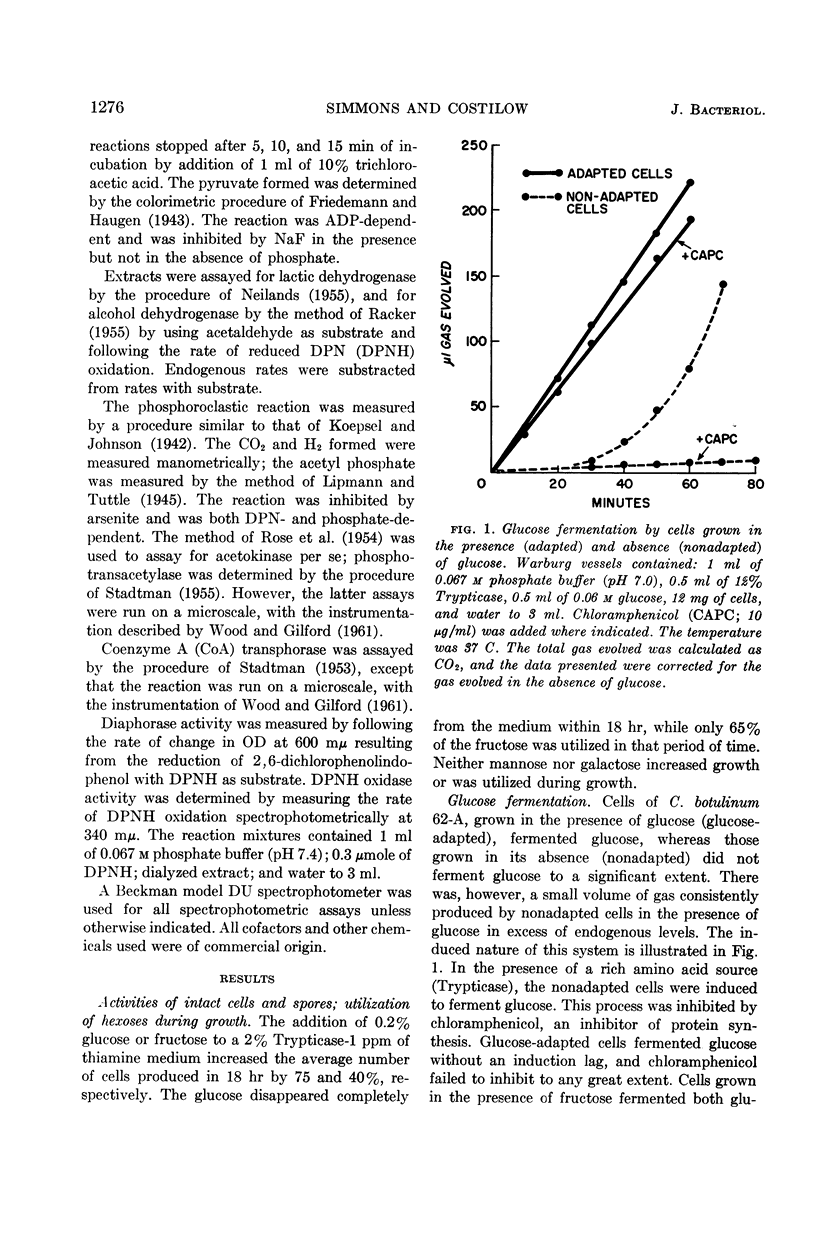
Abstract
Simmons, R. J. (Michigan State University, East Lansing), and R. N. Costilow. Enzymes of glucose and pyruvate catabolism in cells, spores, and germinated spores of Clostridium botulinum. J. Bacteriol. 84:1274–1281. 1962.—An investigation was made of the enzymes of vegetative cells, spores, and germinated spores of Clostridium botulinum 62-A to elucidate a pathway of glucose metabolism. Manometric studies were conducted with intact cells, and various enzymes and enzyme systems were assayed in cell-free and spore-free extracts by use of spectrophotometric and colorimetric procedures. Glucose fermentation was found to be inducible; glucokinase was the controlling enzyme. All other enzymes of the Embden-Meyerhof-Parnas (EMP) pathway were found in both induced and non-induced cells, but they were in relatively low concentrations in the latter. This, plus the fact that no glucose-6-phosphate dehydrogenase was detected, led to the conclusion that glucose is catabolized primarily by the EMP system. A number of glycolytic enzymes were also found in extracts of spores and germinated spores of this organism, but the activities were extremely low as compared with activities in cell extracts. A phosphoroclastic-type reaction was readily demonstrated in both glucose-adapted and non-adapted cells, but not in spores and germinated spores. However, both acetokinase and phosphotransacetylase, as well as coenzyme A transphorase, were detected in spores and germinated-spore extracts, although at very low activity levels as compared with cell extracts. The specific activity of diaphorase in spore extracts was about one-half that of corresponding cell extracts, and the activity of reduced diphosphopyridine nucleotide (DPNH) oxidase was actually higher in the spore extracts. In addition, the DPNH oxidase in spore extracts was considerably more heat-stable than that in extracts of cells or germinated spores.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BACH J. A., SADOFF H. L. Aerobic sporulating bacteria. I. Glucose dehydrogenase of Bacillus cereus. J Bacteriol. 1962 Apr;83:699–707. doi: 10.1128/jb.83.4.699-707.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARD R. C., GUNSALUS I. C. Glucose metabolism of Clostridium perfringens: existence of metallo-aldolase. J Bacteriol. 1950 Mar;59(3):387–400. doi: 10.1128/jb.59.3.387-400.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHURCH B. D., HALVORSON H. Intermediate metabolism of aerobic spores. I. Activation of glucose oxidation in spores of Bacillus cereus var terminalis. J Bacteriol. 1957 Apr;73(4):470–476. doi: 10.1128/jb.73.4.470-476.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COSTILOW R. N. Fermentative activities of control and radiation-"killed" spores of Clostridium botulinum. J Bacteriol. 1962 Dec;84:1268–1273. doi: 10.1128/jb.84.6.1268-1273.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CYNKIN M. A., GIBBS M. Metabolism of pentoses by clostridia. II. The fermentation of C14-labeled pentoses by Clostridium per fringens, Clostridium beijerinckii, and Clostridium butylicum. J Bacteriol. 1958 Mar;75(3):335–338. doi: 10.1128/jb.75.3.335-338.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton C. E. The Utilization of Amino Acids and of Glucose by Clostridium botulinum. J Bacteriol. 1940 May;39(5):485–497. doi: 10.1128/jb.39.5.485-497.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOI R. H., HALVORSON H. Mechanism of dipicolinic acid stimulation of the soluble reduced diphosphopyridine nucleotide oxidase of spores. J Bacteriol. 1961 Apr;81:642–648. doi: 10.1128/jb.81.4.642-648.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOLIN M. I. Oxidation of reduced diphosphopyridine nucleotide by Clostridium perfringens. I. Relation of peroxide to the overall reaction. J Bacteriol. 1959 Apr;77(4):383–392. doi: 10.1128/jb.77.4.383-392.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOLIN M. I. Oxidation of reduced diphosphopyridine nucleotide by Clostridium perfringens. II. Purification of the oxidase: relation to cytochrome c reductase. J Bacteriol. 1959 Apr;77(4):393–402. doi: 10.1128/jb.77.4.393-402.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEIN H. P. Some properties of the hexokinase of Pseudomonas putrefaciens. J Bacteriol. 1953 Dec;66(6):650–655. doi: 10.1128/jb.66.6.650-655.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINSON H. S., HYATT M. T. Some effects of heat and ionizing radiation on spores of Bacillus megaterium. J Bacteriol. 1960 Oct;80:441–451. doi: 10.1128/jb.80.4.441-451.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARTINEZ R. J., RITTENBERG S. C. Glucose dissimilation by Clostridium tetani. J Bacteriol. 1959 Feb;77(2):156–163. doi: 10.1128/jb.77.2.156-163.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAEGE L. M., GIBBS M., BARD R. C. Fermentation of C14-labeled glucose by Clostridium perfringens. J Bacteriol. 1956 Jul;72(1):65–67. doi: 10.1128/jb.72.1.65-67.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSE I. A., GRUNBERG-MANAGO M., KOREY S. R., OCHOA S. Enzymatic phosphorylation of acetate. J Biol Chem. 1954 Dec;211(2):737–756. [PubMed] [Google Scholar]
- SALTMAN P. Hexokinase in higher plants. J Biol Chem. 1953 Jan;200(1):145–154. [PubMed] [Google Scholar]
- SHANKAR K., BARD R. C. Effect of metallic tons on the growth, morphology, and metabolism of Clostridium perfringens. I. Magnesium. J Bacteriol. 1955 Apr;69(4):436–443. doi: 10.1128/jb.69.4.436-443.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STADTMAN E. R. The coenzyme A transphorase system in Clostridium kluyveri. J Biol Chem. 1953 Jul;203(1):501–512. [PubMed] [Google Scholar]
- STEWART B. T., HALVORSON H. O. Studies on the spores of aerobic bacteria. I. The occurrence of alanine racemase. J Bacteriol. 1953 Feb;65(2):160–166. doi: 10.1128/jb.65.2.160-166.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOD W. A., GILFORD S. R. A system for automatic recording of absorbancy and its application to enzyme-catalyzed reactions. Anal Biochem. 1961 Dec;2:589–600. doi: 10.1016/0003-2697(61)90026-4. [DOI] [PubMed] [Google Scholar]


