Abstract
Background
Mixed chimerism induces donor-specific tolerance to composite tissue allotransplants (CTA). In the present studies, we used a nonmyeloablative conditioning approach to establish chimerism and promote CTA acceptance.
Methods
WF (RT1Au) rats were conditioned with 600-300 cGy total body irradiation (TBI, day-1), 100 × 106 T cell-depleted ACI (RT1Aabl) bone marrow cells were transplanted day 0, followed by a 11-day course of tacrolimus and one dose of anti-lymphocyte serum (day 10). Heterotopic osteomyocutaneous flap transplantation was performed 4-6 weeks after bone marrow transplantation.
Results
Mixed chimerism was initially achieved in almost all recipients, but long-term acceptance of CTA was only achieved in rats treated with 600 cGy TBI. When anti-αβ-TCR mAb (day-3) was added into the regimens, donor chimerism was similar to recipients preconditioned without anti-αβ-TCR mAb. However, the long-term CTA survival was significantly improved in chimeras receiving ≥ 300 cGy TBI plus anti-αβ-TCR mAb. Higher levels of donor chimerism were associated with CTA acceptance. The majority of flap-acceptors lost peripheral blood (PB) chimerism within 6 months. However, donor chimerism persisted in transplanted bone at significantly higher levels compared to other hematopoietic compartments. The compartment donor chimerism may be responsible for the maintenance of tolerance to CTA. Long-term acceptors were tolerant to a donor skin graft challenge even in the absence of PB chimerism.
Conclusions
Mixed chimerism established by nonmyeloablative conditioning induces long-term acceptance of CTA which is associated with persistent chimerism preferentially in transplanted donor bone.
Keywords: tolerance, composite tissue allotransplantation, mixed chimerism, nonmyeloablative conditioning, compartment chimerism
Introduction
The field of reconstructive transplantation is expanding rapidly (1, 2). Composite tissue allotransplants (CTA) of hand, arm, face, larynx, esophagus, vascularized knee, and abdominal wall have been successfully performed and are now accepted as a viable therapeutic option (1, 2). These procedures have been shown to improve the quality of life of large numbers of individuals suffering from tissue defects (3-5). However, the toxicity of chronic immunosuppression, most notably opportunistic infection, diabetes, nephrotoxicity, malignancy, osteoporosis, and concerns regarding chronic rejection have limited the widespread application of CTA (6). The induction of tolerance would significantly reduce the toxicity and expand the clinical feasibility of CTA.
In 1985, Ildstad et al. reported that mixed allogeneic chimerism induces tolerance to donor-specific skin grafts (7). Mixed chimeras exhibit superior immunocompetence due to the presence of recipient antigen presenting cells to which lymphocytes of both recipient and donor origin are restricted (8). Mixed chimerism was originally achieved through myeloablative conditioning followed by transplantation of a mixture of T cell depleted (TCD) syngeneic and allogeneic bone marrow cells (9). More recently, nonmyeloablative conditioning has been used to establish mixed chimerism, substantially reducing the toxicity of the conditioning approach (10-15). Currently, patients with hematologic malignancy and co-morbidities are transplanted using nonmyeloablative conditioning routinely on an outpatient basis (16). In the present studies, we explored whether nonmyeloablative conditioning can promote long-term acceptance of highly antigenic CTA using a strong MHC-disparate donor/recipient rat strain combination. We report here that chimerism established by nonmyeloablative conditioning is sufficient to promote long-term acceptance of hind limb CTA. Moreover, although chimerism in the peripheral blood (PB) was only transient in the majority of chimeras, donor osteomyocutaneous flaps in some chimeras were accepted for up to 6 months of follow-up and tolerance was maintained even after a second donor skin graft challenge. To study the dissociation between PB chimerism and tolerance, donor chimerism in hematopoietic compartments was investigated. A predominance of donor chimerism was present in the donor bone marrow compartment and, to a lesser extent, recipient bone and spleen of long-term acceptors. These findings suggest that donor bone marrow preferentially homes to the donor hematopoietic microenvironment, and that chimerism in the donor bone, although absent in PB, is sufficient to induce durable tolerance in CTA. These findings may unify the perceived disparity between the significance of microchimerism and macrochimerism in immunomodulating the recipient immune response to allografts and suggest that various hematopoietic compartments should be evaluated in testing for chimerism (17).
Materials and Methods
Animals
6- to 10-week-old Wistar Furth (WF; RT1Au) and ACI (RT1Aabl) male rats were purchased from Harlan Sprague Dawley (Indianapolis, IN). Animals were housed in a barrier facility at the Institute for Cellular Therapeutics.
Preparation of mixed allogeneic chimeras
WF recipients were conditioned with varying doses of total body irradiation (TBI) on day -1. On day 0 donor ACI bone marrow was prepared as previously described (18). Briefly, the long bones of donor rats were flushed with Medium 199 (Gibco, Grand Island, NY) containing 2 μg/ml of gentamicin (chimera medium), and the bone marrow cells filtered through sterile nylon mesh (100 μm pores), washed, and incubated with mouse anti-rat αβ-TCR (prepared in our laboratory) and γδ-TCR mAb (Mouse IgG1, 1.0 mg/ml, R73; Serotec, Washington, D.C.) at 4°C for 30 min. The bone marrow was washed twice in chimera medium and finally combined with sheep anti-mouse IgG-coated magnetic beads (Dynabeads M-450; Dynal, Lake Success, NY) at a bead:cell ratio of 4:1 and gently agitated at 4°C for 1 h. The flask containing the sensitized marrow was then exposed to a hand-held magnet (DYNAL MPC-1; Dynal A.S., N-0212 Oslo, Norway) for 3 min to negatively select the TCR+ cells. The supernatant was eluted, the cells resuspended in chimera medium at a concentration of 100 × 106 cells/ml, and the adequacy of T cell-depletion confirmed using flow cytometry (FACSAria or LSR; Becton Dickinson, San Jose, CA), staining marrow cells with anti-αβ-TCR and anti-γδ-TCR followed by a secondary goat anti-mouse IgG1-FITC (Southern Biotechnology Associates, Birmingham, AL) before and after depletion. Recipient WF rats were injected with 100 × 106 T TCD bone marrow cells in 1 ml chimera medium via penile vein. All groups received tacrolimus (Fujisawa USA, Deerfield, IL) at a dose of 1 mg/kg/day intraperitoneally (IP) on days 0 through 10 and a single dose of 0.5 ml (5 mg) rabbit anti-rat lymphocyte serum (ALS) IP (Research Diagnostics Inc., Flanders, NJ) on day 10. Some WF recipients were also treated with anti-αβ-TCR mAb (made in our laboratory) IP on day -3. Anti-αβ-TCR mAb was unpurified and concentrated by ammonia sulfite precipitation. Therefore, the dosage of this mAb was determined by the volume. The in vivo dose titration was performed and found that 1.5 ml per rat was the optimal dose required to deplete recipient αβ-TCR+ T cells.
Composite tissue allotransplantation
A heterotopic osteomyocutaneous flap which preserves function of the recipient native hindlimb yet contains all components of the hind limb allograft was used to assess tolerance (19). This approach avoids the relative long operative time, high rate of technical graft failure, and high morbidity and mortality experienced in functional orthotopic hind limb CTA (20-23). Circumferential incisions in the naïve donor ACI lower limb were made at the ankle joint and at the level of the inguinal ligament, and connected by a longitudinal incision along the medial leg and thigh. The femoral vessels were dissected from the inguinal ligament to their bifurcation into superficial and deep artery and vein, and divided proximally at the level of the inguinal ligament along with the femoral nerve. The femur and surrounding musculature were divided just proximal to the knee joint, and the tibia, fibula and surrounding muscles were divided proximal to the ankle joint. The resulting flap was flushed with heparinized saline (10 IU/ml) through the femoral artery to prevent clotting and through the femur and tibia to flush out donor bone marrow. The flap was wrapped in moist gauze and kept on ice during preparation of the recipient.
An oblique incision overlying the inguinal ligament was made in recipient WF rats and the femoral vessels were dissected from the inguinal ligament to their bifurcation and divided distally, preserving the proximal deep branches to the native hind limb. A second incision was made dorsally proximal to the hip joint for seating of the flap, which was anchored to the recipient skin. The donor femoral vessels were tunneled anteriorly and anastomosed to the recipient vessels under an operating microscope with 10-0 suture using standard end-to-end microsurgical technique. Nerve repair was not performed. Animals were monitored daily for the first two weeks then weekly for signs of rejection or graft-versus-host-disease (GVHD). CTA acceptance was defined as integration of the visible skin paddle into surrounding skin, with growth of black hair. Rejection was defined as loss of the skin paddle with an evolution from petechiae to eschar. GVHD was defined clinically by erythema of the paws, abdominal skin, and ears with weight loss. The diagnosis of GVHD by these clinical signs had been confirmed by histology in a previous study (24).
Chimerism and multilineage typing
Peripheral blood was collected into heparinized eppendorf tubes from the tail vein of experimental animals monthly after bone marrow transplantation (BMT) for 6 months (25). 40-50 μl of PB was incubated with the following multi-lineage panel of antibodies for donor chimerism and multilineage analysis: tube 1: anti-RT1Aabl-FITC, anti-CD45RA-PE, anti-CD8a-PerCP (Pharmingen, San Diego, CA), anti-NK161-APC (Caltag, Burlingame, CA); tube 2: anti-RT1Aabl-FITC, anti-αβ-TCR-PerCP, anti-CD4-APC (Pharmingen); and tube 3: anti-RT1Aabl-FITC, anti-OX62-PE (Serotec, Raleigh, NC), anti-CD11b/c-APC (Caltag), at 4°C for 30 min. Samples were washed twice, resuspended in FACS medium and analyzed on a FACSCalibur or LSR flow cytometer (Becton Dickinson, Mountainview, CA), for percentage donor B cells, T cells, dendritic cells, and monocytes. For compartment donor chimerism study PB, spleen, thymus, bone marrow from transplanted bone, and bone marrow from native bone (contralateral femur) were collected at the time of euthanasia and brought to single cell suspension for donor chimerism analysis.
Skin graft placement
Full thickness skin grafts were harvested from tail skin of donor ACI rats and cut into 2 × 1 cm segments (18). The recipient bed was prepared in the mid-scapular region, taking care to preserve the panniculus carnosus, and grafts were sutured into place using a continuous 2-0 vicryl suture, then dressed with petroleum jelly, gauze, dry gauze, and a circumferential layer of coban tape. Dressings were removed after five days and the skin grafts monitored daily thereafter. Rejection was defined as complete when no residual viable graft could be detected.
Hematoxylin-Eosin (H & E) staining
Skin and muscle tissues were freshly embedded in OCT embedding medium (Tissue-Tek® OCT compound 4853, Electron Microscopy Science, Hatfield, PA) by using dry ice-cooled isopentane bath. Serial sections (50 μm interval, 6 μm thickness/each section) were obtained and mounted on superfrost plus slides (12-550-15, Fisher Scientific, Pittsburgh, PA). Finally, sections were stained with H & E using standard procedures to evaluate morphology.
Statistics
Unpaired students t-test was used for the statistical analysis. Statistical significance was given for P values <0.05. Data were shown as mean ± standard error.
Results
Dissociation between peripheral blood donor chimerism and tolerance to hindlimb composite tissue transplants
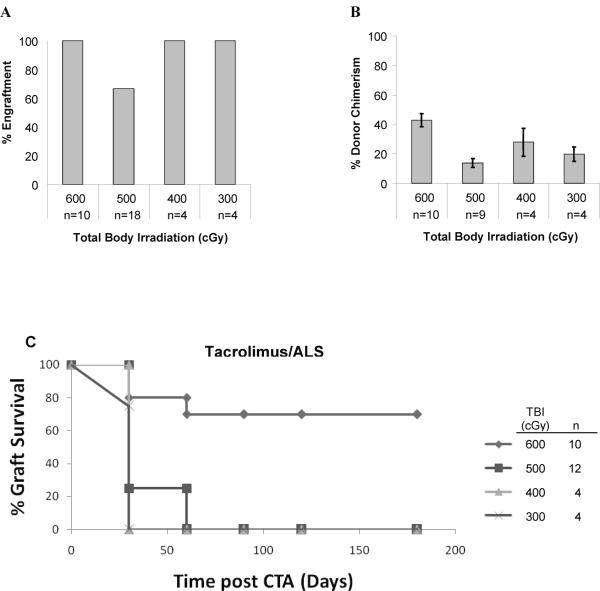
WF recipients were conditioned with from 600 cGy to 300 cGy TBI on day -1, transplanted with 100 × 106 TCD ACI donor bone marrow cells on day 0, then treated with tacrolimus (1 mg/kg/d) on days 0-10 and a single dose of ALS on day 10 (Fig. 1). Engraftment after BMT was defined as donor chimerism >1% in the PB lymphoid gate and was first assessed one month after BMT. In rats treated with tacrolimus/ALS, engraftment occurred in 10 of 10 (100%) of animals conditioned with 600 cGy, 12 of 18 (66.7%) animals conditioned with 500 cGy, 4 of 4 (100%) animals with 400 cGy, and 4 of 4 (100%) animals with 300 cGy, respectively (Fig. 2A). The mean percentage donor chimerism in PB correlated with the intensity of conditioning (Fig. 2B). Animals treated with 600 cGy TBI had significantly higher (P < 0.0001) mean donor chimerism at one month (42.7% ± 4.4%) compared to those treated with 500 cGy (15.9% ± 2.7%), 400 cGy (27.9% ± 9.5%), or 300 cGy (19.8% ± 4.9%), respectively. Multilineage engraftment was present in all chimeras tested between 1 to 2 months after BMT (Table 1, Group A), including T cells (CD4+ and CD8+), B cells (CD45RA+), NK cells (NK161+), dendritic cells (OX62+) and macrophages (CD11b/c+).
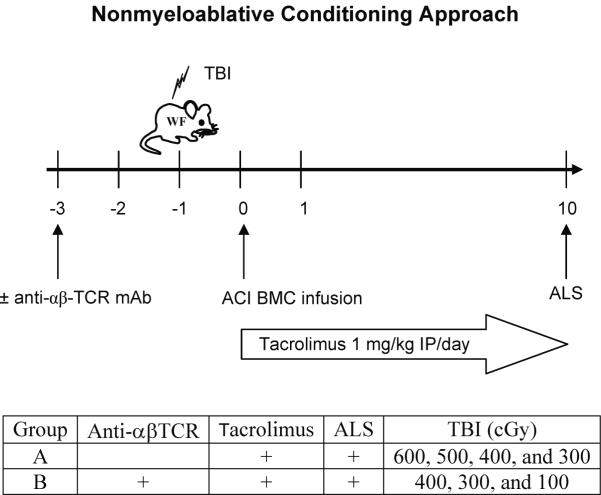
Figure 1. Nonmyeloablative conditioning approach.
WF rats were conditioned with varying doses of TBI (600 to 100 cGy) on day -1, transplanted with 100 × 106 TCD ACI donor BMC on day 0, administered tacrolimus at 1 mg/kg/day (days 0-10), and a single dose of ALS on day 10. In a second experimental group, anti-αβ-TCR mAb preconditioning (on day -3) was added to the tacrolimus/ALS-based conditioning strategy.
Figure 2. Dissociation between peripheral blood donor chimerism and CTA tolerance.
WF recipient rats were treated with 600 (n = 10), 500 (n = 18), 400 (n = 4), or 300 (n = 4) cGy of TBI on day -1, received 100 × 10 6 TCD bone marrow cells from ACI donors on day 0, tacrolimus IP on days 0-10 and a single dose of ALS on day 10. Percentage engraftment was assessed by flow cytometric staining for the donor MHC class I marker RT1Aabl. Engraftment was defined as >1% donor cells in the lymphoid gate. (A) The percentage of animals with PB donor chimerism for a given TBI dose at one month. (B) The mean level of donor chimerism in animals that engrafted. Only animals with engraftment are included. All chimeric animals underwent heterotopic osteomyocutaneous flap allotransplantation 4-6 weeks after BMT. Survival of the CTA was defined as integration of the skin flap into surrounding skin with growth of black donor hair. Rejection was defined as loss of the skin paddle with an evolution from petechiae to eschar. The CTA status was assessed daily for the first two weeks and then weekly thereafter up to 6 months (C).
Multilineage Analysisa
| Group | n | % donor | % in donor (RT1 Aab1+) lymphoid gate b | |||||
|---|---|---|---|---|---|---|---|---|
| T cells | B cells | NK cells | DC | Macrophage | ||||
| CD4 | CD8 | CD45RA | NK161 | OX62 | CD11b/c | |||
| A | 18 | 34.3±14.3 | 36.9±15.7 | 26.3±12.9 | 25.7±15.1 | 17.8±8.1 | 6.5±5.7 | 24.3±11.8 |
| B | 12 | 30.7±6.6 | 29.9±16.9 | 24.8±15.1 | 43.4±13.8 | 16.1±8.3 | 11.6±14.0 | 20.6±11.5 |
A: Conditioned with tacrolimus/ALS and TBI from 600 to 300 cGy.
B: Conditioned with anti-αβ-TCR/tacrolimus/ALS and TBI from 400 to 300 cGy.
Multilineage typing was performed in mice between 2 and 3 mo after 2nd BMT.
Mean ± SD percentage of donor cells in donor lymphoid gate.
Animals that were chimeric at 1 month underwent heterotopic osteomyocutaneous flap transplantation 4-6 weeks after BMT (Fig. 2C). 70% of the 10 animals conditioned with 600 cGy accepted their CTA long-term (> 6 months). Although the grafts were prolonged in acceptance, none of chimeras preconditioned with 500, 400 and 300 cGy TBI accepted their grafts long-term. All grafts were rejected within 60 days in animals with 500 cGy and within 30 days in rats with 400 or 300 cGy TBI, although there were significant levels of donor chimerism detectable in PB at the time of CTA performance. All chimeras lost their donor chimerism within 5 months.
Preconditioning with anti-αβ-TCR mAb promotes acceptance of CTA
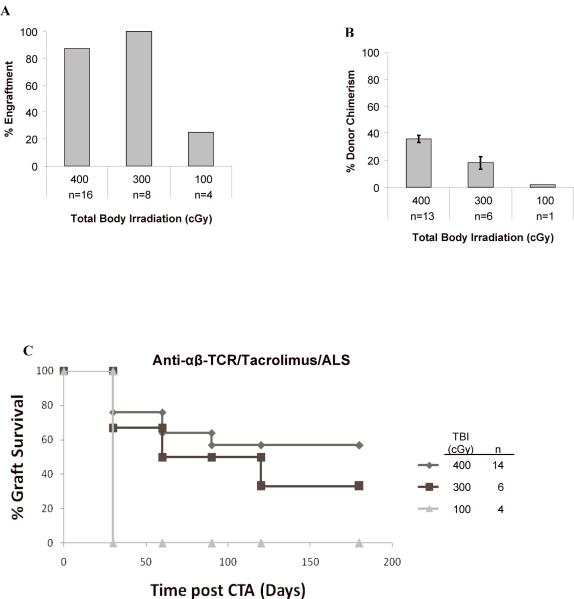
Anti-αβ-TCR was added to tacrolimus/ALS-based conditioning to promote graft acceptance, and animals were irradiated with 400, 300, and 100 cGy TBI. 14 of 16 (87.5%) rats treated with 400 cGy TBI, 6 of 6 (100%) rats treated with 300 cGy TBI, and 1 of 4 (25%) rats treated with 100 cGy TBI engrafted in this cohort (Fig. 3A). The level of PB chimerism at one month was significantly higher for those animals treated with 400 cGy TBI compared to 300 cGy TBI (35.0% ± 2.6% vs 18.0% ± 4.5%; P = 0.0026) (Fig. 3B). Low donor chimerism (1.8%) was achieved in the single animal treated with 100 cGy that engrafted. When comparing the two groups without or with anti-αβ-TCR mAb for a given TBI dose, there was a significantly greater (P < 0.0001) level of donor chimerism in rats conditioned with anti-αβ-TCR mAb and 400 cGy TBI, but this was not seen at 300 cGy TBI. The engraftment was multilineage in all chimeras tested between 1 to 2 months after BMT (Table 1, Group B).
Figure 3. Anti-αβ-TCR preconditioning promotes long-term acceptance of heterotopic osteomyocutaneous flaps.
Anti-αβ-TCR mAb preconditioning (day -3) was added to the tacrolimus/ALS-based conditioning and animals received 400 (n = 14), 300 (n = 6), or 100 (n = 4) cGy of TBI. Engraftment was assessed by flow cytometric staining for the donor MHC class I marker RT1Aabl. Percentage of engrafted animals at 1 month (A) and level of donor chimerism in animals that engrafted at 1 month (B) are shown. Chimeric animals underwent heterotopic osteomyocutaneous flap allotransplantation 4-6 weeks after BMT. The flap graft status was assessed daily for the first two weeks and then weekly thereafter up to 6 months (C).
The addition of anti-αβ-TCR mAb to the tacrolimus/ALS-based preconditioning enhanced CTA acceptance when compared with the recipients conditioned without anti-αβ-TCR mAb but with same TBI doses: 300 and 400 cGy. Eight of 14 animals (57.1%) that received anti-αβ-TCR and 400 cGy TBI and 2 of 6 animals (33.3%) that received anti-αβ-TCR and 300 cGy TBI accepted their CTA long term (Fig. 3C). Therefore, immune-based conditioning with anti-αβ-TCR mAb to control host-versus-graft alloreactivity significantly enhances CTA graft acceptance, as no long-term CTA acceptance was achieved in animals 400 or 300 cGy TBI without anti-αβ-TCR mAb preconditioning.
Higher levels of donor chimerism were associated with CTA acceptance
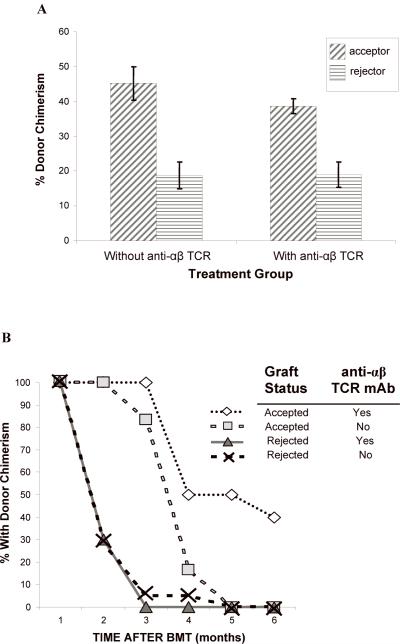
There was a correlation between higher levels of donor chimerism at one month after BMT and graft acceptance in animals from all groups that accepted flap allografts compared to animals that rejected their flaps (P < 0.005) (Fig. 4A). In the group conditioned with TBI plus tacrolimus/ALS, the 7 long-term flap acceptors had 45.2 ± 4.7% donor PB chimerism at the time of CTA. These were significantly higher levels compared to rats that rejected their allografts (18.8 ± 3.5%; P = 0.0012). In the group preconditioned with anti-αβ-TCR/TBI/tacrolimus/ALS, the 10 long-term flap acceptors also had significantly higher donor PB chimerism at the time of CTA (38.6 ± 2.1%) compared to rejecters (18.9 ± 3.6%; P = 0.0002). In following the kinetics of donor chimerism after CTA, we found that none of the 6 long-term acceptors conditioned with 600 cGy/tacrolimus/ALS retained PB chimerism for greater than five months after BMT. Four of 10 acceptors conditioned with anti-αβ-TCR/tacrolimus/ALS plus 400 cGy TBI retained PB chimerism (Fig. 4B). The two long-term acceptors conditioned with anti-αβ-TCR/tacrolimus/ALS plus 300 cGy TBI lost their donor chimerism within 2 months. No clinical signs of GVHD were observed after BMT and CTA in long-term follow-up.
Figure 4. (A, B) The level of donor chimerism is predictive of flap acceptance.
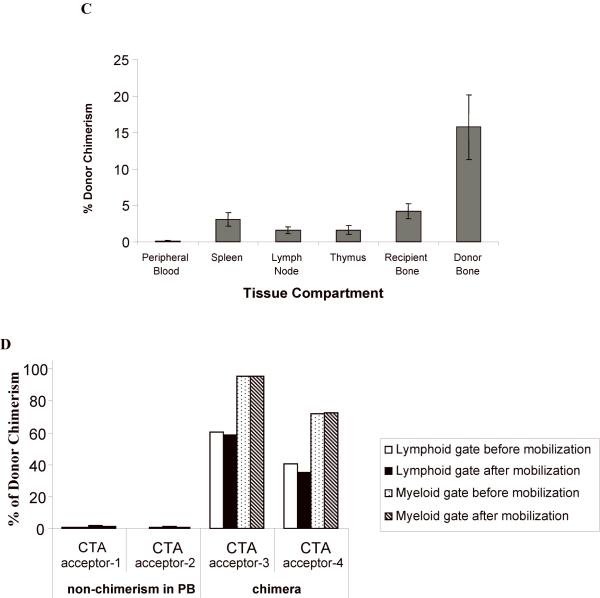
Animals were followed for at least five months after CTA graft placement to monitor chimerism status and graft acceptance. There was a higher percentage donor chimerism at 1 month in CTA acceptors vs. rejectors in both treatment groups (A). PB donor chimerism was monitored monthly up to six months (B). The percent of animals that retained engraftment was defined as those animals that exhibited donor lymphoid chimerism above 1% at each time point. (C) Compartment chimerism in flap acceptors. Five rats that had accepted their heterotopic osteomyocutaneous flap for a minimum of six months underwent hematolymphopoietic compartment chimerism testing and multilineage analysis. The PB, spleen, thymus, mesenteric lymph nodes, donor bone, and recipient bone (contralateral femur) were harvested (> 6 months after CTA). One million cells were stained with the donor MHC class I marker: anti-RT1Aabl-FITC. The percent donor chimerism is shown as the percentage of donor cells in the lymphoid gate in the different tissue compartments. (D) Mobilization does not change chimerism in PB in long-term acceptors. Two long-term acceptors with PB donor chimerism and two long-term acceptors without PB donor chimerism received FL treatment (200 μg/kg) for 10 days and G-CSF (150 μg/kg) for 7 days (57). The donor cells were enumerated by staining of anti-donor ACI MHC class-I (anti-RT1Aabl FITC) 24 hours after completing the FL and G-CSF doses. The percentage of donor cells was analyzed in lymphoid or myeloid gates.
Donor chimerism persists preferentially in transplanted bone after it is lost from PB
In order to study the dissociation between PB chimerism and tolerance, donor chimerism in selected hematopoietic compartments was investigated. Five rats that were CTA acceptors for >6 months were euthanized and evaluated for chimerism in various hematolymphopoietic tissue compartments (Fig. 4C). None of these 5 had PB chimerism ≥ 1%. Strikingly, all animals exhibited significantly higher levels of chimerism in non-PB hematolymphopoietic compartments compared to PB. The mean donor chimerism in donor bone was 15.7 ± 4.5%, which was significantly higher than the donor chimerism in PB (0.09 ± 0.06%; P = 0.0079). Donor chimerism in recipient bone was also significantly higher (4.2 ± 1.0%; P = 0.004) compared with PB but lower than donor bone (P = 0.0358). In spleen, mesenteric lymph nodes, and thymus, statistically significantly higher levels of donor chimerism were present compared to PB (3.1 ± 0.91%, 1.6 ± 0.47%, and 1.6 ± 0.60%, respectively) (P = 0.011, P = 0.014, and P = 0.036, respectively).
The effect of mobilization on donor cells in peripheral blood
Because the majority of long-term CTA acceptors lost donor chimerism in their PB, but retained donor chimerism preferentially in the bone marrow compartments, we evaluated whether the mobilization of these long-term acceptors with FL and G-CSF would temporarily increase PB donor chimerism (Fig. 4D). Two long-term acceptors with PB donor chimerism and two long-term acceptors without PB donor chimerism received FL treatment (200 μg/kg) for 10 days and G-CSF (150 μg/kg) for 7 days. Animals with no donor PB chimerism prior to mobilization did not gain donor chimerism in PB after mobilization. Animals with donor PB chimerism had 60.5% and 40.5% donor chimerism in lymphoid gate and 95.4% and 71.6% in myeloid gate prior to mobilization. After mobilization, they showed similar donor chimerism in lymphoid gate (58.3% and 35.3%) and in myeloid gate (95.6% and 72.4%).
Skin graft challenge in long term acceptors of CTA
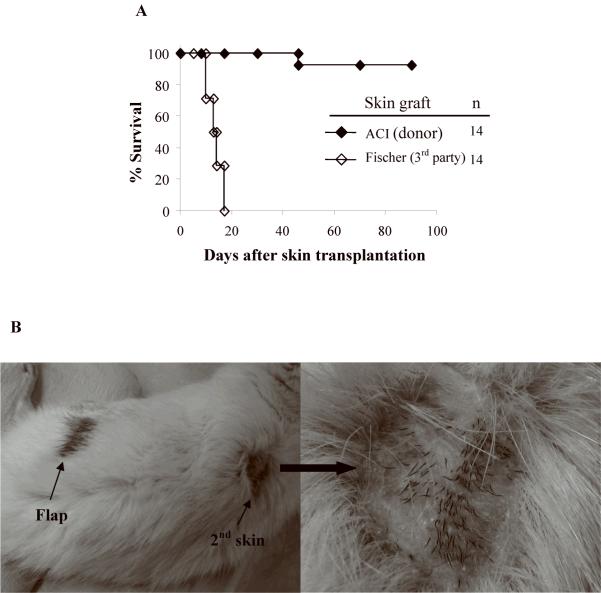
Acceptance of a second donor-derived skin graft is considered the gold standard for assessing central deletional tolerance (26, 27). To test the robustness of tolerance, CTA tolerant rats received second-set full-thickness donor skin grafts 4-6 months after flap transplantation (Fig. 5A, B). 13 of 14 accepted the skin grafts for up to 90 days follow-up. All third-party allogeneic skin grafts from Fischer rats were promptly rejected. Placement of the skin graft did not induce rejection or any changes in the CTA.
Figure 5. (A, B) Second set donor skin graft challenge in long-term acceptors of composite tissue allograft.
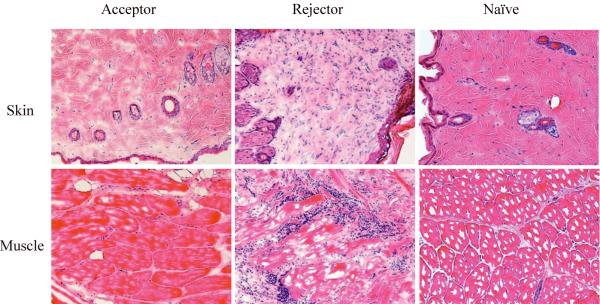
14 CTA-tolerant rats received second-set donor (ACI) skin grafts 4 to 6 months after CTA. Full thickness skin grafts were harvested from tail skin of donor (ACI) or third-party (Fischer) rats and 2x1cm segments of each were transplanted. Skin graft survival was monitored up to 90 days (A). Rejection was defined as complete when no residual viable graft could be detected. A representative photo of an accepted second set donor skin graft is shown (B). (C) Histologic findings from biopsies of skin and muscle. The biopsies of skin or muscle of transplanted flaps from rejecters, acceptors, or naïve ACI donor were embedded in OCT, sectioned in cryostat and stained with hematoxylin and eosin. Tissues from naïve recipients served as controls. A representative photograph taken from the sections of each group is presented. Results are from two experiments (magnification 200x). The biopsies from acceptors were devoid of lymphocytic infiltrates and exhibited normal architecture. In contrast, there were dense lymphocytic infiltrates and loss of tissue integrity in the rejecting muscle and skin from rejectors.
Histologic findings from long-term acceptors and rejectors
To evaluate histological changes of the transplanted flaps, biopsy specimens of the donor flap skin and muscle were obtained from both rejected recipients (during rejection) and long-term acceptors. Tissues from naïve recipients served as controls. Histological examination with H & E staining showed a moderate infiltration of the dermal stroma by numerous mononuclear cells and destruction of architecture in the skin biopsies of rejected animals. We also observed an extensive infiltration of mononuclear lymphatic cells in the muscle biopsies of rejected rats. In contrast, there was no infiltration of mononuclear cells in the skin and muscle biopsies of acceptor rats.
Discussion
The field of reconstructive transplantation is rapidly expanding (28). It is estimated that 7 million individuals in the United States alone could benefit from CTA (29). However, the toxicity of the immunosuppressive agents required to prevent rejection has impaired the widespread application of CTA (30). In the successful hand transplants performed to date, hypertension, impaired renal function, infection, insulin dependent diabetes mellitus, and osteonecrosis have occurred (reviewed in (31). Moreover, concern over the nephrotoxicity that occurs due to chronic calcineurin inhibitor use has raised concerns for this patient population. Approximately 47% of cardiac allograft recipients develop renal compromise by 5 years due to the toxicity of the calcineurin inhibitors, and some progress to renal failure (32). The induction of tolerance to the transplanted tissue would overcome these limitations. Mixed chimerism induces tolerance to solid organ and cellular transplants (9, 15). While tolerance has been established nonmyeloablatively for solid organ allografts (15), whether similar graft acceptance would occur for what has been postulated to be a highly antigenic CTA complex using reduced-intensity conditioning has remained controversial (33). Moreover, the degree of chimerism and optimal approach for conditioning required to promote CTA graft acceptance has remained controversial, especially in robust donor/recipient rat strain combinations that are more comparable with the human experience (34, 35).
In the present studies, we found that nonmyeloablative conditioning is sufficient to induce tolerance to CTA in a robust MHC-disparate rat strain combination. Allogeneic chimerism was established in rats conditioned nonmyeloablatively with tacrolimus/ALS and TBI doses ranging from 300 to 600 cGy. However, only chimeras that received 600 cGy of TBI accepted their flap allografts long term, even though they eventually lost their donor PB chimerism. None of the chimeras preconditioned with 500, 400 and 300 cGy TBI accepted their flap grafts long term, although there were significant levels of donor chimerism in PB at the time of CTA performance. The presence of hemopoietic chimerism has been reported not always reliably to confer tolerance in clinical with microchimerism and in experimental models with macrochimerism (15, 36-38). In a radiation-based tolerance model, the production of both class II Ag-positive cells and T cells was required for tolerance induction. When class II knockout (KO) mice and T cell KO mice were used as bone marrow donors, chimerism was achieved, but tolerance was not associated with this chimerism (38). In a nonradiation-based protocol using antilymphocyte serum and rapamycin, donor class II Ag-positive cells, but not T and/or B cells, were required for tolerance induction (39, 40). We recently reported that at threshold levels of conditioning with recipient T cell depletion, chimerism remained stable only if the animals exhibited donor T-cell engraftment in spite of high levels of donor chimerism, suggesting that donor T cells play an important role in the maintenance of chimerism and induction of donor-specific tolerance in this model (15, 41). The reason for the dissociation of chimerism and tolerance in our current CTA rat model is not clear. Although significant levels of donor chimerism were achieved, the immune balance between donor and recipient apparently was not achieved, resulting in loss of donor chimerism and tolerance to CTA.
The addition of anti-αβ-TCR mAb preconditioning to the tacrolimus/ALS-based regimen significantly increased the rate of CTA acceptance and durability of significant levels of chimerism in the PB. The majority of chimeras that received as little as 400 or 300 cGy TBI in this group accepted their CTA. However, in striking contrast with the findings reported by Siemionow et al. in which preconditioning of Lewis recipients with a 7 day course of anti-TCR mAb plus cyclosporine allowed chimerism and tolerance to be induced to CTA without irradiation (35, 42), these protocols were not sufficient to promote graft/host tolerance without additional TBI in a more robust donor/recipient histocompatibility disparity. These findings point to the importance of examining outcomes in more than one strain combination before translation to the clinic.
Our data suggest that lymphodepletion of recipient T cells in vivo with anti-αβ-TCR mAb influences the mechanism and robustness of tolerance induced. As a result, the same levels of mixed chimerism achieved early after transplant can lead to substantially different outcomes with respect to CTA acceptance. Lymphodepletion of recipient T cells also appeared to have an effect on the duration of chimerism, in that the four animals in our study that retained high levels of chimerism long-term all received anti-αβ-TCR lymphodepletion preconditioning. It has been demonstrated that lymphodepletion of recipients of solid organ grafts with Campath or anti-lymphocyte globulin allows lower levels of immunosuppression to be administered, and often results in steroid-sparing immunosuppression (43, 44). A similar beneficial effect appears to occur in nonmyeloablative conditioning for establishing mixed donor:host chimerism. A recent report in haploidentical nonmyeloablatively conditioned humans using cyclophosphamide (50 mg/kg/day) on day +3 ± +4, following hematopoietic stem cell transplantation, found that engraftment was enhanced. This was attributed to targeting of host-versus-graft alloreactive cells by cyclophosphamide (16). Similarly, lymphodepletion appears to promote BMC acceptance and CTA graft acceptance.
The persistence of CTA tolerance after loss of PB chimerism is an unexpected finding and may unify the debate regarding the significance of and differences between macrochimerism and microchimerism in promoting graft acceptance (17, 45). To define the mechanism for the dissociation between PB donor chimerism and tolerance to CTA, we analyzed the presence of donor-derived cells in selected hematopoietic compartments. We found that donor chimerism was present at significantly higher levels in the transplanted bone compared to other tissues of all composite tissue graft acceptors. One could hypothesize a number of possible underlying mechanisms for graft acceptance. A vascularized bone graft may provide a niche for the persistence of donor lymphoid cells, which could act as a constant tolerizing source, producing the systemic microchimerism observed in our current findings, confirming a recent report from Siemionow et al. (42). On the other hand, the composite tissue allograft acceptance we see could be independent of the vascularized bone transplant and may have been induced at the time of BMT, with the donor lymphoid chimerism in the transplanted bone not having a causal effect on tolerance but being simply an effect of transplantation of vascularized bone. One argument for our composite tissue allograft acceptance in the absence of PB chimerism would be that we have not achieved true tolerance but instead prope tolerance (46). To determine the potential mechanism for graft prolongation in the absence of PB chimerism (deletion vs. anergy), we performed a skin graft syngeneic to the CTA donor. In the absence of central deletional tolerance, a second skin graft challenge may induce rejection of both grafts. The fact that 13 of 14 second-set skin grafts placed on rats with CTA acceptance were accepted and none induced rejection of the CTA is suggestive of deletional tolerance as the underlying mechanism of graft acceptance. These data are surprising and indicate that robust tolerance to what is believed to be one of the most antigenic tissue complexes can occur for CTA in the absence of persistent PB chimerism.
The minimum level of PB mixed chimerism necessary to achieve tolerance and whether it must be persistent is highly relevant to clinical practice and remains controversial (17, 45). It was recently reported that renal transplant subjects enrolled in a nonmyeloablative protocol intended to establish donor macrochimerism and tolerance were successfully weaned from immunosuppression in spite of the fact that donor PB chimerism was only transient, lasting for ≤ 2 weeks (47). Moreover, infusion of donor bone marrow in unconditioned heart and lung transplant recipients at the time of transplantation is associated with a significant reduction in chronic rejection long term (48-50). The microchimerism first reported by Starzl et al. (51), which was postulated to result in bidirectional host-versus-graft and graft-versus-host alloreactivity that resulted in clonal exhaustion (52), may in fact represent a continuum of the beneficial effects that transplanted donor hematolymphopoietic cells may have on promoting graft acceptance. One may hypothesize that the presence of bone marrow cells induces a regulatory feedback loop that promotes CTA acceptance. In our own clinical hand transplant experience, lymphodepletion induction has allowed steroid-free maintenance therapy with FK506 and MMF similar to that for maintaining renal allografts, with rejection readily controlled with additional topical treatment alone in most cases (53). Moreover, chronic rejection has not been observed in hand transplant recipients in up to 10 years follow-up and is difficult to induce in animal models (54, 55). Both observations are in distinct contrast to the prediction that hand graft acceptance would be difficult to achieve due to the high antigenic burden (56).
Our current finding that donor chimerism is significantly enhanced in transplanted donor bone in tolerant animals who had lost their donor-chimerism in PB suggests a source for microchimerism in PB and a site to maintain graft acceptance. One could hypothesize that this tissue unit exerts a regulatory effect on the bidirectional host-versus-graft and graft-versus-host response. We are currently evaluating the kinetic of Treg induction in these recipients. If the mechanism of persistent CTA acceptance in the absence of PB chimerism can be elucidated, this could create a framework for the reliable induction of tolerance with the establishment of a certain targeted level of transient chimerism by using specific tolerogenic cell subsets and allowing minimal toxicity of the tolerizing regimen. Due to a more than 1 month delay of CTA in chimeras, this has made our current sequential CTA protocol not applicable in the clinical scenario of standard deceased donor organ donation. In order to establish a clinically relevant CTA model in rat, both the BMT and CTA should be performed simultaneously. In our continuing studies, we have been working to establish a model in which BMT and CTA are performed simultaneously.
Acknowledgements
The authors thank Dr. Paula Chilton for helpful comments, Carolyn DeLautre for manuscript preparation, and the staff of the animal facility at the University of Louisville for outstanding animal care.
This research was supported in part by NIH RO1 HL63442, NIH R01 DK069766, NIH R01 HL076794; The Juvenile Diabetes Research Foundation; the W. M. Keck Foundation; The Department of Defense: Office of Naval Research; and The Department of Defense: Office of Army Research (Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the Office of Army Research. This publication was made possible by Award No.W81XWH-07-1-0185 from the Office of Army Research); the National Foundation to Support Cell Transplant Research; the Commonwealth of Kentucky Research Challenge Trust Fund; the W. M. Keck Foundation; and The Jewish Hospital Foundation
Abbreviations
- ALS
anti-rat lymphocyte serum
- CTA
composite tissue allotransplants
- PB
peripheral blood
- TBI
total body irradiation
- TCD
T cell depleted
Footnotes
The above authors have no conflict of interest to declare.
References
- 1.Composite tissue allograft. World Scientific Publishing Co.; Hackensack: 2006. [Google Scholar]
- 2.Siemionow M, Bozkurt M, Kulahci Y. Current status of composite tissue allotransplantation. Handchir Mikrochir Plast Chir. 2007;39:145. doi: 10.1055/s-2007-965233. [DOI] [PubMed] [Google Scholar]
- 3.Barker JH, Breidenbach WC, Hewitt CW. Proceedings of the second international symposium on composite tissue allotransplantation; Wiley-Liss, Inc.; 2000. pp. 357–465. [DOI] [PubMed] [Google Scholar]
- 4.Levi DM, Tzakis AG, Kato T, et al. Transplantation of the abdominal wall. Lancet. 2003;361:2173. doi: 10.1016/S0140-6736(03)13769-5. [DOI] [PubMed] [Google Scholar]
- 5.Birchall MA, Lorenz RR, Berke GS, et al. Laryngeal transplantation in 2005: a review. Am J Transplant. 2006;6:20. doi: 10.1111/j.1600-6143.2005.01144.x. [DOI] [PubMed] [Google Scholar]
- 6.Bloom RD, Goldberg LR, Wang AY, Faust TW, Kotloff RM. An overview of solid organ transplantation. Clin Chest Med. 2005;26:529. doi: 10.1016/j.ccm.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Ildstad ST, Wren SM, Bluestone JA, Barbieri SA, Sachs DH. Characterization of mixed allogeneic chimeras: Immunocompetence, in vitro reactivity and genetic specificity of tolerance. J Exp Med. 1985;162:231. doi: 10.1084/jem.162.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colson YL, Tripp RA, Doherty PC, et al. Antiviral cytotoxic activity across a species barrier in mixed xenogeneic chimeras: functional restriction to host MHC. J Immunol. 1998;160:3790. [PubMed] [Google Scholar]
- 9.Ildstad ST, Sachs DH. Reconstitution with syngeneic plus allogeneic or xenogeneic bone marrow leads to specific acceptance of allografts or xenografts. Nature. 1984;307:168. doi: 10.1038/307168a0. [DOI] [PubMed] [Google Scholar]
- 10.Colson YL, Wren SM, Schuchert MJ, et al. A nonlethal conditioning approach to achieve durable multilineage mixed chimerism and tolerance across major, minor, and hematopoietic histocompatibility barriers. J Immunol. 1995;155:4179–4188. [PubMed] [Google Scholar]
- 11.Gammie JS, Li S, Colson YL, et al. A partial conditioning strategy for achieving mixed chimerism in the rat: Tacrolimus and anti-lymphocyte serum substantially reduce the minimum radiation dose for engraftment. Exp Hematol. 1998;26:927. [PubMed] [Google Scholar]
- 12.Li H, Colson YL, Ildstad ST. Mixed allogeneic chimerism achieved by lethal and nonlethal conditioning approaches induces donor-specific tolerance to simultaneous islet allografts. Transplantation. 1995;60:523. doi: 10.1097/00007890-199509270-00001. [DOI] [PubMed] [Google Scholar]
- 13.Li H, Inverardi L, Molano RD, Pileggi A, Ricordi C. Nonlethal conditioning for the induction of allogeneic chimerism and tolerance to islet allografts. Transplantation. 2003;75:966. doi: 10.1097/01.TP.0000058516.74246.71. [DOI] [PubMed] [Google Scholar]
- 14.Aksentijevich I, Sharabi Y, Sundt TM, III, Sachs DH, Sykes M. Humoral tolerance in mixed xenogeneic chimeras prepared by a nonmyeloablative conditioning regimen. Transplant Proc. 1991;23:880. [PubMed] [Google Scholar]
- 15.Xu H, Chilton PM, Huang Y, Schanie CL, Ildstad ST. Production of donor T cells is critical for induction of donor-specific tolerance and maintenance of chimerism. J Immunol. 2004;172:1463. doi: 10.4049/jimmunol.172.3.1463. [DOI] [PubMed] [Google Scholar]
- 16.Luznik L, O'Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Starzl TE, Murase N. Microchimerism, macrochimerism, and tolerance. Clin Transplant. 2000;14:351. doi: 10.1034/j.1399-0012.2000.t01-1-140412.x. [DOI] [PubMed] [Google Scholar]
- 18.Colson YL, Zadach K, Nalesnik M, Ildstad ST. Mixed allogeneic chimerism in the rat. Donor-specific transplantation tolerance without chronic rejection for primarily vascularized cardiac allografts. Transplantation. 1995;60:971. [PubMed] [Google Scholar]
- 19.Adamson LA, Huang W, Breidenbach WC, et al. A modified model of hindlimb osteomyocutaneous flap for the study of tolerance to composite tissue allotransplantation. Microsurgery. 2007;27:630. doi: 10.1002/micr.20414. [DOI] [PubMed] [Google Scholar]
- 20.Shapiro RI, Cerra FB. A model for reimplantation and transplantation of a complex organ: the rat hind limb. J Surg Res. 1978;24:501. doi: 10.1016/0022-4804(78)90048-3. [DOI] [PubMed] [Google Scholar]
- 21.Hiotis SP, Wnuk KL, Blumenthals WA, Halaris SA, Good RA. Orthotopic hindlimb transplantation in the rat: a technically challenging but useful animal model for solid organ engraftment. Transplant Proc. 1999;31:1567. doi: 10.1016/s0041-1345(99)00039-1. [DOI] [PubMed] [Google Scholar]
- 22.Lipson RA, Kawano H, Halloran PF, McKee NH, Pritzker KP, Langer F. Vascularized limb transplantation in the rat. I. Results with syngeneic grafts. Transplantation. 1983;35:293. doi: 10.1097/00007890-198304000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Ulusal AE, Ulusal BG, Hung LM, Wei FC. Heterotopic hindlimb allotransplantation in rats: An alternative model for immunological research in composite-tissue allotransplantation. Microsurgery. 2005;25:410. doi: 10.1002/micr.20139. [DOI] [PubMed] [Google Scholar]
- 24.Foster RD, Pham S, Li S, Aitouche A. Long-term acceptance of composite tissue allografts through mixed chimerism and CD28 blockade. Transplantation. 2003;76:988. doi: 10.1097/01.TP.0000079827.91675.A3. [DOI] [PubMed] [Google Scholar]
- 25.Gammie JS, Li S, Demetris AJ, Zeevi A, Ildstad ST, Pham SM. Tacrolimus-based partial conditioning produces stable mixed lymphohematopoietic chimerism and tolerance for cardiac allografts. Circulation. 1998;98:II163. discussion II168-II168;discussion II169. [PubMed] [Google Scholar]
- 26.Billingham RE, Brent L, BROWN JB, Medawar PB. Time of onset and duration of transplantation immunity. Transplant Bull. 1959;6:410. doi: 10.1097/00006534-195910000-00035. [DOI] [PubMed] [Google Scholar]
- 27.Graff RJ, Silvers WK, Billingham RE, Hildemann WH, Snell GD. The cumulative effect of histocompatibility antigens. Transplantation. 1966;4:605. doi: 10.1097/00007890-196609000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Tobin GR, Breidenbach WC, III, Pidwell DJ, Ildstad ST, Ravindra KV. Transplantation of the hand, face, and composite structures: evolution and current status. Clin Plast Surg. 2007;34:271. doi: 10.1016/j.cps.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Gander B, Brown CS, Vasilic D, et al. Composite tissue allotransplantation of the hand and face: a new frontier in transplant and reconstructive surgery. Transpl Int. 2006;19:868. doi: 10.1111/j.1432-2277.2006.00371.x. [DOI] [PubMed] [Google Scholar]
- 30.Pidwell DJ, Burns C. The immunology of composite tissue transplantation. Clin Plast Surg. 2007;34:303. doi: 10.1016/j.cps.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Kvernmo HD, Gorantla VS, Gonzalez RN, Breidenbach WC., III Hand transplantation. A future clinical option? Acta Orthop. 2005;76:14. doi: 10.1080/00016470510030283. [DOI] [PubMed] [Google Scholar]
- 32.Garrido IP, Crespo-Leiro MG, Paniagua MJ, et al. Independent predictors of renal dysfunction after heart transplantation in patients with normal pretransplant renal function. J Heart Lung Transplant. 2005;24:1226. doi: 10.1016/j.healun.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 33.Brenner MJ, Tung TH, Jensen JN, Mackinnon SE. The spectrum of complications of immunosuppression: is the time right for hand transplantation? J Bone Joint Surg Am. 2002;84-A:1861. [PubMed] [Google Scholar]
- 34.Foster RD, Ascher NL, McCalmont TH, Neipp M, Anthony JP, Mathes SJ. Mixed allogeneic chimerism as a reliable model for composite tissue allograft tolerance induction across major and minor histocompatibility barriers. Transplantation. 2001;72:791. doi: 10.1097/00007890-200109150-00009. [DOI] [PubMed] [Google Scholar]
- 35.Siemionow MZ, Izycki DM, Zielinski M. Donor-specific tolerance in fully major histocompatibility major histocompatibility complex-mismatched limb allograft transplants under an anti-alphabeta T-cell receptor monoclonal antibody and cyclosporine A protocol. Transplantation. 2003;76:1662. doi: 10.1097/01.TP.0000105343.49626.6F. [DOI] [PubMed] [Google Scholar]
- 36.Hisanaga M, Hundrieser J, Boker K, et al. Development, stability, and clinical correlations of allogeneic microchimerism after solid organ transplantation. Transplantation. 1996;61:40. doi: 10.1097/00007890-199601150-00010. [DOI] [PubMed] [Google Scholar]
- 37.Elwood ET, Larsen CP, Maurer DH, et al. Microchimerism and rejection in clinical transplantation. Lancet. 1997;349:1358. doi: 10.1016/s0140-6736(96)09105-2. [DOI] [PubMed] [Google Scholar]
- 38.Umemura A, Morita H, Chang L,X, Tahan S, Monaco AP, Maki T. Dissociation of hemopoietic chimerism and allograft tolerance after allogeneic bone marrow transplantation. J Immunol. 2001;167:3043. doi: 10.4049/jimmunol.167.6.3043. [DOI] [PubMed] [Google Scholar]
- 39.Umemura A, Monaco AP, Maki T. Donor MHC class II antigen is essential for induction of transplantation tolerance by bone marrow cells. J Immunol. 2000;164:4452. doi: 10.4049/jimmunol.164.9.4452. [DOI] [PubMed] [Google Scholar]
- 40.Umemura A, Monaco AP, Maki T. Expression of MHC class II antigen is essential in tolerance induction by donor bone marrow cell in antilymphocyte serum-treated and rapamycin-treated mice. Transplant Proc. 2001;33:148. doi: 10.1016/s0041-1345(00)01947-3. [DOI] [PubMed] [Google Scholar]
- 41.Xu H, Chilton PM, Huang Y, Schanie CL, Yan J, Ildstad ST. Addition of cyclophosphamide to T-cell depletion based nonmyeloablative conditioning allows donor T-cell engraftment and clonal deletion of alloreactive host T-cells after bone marrow transplantation. Transplantation. 2007;83:954. doi: 10.1097/01.tp.0000258679.18684.b0. [DOI] [PubMed] [Google Scholar]
- 42.Siemionow M, Klimczak A, Unal S, Agaoglu G, Carnevale K. Hematopoietic stem cell engraftment and seeding permits multi-lymphoid chimerism in vascularized bone marrow transplants. Am J Transplant. 2008;8:1163. doi: 10.1111/j.1600-6143.2008.02241.x. [DOI] [PubMed] [Google Scholar]
- 43.Gallon LG, Winoto J, Chhabra D, Parker MA, Leventhal JR, Kaufman DB. Long-term renal transplant function in recipient of simultaneous kidney and pancreas transplant maintained with two prednisone-free maintenance immunosuppressive combinations: tacrolimus/mycophenolate mofetil versus tacrolimus/sirolimus. Transplantation. 2007;83:1324. doi: 10.1097/01.tp.0000264189.58324.91. [DOI] [PubMed] [Google Scholar]
- 44.Shapiro AM, Lakey JR, Ryan EA, et al. Edmonton Protocol - Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 45.Ildstad ST, Breidenbach WC. Tolerance to organ transplants: is chimerism bringing it closer than we think? Curr Opin Organ Transplant. 2007;12:329. [Google Scholar]
- 46.Calne R, Moffatt SD, Friend PJ, et al. Prope tolerance with induction using Campath 1H and low-dose cyclosporin monotherapy in 31 cadaveric renal allograft recipients. Nippon Geka Gakkai Zasshi. 2000;101:301. [PubMed] [Google Scholar]
- 47.Elster EA, Hale DA, Mannon RB, Cendales LC, Swanson SJ, Kirk AD. The road to tolerance: renal transplant tolerance induction in nonhuman primate studies and clinical trials. Transpl Immunol. 2004;13:87. doi: 10.1016/j.trim.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 48.Pham SM, Rao AS, Zeevi A, et al. Effects of donor bone marrow infusion in clinical lung transplantation. Ann Thorac Surg. 2000;69:345. doi: 10.1016/s0003-4975(99)01471-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pham SM, Rao AS, Zeevi A, et al. A clinical trial combining donor bone marrow infusion and heart transplantation: intermediate-term results. J Thorac Cardiovasc Surg. 2000;119:673. doi: 10.1016/S0022-5223(00)70001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li S, Salgar SK, Thanikachalam M, et al. CTLA4-Ig-based conditioning regimen to induce tolerance to cardiac allografts. J Surg Res. 2006;136:238. doi: 10.1016/j.jss.2006.05.032. [DOI] [PubMed] [Google Scholar]
- 51.Starzl TE. Chimerism and tolerance in transplantation. Proc Natl Acad Sci USA. 2004;101(Suppl 2):14607. doi: 10.1073/pnas.0404829101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Starzl TE, Zinkernagel RM. Antigen localization and migration in immunity and tolerance. N Engl J Med. 1998;339:1905. doi: 10.1056/NEJM199812243392607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ildstad ST, Rahhal D, Ravindra K, Buell J, Breidenbach WC. Immunologic Monitoring and Kinetic of Graft-Infiltrating Treg in Long-Term Hand Transplant Recipients. American Transplant Congress; Toronto, ON, Canada. May 31-June 4, 2008. [Google Scholar]
- 54.Breidenbach WC, Gonzales NR, Kaufman CL, Klapheke M, Tobin GR, Gorantla VS. Outcomes of the first 2 American hand transplants at 8 and 6 years posttransplant. J Hand Surg [Am] 2008;33:1039. doi: 10.1016/j.jhsa.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 55.Swearingen B, Xu H, Breidenbach WC, Ildstad ST. The science of composite tissue allotransplantation. Transplantation. 2008;86:627. doi: 10.1097/TP.0b013e318184ca6a. PMC Journal - In Process. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jones NF, Hansen SL, Bates SJ. Toe-to-hand transfers for congenital anomalies of the hand. Hand Clin. 2007;23:129. doi: 10.1016/j.hcl.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 57.Neipp M, Zorina T, Domenick MA, Exner BG, Ildstad ST. Effect of FLT3 ligand and granulocyte colony-stimulating factor on expansion and mobilization of facilitating cells and hematopoietic stem cells in mice: kinetics and repopulating potential. Blood. 1998;92:3177. [PubMed] [Google Scholar]