Abstract
BReast CAncer 1 (BRCA1) was initially identified as one of the genes conferring genetic predisposition to both breast and ovarian cancer. One of the interesting aspects of BRCA1 linked cancers is the observed specificity for oestrogen responsive tissues such as breast and ovary. Recent advances in our understanding of BRCA1 linked breast cancers have revealed a complex relationship between BRCA1 and oestrogen receptor alpha (ERα) signalling. Oestrogen stimulation increases expression of BRCA1 at the mRNA and protein level and conversely BRCA1 functions to both induce ERα mRNA expression and act as a negative regulator of ERα signalling. Here we review the relationship between BRCA1 and ERα and discuss the use of antioestrogen therapies in the treatment of BRCA1 mutation carriers.
Background
Introduction
Approximately 3-5% of breast cancers arise as a consequence of highly penetrant mutations in the BRCA1 tumour suppressor gene (1). BRCA1 mutation carriers have a 50-80% risk of developing breast cancer and a 16-40% risk of developing ovarian cancer by age 70 (1-3). In addition, carriers are at an increased risk of developing uterine and cervical cancers (4, 5). To date approximately 300 mutations within the BRCA1 gene have been identified including small insertions, deletions and nonsense mutations most of which lead to a functionally inactive protein (6-8). A number of studies have also demonstrated epigenetic inactivation of BRCA1 in sporadic breast cancer suggesting it may play a greater role than previously suggested (9-12).
BRCA1 has been implicated in a number of important cellular functions including DNA damage repair, cell cycle checkpoint control, apoptosis and transcriptional regulation (13). The only known enzymatic activity linked to BRCA1 is its ability to function as an E3 ligase in association with its binding partner, BARD1, and it has therefore been suggested that this E3 ligase activity may underpin many of the functions ascribed to BRCA1 (14).
One of the most interesting aspects of BRCA1 biology is the apparent specificity for hormonally regulated tissues such as breast and ovary, despite performing an apparently fundamental role in all cell types. This has led to speculation as to the potential relationship between BRCA1 and hormonal signalling in breast cancer. Paradoxically, approximately 90% of BRCA1 linked tumours are ERα negative, and similar to ERα deficient tumours have a poor prognosis (15). ERα negativity has also been reported to be a positive predictor of BRCA1 mutation status as many of the characteristics of ERα negative tumours are also evident for BRCA1 mutant tumours (16, 17). Furthermore ERα negativity is associated with reduced BRCA1 expression and there appears to be a correlation between the expression levels of BRCA1 and ERα mRNA levels in sporadic breast cancers (18-20). Further information on the relationship between BRCA1-linked tumours and the various subtypes of breast cancer has been elucidated from microarray studies. Microarray-based expression profiling has demonstrated that breast tumours can be classified into at least five major subtypes including ERα positive luminal A & B subgroups, a HER2 positive subgroup, an ERα and Her2 negative subgroup and a basal-like subgroup in which tumours are generally triple negative for ER/PR/HER2 (21-23). Significantly, BRCA1 mutant tumours were shown to cluster with the ERα negative basal-like subgroup which display the worst overall prognosis (23).
E2 regulation of BRCA1 expression
The most potent and abundant oestrogen found in women is estrodiol (E2); however oestrone (E1) and oestriol (E3) also circulate throughout the body. They exert their effects by binding to the oestrogen receptors; ERα and ERβ, both ligand activated transcription factors. ERα is thought to be the most important in breast cancer development, and is a predictive marker for antioestrogen response in the clinic. The rest of this review will therefore focus on ERα.
Initial evidence to suggest interplay between ERα and BRCA1 came from mice studies, which, demonstrated that BRCA1 levels increase dramatically during puberty and pregnancy when E2 levels increase. In addition, expression of BRCA1 was shown to be induced following treatment of ovariectomised animals with E2 (24, 25). The mechanism through which E2 regulates BRCA1 mRNA expression however has been more contentious.
Early studies suggested that E2 regulation of BRCA1 was indirect based on the delayed kinetics of induction and the fact that induction could be blocked by cycloheximide indicating that new protein synthesis was required (19, 20). A more recent study however demonstrated an alternative model of regulation whereby ERα and its cofactor p300 are recruited to an AP-1 site on the BRCA1 promoter following E2 stimulation (26). Subsequent studies demonstrated that E2 stimulation of BRCA1 mRNA expression was also dependent on occupancy of the BRCA1 promoter by the unliganded aromatic hydrocarbon receptor (AhR) in complex with ligand-bound ERα (Fig 1.) (27). Although there are sequences resembling oestrogen responsive elements (ERE's) on the BRCA1 promoter they may not be directly responsive to E2 stimulation. It appears likely that E2 regulation of BRCA1 mRNA expression is highly complex involving a variety of ERα cofactors that may compete for ERα binding or indeed for BRCA1 promoter occupancy. The biological significance of the coordinated induction of BRCA1 expression following E2 stimulation is not yet clear but it may reflect a feedback mechanism required to control the proliferative effects of oestrogens and as such may provide one explanation for the tissue specificity observed in BRCA1 linked tumours.
Figure 1.
Overview of the regulatory interplay between BRCA1 and ERα. Oestrogen stimulation increases the expression of BRCA1 mRNA levels through mechanism potentially involving both p300 and AhR. BRCA1 in turn regulates ERα at both the mRNA and protein levels. BRCA1 regulates expression of ERα mRNA in an OCT1 dependent manner. In addition BRCA1 can compete with p300 and Cyclin D for binding to ERα and negatively regulate ERα mediated transactivation of its downstream target genes.
BRCA1 regulation of ERα signalling
Consistent with the concept that BRCA1 may function as part of a feedback mechanism to regulate oestrogen signalling BRCA1 was shown to interact with and inhibit ERα mediated transactivation following oestrogen stimulation. Specifically it was demonstrated that co-transfection of wild-type BRCA1 with ERα blocked the ability of ERα to transactivate reporter constructs under the control of ERE's. In contrast most cancer associated mutations of BRCA1 lack the ability to repress ERα signalling (28). This was an important finding as it suggested that BRCA1 could function as a brake on ERα driven proliferation and demonstrated that BRCA1 mutation released this brake. Consistent with this it was reported that BRCA1 could abrogate the induction of over 90% of known E2 inducible genes (29). Initial studies suggested that BRCA1 functioned to block ERα transactivation following oestrogen stimulation, however BRCA1 was also shown to block ligand-independent ERα mediated transcriptional activity (30). The mechanism through which BRCA1 inhibits ERα mediated transcriptional activity is postulated to occur through an oestrogen independent interaction between the N-terminus of BRCA1 and the C-terminal activation domain (AF-2) with the C-terminus of BRCA1 suggested to function as a transcriptional repression domain (31). It was subsequently demonstrated that BRCA1 may affect ERα transcriptional activation by de-regulation of p300 a well characterized ERα coactivator. Indeed it was shown the BRCA1 and p300 are likely to compete for the same binding site on ERα and that overexpression of p300 could reverse BRCA1 mediated repression of ERα (32). Interestingly Cyclin D has also been reported to compete with BRCA1 for ERα binding and to reverse BRCA1 mediated repression of ERα transactivaton (Fig 1). It is worth considering the consequence of BRCA1 mediated repression of ERα signalling. ERα regulates a complex network of pathways essential for the proliferation and differentiation of both breast and ovarian tissue. The direct role played by BRCA1 in the repression of ERα mediated transcription would be expected to attenuate the proliferative capacity of oestrogens. For example, the transcriptional activation and secretion of Vascular Endothelial Growth Factor (VEGF), an oestrogen inducible gene implicated in tumour growth and angiogenesis, is severely impaired by functional BRCA1 (33).
BRCA1 Transcriptionally Regulates ERα
One may presume from the data above that loss of BRCA1 function would promote increased ERα signalling, resulting in increased proliferation and potentially malignant transformation. However, as mentioned above the majority of BRCA1 mutant tumours do not express ERα (16, 34, 35). We recently presented data to explain this apparent paradox and provided a model to explain the high percentage of ERα deficiency observed in BRCA1 linked tumours. In a further twist to the story we demonstrated that BRCA1 could also transcriptionally induce ERα mRNA expression (36). The ability of BRCA1 to induce expression of ERα was dependent on the transcription factor Oct-1, which was required to recruit BRCA1 to the ERα promoter. Interestingly, ERα itself was not required even though ERα has been shown to autoregulate at the mRNA level. As part of the study we demonstrated that the BRCA1 mutant, ERα deficient cell line HCC1937 became ERα positive following reconstitution of these cells with exogenous wildtype BRCA1. Similarly it was shown that inactivation of endogenous BRCA1 in T47D or MCF7 cells using a siRNA approach resulted in a loss of endogenous ERα expression. Finally we demonstrated that inhibition of endogenous BRCA1 rendered both T47D and MCF7 cells resistant to the antioestrogen fulvestrant an effect that could be rescued by overexpression of exogenous ERα. We therefore proposed a model whereby both alleles of BRCA1 are lost through mutation and subsequent LOH at a relatively early stage in BRCA1 linked breast and ovarian cancers; this has the added effect of transitioning cells from an ERα positive to an ERα negative genotype. Since ERα plays a central role in maintaining the luminal phenotype, this data may help explain in part the wider link between BRCA1 deficiency and the basal-like subtype of breast cancer. This is consistent with the recent report that BRCA1 may play a fundamentally important role in the regulation of mammary stem/progenitor cell fate (37). It was demonstrated that BRCA1 expression was required for the differentiation of ERα negative progenitor cells to ERα positive luminal cells. The report also demonstrated that inhibition of endogenous BRCA1 in primary breast epithelial cells led to an increase in the number of cells expressing the progenitor cell marker ALDH1 and a reduction in the number of cells expressing luminal epithelial markers and ERα (37). Taken together these data provide a potential explanation for the distinctive histopathological phenotype of BRCA1 mutant tumours. Interestingly there is some indication that a proportion of sporadic basal breast cancer tumours arising in non-BRCA1 mutation carriers may actually have underlying defects in BRCA1 function which may account for their basal phenotype (38).
Clinical-Translational Advances
Can we target ERα for Cancer Prevention in BRCA1 Mutation Carriers?
In the absence of better cancer preventative measures, patients who carry a BRCA1 mutation are often offered prophylactic surgical removal of ovarian and breast tissue (39). A less severe primary preventative strategy such as an oral medication is highly desirable. In the case of sporadic breast cancer, tamoxifen has been shown to reduce the risk of breast cancer by approximately 50% (40). BRCA1 mutation carriers however, do not seem to receive the same degree of protection (41). From these data it would appear that BRCA1-linked cancers arise in a hormonally independent manner. In contrast however, removal of ovarian tissue in pre-menopausal BRCA1 mutation carriers has been shown to reduce the risk of breast cancer by approximately half, clearly implicating oestrogen in breast cancer development (42). How then can these seemingly paradoxical observations be explained in light of our current understanding of BRCA1 and ERα function?
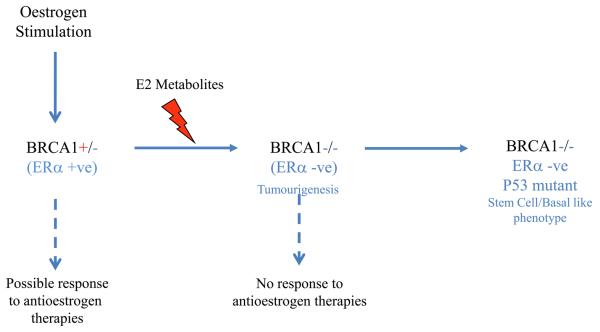
Preclinical models indicate that loss of BRCA1 function is accompanied by a loss of ERα expression. Assuming that loss of heterozgosity at the BRCA1 locus is a relatively early event in carcinogenesis, it would be expected that ERα would also be lost and the developing tumour would be hormonally independent (Fig 2). This would explain the failure of tamoxifen as a chemo preventative agent in these patients. Why then does oophorectomy protect against breast cancer in these patients? One possible explanation is that oestrogen metabolites can be genotoxic in their own right. This hypothesis has been supported by a number of epidemiological studies that have confirmed the carcinogenic effect of prolonged exposure to estrogens (43, 44). The reaction of specific oestrogen metabolites such as catechol estrogens-3-4quinones with DNA results in the formation of depurinating adducts which are mutagenic (44). It is possible that the consequent accumulation of mutagenic metabolites in ERα responsive tissues such as breast increases the statistical likelihood of losing the second BRCA1 allele in BRCA1 mutant carriers thereby initiating tumour formation. Oophorectomy reduces the levels of oestrogen in pre-menopausal women, thereby reducing the levels of genotoxic metabolites. Tamoxifen however, does not reduce oestrogen levels and would not be predicted to protect against cancer in mutation carriers, as is observed in the clinic. Effective breast cancer prophylaxis may therefore require ovarian suppression either through surgical resection or through the administration of gonadotrophin antagonists such as goserilin. However, it is important to note that BRCA1 mutant carriers are at risk of ovarian cancer and in the absence of an effective prophylactic approach, removal of the ovaries would still be preferable. In post-menopausal women the primary source of oestrogen is generated through the aromatase pathway in adipose and muscle tissue (45). It is possible therefore that unlike tamoxifen, aromatase inhibitors, such as anastrozole, may be effective as a breast cancer preventative agent in BRCA1 mutation carriers who have undergone natural or surgical menopause as these agents block production of oestrogen and thereby are likely to prevent production of secondary carcinogenic metabolites.
Figure 2.
BRCA1 mutant carriers retain a single copy of functional BRCA1 and therefore may derive a preventative benefit from some antiestrogen therapies such as aromatase inhibitors. Mutagenic oestrogen metabolites may accelerate loss of the second BRCA1 allele resulting in loss of ERα expression and possible de-differentiation towards a more basal/stem cell like phenotype.
Conclusions
The continuing investigation of the complex relationship between BRCA1 and ERα has provided potential answers to help understand some of the important clinical facts, such as the ERα deficiency observed in BRCA1 linked breast cancer. In addition to the potential ability of oestrogen metabolites to induce loss of the second BRCA1 allele it has also been suggested that oestrogen may somehow facilitate the survival of BRCA1 deficient cells in hormonally responsive tissue. While this may be a reasonable hypothesis for BRCA2 linked breast cancers it is unlikely to be the case for BRCA1 linked cancers as they are ERα deficient and unlikely to gain a selective proliferative advantage from oestrogen. Another possibility is that BRCA1 may function as a specific regulator of cell fate in hormonally responsive tissues. Loss of BRCA1 may result in the de-differentiation of cells towards a more resilient basal/stem cell like genotype. These de-differentiation breast cells may be capable of surviving the genomic instability caused by loss of BRCA1 potentially by selecting for concurrent p53 loss as is observed in the majority of BRCA1 deficient tumours. While the underlying molecular basis for the tissue specificity observed for BRCA1 linked tumours still remains to be resolved, it is likely to be highly complex and dependent on known/unknown functions of both BRCA1 and ERα.
Acknowledgments
Funding Bodies:
Julia Gorski: R&D Office N I
Richard Kennedy: Almac diagnostics
Alison Hosey: Action Cancer
Paul Harkin: Cancer Research UK, Breast Cancer Campaign.
References
- 1.Ford D, Easton DF, Bishop DT, Narod SA, Goldgar DE. Risks of cancer in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Lancet. 1994;343:692–5. doi: 10.1016/s0140-6736(94)91578-4. [DOI] [PubMed] [Google Scholar]
- 2.Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1998;62:676–89. doi: 10.1086/301749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Easton DF, Ford D, Bishop DT. Breast and ovarian cancer incidence in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Am J Hum Genet. 1995;56:265–71. [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson D, Easton DF. Cancer Incidence in BRCA1 mutation carriers. J Natl Cancer Inst. 2002;94:1358–65. doi: 10.1093/jnci/94.18.1358. [DOI] [PubMed] [Google Scholar]
- 5.Brose MS, Rebbeck TR, Calzone KA, Stopfer JE, Nathanson KL, Weber BL. Cancer risk estimates for BRCA1 mutation carriers identified in a risk evaluation program. J Natl Cancer Inst. 2002;94:1365–72. doi: 10.1093/jnci/94.18.1365. [DOI] [PubMed] [Google Scholar]
- 6.Miki Y, Swensen J, Shattuck-Eidens D, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- 7.Simard J, Tonin P, Durocher F, et al. Common origins of BRCA1 mutations in Canadian breast and ovarian cancer families. Nat Genet. 1994;8:392–8. doi: 10.1038/ng1294-392. [DOI] [PubMed] [Google Scholar]
- 8.Friedman LS, Ostermeyer EA, Szabo CI, et al. Confirmation of BRCA1 by analysis of germline mutations linked to breast and ovarian cancer in ten families. Nat Genet. 1994;8:399–404. doi: 10.1038/ng1294-399. [DOI] [PubMed] [Google Scholar]
- 9.Seery LT, Knowlden JM, Gee JM, et al. BRCA1 expression levels predict distant metastasis of sporadic breast cancers. Int J Cancer. 1999;84:258–62. doi: 10.1002/(sici)1097-0215(19990621)84:3<258::aid-ijc10>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 10.Esteller M, Silva JM, Dominguez G, et al. Promoter hypermethylation and BRCA1 inactivation in sporadic breast and ovarian tumors. J Natl Cancer Inst. 2000;92:564–9. doi: 10.1093/jnci/92.7.564. [DOI] [PubMed] [Google Scholar]
- 11.Butcher DT, Rodenhiser DI. Epigenetic inactivation of BRCA1 is associated with aberrant expression of CTCF and DNA methyltransferase (DNMT3B) in some sporadic breast tumours. Eur J Cancer. 2007;43:210–9. doi: 10.1016/j.ejca.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Stone C, McCabe N, Ashworth A. X-chromosome inactivation: X marks the spot for BRCA1. Curr Biol. 2003;13:R63–4. doi: 10.1016/s0960-9822(02)01430-6. [DOI] [PubMed] [Google Scholar]
- 13.Mullan PB, Quinn JE, Harkin DP. The role of BRCA1 in transcriptional regulation and cell cycle control. Oncogene. 2006;25:5854–63. doi: 10.1038/sj.onc.1209872. [DOI] [PubMed] [Google Scholar]
- 14.Ruffner H, Joazeiro CA, Hemmati D, Hunter T, Verma IM. Cancer-predisposing mutations within the RING domain of BRCA1: loss of ubiquitin protein ligase activity and protection from radiation hypersensitivity. Proc Natl Acad Sci U S A. 2001;98:5134–9. doi: 10.1073/pnas.081068398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lakhani SR, Van De Vijver MJ, Jacquemier J, et al. The pathology of familial breast cancer: predictive value of immunohistochemical markers estrogen receptor, progesterone receptor, HER-2, and p53 in patients with mutations in BRCA1 and BRCA2. J Clin Oncol. 2002;20:2310–8. doi: 10.1200/JCO.2002.09.023. [DOI] [PubMed] [Google Scholar]
- 16.Lakhani SR, Reis-Filho JS, Fulford L, et al. Prediction of BRCA1 status in patients with breast cancer using estrogen receptor and basal phenotype. Clin Cancer Res. 2005;11:5175–80. doi: 10.1158/1078-0432.CCR-04-2424. [DOI] [PubMed] [Google Scholar]
- 17.Foulkes WD, Stefansson IM, Chappuis PO, et al. Germline BRCA1 mutations and a basal epithelial phenotype in breast cancer. J Natl Cancer Inst. 2003;95:1482–5. doi: 10.1093/jnci/djg050. [DOI] [PubMed] [Google Scholar]
- 18.Ding SL, Sheu LF, Yu JC, et al. Expression of estrogen receptor-alpha and Ki67 in relation to pathological and molecular features in early-onset infiltrating ductal carcinoma. J Biomed Sci. 2004;11:911–9. doi: 10.1007/BF02254376. [DOI] [PubMed] [Google Scholar]
- 19.Spillman MA, Bowcock AM. BRCA1 and BRCA2 mRNA levels are coordinately elevated in human breast cancer cells in response to estrogen. Oncogene. 1996;13:1639–45. [PubMed] [Google Scholar]
- 20.Marks JR, Huper G, Vaughn JP, et al. BRCA1 expression is not directly responsive to estrogen. Oncogene. 1997;14:115–21. doi: 10.1038/sj.onc.1200808. [DOI] [PubMed] [Google Scholar]
- 21.Perou CM, Jeffrey SS, van de Rijn M, et al. Distinctive gene expression patterns in human mammary epithelial cells and breast cancers. Proc Natl Acad Sci U S A. 1999;96:9212–7. doi: 10.1073/pnas.96.16.9212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sorlie T, Tibshirani R, Parker J, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100:8418–23. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marquis ST, Rajan JV, Wynshaw-Boris A, et al. The developmental pattern of Brca1 expression implies a role in differentiation of the breast and other tissues. Nat Genet. 1995;11:17–26. doi: 10.1038/ng0995-17. [DOI] [PubMed] [Google Scholar]
- 25.Lane TF, Deng C, Elson A, Lyu MS, Kozak CA, Leder P. Expression of Brca1 is associated with terminal differentiation of ectodermally and mesodermally derived tissues in mice. Genes Dev. 1995;9:2712–22. doi: 10.1101/gad.9.21.2712. [DOI] [PubMed] [Google Scholar]
- 26.Jeffy BD, Hockings JK, Kemp MQ, et al. An estrogen receptor-alpha/p300 complex activates the BRCA-1 promoter at an AP-1 site that binds Jun/Fos transcription factors: repressive effects of p53 on BRCA-1 transcription. Neoplasia. 2005;7:873–82. doi: 10.1593/neo.05256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hockings JK, Thorne PA, Kemp MQ, Morgan SS, Selmin O, Romagnolo DF. The ligand status of the aromatic hydrocarbon receptor modulates transcriptional activation of BRCA-1 promoter by estrogen. Cancer Res. 2006;66:2224–32. doi: 10.1158/0008-5472.CAN-05-1619. [DOI] [PubMed] [Google Scholar]
- 28.Fan S, Wang J, Yuan R, et al. BRCA1 inhibition of estrogen receptor signaling in transfected cells. Science. 1999;284:1354–6. doi: 10.1126/science.284.5418.1354. [DOI] [PubMed] [Google Scholar]
- 29.Xu J, Fan S, Rosen EM. Regulation of the estrogen-inducible gene expression profile by the breast cancer susceptibility gene BRCA1. Endocrinology. 2005;146:2031–47. doi: 10.1210/en.2004-0409. [DOI] [PubMed] [Google Scholar]
- 30.Zheng L, Annab LA, Afshari CA, Lee WH, Boyer TG. BRCA1 mediates ligand-independent transcriptional repression of the estrogen receptor. Proc Natl Acad Sci U S A. 2001;98:9587–92. doi: 10.1073/pnas.171174298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fan S, Ma YX, Wang C, et al. Role of direct interaction in BRCA1 inhibition of estrogen receptor activity. Oncogene. 2001;20:77–87. doi: 10.1038/sj.onc.1204073. [DOI] [PubMed] [Google Scholar]
- 32.Fan S, Ma YX, Wang C, et al. p300 Modulates the BRCA1 inhibition of estrogen receptor activity. Cancer Res. 2002;62:141–51. [PubMed] [Google Scholar]
- 33.Kawai H, Li H, Chun P, Avraham S, Avraham HK. Direct interaction between BRCA1 and the estrogen receptor regulates vascular endothelial growth factor (VEGF) transcription and secretion in breast cancer cells. Oncogene. 2002;21:7730–9. doi: 10.1038/sj.onc.1205971. [DOI] [PubMed] [Google Scholar]
- 34.Johannsson OT, Idvall I, Anderson C, et al. Tumour biological features of BRCA1-induced breast and ovarian cancer. Eur J Cancer. 1997;33:362–71. doi: 10.1016/s0959-8049(97)89007-7. [DOI] [PubMed] [Google Scholar]
- 35.Verhoog LC, Brekelmans CT, Seynaeve C, et al. Survival and tumour characteristics of breast-cancer patients with germline mutations of BRCA1. Lancet. 1998;351:316–21. doi: 10.1016/s0140-6736(97)07065-7. [DOI] [PubMed] [Google Scholar]
- 36.Hosey AM, Gorski JJ, Murray MM, et al. Molecular basis for estrogen receptor alpha deficiency in BRCA1-linked breast cancer. J Natl Cancer Inst. 2007;99:1683–94. doi: 10.1093/jnci/djm207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu S, Ginestier C, Charafe-Jauffret E, et al. BRCA1 regulates human mammary stem/progenitor cell fate. Proc Natl Acad Sci U S A. 2008;105:1680–5. doi: 10.1073/pnas.0711613105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turner N, Tutt A, Ashworth A. Hallmarks of ‘BRCAness’ in sporadic cancers. Nat Rev Cancer. 2004;4:814–9. doi: 10.1038/nrc1457. [DOI] [PubMed] [Google Scholar]
- 39.Meijers-Heijboer H, Brekelmans CT, Menke-Pluymers M, et al. Use of genetic testing and prophylactic mastectomy and oophorectomy in women with breast or ovarian cancer from families with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2003;21:1675–81. doi: 10.1200/JCO.2003.09.052. [DOI] [PubMed] [Google Scholar]
- 40.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–88. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 41.Narod SA, Brunet JS, Ghadirian P, et al. Tamoxifen and risk of contralateral breast cancer in BRCA1 and BRCA2 mutation carriers: a case-control study. Hereditary Breast Cancer Clinical Study Group. Lancet. 2000;356:1876–81. doi: 10.1016/s0140-6736(00)03258-x. [DOI] [PubMed] [Google Scholar]
- 42.Eisen A, Lubinski J, Klijn J, et al. Breast cancer risk following bilateral oophorectomy in BRCA1 and BRCA2 mutation carriers: an international case-control study. J Clin Oncol. 2005;23:7491–6. doi: 10.1200/JCO.2004.00.7138. [DOI] [PubMed] [Google Scholar]
- 43.Folkerd EJ, Martin LA, Kendall A, Dowsett M. The relationship between factors affecting endogenous oestradiol levels in postmenopausal women and breast cancer. The Journal of steroid biochemistry and molecular biology. 2006;102:250–5. doi: 10.1016/j.jsbmb.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 44.Kaaks R, Rinaldi S, Key TJ, et al. Postmenopausal serum androgens, oestrogens and breast cancer risk: the European prospective investigation into cancer and nutrition. Endocrine-related cancer. 2005;12:1071–82. doi: 10.1677/erc.1.01038. [DOI] [PubMed] [Google Scholar]
- 45.Miller WR. Aromatase inhibitors: mechanism of action and role in the treatment of breast cancer. Semin Oncol. 2003;30:3–11. doi: 10.1016/s0093-7754(03)00302-6. [DOI] [PubMed] [Google Scholar]