.28–1.75) | 0.81 (0.32–2.03) |
| Asian | 0.75 (0.30–1.88) | 0.82 (0.32–2.06) |
| Hispanic | 0.60 (0.22–1.64) | 0.65 (0.24–1.80) |
| Obesity (BMI >30 kg/m2) | 0.99 (0.70–1.49) | 1.10 (0.76–1.58) |
| Neck circumference (per 5-cm increase) | 1.12 (0.87–1.44) | 1.27 (0.98–1.65) |
| Waist circumference (per 5-cm increase) | 1.02 (0.96–1.09) | 1.04 (0.97–1.11) |
| Socioeconomic status (range 1–10, per 1-unit increase) | 1.00 (0.91–1.09) | 1.00 (0.92–1.09) |
| Current smoker | 0.23 (0.03–1.69) | 0.28 (0.04–2.02) |
| Alcohol use (per category increase) | 0.93 (0.86–1.26) | 0.94 (0.86–1.02) |
| Comorbid factors | ||
| Hypertension | 0.95 (0.72–1.69) | 0.93 (0.70–1.24) |
| Diabetes mellitus | 1.12 (0.75–0.68) | 1.14 (0.75–1.72) |
| Cardiovascular disease | 1.30 (0.97–1.74) | 1.17 (0.87–1.58) |
| Heart failure | 2.05 (1.28–3.30)‡ | 1.88 (1.17–3.04)‡ |
| Stroke | 1.16 (0.58–2.33) | 1.08 (0.54–2.19) |
Unadjusted univariate models presented.
Models adjusted for age, race, and body mass index (BMI) except age, race, and obesity measures, which were adjusted for respective other subject characteristics.
Statistically significant.
Prevalence of SDB Using the AASM Definition
The prevalence of SDB was 21.4% when SDB was defined using the AASM definition (i.e., a stricter amplitude threshold for scoring hypopneas) and a RDI cutoff of 15. Bland-Altman plots showed that a small but systematically larger RDI was found when using the SHHS- than when using the AASM-based hypopnea definitions (Figure 3), although models using the AASM criteria to define SDB showed almost identical patterns of associations as observed when using the SHHS criteria.
Figure 3.
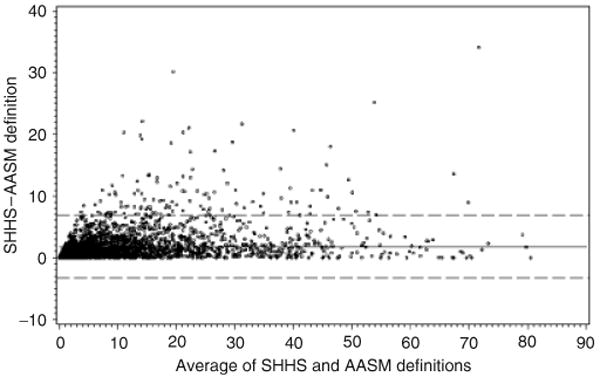
Bland-Altman Plot for the Sleep Heart Health Study (SHHS) versus American Academy of Sleep Medicine (AASM)-defined hypopneas to determine sleep apnea severity. The dashed lines represent the 95% confidence intervals of the mean difference; the solid line represents the mean difference.
Discussion
We quantified the prevalence of SDB in older men in a community-based sample and sought to determine whether SDB was associated with a similar distribution of symptoms and risk and comorbid factors as has been previously reported in other population groups. Although the prevalence of SDB varied somewhat according to definition, a high proportion (21–26%) of this older male population sampled from the community was classified with moderate or severe SDB (defined as RDI ≥15). The findings of a statistically significant P-value for trend observed with SDB defined as RDI of 15 or greater and increasing age quartile supports the justification and rationale for the use of the RDI cutoff of 15 to define SDB; a relationship that, conversely, was not observed with SDB defined as RDI of 5 or greater. Regardless of whether the SHHS or AASM definition was used, SDB prevalence estimates were almost three times as high as what has been reported in individuals aged 30 to 60 studied in the Wisconsin Cohort Study.1 Nevertheless, prevalence of SDB in the MrOS cohort is similar to the prevalence observed in male participants in the SHHS (mean age ∼ 65, i.e., 25% prevalence)11 and in a subgroup of men aged 65 and older participating in a Pennsylvania-based cohort study.28 In aggregate, the consistency of estimates across studies in older men, and differences in estimates made in younger populations, strongly supports the vulnerability of older men to this disorder and suggests that population differences across these large cohort studies1,11,28 mainly reflect differences in age and sex distributions rather than methodological approaches to defining SDB.
It was also demonstrated that SDB was associated with a qualitatively similar complex of associated symptoms and comorbid factors as previously reported for middle-aged adults, including snoring, excessive daytime sleepiness, greater body mass and central obesity, and hypertension. Furthermore, it was found that these associations were robust to the use of different hypopnea definitions (comparing SHHS and AASM criteria), providing further evidence that the observed associations were not dependent on hypopnea scoring approaches. However, the relative strength of associations between SDB and snoring and obesity were not as strong as that reported for younger populations.1 The noted reduction in the strength of these associations in this older cohort may be due to a variety of reasons, including survivorship effect, underreporting of symptoms such as snoring, competing risks, and differences in pathophysiology related to aging.
Snoring was associated with twice the odds of SDB in this sample, compared with a five times greater odds of SDB in the younger Wisconsin Cohort Study.1 The weaker association with snoring is consistent with data showing a negative correlation between snoring prevalence and age.11,29 It has been postulated that this negative relationship could be attributable to underreporting of snoring status due to the unavailability of a bed partner, hearing loss, dementia, and other age-related changes. Age-related upper airway muscle weakness also may reduce the ability of older individuals to generate sufficiently turbulent flow to generate the sounds characteristic of loud snoring.
There have been conflicting data regarding the role of obesity as a risk factor for SDB in older adults.5,9,11,30,31 It has been postulated that, in older adults, age-associated diseases and frailty causing unintentional weight loss may explain weaker associations between SDB and indices of obesity, altering the risk factor milieu of older adults.32,33 For instance, in the SHHS, men who lost large amounts of weight over a 5-year period had a high incidence of SDB,7 although the results of the current study suggest that obesity, including central obesity, is associated with greater odds of SDB. Nevertheless, the strength of these associations (OR for obesity ∼ 2.0) is approximately half that reported in younger cohorts.9,12
SDB was associated with 50% greater odds of sleepiness in the older male participants in the current study. The magnitude of this association is similar to that reported in the SHHS, demonstrating that 46% of participants with an RDI of 15 or greater had sleepiness symptoms using the same definition of sleepiness used in the current analysis (ESS score > 10).34 Although the specificity and sensitivity of daytime sleepiness may be less in older people with many comorbidities, the evidence for significantly greater odds of daytime sleepiness with SDB underscores the importance of assessing this symptom in relationship to SDB in older people.
By examining the strength of the associations between risk factors and comorbidities according to definitions that differentiated central from obstructive events, these analyses provided further evidence that CAs and OAs were associated with a distinct constellation of risk factors and outcomes. In particular, an elevated CAI was associated with advancing age and with heart failure but not with indices of obesity. This observation in a large cohort is consistent with the close association between central events and periodic breathing, which is more common in older individuals with underlying cerebrovascular and cardiac dysfunction and delayed circulatory times.35
Weaker associations between OA or CA and hypertension, CVD, and stroke were observed than with SDB defined according to the RDI. This may be due to the lower frequency of exclusively apneic events and use of different threshold in each series of models (≥5 for apneic indices and ≥15 for the RDI).
Novel findings of associations between race and SDB were noted, with Asians demonstrating a greater risk for SDB. The high prevalence of SDB in older Asian men is consistent with growing data from Asia indicating rates of SDB that are equivalent to or higher than that observed in Western cultures, even though obesity rates are lower.36–38 It has been postulated that, in Asians, craniofacial risk factors contributing to a brachycephalic head form and reduced airway dimensions may contribute to SDB in the absence of obesity.
Although some research suggests that cardiovascular consequences of SDB may be less severe in older than in younger adults,13,14 the overall results of the current study suggest that, despite the greater background cardiovascular risk in older men, there remains a significant association between SDB and CVD.
The strengths of the present study include the large community-based sample (enhancing generalizability to other samples of older men); the careful consideration and collection of detailed covariate data, which was collected at the time of the sleep study visit; and standardized data collection with prospectively designed scoring approaches that allowed alternative definitions to be evaluated. A limitation of this study is the availability of only a single night of polysomnography, although night-to-night reliability for the RDI determined using home polysomnography is high.23 In addition, the cross-sectional design of this study precludes inferring causality. Future analyses will address the association between SDB and incident comorbidities and will permit a more-specific evaluation of potential causal pathways. Lack of Hispanic sample representation and therefore lack of generalizability to the Hispanic population should be noted. Another limitation is the lack of sample size to detect associations with isolated central apnea without obstructive apnea contribution and the correlates of interest.
In summary, this study of a large community-based sample showed that older men have a high prevalence of sleep disorders. Furthermore, in this sample, various indices of sleep-related breathing disturbances were associated with hypertension, heart failure, CVD, and stroke. If the latter associations are causal, then the population attributable risk for the latter conditions would be high, underscoring the need to develop better screening, diagnostic, and treatment approaches appropriate for older populations. Neither the prevalence estimates nor the associations with a broad group of disease correlates varied substantively according to hypopnea definition. These data emphasize the relevance of using standard SDB risk factors in screening older male populations and highlight the importance of sleepiness as a corollary factor that is useful in identifying subgroups that may be at highest risk for associated comorbidities. Having established strong cross-sectional associations, future work now is needed to address the effect of treating sleep disorders on health outcomes in this high-risk population.
Acknowledgments
The authors would like to gratefully acknowledge the administrative assistance of Ms. Liezl Concepcion and the Case Western Reserve University Reading Center staff: Susan Surovec, Nancy Scott, Jean Arnold, Rawan Salem, Joanna Romaniuk, and Sinziana Seacian.
The authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This work was performed at the San Francisco Coordinating Center, University of California, San Francisco, and Case Western Reserve University.
An abstract of this manuscript was presented at the American College of Chest Physicians meeting, Salt Lake City, Utah, October 2006.
Author Contributions: Reena Mehra: lead analysis concept and design, lead interpretation of data, and preparation of manuscript. Katie L. Stone: lead study concept and design for cohort, assisted with interpretation of data and preparation of manuscript. Terri Blackwell: analysis and interpretation of data and assistance with manuscript preparation. Sonia Ancoli-Israel: assistance with study concept and design for cohort, interpretation of data, and preparation of manuscript. Thuy-Tien L. Dam and Marcia L. Stefanick: assistance with interpretation of data and preparation of manuscript. Susan Redline: assistance with study concept and design for cohort and this particular analysis, assistance with interpretation of data and preparation of manuscript.
Financial Disclosure: The MrOS Study and the ancillary MrOS Sleep Study are supported by the National Institutes of Health. The following institutes provide support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the National Institute on Aging, and the National Cancer Institute, under the following grant numbers: U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, and M01 RR000334. The National Heart, Lung, and Blood Institute (NHLBI) provides funding for the MrOS Sleep Study “Outcomes of Sleep Disorders in Older Men” under the following grant numbers: R01 HL071194, R01 HL070848, R01 HL070847, R01 HL070842, R01 HL070841, R01 HL070837, R01 HL070838, and R01 HL070839. Other funding sources include the American Heart Association National Scientist Development Award (0530188N), Association of Subspecialty Professors, CHEST Foundation of the American College of Chest Physicians T. Franklin Williams Geriatric Development Research Award, and K23 HL079114-01A2 from the NHLBI.
Sponsor's Role: The funding institutes had no role in the collection, analysis, or interpretation of the data or in the decision to submit the manuscript for publication.
References
- 1.Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Ancoli-Israel S. Epidemiology of Sleep Disorder: Clinics in Geriatric Medicine. Philadelphia: W.B. Saunders; 1989. [PubMed] [Google Scholar]
- 3.Ancoli-Israel S, Klauber MR, Stepnowsky C, et al. Sleep-disordered breathing in African-American elderly. Am J Respir Crit Care Med. 1995;152(6 Part 1):1946–1949. doi: 10.1164/ajrccm.152.6.8520760. [DOI] [PubMed] [Google Scholar]
- 4.Ancoli-Israel S, Kripke DF, Klauber MR, et al. Morbidity, mortality and sleep-disordered breathing in community dwelling elderly. Sleep. 1996;19:277–282. doi: 10.1093/sleep/19.4.277. [DOI] [PubMed] [Google Scholar]
- 5.Ancoli-Israel S, Kripke DF, Klauber MR, et al. Sleep-disordered breathing in community-dwelling elderly. Sleep. 1991;14:486–495. doi: 10.1093/sleep/14.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ancoli-Israel S, Kripke DF, Klauber MR, et al. Natural history of sleep disordered breathing in community dwelling elderly. Sleep. 1993;16(Suppl 8):S25–S29. doi: 10.1093/sleep/16.suppl_8.s25. [DOI] [PubMed] [Google Scholar]
- 7.Newman AB, Foster G, Givelber R, et al. Progression and regression of sleep-disordered breathing with changes in weight: The sleep heart health study. Arch Intern Med. 2005;165:2408–2413. doi: 10.1001/archinte.165.20.2408. [DOI] [PubMed] [Google Scholar]
- 8.Redline S, Schluchter MD, Larkin EK, et al. Predictors of longitudinal change in sleep-disordered breathing in a nonclinic population. Sleep. 2003;26:703–709. doi: 10.1093/sleep/26.6.703. [DOI] [PubMed] [Google Scholar]
- 9.Ancoli-Israel S, Gehrman P, Kripke DF, et al. Long-term follow-up of sleep disordered breathing in older adults. Sleep Med. 2001;2:511–516. doi: 10.1016/s1389-9457(00)00096-4. [DOI] [PubMed] [Google Scholar]
- 10.Ancoli-Israel S, Coy T. Are breathing disturbances in elderly equivalent to sleep apnea syndrome? Sleep. 1994;17:77–83. doi: 10.1093/sleep/17.1.77. [DOI] [PubMed] [Google Scholar]
- 11.Young T, Shahar E, Nieto FJ, et al. Predictors of sleep-disordered breathing in community-dwelling adults: The sleep heart health study. Arch Intern Med. 2002;162:893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 12.Young T. Sleep-disordered breathing in older adults: Is it a condition distinct from that in middle-aged adults? Sleep. 1996;19:529–530. doi: 10.1093/sleep/19.7.529. [DOI] [PubMed] [Google Scholar]
- 13.Haas DC, Foster GL, Nieto FJ, et al. Age-dependent associations between sleep-disordered breathing and hypertension: Importance of discriminating between systolic/diastolic hypertension and isolated systolic hypertension in the sleep heart health study. Circulation. 2005;111:614–621. doi: 10.1161/01.CIR.0000154540.62381.CF. [DOI] [PubMed] [Google Scholar]
- 14.Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: The sleep heart health study. Am J Respir Crit Care Med. 2006;173:910–916. doi: 10.1164/rccm.200509-1442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: Cross-sectional results of the sleep heart health study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 16.Mehra R, Blackwell T, Ancoli Israel S, et al. Prevalence and Correlates of Sleep-Disordered Breathing in Older Men: The MrOS Sleep Study. American College of Chest Physicians; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study — a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–585. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the Osteoporotic Fractures in Men Study (MrOS) Contemp Clin Trials. 2005;26:557–568. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Redline S, Sanders MH, Lind BK, et al. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep heart health research group. Sleep. 1998;21:759–767. [PubMed] [Google Scholar]
- 20.Johns MW. A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 21.Johns MW. Sensitivity and specificity of the multiple sleep latency test (MSLT), the maintenance of wakefulness test and the Epworth Sleepiness Scale: Failure of the MSLT as a gold standard. J Sleep Res. 2000;9:5–11. doi: 10.1046/j.1365-2869.2000.00177.x. [DOI] [PubMed] [Google Scholar]
- 22.The Report of an American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 23.Whitney CW, Gottlieb DJ, Redline S, et al. Reliability of scoring respiratory disturbance indices and sleep staging. Sleep. 1998;21:749–757. doi: 10.1093/sleep/21.7.749. [DOI] [PubMed] [Google Scholar]
- 24.Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults — the evidence report. National Institutes of Health. Obes Res. 1998;2(6 Suppl):51S–209S. [PubMed] [Google Scholar]
- 25.Report of a WHO Consultation on Obesity. Geneva: World Health Organization; Obesity: Preventing and Managing the Global Epidemic. [PubMed] [Google Scholar]
- 26.Callaway CW, Buchard C. Circumferences. In: Lohman TG, Roche AF, Martorell R, editors. Anthropometric Standardization Reference Manual. Champaign, IL: Human Kinetic Books; 1988. pp. 41–45. [Google Scholar]
- 27.Adler NE, Epel ES, Castellazzo G, et al. Relationship of subjective and objective social status with psychological and physiological functioning: Preliminary data in healthy white women. Health Psychol. 2000;19:586–592. doi: 10.1037//0278-6133.19.6.586. [DOI] [PubMed] [Google Scholar]
- 28.Bixler EO, Vgontzas AN, Ten Have T, et al. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157:144–148. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- 29.Enright PL, Newman AB, Wahl PW, et al. Prevalence and correlates of snoring and observed apneas in 5,201 older adults. Sleep. 1996;19:531–538. doi: 10.1093/sleep/19.7.531. [DOI] [PubMed] [Google Scholar]
- 30.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56A:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 31.Vanitallie TB. Frailty in the elderly: Contributions of sarcopenia and visceral protein depletion. Metabolism. 2003;52(10 Suppl 2):22–26. doi: 10.1016/s0026-0495(03)00297-x. [DOI] [PubMed] [Google Scholar]
- 32.Kapur VK, Baldwin CM, Resnick HE, et al. Sleepiness in patients with moderate to severe sleep-disordered breathing. Sleep. 2005;28:472–477. doi: 10.1093/sleep/28.4.472. [DOI] [PubMed] [Google Scholar]
- 33.Arzt M, Bradley TD. Treatment of sleep apnea in heart failure. Am J Respir Crit Care Med. 2006;173:1300–1308. doi: 10.1164/rccm.200511-1745PP. [DOI] [PubMed] [Google Scholar]
- 34.Hida W, Shindoh C, Miki H, et al. Prevalence of sleep apnea among Japanese industrial workers determined by a portable sleep monitoring system. Respiration. 1993;60:332–337. doi: 10.1159/000196231. [DOI] [PubMed] [Google Scholar]
- 35.Ip MS, Lam B, Lauder IJ, et al. A community study of sleep-disordered breathing in middle-aged Chinese men in Hong Kong. Chest. 2001;119:62–69. doi: 10.1378/chest.119.1.62. [DOI] [PubMed] [Google Scholar]
- 36.Kim J, In K, Kim J, et al. Prevalence of sleep-disordered breathing in middle-aged Korean men and women. Am J Respir Crit Care Med. 2004;170:1108–1113. doi: 10.1164/rccm.200404-519OC. [DOI] [PubMed] [Google Scholar]
- 37.Ohta Y, Kawakami Y, Takishima T, et al. Sleep-disordered breathing in Japan: An overview. Nihon Kyobu Shikkan Gakkai Zasshi. 1993;31(Suppl):12–18. [PubMed] [Google Scholar]
- 38.Udwadia ZF, Doshi AV, Lonkar SG, et al. Prevalence of sleep-disordered breathing and sleep apnea in middle-aged urban Indian men. Am J Respir Crit Care Med. 2004;169:168–173. doi: 10.1164/rccm.200302-265OC. [DOI] [PubMed] [Google Scholar]