Abstract
Receptor protein tyrosine phosphatases (RPTPs) control many aspects of nervous system development. At the Drosophila neuromuscular junction (NMJ), regulation of synapse growth and maturation by the RPTP LAR depends on catalytic phosphatase activity and on the extracellular ligands Syndecan and Dally-like. We show here that the function of LAR in controlling R7 photoreceptor axon targeting in the visual system differs in several respects. The extracellular domain of LAR important for this process is distinct from the domains known to bind Syndecan and Dally-like, suggesting the involvement of a different ligand. R7 targeting does not require LAR phosphatase activity, but instead depends on the phosphatase activity of another RPTP, PTP69D. In addition, a mutation that prevents dimerization of the intracellular domain of LAR interferes with its ability to promote R7 targeting, although it does not disrupt phosphatase activity or neuromuscular synapse growth. We propose that LAR function in R7 is independent of its phosphatase activity, but requires structural features that allow dimerization and may promote the assembly of downstream effectors.
Keywords: synapse, neuromuscular junctions, dimerization, wedge
Receptor protein tyrosine phosphatases (RPTPs) are required for nervous system development in both vertebrates and invertebrates (1). Of the six Drosophila RPTPs, Leukocyte antigen-related (LAR) has been studied in most detail due to its non-redundant role in several developmental processes. In Lar mutant embryos, motor neurons in the intersegmental nerve b (ISNb) fail to innervate the appropriate muscles and aberrantly track along the ISN (2). LAR has two distinct functions at the synapses formed by larval motor neurons on their target muscles. Synapse size as defined by the number of synaptic boutons present at these larval neuromuscular junctions (NMJs) is proportional to Lar dosage; and LAR controls active zone morphogenesis and thus synaptic strength (3). In the visual system, LAR enables photoreceptor axons to establish connections to the correct synaptic partners. Photoreceptors R1–R6 project into the lamina, where the axons from a single ommatidium defasciculate and connect to six different laminar cartridges; this defasciculation requires Lar (4). Photoreceptors R7 and R8, which mediate color vision, project beyond the lamina to terminate in two distinct layers of the medulla, R8 in M3 and R7 in the deeper M6 layer (5). In Lar mutants, most R7 axons terminate inappropriately in M3, the same layer as R8 (4, 6).
LAR and its vertebrate homologues PTPσ and PTPδ are type IIa RPTPs, which have two intracellular phosphatase domains (D1 and D2) and extracellular Ig (Ig) and fibronectin type III (FNIII) domains. The membrane-distal D2 domains of such RPTPs show no phosphatase activity on artificial substrates in vitro (7–9). Nevertheless, the LAR D2 domain is essential for R7 targeting, where it may act by recruiting the scaffolding protein Liprin-α (10) or regulating the activity of the D1 domain (8). An important class of ligands for type IIa RPTPs are heparan sulfate proteoglycans (HSPGs) such as Agrin and Collagen XVIII, which bind to PTPσ (11), and Syndecan (Sdc) and Dally-like (Dlp), which control the activity of Drosophila LAR in motor neurons (12, 13). Sdc and Dlp both bind to the Ig domains of LAR, but Sdc promotes LAR activity while Dlp antagonizes it (12, 13). Of the nine LAR FNIII domains, only the fifth has known binding partners, the Laminin-Nidogen extracellular matrix complex and a small alternatively spliced secreted isoform of LAR itself (14, 15).
Ligand binding can control RPTP activity by regulating interactions between receptor molecules; for example, the cytokine Pleiotrophin inhibits the activity of RPTPβ by inducing its oligomerization (16). Numerous RPTPs have been shown to form dimers in cultured cells (17–20). The crystal structure of the D1 phosphatase domain of RPTPα shows a dimer in which a wedge from the juxtamembrane region of one monomer blocks the active site of the other monomer (21), providing a possible mechanism for dimerization-induced inhibition. Mutations in this wedge reduce RPTPα dimerization, restore activity to forms of the RPTP CD45 or RPTPα forced to dimerize by changes in their extracellular domains, and increase the activity of CD45 in vivo (18, 22–25). Ligand binding may also have effects other than controlling phosphatase activity, since there are reports of phosphatase-independent functions for RPTPs; for instance, PTPμ mediates cell adhesion independently of its intracellular domain (26, 27), and the intracellular domain of ICA512 acts as a transcription factor (28).
We show here that R7 targeting requires the three membrane-proximal FNIII domains of LAR, rather than the Ig domains, suggesting the involvement of a ligand other than Sdc or Dlp. Unlike NMJ growth, R7 targeting does not require catalytic residues of the phosphatase domains. We also report that a mutation in the wedge inhibits dimerization of the LAR intracellular domain. This mutant form of LAR retains phosphatase activity and the ability to promote NMJ growth, but is not functional in R7. These results show that LAR function in R7 targeting is independent of its phosphatase activity, but requires a conformation dependent on the wedge domain.
Results
Phosphatase Activity Is Not Required for LAR Function in R7 Targeting.
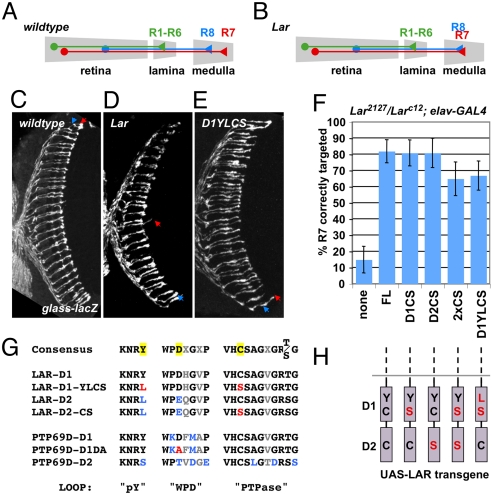
Although RPTPs are generally thought to act by dephosphorylating substrates, several studies support the existence of phosphatase-independent functions (26–28). Phosphatase activity of LAR is essential for its function in regulating NMJ size (13) and for its ability to cause motor axon guidance defects when ectopically expressed (12), but not to restore viability to Lar mutants (8). In the absence of LAR, most R7 photoreceptor axons terminate at the more superficial R8 layer (4, 6) (Fig. 1 A–D). This mistargeting can be strongly rescued by pan-neuronal expression of wild-type UAS-LAR driven by elav-GAL4 (Fig. 1F). To determine whether the function of LAR in R7 targeting requires its catalytic activity, we tested whether Lar mutants could be rescued by UAS-LAR transgenes containing point mutations that change the catalytic cysteine to serine in the D1 (LAR-D1CS), the D2 (LAR-D2CS) or both phosphatase domains (LAR-2XCS) (Fig. 1 G and H) (8). Surprisingly, transgenes with either or both cysteines mutated strongly rescued R7 targeting in a Lar null mutant background; indeed, phosphatase-dead LAR-D1CS was as active as wild-type LAR in this assay (Fig. 1F). Nevertheless, the cytoplasmic domain of LAR is essential for R7 targeting (6, 10), indicating that LAR is not simply an extracellular adhesion molecule, but has an intracellular function.
Fig. 1.
Phosphatase activity of LAR is dispensable for R7 targeting. (A and B) Schematic representation of the photoreceptor axon projection pattern in wild-type (A) and Lar mutants (B). (C–E) Horizontal sections of adult heads carrying glass-lacZ to label all photoreceptors, stained with anti-β-galactosidase. Arrowheads indicate the target layers of R8 (blue) and R7 (red). (C) Wild-type; (D) Larc12/Lar2127; (E) Larc12/Lar2127, elav-GAL4; UAS-LARD1YLCS/+. (F) Horizontal sections like those shown in (C–E) were scored for R7 targeting defects. In Larc12/Lar2127, elav-GAL4 mutants, only 15% of R7 axons project beyond the R8 layer, while addition of the indicated UAS-LAR transgenes significantly increased this percentage (FL 82%, D1CS 83%, D2CS 81%, 2xCS 65%, D1YLCS 67%). A schematic of the LAR phosphatase domains with the introduced point mutations in red is shown in (H). (G) Sequence comparison of highly conserved structural motifs in domains D1 and D2 of Drosophila LAR and PTP69D with the corresponding consensus sequences (32). Variable amino acid residues are shown in gray, residues deviating from the consensus in blue, and residues mutated in this study are highlighted yellow in the consensus sequences and shown in red in the construct sequences.
Mutating the catalytic cysteine might create a substrate-trapping form of the enzyme, which could bind the substrate in its active site without dephosphorylating and releasing it (29, 30). By removing phosphorylated substrate from circulation, such a substrate-trap could mimic the function of the wild-type enzyme. To test this hypothesis, we mutated a conserved tyrosine residue in the phosphotyrosine recognition loop, which interacts with phosphotyrosine to stabilize substrate binding in the active site (31, 32). The presence of a leucine at this position in the LAR D2 domain (Fig. 1G) is one of the two amino acid substitutions that render the domain catalytically inactive (33). To prevent prolonged substrate binding in the active site of LAR-D1CS, we changed the tyrosine in the LAR D1 domain (Y1504) to leucine. However, this UAS-LAR-D1YLCS transgene (Fig. 1 G and H) still strongly rescued R7 targeting in Lar mutants (Fig. 1 E and F), suggesting that LAR-D1CS promotes R7 targeting through a mechanism other than substrate sequestration.
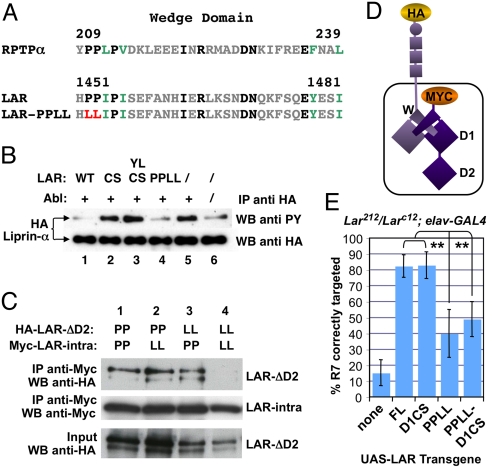
The LAR-D1CS protein used in this and previous studies was shown to lack all phosphatase activity in vitro on an artificial substrate (8). We wanted to test its activity on a substrate relevant for LAR function in vivo. The intracellular LAR-binding protein Liprin-α acts downstream of LAR in NMJ synapse growth and photoreceptor axon targeting, and is tyrosine phosphorylated in S2R+ cells (3, 10, 34). To facilitate the detection of LAR phosphatase activity in S2R+ cells, we increased the tyrosine phosphorylation of HA-tagged Liprin-α by coexpressing the Abelson (Abl) protein kinase (Fig. 2B), chosen because abl antagonizes Lar in the context of embryonic motor axon guidance (35). Expression of the intracellular domain of wild-type LAR decreased Liprin-α tyrosine phosphorylation in the presence of Abl (Fig. 2B). As predicted, intracellular LAR constructs carrying the D1CS mutation alone or in combination with the D1YL mutation did not reduce Liprin-α tyrosine phosphorylation (Fig. 2B). LAR-D1CS is thus catalytically inactive on a functionally relevant substrate in Drosophila cells.
Fig. 2.
The wedge domain of LAR contributes to dimerization and R7 targeting. (A) Sequence comparison of the Drosophila LAR and human RPTPα wedge domains. Identical residues are shown in black, similar amino acids in green and divergent residues in gray. Prolines 1,452/1,453 mutated to leucine in LAR-PPLL are marked in red. (B) Wild-type LAR and LAR-PPLL, but not LAR-D1CS or LAR-D1YLCS, are catalytically active in cell culture. HA-tagged Liprin-α was immunoprecipitated from S2R+ cells transfected with the indicated constructs and its tyrosine phosphorylation was assayed by Western blot (WB) with anti-phosphotyrosine (pY), normalized to anti-HA in the immunoprecipitate. Co-transfection of Abl increased Liprin-α pY levels (compare lane 5 to 6). In the presence of Abl (lanes 1–5), co-transfection of the intracellular domain of wild-type LAR (1) or LAR-PPLL (4) reduced pY levels on Liprin-α. Co-transfection of the intracellular domain of LAR-D1CS (2) or LAR-D1YLCS (3) did not reduce Liprin-α pY levels. (C) Co-immunoprecipitation of the Myc-tagged intracellular domain of LAR and HA-tagged LAR lacking the D2 domain expressed in S2R+ cells. Wild-type (lanes 1 and 3) or PPLL mutant Myc-LAR-intra (lanes 2 and 4) was pulled down from cell lysates using anti-Myc antibody. Levels of co-immunoprecipitated wild-type (lanes 1 and 2) or PPLL mutant (lanes 3 and 4) HA-LARΔD2 were assayed by WB (top row). After normalization to both immunoprecipitated Myc-LAR-intra (2nd row) and HA-LARΔD2 in the input (third row), there was a negligible difference in wild-type HA-LARΔD2 co-immunoprecipitated with PPLL mutant Myc-LAR-intra (2) compared to wild-type Myc-LAR-intra (1). There was a 5-fold decrease in PPLL mutant HA-LARΔD2 co-immunoprecipitated with PPLL mutant Myc-LAR-intra (4) compared to wild-type Myc-LAR-intra (3). (D) Schematic of a putative dimer formed by the tagged LAR constructs used in (C), indicating the wedge domain (W). (E) Quantification of R7 targeting defects in horizontal sections of adult heads. While elav-GAL4-driven expression of wild-type LAR (82%) or LAR-D1CS (83%) strongly rescues R7 targeting in Lar null mutants (15%), wedge mutant LAR-PPLL has a reduced ability to promote R7 targeting (40%), which is not due to hyperactivity since catalytically inactive LAR-PPLL-D1CS shows a similarly low percentage of correctly targeted R7 axons (49%). Double asterisks indicate statistical significance (P < 0.0001).
Phosphatase Activity Necessary for R7 Targeting Is Provided by PTP69D.
The results above suggest that R7 targeting does not require LAR phosphatase activity. However, LAR is not the only RPTP involved in this process; loss of the other Drosophila type IIa RPTP, PTP69D, from photoreceptors also causes R7 to terminate in the R8 layer (36)(Fig. S1A). Mutations that inactivate the remaining neuronally expressed RPTPs, PTP10D, PTP52F, and PTP99A, do not affect innervation of the R7 target layer (Fig. S2). To test whether R7 targeting requires the phosphatase activity of PTP69D, we used a UAS-PTP69D transgene with the catalytic aspartic acid in the WPD loop, which is essential for phosphatase activity of PTP69D and other tyrosine phosphatases (7, 30), mutated to alanine (PTP69D-D1DA) (Fig. 1G) (37). PTP69D-D1DA had a significantly lower ability to rescue targeting of Ptp69D mutant R7 photoreceptors than wild-type PTP69D (Fig. S1E), indicating a requirement for PTP69D phosphatase activity.
We further found that removal of PTP69D created a requirement for the phosphatase activity of LAR. Overexpression of LAR in photoreceptors mutant for Ptp69D can rescue R7 targeting (6). Although catalytically inactive LAR-D1CS rescued R7 targeting to the same extent as wild-type LAR in Lar mutants (Fig. S1E), wild-type LAR had a significantly greater ability to promote R7 targeting than LAR-D1CS in photoreceptors singly mutant for Ptp69D or doubly mutant for both Ptp69D and Lar (Fig. S1 B–E). LAR is thus capable of dephosphorylating substrates critical for R7 targeting, although in wild-type animals the necessary phosphatase activity is provided by PTP69D.
The Wedge Domain Is Required for LAR Dimerization and R7 Targeting.
At the NMJ, a switch from activation of LAR by the ligand Sdc to inhibition of its phosphatase activity by Dlp is thought to allow motor neurons to progress from synapse growth to active zone morphogenesis (13). Since Sdc is present on photoreceptors (38), LAR is likely to be active in R7 unless downregulated by an inhibitory ligand. A constitutively active form of LAR would enable us to test the importance of LAR inhibition. Since forced or ligand-induced dimerization inhibits the phosphatase activity of several RPTPs (16, 23, 39), we investigated the effect of dimerization on LAR activity.
Two differently tagged LAR constructs, one of which lacked the extracellular and transmembrane domains, and the other the D2 domain, could be co-immunoprecipitated from S2R+ cells (Fig. 2 C and D), showing that intracellular domain interactions promote LAR dimerization. Dimerization and inactivation of RPTPα can be prevented by mutating two conserved proline residues in the juxtamembrane wedge domain (Fig. 2A) (18, 21, 23). We mutated the wedge proline residues (PP) in Drosophila LAR to leucines (LL) to determine their effect on dimerization (Fig. 2A). Mutating the prolines in both monomers strongly reduced their co-immunoprecipitation; however, the presence of prolines in either monomer was sufficient for dimerization (Fig. 2C).
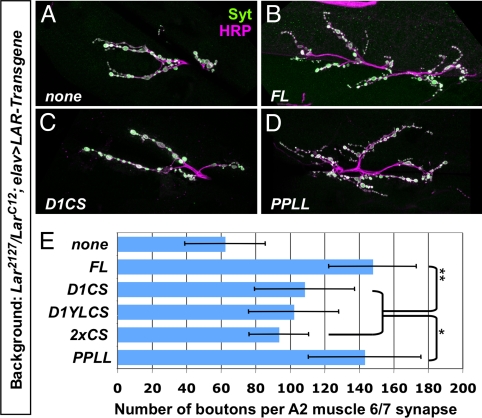
We next determined the effect of the wedge mutations on LAR catalytic activity. We found that the intracellular domain of LAR carrying the proline to leucine mutations (LAR-PPLL) was as active as wild-type LAR in reducing Abl-induced tyrosine phosphorylation of Liprin-α (Fig. 2B). We used NMJ synapse growth as an assay for phosphatase activity in vivo. A previous report found that LAR-2XCS did not promote significant synapse growth in a Lar mutant background (13). We confirmed the importance of LAR phosphatase activity at the NMJ using UAS-LAR-D1CS, its non-substrate-trapping derivative UAS-LAR-D1YLCS, and UAS-LAR-2XCS. Neuronal expression of these constructs only slightly increased NMJ synapse growth in a Lar mutant background (Fig. 3A, C, and E). The requirement for LAR phosphatase activity at the NMJ is not due to absence of PTP69D, since Ptp69D mutants also show reduced synapse size (Fig. S3). Neuronally expressed UAS-LAR-PPLL promoted synapse growth in Lar mutants to the same extent as wild-type UAS-LAR (Fig. 3 B, D, and E), indicating that LAR-PPLL can dephosphorylate the substrates relevant for NMJ growth.
Fig. 3.
NMJ growth requires LAR catalytic activity, but not an intact wedge domain. (A–D) Neuromuscular junctions at muscle 6/7 in segment A2 of filleted third-instar larvae were stained with anti-HRP (magenta) to label all neuronal membranes and anti-Syt (green) to label synaptic boutons. (A) Larc12/Lar2127, elav-GAL4; (B) Larc12/Lar2127, elav-GAL4; UAS-LAR/+; (C) Larc12/Lar2127, elav-GAL4; UAS-LAR-D1CS/+; (D) Larc12/Lar2127, elav-GAL4; UAS-LARPPLL/+. (E) Quantification of bouton number in segment A2 at the muscle 6/7 synapse. Compared to the number of boutons in Lar null mutants (63 ± 23), wild-type LAR (148 ± 25) and wedge mutant LAR-PPLL (143 ± 36) promote equivalent levels of synapse growth. Catalytically inactive forms of LAR show less ability to promote synapse growth (LAR-D1CS: 108 ± 29, LAR-D1YLCS: 102 ± 26, LAR-2XCS: 94 ± 17). Statistically significant differences are indicated by ** (P < 0.0001) or * (P < 0.005).
Both assays indicate that LAR-PPLL is catalytically active; to address whether its activity is constitutive, we misexpressed LAR-PPLL and wild-type LAR in the wing imaginal disc, which does not require Lar for its normal development. Ectopic expression of LAR disrupted wing vein formation (Fig. S4), possibly by antagonizing the epidermal growth factor receptor, a receptor tyrosine kinase responsible for vein formation (40). Phosphatase activity is required for this function of LAR, since expression of LAR-2XCS did not cause vein defects (Fig. S4). Ectopic expression of LAR-PPLL caused a greater frequency and severity of vein formation defects than wild-type LAR (Fig. S4), suggesting an increase in its phosphatase activity.
We next determined the effect of LAR-PPLL on R7 targeting. In a wild-type background, expression of LAR-PPLL in R7 had no apparent effect (Fig. S5), perhaps due to its dimerization with and inhibition by endogenous LAR. However, UAS-LAR-PPLL had much less ability to rescue R7 targeting in Lar mutants than wild-type UAS-LAR (Fig. 2E), although it was correctly localized to photoreceptor growth cones (Fig. S6). If this R7 targeting defect reflected a detrimental effect of constitutive phosphatase activity, then inactivating the phosphatase domain of LAR-PPLL by introducing the D1CS mutation should restore its R7 targeting function. However, in a Lar mutant background UAS-LAR-PPLL-D1CS showed weak R7 rescuing activity similar to UAS-LAR-PPLL, rather than strong rescuing activity like UAS-LAR-D1CS (Fig. 2E). Since eliminating its phosphatase activity does not restore the function of LAR-PPLL, it is unlikely that the role of the wedge prolines is to allow inhibition of LAR phosphatase activity. Taken together, these findings show that LAR phosphatase activity neither promotes nor inhibits R7 targeting, and are consistent with a non-catalytic role for LAR in this process.
LAR Function in R7 May Be Controlled by an Uncharacterized Ligand.
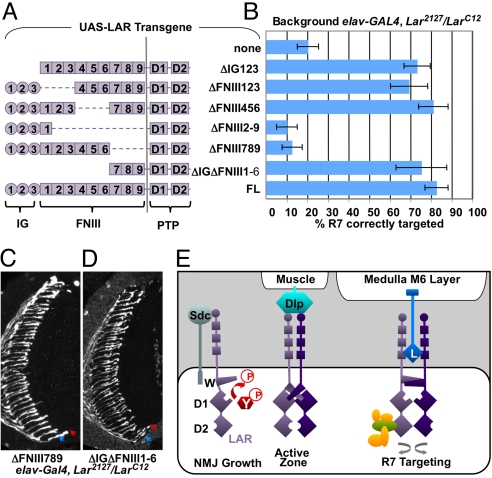
The HSPGs Sdc and Dlp are thought to regulate LAR phosphatase activity in NMJ morphogenesis and motor axon guidance (12, 13). It is unclear whether the same ligands control the phosphatase-independent function of LAR in R7 targeting, since sdc and dlp mutations have complex effects on photoreceptor projection patterns (38). Sdc and Dlp bind exclusively to the three Ig domains of LAR (12, 13). Since a UAS-LAR transgene lacking the three Ig domains (8) strongly rescued R7 targeting in a Lar mutant background (Fig. 4 A and B), Sdc and Dlp are unlikely to be the relevant ligands for this process.
Fig. 4.
R7 targeting requires only FNIII domains 7–9 in the LAR extracellular domain. (A) A schematic of the different LAR extracellular deletion constructs. Squares indicate FNIII domains and circles Ig domains. (B) Quantification of R7 targeting defects. In Larc12/Lar2127, elav-GAL4 mutants only 20% of R7 axons are correctly targeted. Pan-neuronal expression of the UAS-LAR extracellular deletion constructs rescues R7 targeting if they retain FNIII domains 789 (ΔIg123 73%, ΔFNIII123 69%, ΔFNIII456 81%, ΔIgΔFNIII1–6 75%, FL 83%). Constructs lacking FNIII789 do not rescue R7 targeting (ΔFNIII2–9 10%, ΔFNIII789 13%). (C and D) Horizontal sections of adult heads carrying glass-lacZ and stained with anti-β-galactosidase. Arrowheads indicate the terminal layers of R8 (blue) and R7 (red). (C) Larc12/Lar2127, elav-GAL4; UAS-LARΔFNIII789/+. (D) Larc12/Lar2127, elav-GAL4; UAS-LARΔIgΔFNIII1–6/+. (E) Distinct mechanisms of LAR signaling in Drosophila neurons. LAR (purple) may promote synapse growth as a catalytically active monomer with an unpaired wedge domain (W) that can dephosphorylate tyrosine-phosphorylated substrates (pY, red). The HSPG ligand Sdc acts in motor neurons to increase LAR activity by binding to its Ig domains (circle). Active zone morphogenesis requires competitive binding of the inhibitory HSPG ligand Dlp expressed in muscle cells to the Ig domains. Dlp inhibits LAR phosphatase activity, perhaps by inducing dimerization through insertion of the wedge domain of one monomer into the active site of the other. R7 targeting requires binding of an unknown ligand (L) to FNIII domains 7–9 (square), and an intact wedge domain. Dimerization mediated by the wedge domain may result in conformational changes that allow binding of a transmembrane ligand presented by the medulla target cells, or an intracellular adaptor protein (green). This scaffolding function of LAR might increase adhesion to target neurons through interactions with an intracellular protein network (yellow).
In contrast, deletion of 8 of the 9 FNIII domains (LARΔFNIII2–9) (8) strongly reduced the ability of LAR to rescue R7 targeting (Fig. 4 A and B). We used deletion constructs lacking subsets of FNIII domains to analyze whether individual FNIII domains differ in their ability to promote R7 targeting. R7 targeting was rescued by neuronal expression of constructs lacking either FNIII domains 1–3 or FNIII domains 4–6 (Fig. 4 A and B). Only loss of the three most proximal FNIII domains, 7–9, caused a decrease in rescuing activity similar to deletion of domains 2–9 (Fig. 4 A–C). To test whether these domains were not only necessary, but also sufficient, to promote R7 targeting, we generated a UAS-LAR transgene with an extracellular domain solely composed of FNIII domains 7–9 (LARΔIgΔFNIII1–6). Neuronal expression of this construct strongly rescued R7 targeting (Fig. 4 A, B, and D). FNIII domains 7–9 are therefore sufficient to promote R7 targeting, and are likely to bind a novel ligand that regulates the non-catalytic function of LAR. The function of LAR in R7 thus differs from its function at the NMJ in three respects: its independence of intrinsic phosphatase activity, its requirement for an intact wedge domain, and its regulation by a distinct extracellular ligand.
Discussion
We have shown that a catalytically inactive form of LAR (LAR-D1CS) can promote R7 targeting. Its function in R7 is not due to substrate trapping, since it does not require the tyrosine residue that stabilizes substrate binding in the active site (31). Conversely, a mutant form of LAR that retains phosphatase activity in vitro and phosphatase-dependent functions in vivo (LAR-PPLL) fails to promote R7 targeting. This defect is not due to unregulated LAR phosphatase activity, because mutating the active site of LAR-PPLL does not restore its function in R7. We therefore conclude that R7 targeting is independent of LAR catalytic activity. In contrast, the phosphatase activity of the RPTP PTP69D is required for this process. Our rescue experiments suggest that LAR has retained the ability to dephosphorylate substrates of PTP69D, perhaps because it acts on the same molecules in other processes such as NMJ synapse growth. PTP69D overexpression does not rescue R7 targeting in Lar mutants (6), suggesting that LAR has evolved a second function.
Several elements of the LAR protein are essential for its non-catalytic function in R7. Within the extracellular domain, FNIII domains 7–9 are necessary and sufficient to promote R7 targeting. The identified ligands for Drosophila and vertebrate LAR do not interact with these domains, suggesting the involvement of an unknown ligand (Fig. 4E). In Lar mutants, R7 axons initially project beyond R8, but then retract back to the R8 layer (4, 6), suggesting that LAR stabilizes adhesion between R7 and its target cells. Interaction with a transmembrane rather than a secreted ligand would best direct the formation of stable contacts with specific medulla target neurons. Interestingly, the transmembrane protein Netrin-G ligand-3 has recently been shown to interact with vertebrate LAR, although its binding site on LAR is unknown (41). While interactions of the Ig domains with the HSPGs Sdc and Dlp regulate the catalytic activity of LAR, ligand binding to the FNIII domains might induce conformational changes that reveal binding sites for intracellular adaptor proteins, or control proteolytic cleavage, which regulates the association of vertebrate LAR with β-catenin (42). Ligand binding might also localize LAR to a specific region in the R7 growth cone.
In the intracellular domain, the two juxtamembrane proline residues required for R7 targeting are homologous to prolines that stabilize the wedge domain of RPTPα, which mediates catalytic inhibition through dimerization (18, 21, 23). Although LAR function in R7 is phosphatase-independent, it may require dimerization (Fig. 4E). Our co-immunoprecipitation assays support the existence of LAR homodimers in Drosophila cells, consistent with reports of RPTP dimers in vertebrate cell types (17–20). However, structural studies of LAR family RPTPs suggest that steric hindrance by the D2 domains would prevent the wedge-mediated mode of dimerization (33, 43). Mutations in the wedge prolines may affect LAR dimerization because the relative orientations of D1 and D2 are flexible in vivo, or because the wedge contributes to dimerization mediated by interactions between D1 and D2 domains (20, 43, 44), or their effect may be indirect. Consistent with an essential role for dimerization, a peptide homologous to the wedge specifically inhibits the biological functions of LAR in vertebrate cells (45). Interestingly, a wedge mutation in the mouse RPTP CD45 has opposite effects on T-cell response in central and peripheral T cells (46). This suggests that RPTPs other than LAR may also signal in both catalytic and non-catalytic modes that are affected differently by changes in the wedge domain.
Dimerization could affect the ability of LAR to bind either downstream signal transduction molecules or upstream ligands (17). Ligand-regulated binding of adaptor proteins, such as Liprin-α, might assemble a protein network that mediates functions independent of LAR phosphatase activity (Fig. 4E). Most intracellular binding partners of LAR, including Abl, Enabled and Liprin-α, interact with the catalytically inactive D2 domain (3, 10, 35), which is essential for R7 targeting (10). In vertebrates, large PTPs that contain protein interaction domains such as FERM or PDZ domains can act independently of phosphatase activity (47). Perhaps the catalytically inactive PTP-D2 domains of LAR and other RPTPs have evolved to act as protein docking sites.
We show here that LAR uses two very different modes of signaling at the NMJ and in R7. R7 targeting employs a ligand distinct from the HSPGs Sdc and Dlp that control LAR activity at the NMJ (13). While the catalytic activity of LAR is required to promote NMJ synapse growth (13), it is dispensable for R7 targeting. Inhibition of LAR catalytic activity also plays no role in R7 targeting, although it is essential for active zone morphogenesis (13). Finally, an intact wedge domain is necessary for LAR to function in R7, but not in NMJ growth. Synapse growth and R7 targeting are likely to involve different cellular responses to LAR signaling. The R7 targeting defect reflects reduced adhesion to target neurons (4, 6), while NMJ growth is achieved by sprouting and extension of new axonal branches. The two signaling modes probably require distinct downstream factors, in addition to common components such as Liprin-α (3, 6, 10, 34, 48). The different requirements for LAR structural motifs make it possible to selectively interfere with only a subset of LAR-dependent processes. Since LAR and other RPTPs are implicated in various human diseases (49–51), differential usage of signaling modes may prove important for the development of selective pharmaceutical reagents.
Materials and Methods
Genetics.
UAS-HA-LAR transgenic flies were made with the construct described in (10). UAS-LAR extracellular deletion constructs (ΔIg123, ΔFNIII123, ΔFNIII456, ΔFNIII789 and ΔFNIII2–9) and active site mutations (D1CS, D2CS, and 2xCS) were described in (8). Other stocks used were Lar2127, LarC12 and UAS-LAR (6), glass-lacZ (52), elav-GAL4, and sev-GAL4 (Bloomington Drosophila Stock Center).
Transgenes and Expression Constructs.
To make UAS-HA-LAR-ΔIg123ΔFNIII1–6, the signal sequence from Wingless followed by three copies of the HA epitope tag (36) was fused in frame to the N terminus of FNIII domain 7 of LAR. This construct contains LAR amino acids 907–2,029. UAS-HA-LAR contains LAR amino acids 33–2,029 (10). UAS-HA-LAR-ΔD2 was derived from this construct using PCR to delete amino acids 1,811–2,024 of LAR. pPAC-Myc-LARintra was made by adding five copies of the Myc epitope tag to the N terminus of the LAR intracellular domain (LAR amino acids 1,415–2,029) in the pPAC-PL vector. Mutagenic primers were used to introduce the point mutations D1-C1670S, D1-Y1504L and PP1452/1453LL into the corresponding LAR constructs by PCR. The Abl-Myc sequence inserted into pPAC-PL to generate pPAC-Abl-Myc was from pMET-Abl-Myc (53).
Immunohistochemistry.
Primary antibodies used were rabbit anti-β-galactosidase (1:2,500; Cappel) and rabbit anti-Synaptotagmin (Syt; 1:2,000; a gift from H. Bellen). Primary antibody incubations were performed overnight at 4 °C. Fluorescently conjugated secondary antibodies and goat anti-horseradish peroxidase (HRP; Jackson ImmunoResearch) were used at 1:250 for 2 h at room temperature. Fluorescent images were collected on a Zeiss LSM510 confocal microscope. Adult head sections were stained as described (36). Larval NMJs were stained as described (3).
Quantification of Phenotypic Defects.
R7 targeting was quantified as previously described (10). Larval fillets stained with anti-HRP and anti-Syt were used to obtain maximum intensity projections of stacks of confocal images of the muscle 6/7 synapse in abdominal segment 2. Boutons per synapse were counted blind in groups of 2–5 genotypes. Statistical analysis of all quantifications used Microsoft Excel and Student's t-test (http://www.physics.csbsju.edu/stats/t test.html). Error bars indicate standard deviations. One transgenic line per construct was tested with the following exceptions: data from more than one line were pooled for LAR-YLD1CS (2×), LAR-PPLL (2×), LAR-PPLLD1CS (3×), LAR-ΔIg123ΔFNIII1–6 (3×), and LAR-WT (2× only in Fig. 3).
Co-Immunoprecipitation (Co-IP) and Phospho-Tyrosine (pY) Detection.
Culturing and transfection of S2R+ cells was performed as described (10). Forty-eight hours after transient transfection, S2R+ cells were rinsed briefly in PBS (coIP) or ddH2O (pY) and lysed in ice-cold IP buffer [50 mM Tris, pH 7.5, 150 mM NaCl, 1 mM NaF, and 1% Nonidet P-40 with protease inhibitors (Roche)] including 1 mM EDTA for pY detection. Proteins were immunoprecipitated with 2 μg mouse anti-Myc 9E10 (Santa Cruz Biotechnology) or 1.5 μg rat anti-HA 3F10 (Roche). Protein-G agarose beads (Roche) were used for precipitation according to the manufacturer's instructions. Proteins were separated by SDS/PAGE and probed with mouse anti-Myc 9E10 (1:8,000), rat anti-HA 3F10 (1:1,000), or mouse anti p-Tyr (PY99)-HRP (Santa Cruz Biotechnology; 1:500) in Tris-buffered saline/0.2% Tween 20 with 5% BSA, followed by HRP-coupled donkey anti-mouse or anti-rat (1:8,000; Jackson ImmunoResearch). Signal was detected by enhanced chemiluminescence (Pierce). For signal quantification, scanned image files were analyzed using the Photoshop histogram tool to measure band intensity.
Additional methods are described in SI Text.
Supplementary Material
Acknowledgments.
We thank Hugo Bellen (Baylor College of Medicine), Catherine Collins (University of Michigan), Barry Dickson (Institute of Molecular Pathology, Vienna), David Forsthoefel (Ohio State University), Paul Garrity (Brandeis University), Mark Seeger (Ohio State University), Helen Sink (NYU School of Medicine), David Van Vactor (Harvard Medical School), the Bloomington Drosophila stock center, and the Developmental Studies Hybridoma Bank for fly stocks, reagents and technical advice and Sergio Astigarraga, Katrin Deininger, Claude Desplan, Thomas Hurd, Kevin Legent, Sylvie Ozon Rickman, Jean-Yves Roignant, and Josie Steinhauer for critical comments on the manuscript. This work was supported by March of Dimes Birth Defects Foundation Grant 1-FY04-101.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0903961106/DCSupplemental.
References
- 1.Johnson KG, Van Vactor D. Receptor protein tyrosine phosphatases in nervous system development. Physiol Rev. 2003;83:1–24. doi: 10.1152/physrev.00016.2002. [DOI] [PubMed] [Google Scholar]
- 2.Krueger NX, et al. The transmembrane tyrosine phosphatase DLAR controls motor axon guidance in Drosophila. Cell. 1996;84:611–622. doi: 10.1016/s0092-8674(00)81036-3. [DOI] [PubMed] [Google Scholar]
- 3.Kaufmann N, DeProto J, Ranjan R, Wan H, Van Vactor D. Drosophila liprin-alpha and the receptor phosphatase Dlar control synapse morphogenesis. Neuron. 2002;34:27–38. doi: 10.1016/s0896-6273(02)00643-8. [DOI] [PubMed] [Google Scholar]
- 4.Clandinin TR, et al. Drosophila LAR regulates R1–R6 and R7 target specificity in the visual system. Neuron. 2001;32:237–248. doi: 10.1016/s0896-6273(01)00474-3. [DOI] [PubMed] [Google Scholar]
- 5.Clandinin TR, Zipursky SL. Making connections in the fly visual system. Neuron. 2002;35:827–841. doi: 10.1016/s0896-6273(02)00876-0. [DOI] [PubMed] [Google Scholar]
- 6.Maurel-Zaffran C, Suzuki T, Gahmon G, Treisman JE, Dickson BJ. Cell-autonomous and -nonautonomous functions of LAR in R7 photoreceptor axon targeting. Neuron. 2001;32:225–235. doi: 10.1016/s0896-6273(01)00471-8. [DOI] [PubMed] [Google Scholar]
- 7.Marlo JE, Desai CJ. Loss of phosphatase activity in Ptp69D alleles supporting axon guidance defects. J Cell Biochem. 2006;98:1296–1307. doi: 10.1002/jcb.20862. [DOI] [PubMed] [Google Scholar]
- 8.Krueger NX, et al. Functions of the ectodomain and cytoplasmic tyrosine phosphatase domains of receptor protein tyrosine phosphatase Dlar in vivo. Mol Cell Biol. 2003;23:6909–6921. doi: 10.1128/MCB.23.19.6909-6921.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Streuli M, Krueger NX, Tsai AY, Saito H. A family of receptor-linked protein tyrosine phosphatases in humans and Drosophila. Proc Natl Acad Sci USA. 1989;86:8698–8702. doi: 10.1073/pnas.86.22.8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hofmeyer K, Maurel-Zaffran C, Sink H, Treisman JE. Liprin-alpha has LAR-independent functions in R7 photoreceptor axon targeting. Proc Natl Acad Sci USA. 2006;103:11595–11600. doi: 10.1073/pnas.0604766103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aricescu AR, McKinnell IW, Halfter W, Stoker AW. Heparan sulfate proteoglycans are ligands for receptor protein tyrosine phosphatase sigma. Mol Cell Biol. 2002;22:1881–1892. doi: 10.1128/MCB.22.6.1881-1892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fox AN, Zinn K. The heparan sulfate proteoglycan syndecan is an in vivo ligand for the Drosophila LAR receptor tyrosine phosphatase. Curr Biol. 2005;15:1701–1711. doi: 10.1016/j.cub.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 13.Johnson KG, et al. The HSPGs Syndecan and Dallylike bind the receptor phosphatase LAR and exert distinct effects on synaptic development. Neuron. 2006;49:517–531. doi: 10.1016/j.neuron.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 14.Yang T, et al. Leukocyte antigen-related protein tyrosine phosphatase receptor: A small ectodomain isoform functions as a homophilic ligand and promotes neurite outgrowth. J Neurosci. 2003;23:3353–3363. doi: 10.1523/JNEUROSCI.23-08-03353.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Grady P, Thai TC, Saito H. The laminin-nidogen complex is a ligand for a specific splice isoform of the transmembrane protein tyrosine phosphatase LAR. J Cell Biol. 1998;141:1675–1684. doi: 10.1083/jcb.141.7.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukada M, et al. Protein tyrosine phosphatase receptor type Z is inactivated by ligand-induced oligomerization. FEBS Lett. 2006;580:4051–4056. doi: 10.1016/j.febslet.2006.06.041. [DOI] [PubMed] [Google Scholar]
- 17.Lee S, et al. Dimerization of protein tyrosine phosphatase {sigma} governs both ligand binding and isoform specificity. Mol Cell Biol. 2007;27:1795–1808. doi: 10.1128/MCB.00535-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang G, den Hertog J, Hunter T. Receptor-like protein tyrosine phosphatase alpha homodimerizes on the cell surface. Mol Cell Biol. 2000;20:5917–5929. doi: 10.1128/mcb.20.16.5917-5929.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groen A, Overvoorde J, van der Wijk T, den Hertog J. Redox regulation of dimerization of the receptor protein-tyrosine phosphatases RPTPalpha, LAR, RPTPmu and CD45. FEBS J. 2008;275:2597–2604. doi: 10.1111/j.1742-4658.2008.06407.x. [DOI] [PubMed] [Google Scholar]
- 20.Blanchetot C, Tertoolen LG, Overvoorde J, den Hertog J. Intra- and intermolecular interactions between intracellular domains of receptor protein-tyrosine phosphatases. J Biol Chem. 2002;277:47263–47269. doi: 10.1074/jbc.M205810200. [DOI] [PubMed] [Google Scholar]
- 21.Bilwes AM, den Hertog J, Hunter T, Noel JP. Structural basis for inhibition of receptor protein-tyrosine phosphatase-alpha by dimerization. Nature. 1996;382:555–559. doi: 10.1038/382555a0. [DOI] [PubMed] [Google Scholar]
- 22.Majeti R, Bilwes AM, Noel JP, Hunter T, Weiss A. Dimerization-induced inhibition of receptor protein tyrosine phosphatase function through an inhibitory wedge. Science. 1998;279:88–91. doi: 10.1126/science.279.5347.88. [DOI] [PubMed] [Google Scholar]
- 23.Jiang G, et al. Dimerization inhibits the activity of receptor-like protein-tyrosine phosphatase-alpha. Nature. 1999;401:606–610. doi: 10.1038/44170. [DOI] [PubMed] [Google Scholar]
- 24.Hermiston ML, Tan AL, Gupta VA, Majeti R, Weiss A. The juxtamembrane wedge negatively regulates CD45 function in B cells. Immunity. 2005;23:635–647. doi: 10.1016/j.immuni.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Majeti R, et al. An inactivating point mutation in the inhibitory wedge of CD45 causes lymphoproliferation and autoimmunity. Cell. 2000;103:1059–1070. doi: 10.1016/s0092-8674(00)00209-9. [DOI] [PubMed] [Google Scholar]
- 26.Brady-Kalnay SM, Flint AJ, Tonks NK. Homophilic binding of PTP mu, a receptor-type protein tyrosine phosphatase, can mediate cell-cell aggregation. J Cell Biol. 1993;122:961–972. doi: 10.1083/jcb.122.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gebbink MF, et al. Cell-cell adhesion mediated by a receptor-like protein tyrosine phosphatase. J Biol Chem. 1993;268:16101–16104. [PubMed] [Google Scholar]
- 28.Mziaut H, et al. Synergy of glucose and growth hormone signaling in islet cells through ICA512 and STAT5. Nat Cell Biol. 2006;8:435–445. doi: 10.1038/ncb1395. [DOI] [PubMed] [Google Scholar]
- 29.Allard JD, Herbst R, Carroll PM, Simon MA. Mutational analysis of the SRC homology 2 domain protein-tyrosine phosphatase Corkscrew. J Biol Chem. 1998;273:13129–13135. doi: 10.1074/jbc.273.21.13129. [DOI] [PubMed] [Google Scholar]
- 30.Flint AJ, Tiganis T, Barford D, Tonks NK. Development of “substrate-trapping” mutants to identify physiological substrates of protein tyrosine phosphatases. Proc Natl Acad Sci USA. 1997;94:1680–1685. doi: 10.1073/pnas.94.5.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jia Z, Barford D, Flint AJ, Tonks NK. Structural basis for phosphotyrosine peptide recognition by protein tyrosine phosphatase 1B. Science. 1995;268:1754–1758. doi: 10.1126/science.7540771. [DOI] [PubMed] [Google Scholar]
- 32.Andersen JN, et al. Structural and evolutionary relationships among protein tyrosine phosphatase domains. Mol Cell Biol. 2001;21:7117–7136. doi: 10.1128/MCB.21.21.7117-7136.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nam HJ, Poy F, Krueger NX, Saito H, Frederick CA. Crystal structure of the tandem phosphatase domains of RPTP LAR. Cell. 1999;97:449–457. doi: 10.1016/s0092-8674(00)80755-2. [DOI] [PubMed] [Google Scholar]
- 34.Choe KM, Prakash S, Bright A, Clandinin TR. Liprin-alpha is required for photoreceptor target selection in Drosophila. Proc Natl Acad Sci USA. 2006;103:11601–11606. doi: 10.1073/pnas.0601185103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wills Z, Bateman J, Korey CA, Comer A, Van Vactor D. The tyrosine kinase Abl and its substrate enabled collaborate with the receptor phosphatase Dlar to control motor axon guidance. Neuron. 1999;22:301–312. doi: 10.1016/s0896-6273(00)81091-0. [DOI] [PubMed] [Google Scholar]
- 36.Newsome TP, Asling B, Dickson BJ. Analysis of Drosophila photoreceptor axon guidance in eye-specific mosaics. Development. 2000;127:851–860. doi: 10.1242/dev.127.4.851. [DOI] [PubMed] [Google Scholar]
- 37.Garrity PA, et al. Retinal axon target selection in Drosophila is regulated by a receptor protein tyrosine phosphatase. Neuron. 1999;22:707–717. doi: 10.1016/s0896-6273(00)80730-8. [DOI] [PubMed] [Google Scholar]
- 38.Rawson JM, et al. The heparan sulfate proteoglycans Dally-like and Syndecan have distinct functions in axon guidance and visual-system assembly in Drosophila. Curr Biol. 2005;15:833–838. doi: 10.1016/j.cub.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 39.Desai DM, Sap J, Schlessinger J, Weiss A. Ligand-mediated negative regulation of a chimeric transmembrane receptor tyrosine phosphatase. Cell. 1993;73:541–554. doi: 10.1016/0092-8674(93)90141-c. [DOI] [PubMed] [Google Scholar]
- 40.Roch F, Jimenez G, Casanova J. EGFR signaling inhibits Capicua-dependent repression during specification of Drosophila wing veins. Development. 2002;129:993–1002. doi: 10.1242/dev.129.4.993. [DOI] [PubMed] [Google Scholar]
- 41.Woo J, et al. Trans-synaptic adhesion between NGL-3 and LAR regulates the formation of excitatory synapses. Nat Neurosci. 2009;12:428–437. doi: 10.1038/nn.2279. [DOI] [PubMed] [Google Scholar]
- 42.Haapasalo A, et al. Presenilin/gamma-secretase-mediated cleavage regulates association of leukocyte-common antigen-related (LAR) receptor tyrosine phosphatase with beta-catenin. J Biol Chem. 2007;282:9063–9072. doi: 10.1074/jbc.M611324200. [DOI] [PubMed] [Google Scholar]
- 43.Barr AJ, et al. Large-scale structural analysis of the classical human protein tyrosine phosphatome. Cell. 2009;136:352–363. doi: 10.1016/j.cell.2008.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wallace MJ, Fladd C, Batt J, Rotin D. The second catalytic domain of protein tyrosine phosphatase delta (PTP delta) binds to and inhibits the first catalytic domain of PTP sigma. Mol Cell Biol. 1998;18:2608–2616. doi: 10.1128/mcb.18.5.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xie Y, et al. PTP wedge domain peptides: A novel approach for inhibition of PTP function and augmentation of PTK function. J Biol Chem. 2006;281:16482–16492. doi: 10.1074/jbc.M603131200. [DOI] [PubMed] [Google Scholar]
- 46.Hermiston ML, et al. Differential impact of the CD45 juxtamembrane wedge on central and peripheral T cell receptor responses. Proc Natl Acad Sci USA. 2009;106:546–551. doi: 10.1073/pnas.0811647106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wansink DG, et al. Mild impairment of motor nerve repair in mice lacking PTP-BL tyrosine phosphatase activity. Physiol Genomics. 2004;19:50–60. doi: 10.1152/physiolgenomics.00079.2004. [DOI] [PubMed] [Google Scholar]
- 48.Newsome TP, et al. Trio combines with dock to regulate Pak activity during photoreceptor axon pathfinding in Drosophila. Cell. 2000;101:283–294. doi: 10.1016/s0092-8674(00)80838-7. [DOI] [PubMed] [Google Scholar]
- 49.Langberg EC, Gu HF, Nordman S, Efendic S, Ostenson CG. Genetic variation in receptor protein tyrosine phosphatase sigma is associated with type 2 diabetes in Swedish Caucasians. Eur J Endocrinol. 2007;157:459–464. doi: 10.1530/EJE-07-0114. [DOI] [PubMed] [Google Scholar]
- 50.Muise AM, et al. Protein-tyrosine phosphatase sigma is associated with ulcerative colitis. Curr Biol. 2007;17:1212–1218. doi: 10.1016/j.cub.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 51.Schormair B, et al. PTPRD (protein tyrosine phosphatase receptor type delta) is associated with restless legs syndrome. Nat Genet. 2008;40:946–948. doi: 10.1038/ng.190. [DOI] [PubMed] [Google Scholar]
- 52.Moses K, Rubin GM. glass encodes a site-specific DNA-binding protein that is regulated in response to positional signals in the developing Drosophila eye. Genes Dev. 1991;5:583–593. doi: 10.1101/gad.5.4.583. [DOI] [PubMed] [Google Scholar]
- 53.Forsthoefel DJ, Liebl EC, Kolodziej PA, Seeger MA. The Abelson tyrosine kinase, the Trio GEF and Enabled interact with the Netrin receptor Frazzled in Drosophila. Development. 2005;132:1983–1994. doi: 10.1242/dev.01736. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.