Abstract
We have recently shown that sulindac, an anti-inflammatory drug, enhances the killing of cancer cells, but not normal cells, under conditions of oxidative stress, by mechanisms unrelated to its cyclooxygenase (COX) inhibition. To further study the protective effect of sulindac on cells exposed to oxidative stress, we have investigated the effect of sulindac on rat cardiac myocytes subjected to hypoxia/reoxygenation, as well as in a Langendorff model of myocardial ischemia. Low levels of sulindac could protect cardiac myocytes against cell death due to hypoxia/reoxygenation. In the Langendorff model sulindac provided significant protection against cell death, when the drug was fed to the animals before the removal of the heart for the Langendorff procedure. The results indicate that the primary protective effect of sulindac in these experiments does not involve its role as a COX inhibitor. Numerous signaling pathways have been implicated in myocardial protective mechanisms, many of which involve fluctuations in reactive oxygen species (ROS) levels. The results suggest that low levels of sulindac can induce a preconditioning response, triggered by ROS, to protect cardiac tissues against oxidative damage. Blocking of preconditioning pathways by administration of the PKC blocker chelerythrine abrogated the ischemic protection afforded by sulindac. Secondly, after feeding of sulindac, two end-effectors of preconditioning, inducible nitric oxide synthase and heat shock protein 27, were found to be markedly induced in the heart, dependent on PKC. These results suggest that sulindac may have therapeutic potential as a preconditioning agent.
Keywords: cardioprotection, myocardial ischemia
Sulindac is a nonsteroidal anti-inflammatory drug that is capable of inhibiting cyclo-oxygenases (COX) 1 and 2 (1). In addition to its known anti-inflammatory activity there have been numerous studies in recent years on the ability of sulindac and its metabolites to act as potential anti-cancer agents, based on their ability to slow the progression of colorectal polyps to colon cancer, as well as their ability to kill colon and other cancer cells (2, 3). We have recently shown that cancer and normal cells react differently to oxidative stress after exposure to sulindac. Sulindac can enhance the killing of cancer cells when exposed to an oxidizing agent, under conditions in which normal cells are either not affected, or show protection (4, 5).
Our interest in sulindac initially stemmed from the fact that the S epimer of sulindac is a substrate for methionine sulfoxide reductase A (MsrA) (6), which reduces the S epimer of sulindac to sulindac sulfide, the active COX inhibitor (see Fig. S1 for sulindac structure). The Msr system has been shown to be an important cellular protective system against oxidative stress, and may play a role in aging (7–9). The goal of the present studies was to look more closely at the ability of sulindac to protect normal cells against oxidative stress, and to determine whether the Msr system may be involved.
A well studied physiological system is the oxidative damage that occurs to cardiac tissue under conditions of hypoxia and reperfusion. One important physiological mechanism to protect cardiac and other cells against a variety of stresses, including oxidative stress, is preconditioning (10–12). Cells can be preconditioned by exposure to a nonlethal stress, such as limited ischemia, ROS, slightly elevated heat or even exercise (10). There are also pharmacological agents that can precondition cells including cytokines, nitric oxide donors, and opioid receptor agonists (13). The mechanism of preconditioning is complex but appears to involve signaling pathways in which specific isoforms of protein kinase c (PKC) (14, 15), MAP kinases and other kinases are activated leading to the activation of transcription factors such as NFkB and AP-1 (16). The later phase of this process eventually leads to the production of a series of protective proteins including inducible nitric oxide synthase (iNOS), MnSOD, HSPs, and activation of ion channels (10). Compounds that could precondition cells to oxidative stress might have important therapeutic value, since oxidative damage appears to play a major role in age related diseases.
In the present study, we have examined the protective effect of sulindac elicited by hypoxia/reoxygenation in both rat cardiac myocytes in culture and in a Langendorff model of myocardial ischemia, and provide evidence that sulindac can function as a preconditioning agent.
Results
Sulindac Protects Cardiac Myocytes Against Oxidative Damage.
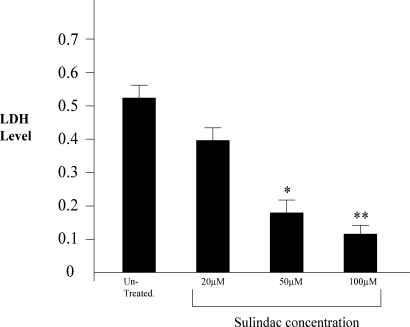
The initial experiments were designed to determine whether sulindac could protect primary neonatal cardiac myocytes in culture against oxidative damage. As described in Materials and Methods and Fig. 1, the cardiac myocytes were exposed to hypoxia and reoxygenation to promote oxidative damage in the presence or absence of sulindac. Cell viability was then assayed by LDH release. As shown in Fig. 1, sulindac protection is dose dependent in myocytes exposed to hypoxia/reoxygenation. Sulindac can significantly reduce the level of LDH released following hypoxia/reoxygenation at concentrations as low as 20 μM. As compared to controls, sulindac at 100 μM reduced LDH release, and presumably cell death, by approximately 4-fold. Higher levels of sulindac did not produce a greater effect. Tunel assays (see Materials and Methods) showed that the cell death was due primarily to apoptosis. After hypoxia/reoxygenation, close to 40% of the control cells were tunel positive whereas <5% of the sulindac treated cells were tunel positive. Based on the cell culture results with cardiac myocytes, studies were initiated to test the effect of sulindac in the intact heart using the Langendorff model.
Fig. 1.
Effect of sulindac in protecting cardiac myocytes. Lactate dehydrogenase (LDH) levels were measured in the media as an index of cell death and analyzed against untreated myocytes. Levels of LDH release are shown as absorbance units. (*, P < 0.05 vs. no drug; n = 6, **, P < 0.01 vs. no drug; n = 6).
Animals Fed Sulindac Are Protected Against Myocardial Damage Due to Ischemia and Reperfusion in the Langendorff Model.
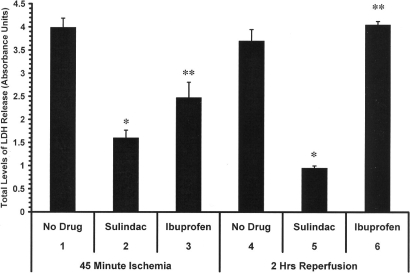
The ex vivo Langendorff procedure is a well established preparation used to investigate heart physiology, ischemia/reperfusion injury, and other cardiovascular insults. Animals were fed diets containing either no drug, sulindac, or ibuprofen at 0.2 mg/day for 48 h. The hearts were removed and exposed to 45 min no flow ischemia and 2 h reperfusion in the Langendorff model, in the absence of drug. As shown in Fig. 2, after 48 h, the levels of LDH released are significantly lower after a 45 min period of no flow ischemia in the hearts from sulindac-fed rats compared to the hearts from animals receiving the no drug diet (Fig. 2, lanes 1 and 2). As also shown in Fig. 2, lane 3, Ibuprofen-fed rats showed some protection after the 45 min ischemic period, although the LDH level was significantly higher than seen with sulindac (compare Fig. 2, lanes 2 and 3). These results suggested that COX inhibition may afford some protection during the ischemic period, which has been noted previously (17). However, during the 2 h reperfusion sulindac markedly protected the heart against oxidative damage as seen by the decrease in LDH levels compared to the no drug control (compare Fig. 2, lanes 4 and 5), whereas ibuprofen showed no protection at all (Fig. 2, lane 6). Infarct size as measured by TTC staining method on the hearts from ibuprofen-fed rats was 66.5%, which is similar to no drug controls (65.28%), whereas the infarct size for the hearts from sulindac-fed animals was 36%.
Fig. 2.
Effect of feeding sulindac and ibuprofen before performing the Langendorff procedure. Animals were fed either no drug, sulindac, or ibuprofen at 0.2 mg/day for 48 h before isolation of the heart for analysis on the Langendorff apparatus. Total LDH levels are shown after 45 min ischemia and 2 h of reperfusion. Animals were fed for 48 h with no drug (lanes 1,4), sulindac 0.2 mg/day (lanes 2,5), or ibuprofen 0.2 mg/day (lanes 3,6) after which each heart was isolated for analysis on the Langendorff apparatus. (*, P < 0.01 compared to no drug control; n = 5, **, P < 0.01 compared to sulindac treated hearts; n = 5).
Sulindac sulfone, the oxidized metabolite of sulindac, which is not a COX inhibitor or a substrate for the Msr enzymes, also gave significant protection in the Langendorff model under the conditions used. Specifically, for the animals fed sulindac sulfone, at the end of 45 min of ischemia total LDH levels were decreased by about 50% and by greater than 55% during the 2 h reperfusion period relative to no drug controls. These LDH values were close to what was observed with sulindac (Fig. S2).
The above results with ibuprofen and sulindac sulfone suggest that although some of the protective effect of sulindac during ischemia may be due to its COX inhibition, the major protective effect, especially during reperfusion, is not due to COX inhibition or to its ability to be a substrate for MsrA.
Evidence That Sulindac Protection Against Ischemic Heart Damage Occurs Through Chemical Preconditioning: Role of PKC.
One of the possibilities to explain the protective effect of sulindac in the Langendorff model was that it might be acting as a preconditioning agent. Since PKC is a known mediator of preconditioning, the effect of inhibiting PKC was determined, as well as the levels of two preconditioning end effectors, iNOS and Hsp27.
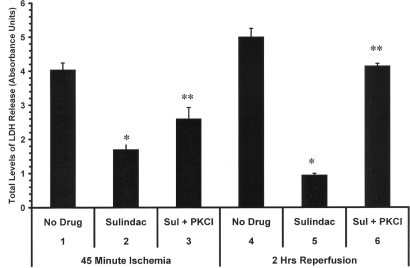
As seen in Fig. 3, lanes 1–3, blocking of PKC activation by daily administration of chelerythrine, a nonselective PKC blocker (lane 3), partly reversed the protection by sulindac during ischemia, as measured by levels of LDH (compare lane 3 to lane 2). It can be seen that chelerythrine treatment along with sulindac resulted in 30% greater cell death after 45 min ischemia than seen with hearts from animals fed sulindac alone. Lanes 4–6 (Fig. 3) show the results after 2 h of reperfusion. These results were more striking since LDH levels released from the hearts of animals that received sulindac plus chelerythrine (lane 6) were close to the values seen in animals that received no drug (lane 4) and approximately four times that seen in animals that received sulindac alone (lane 5). Animals that received only chelerythrine gave results similar to those seen in animals receiving no drug. It should be noted that PKC epsilon is believed to be the PKC isoform that is activated in preconditioning (10). However, the inhibitor used in these studies is known to inhibit all of the PKC isoforms, so we cannot be sure that the effect seen is specifically due to inhibition of PKC epsilon.
Fig. 3.
PKC inhibition reverses the effect of sulindac in the Langendorff heart model. Total LDH levels are shown after 45 min ischemia and 2 h of reperfusion. Animals were fed no drug (lanes 1,4), sulindac 0.2 mg/day for 48 h (lanes 2,5), or injected with chelerythrine (PKCI) 5 mg/kg (I.P., lanes 3,6) just before feeding with sulindac, after which the heart was isolated for analysis on the Langendorff apparatus. (*, P < 0.01 compared to no drug control; n = 5, **, P < 0.01 compared to sulindac treated hearts; n = 5).
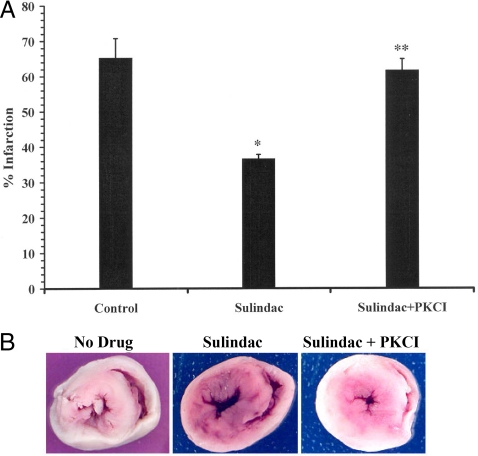
To verify the LDH results the percent of viable and infarcted heart tissue after ischemia/reperfusion was determined using the stain TTC (see Materials and Methods). Fig. 4A shows rats fed sulindac had an average infarction size of 36% whereas, hearts treated with sulindac and chelerythrine had 61.8% infarction, which was similar to control hearts. Representative images of TTC stained hearts show viable (red) vs. infarct (white) in hearts from rats fed no drug, sulindac, and sulindac + chelerythrine (Fig. 4B). It is clear that the hearts from the sulindac-fed animal had significantly more viable cardiac tissue.
Fig. 4.
Infarct size of hearts as measured by TTC staining. (A) Hearts from rats fed no drug, sulindac, or sulindac plus chelerythrine and exposed to ischemia and reperfusion were stained with 1% TTC, then cut transversely into 2-mm sections (see Materials and Methods). Percent infarction was determined using NIH image J analysis software. Graph represents percent of heart that was infarcted. (*, P < 0.01 compared to no drug control; n = 4, **, P < 0.01 compared to sulindac treated hearts; n = 4). (B) Representative sections from langendorff hearts following 45 min ischemia and 2 h reperfusion showing infarcted tissue (white) and viable tissue (red).
Sulindac Induces iNOS and Hsp27 Dependent on PKC and the Role of ROS in Sulindac Preconditioning.
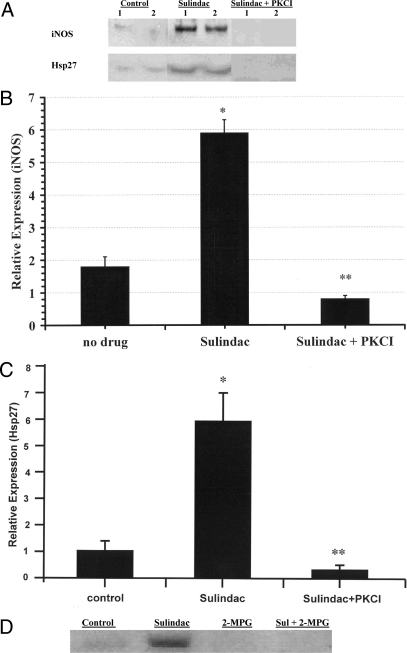
iNOS and Hsp27 are central participants in late phase preconditioning in heart (18). To investigate the possible role of iNOS and Hsp27 in sulindac protection, levels of these proteins expressed in rat myocardium were measured by Western blot in hearts from rats that had been fed 0.2 mg/day sulindac for 48 h (see Materials and Methods). As shown in Fig. 5A (top panel), there was a substantial and significant increase in both iNOS and Hsp27 production following sulindac treatment. It has also been shown that induction of protein in late ischemic preconditioning is dependent upon PKC epsilon activation (19). As also shown in Fig. 5A, the induction of both iNOS and Hsp27 was reversed when chelerythrine was given. Quantitation of the Western blot by densitometric scanning showed about a 3- to 4-fold increase in iNOS and 6-fold increase in Hsp27 expression after sulindac feeding (Fig. 5 B and C). As also shown in Fig. 5 B and C, when animals were injected with chelerythrine the sulindac induced increase in both iNOS and Hsp27 expression was reversed and these proteins remained at “no drug” control levels. These results indicate a likely role of both iNOS and Hsp27 in sulindac mediated ischemic protection. To determine whether iNOS was involved in the sulindac protective effect, animals were injected with the compound 1400W, a specific inhibitor of iNOS. These animals showed essentially no protection with sulindac after ischemia, and after reperfusion this drug resulted in a marked increase in cell death in the presence of sulindac. These results indicate a role of iNOS in the sulindac effect. We have also examined the effect of sulindac on other known end effectors of preconditioning including MnSOD and AKT. Modest increases of less than 2-fold were observed.
Fig. 5.
Sulindac induces expression of iNOS and Hsp27 in the rat myocardium dependent on PKC and ROS. (A) Rats were fed either no drug (control), sulindac 0.2 mg/day for 48 h, or injected (i.p.) with protein kinase C inhibitor chelerythrine (PKCI) 5 mg/kg plus sulindac 0.2 mg per day for 48 h. Results from two typical experiments in which iNOS and Hsp27 expression levels were determined by Western blot analysis as described in Materials and Methods. (B) Average results (n = 5) of iNOS densitometric scanning (*, P < 0.01 compared to no drug control; **, P < 0.01 compared to sulindac treated hearts). (C) Average results (n = 5) of Hsp27 densitometric scanning are presented (*, P < 0.01 compared to no drug control; **, P < 0.01 compared to sulindac treated hearts). (D) Effect of 2-MPG on iNOS levels in heart. Rats were fed either no drug (control); sulindac 0.2 mg/day for 48 h; 2-MPG alone (20 mg/kg, five injections per/day); sulindac 0.2 mg/day for 48 h and also injected (i.p.) with 2-MPG (20 mg/kg, five injections per/day).
Sulindac has recently been shown to increase ROS in cancer cells (4, 5). Since ROS is a known preconditioning trigger, the effect of scavenging ROS by using N-2-mercaptopropionyl glycine (2-MPG) (see Materials and Methods) was determined on the induction of iNOS. A typical experiment showing the effect of 2-MPG on the induction of iNOS by sulindac is shown in Fig. 5D. Levels of iNOS were decreased to control levels in rats fed sulindac that had been injected with the antioxidant 2-MPG. 2-MPG alone had no effect on iNOS levels (Fig. 5D). These results suggest that sulindac triggers preconditioning through the production of sublethal levels of ROS in the myocardium.
Discussion
The studies presented here show that sulindac can protect both rat cardiac myocytes in culture and intact hearts (Langendorff model) against oxidative damage resulting from ischemia/reperfusion. As shown above, feeding of sulindac to rats in vivo followed by removal of the heart, wash-out of the drug and subsequent ischemia and reperfusion resulted in substantial protection against ischemia induced cell death relative to untreated hearts.
The initial hypothesis that led to our testing sulindac was based on the fact that the S epimer of sulindac is a substrate for MsrA (6), an enzyme that has been shown to protect cells against oxidative damage. However, there is no evidence to suggest that the protective effect of sulindac in our experiments is in any way related to the Msr enzyme system, especially since sulindac sulfone, which is not a substrate for MsrA, afforded cardiac protection. The major protective effect, especially during reperfusion, does not appear to be due to the well established role of sulindac as a COX inhibitor. Although another NSAID, ibuprofen, when fed for 2 days did show a weak protective effect during the ischemic period, it had no protective effect during reperfusion, and TTC staining of the heart after ischemia and reperfusion showed as much death in the heart from the ibuprofen-fed animals, as the untreated hearts. In addition, it should be noted that sulindac sulfone is not a COX inhibitor and it provided significant protection in these experiments, as described above. Although we cannot eliminate that some of the sulindac effect during ischemia is due to its COX inhibition, it seems clear that sulindac is protecting the heart in these experiments primarily by another mechanism.
Numerous signaling pathways have been implicated in protective mechanisms, many of which require fluctuations in ROS levels as initiators or mediators. One obvious possibility that was considered is that sulindac induces tissue preconditioning against oxidative damage. The phenomenon of ischemic preconditioning has been described, whereby a short mild ischemic episode was found to protect against a later more severe ischemic event (10, 20). The protective effects we have observed in heart against tissue ischemia following preexposure to sulindac, along with the biochemical data presented on the role of PKC and the expression of iNOS and Hsp27, are consistent with the conclusion that this drug can function as a pharmacological preconditioning agent. Protein kinase C-epsilon is known to be an important mediator of late ischemic preconditioning in heart (21) and administration of the PKC blocker chelerythrine abrogated the ischemic protection afforded by sulindac. Secondly, as noted above, two end-effectors of preconditioning, iNOS and Hsp27, were induced at the protein level by greater than 3- and 6-fold, respectively, after 48 h feeding with sulindac. Furthermore, administration of chelerythrine inhibited iNOS and Hsp27 induction in rats that were fed sulindac.
iNOS is known to be a major contributor to late myocardial ischemic preconditioning. In a mouse model the targeted deletion of the iNOS gene has been shown to prevent the preconditioning induced by a range of stimuli including ischemia, adenosine agonists, and exercise (22). The induction of iNOS by sulindac, concurrently with cardio-protection, is consistent with the reported central role of NO in late preconditioning, which may result in cardio-protection through one of several mechanisms. It was previously demonstrated that NO can nitrosylate caspases causing inhibition of apoptosis. Other mechanisms for NO induced cardio-protection include opening of K(ATP) channels and increasing expression of antioxidant proteins (23). At different stages in preconditioning NO may be generated by either eNOS in day 1 or by iNOS on day 2 (18). There is, however, substantial evidence that iNOS specifically plays an obligatory role in NO generation in late preconditioning by acting as a mediator or effector of preconditioning (10, 24). We have also demonstrated a substantial induction of myocardial Hsp27 after feeding with sulindac and, as shown, this increase was also prevented by administration of chelerythrine. Several studies have demonstrated a cardio-protective function for Hsp27, either through its exogenous overexpression from a transgene, or through its activation in preconditioning (25–27). In addition, this chaperone is known to contribute to preconditioning induced by a range of stimuli including opioids, isoproterinol, and ischemia (28–30). The protective effect of Hsp27 may include an anti-apoptotic role, which has been implicated in studies reporting an interaction of Hsp27 with cytochrome c and inhibition of apoptosome function (31).
In recent studies it was shown that the enhanced killing of cancer cells by sulindac and oxidative stress was associated with increased production of ROS (4, 5). Since ROS has been shown to be a trigger of late phase preconditioning (32) it seemed plausible that the protection afforded by sulindac is through its ability to increase the levels of ROS in the cardiac cells. As shown in Fig. 5D, the induction of, iNOS, was completely abolished in hearts from animals treated with the antioxidant 2-MPG, these results indicate that sulindac can increase the production of ROS which then triggers the preconditioning response. Our data indicate that elevated ROS underlies the late preconditioning effect of sulindac and that downstream PKC activation is also necessary for the molecular changes and protective responses. There is an extensive body of work implicating ROS dependent mechanisms in the triggering of delayed preconditioning by ischemia, exercise or other preconditioning stimuli (33–37). Following the trigger the next step in late preconditioning requires signaling through pathways that involve key protective kinases including PKC epsilon and PKC epsilon/Src containing modules (38, 33). The ability of sulindac to induce preconditioning markers dependent on both PKC activity and ROS is strong evidence that sulindac is functioning as a chemical preconditioning agent.
Sulindac is now known to have a variety of activities. It has been shown to induce the expression of several P450 enzymes (39) and ubiquinone oxido/reductase, a marker for the Phase 2 system (40). Thus, the activity of sulindac as a chemoprotectant could involve a combination of activities, including anti-inflammatory, cell preconditioning, and induction of Phase 1 and Phase 2 enzymes. Interestingly, one other compound, resveratrol, a polyphenol phytoalexin, is also protective against ischemia-reperfusion in kidney, heart, and brain (41).
There are other isolated studies suggesting that sulindac can protect cells against free radical or ROS damage. Sulindac, administered topically or in the drinking water, protected the skin of SKH-1 hairless mice in response to UV light (42). Sulindac has also been reported to decrease age related defects in learning and memory in rats (43) and was found to prevent the depletion of GSH in the hippocampus under conditions of quinolinic acid induced oxidative stress (44).
The protective cardiac effect we are seeing with sulindac in these experiments is contrary to studies that have shown that most, if not all, NSAIDS cause increased risk of heart attacks in humans (45). It should be noted that in the rat feeding experiments described in this study the daily dose of sulindac (0.2 mg/day) was only 10–15%, on a weight basis, of that taken by humans as an anti-inflammatory agent. A significant protective effect was also seen at 0.1 mg/day of sulindac. It may be that the protective effect of sulindac can be achieved at concentrations of the drug below that required for COX inhibition, which should decrease the risk of cardiac damage.
In conclusion sulindac, at low doses, may have excellent potential as a protective agent for the heart against elevated reactive oxygen species associated with ischemia and reperfusion.
Materials and Methods
Cell Culture Studies Using Cardiac Myocytes.
Unless otherwise stated all chemicals were obtained from Sigma-Aldrich. Neonatal rat cardiac myocytes were cultured as previously described (46). The cells were treated with no drug or a range of doses of sulindac and exposed to 24 h hypoxia and 20 h of reoxygenation. LDH was measured using the Cytotox-96 nonradioactive cytotoxicity assay kit (Promega) and the absorbance was read at 490 nm.
In Vivo Feeding of Sulindac Before Performing the Langendorff Procedure.
Spraque Dawley rats (275–325g) were fed 6 g of standard rat food supplemented with sulindac, ibuprofen, or sulindac sulfone at doses of 0.2 mg/day for 48 h before Langendorff or myocardial isolation. Langendorff heart preparation has been described elsewhere (47, 48). Forty-eight hours after the start of drug exposure hearts were excised and analyzed in a Langendorff preparation without further drug exposure. Hearts were equilibrated in KHB buffer for 10 min and then subjected to 45 min ischemia followed by 2 h reperfusion with KHB buffer. The assay for LDH in the samples was carried out as described above.
Blocking of PKC Pathways and Role of Reactive Oxygen Species.
Late phase preconditioning pathways were investigated by introducing the protein kinase C inhibitor Chelerythrine (49) via IP injection at 5 mg/kg body weight. Sulindac was then given to the rats using the in vivo drug exposure protocol (see above). On day 2 rats were fed a second dose of sulindac. Forty-eight hours after the start of sulindac exposure hearts were excised and analyzed in a Langendorff preparation without further drug exposure using 45 min ischemia followed by 2 h reperfusion, as described above. To determine if sulindac induced late phase preconditioning pathways through increasing the levels of ROS, the antioxidant N-2-mercaptopropionyl glycine (20 mg/kg i.p.) was given every 4 h for 24 h. After 24 h, hearts were excised and assayed for iNOS (as described below).
Cell Death and Viability Assays: LDH and TTC Assays for Langendorff Preparation.
Coronary effluent samples (500-μL) from the Langendorff preparation were obtained upon attachment of excised heart, every 15 min before ischemia, immediately after 45 min ischemia, and at 15 min intervals during reperfusion. LDH was measured as described above. Immediately, upon completion the heart was sliced into 2-mm cross-sectional pieces and slices were incubated for 30 min with 1% 2,3,5-triphenyl tetrazolium chloride (TTC) stain in Krebs-Henseleit buffer (KHB) (pH = 7.4) at 37 °C to distinguish between viable (red) and nonviable (white) tissue. Tissue slices were stored overnight in 10% formaldehyde before measurement of infarct size using NIH- Image J software. Tunel assays were based on end labeling of the DNA fragments using the DeadEnd colorimetric TUNEL system (Promega).
Western Blotting Protocol.
Western blot protocol has been previously described elsewhere (42). Rats were fed no drug (control) or 0.2 mg sulindac/day for 2 days. Western blots were carried out using antibodies for actin as a control protein (Promega), Hsp27 (Assay Designs Inc.), iNOS (Santa Cruz Biotechnology Inc.). Western blots were corrected for variations in the procedure by determining levels of beta-actin as an internal control.
Statistical Analysis.
Error bars represent SEM; significance was calculated by using SPSS software. Statistical significance was determined for P ≤ 0.05 unless otherwise stated.
Supplementary Material
Acknowledgments.
We thank Diana Navarro for technical assistance and Dr. Shailaja Kesaraju for helpful advice. The research was funded in part by a Grant in Aid from the American Heart Association (to H.P.) and a Florida State University Research Commercialization Assistance Grant (to H.W.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0911046106/DCSupplemental.
References
- 1.Vane J. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- 2.Taketo MM. Cyclooxygenase-2 inhibitors in tumorigenesis (part I) J Natl Cancer Inst. 1998;90:1529–1536. doi: 10.1093/jnci/90.20.1529. [DOI] [PubMed] [Google Scholar]
- 3.Guldenschuh I, et al. Relationship between APC genotype, polyp distribution, and oral sulindac treatment in the colon and rectum of patients with familial adenomatous polyposis. Dis Colon Rectum. 2001;44:1090–1097. doi: 10.1007/BF02234627. [DOI] [PubMed] [Google Scholar]
- 4.Resnick L, Rabinovitz H, Binninger D, Marchetti M, Weissbach H. Topical sulindac combined with hydrogen peroxide in the treatment of actinic keratoses. J Drugs Dermatol. 2009;8:29–32. [PubMed] [Google Scholar]
- 5.Marchetti M, Resnick L, Gamliel E, Weissbach H, Binninger D. Sulindac enhances the killing of cancer cells exposed to oxidative stress. PLoS One. 2009;4(6):e5804. doi: 10.1371/journal.pone.0005804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Etienne F, Resnick L, Sagher D, Brot N, Weissbach H. Reduction of Sulindac to its active metabolite, sulindac sulfide: Assay and role of the methionine sulfoxide reductase system. Biochem Biophys Res Commun. 2003;312:1005–1010. doi: 10.1016/j.bbrc.2003.10.203. [DOI] [PubMed] [Google Scholar]
- 7.Weissbach H, Resnick L, Brot N. Methionine sulfoxide reductases: History and cellular role in protecting against oxidative damage. Biochim Biophys Acta. 2005;1703:203–212. doi: 10.1016/j.bbapap.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Moskovitz J, et al. Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals. Proc Natl Acad Sci USA. 2001;98:12920–12925. doi: 10.1073/pnas.231472998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruan H, et al. High-quality life extension by the enzyme peptide methionine sulfoxide reductase. Proc Natl Acad Sci USA. 2002;99:2748–2753. doi: 10.1073/pnas.032671199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolli R. The late phase of preconditioning. Circ Res. 2000;87:972–983. doi: 10.1161/01.res.87.11.972. [DOI] [PubMed] [Google Scholar]
- 11.Dawn B, et al. Tumor necrosis factor-alpha does not modulate ischemia/reperfusion injury in naïve myocardium but is essential for the development of late preconditioning. J Mol Cell Cardiol. 2004;37:51–61. doi: 10.1016/j.yjmcc.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 12.Yeh CH, Wang YC, Wu YC, Lin YM, Lin PJ. Ischemic preconditioning or heat shock pretreatment ameliorates neuronal apoptosis following hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 2004;128:203–210. doi: 10.1016/j.jtcvs.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 13.Gross GJ. Role of opioids in acute and delayed preconditioning. J Mol Cell Cardiol. 2003;35:709–718. doi: 10.1016/s0022-2828(03)00135-4. [DOI] [PubMed] [Google Scholar]
- 14.Budas GR, Mochly-Rosen D. Mitochondrial protein kinase C ε (PKCε): Emerging role in cardiac protection from ischaemic damage. Biochem Soc Trans. 2007;35:1052–1054. doi: 10.1042/BST0351052. [DOI] [PubMed] [Google Scholar]
- 15.Raval AP, Dave KR, Mochly-Rosen D, Sick TJ, Pérez-Pinzón MA. Epsilon PKC is required for the induction of tolerance by ischemic and NMDA-mediated preconditioning in the organotypic hippocampal slice. J Neurosci. 2003;23:384–391. doi: 10.1523/JNEUROSCI.23-02-00384.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hausenloy DJ, Yellon DM. Survival kinases in preconditioning and postconditioning. Cardiovasc Res. 2006;70:240–253. doi: 10.1016/j.cardiores.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 17.Gross ER, Hsu AK, Gross GJ. Acute aspirin treatment abolishes, whereas acute ibuprofen treatment enhances morphine-induced cardioprotection: Role of 12-Lipoxygenase. JPET. 2004;310:185–191. doi: 10.1124/jpet.103.064667. [DOI] [PubMed] [Google Scholar]
- 18.Jones SP, Bolli R. The ubiquitous role of nitric oxide in cardioprotection. J Mol Cell Cardiol. 2006;40:16–23. doi: 10.1016/j.yjmcc.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Xuan YT, et al. Biphasic response of cardiac NO synthase isoforms to ischemic preconditioning in conscious rabbits. Am J Physiol Heart Circ Physiol. 2000;279:H2360–H2371. doi: 10.1152/ajpheart.2000.279.5.H2360. [DOI] [PubMed] [Google Scholar]
- 20.Dawson VL, Dawson TM. Neuronal ischaemic preconditioning. Trends Pharmacol Sci. 2000;21:423–424. doi: 10.1016/s0165-6147(00)01560-1. [DOI] [PubMed] [Google Scholar]
- 21.Li RC, et al. PKCepsilon modulates NF-kappaB and AP-1 via mitogen-activated protein kinases in adult rabbit cardiomyocytes. Am J Physiol Heart Circ Physiol. 2000;279:H1679–H1689. doi: 10.1152/ajpheart.2000.279.4.H1679. [DOI] [PubMed] [Google Scholar]
- 22.Guo Y, et al. The late phase of ischemic preconditioning is abrogated by targeted disruption of the inducible NO synthase gene. Proc Natl Acad Sci USA. 1999;96:11507–11512. doi: 10.1073/pnas.96.20.11507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.West MB, et al. Cardiac myocyte-specific expression of inducible nitric oxide synthase protects against ischemia/reperfusion injury by preventing mitochondrial permeability transition. Circulation. 2008;118:1970–1978. doi: 10.1161/CIRCULATIONAHA.108.791533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao T, Xi L, Chelliah J, Levasseur JE, Kukreja RC. Inducible nitric oxide synthase mediates delayed myocardial protection induced by activation of adenosine A(1) receptors: Evidence from gene-knockout mice. Circulation. 2000;102:902–907. doi: 10.1161/01.cir.102.8.902. [DOI] [PubMed] [Google Scholar]
- 25.Efthymiou CA, et al. Heat shock protein 27 protects the heart against myocardial infarction. Basic Res Cardiol. 2004;99:392–394. doi: 10.1007/s00395-004-0483-6. [DOI] [PubMed] [Google Scholar]
- 26.Sanada S, et al. Role of phasic dynamism of p38 mitogen-activated protein kinase activation in ischemic preconditioning of the canine heart. Circ Res. 2001;88:175–180. doi: 10.1161/01.res.88.2.175. [DOI] [PubMed] [Google Scholar]
- 27.Venkatakrishnan CD, et al. Heat shock protects cardiac cells from doxorubicin-induced toxicity by activating p38 MAPK and phosphorylation of small heat shock protein 27. Am J Physiol Heart Circ Physiol. 2006;291:H2680–H2691. doi: 10.1152/ajpheart.00395.2006. [DOI] [PubMed] [Google Scholar]
- 28.Dana A, Jonassen AK, Yamashita N, Yellon DM. Adenosine A(1) receptor activation induces delayed preconditioning in rats mediated by manganese superoxide dismutase. Circulation. 2000;101:2841–2848. doi: 10.1161/01.cir.101.24.2841. [DOI] [PubMed] [Google Scholar]
- 29.Marais E, et al. The temporal relationship between p38 MAPK and HSP27 activation in ischaemic and pharmacological preconditioning. Basic Res Cardiol. 2005;100:35–47. doi: 10.1007/s00395-004-0495-7. [DOI] [PubMed] [Google Scholar]
- 30.Peart JN, Gross ER, Headrick JP, Gross GJ. Impaired p38 MAPK/HSP27 signaling underlies aging-related failure in opioid-mediated cardioprotection. J Mol Cell Cardiol. 2007;42:972–980. doi: 10.1016/j.yjmcc.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murashov AK, et al. Crosstalk between p38, Hsp25, and Akt in spinal motor neurons after sciatic nerve injury. Brain Res Mol Brain Res. 2001;93:199–208. doi: 10.1016/s0169-328x(01)00212-1. [DOI] [PubMed] [Google Scholar]
- 32.Sun JZ, et al. Evidence for an essential role of reactive oxygen species in the genesis of late preconditioning against myocardial stunning in conscious pigs. J Clin Invest. 1996;97:562–576. doi: 10.1172/JCI118449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stein AB, et al. Delayed adaptation of the heart to stress: Late preconditioning. Stroke. 2004;35:2676–2679. doi: 10.1161/01.STR.0000143220.21382.fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joyeux-Faure M, Arnaud C, Godin-Ribuot D, Ribuot C. Heat stress preconditioning and delayed myocardial protection: What is new? Cardiovasc Res. 2003;60:469–477. doi: 10.1016/j.cardiores.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 35.Li H, Wallerath T, Münzel T, Förstermann U. Regulation of endothelial-type NO synthase expression in pathophysiology and in response to drugs. Nitric Oxide. 2002;7:149–164. doi: 10.1016/s1089-8603(02)00111-8. [DOI] [PubMed] [Google Scholar]
- 36.Akita Y, et al. Exercise-induced activation of cardiac sympathetic nerve triggers cardioprotection via redox-sensitive activation of eNOS and upregulation of iNOS. Am J Physiol Heart Circ Physiol. 2007;292:H2051–H2059. doi: 10.1152/ajpheart.01102.2006. [DOI] [PubMed] [Google Scholar]
- 37.Kukreja RC. Essential role of oxygen radicals in delayed pharmacological preconditioning. J Mol Cell Cardiol. 2001;33:1395–1398. doi: 10.1006/jmcc.2001.1422. [DOI] [PubMed] [Google Scholar]
- 38.Vondriska TM, et al. Protein kinase C epsilon-Src modules direct signal transduction in nitric oxide-induced cardioprotection: Complex formation as a means for cardioprotective signaling. Circ Res. 2001;88:1306–1313. doi: 10.1161/hh1201.092994. [DOI] [PubMed] [Google Scholar]
- 39.Ciolino HP, MacDonald CJ, Memon OS, Bass SE, Yeh GC. Sulindac regulates the aryl hydrocarbon receptor-mediated expression of phase 1 metaoblic enzymes in vivo and in vitro. Carcinogenesis. 2006;27:1586–1592. doi: 10.1093/carcin/bgi359. [DOI] [PubMed] [Google Scholar]
- 40.Ciolino HP, Bass SE, MacDonald CJ, Cheng RY, Yeh GC. Sulindac and its metabolites induce carcinogen metabolizing enzymes in human colon cancer cells. Int J Cancer. 2008;122:990–998. doi: 10.1002/ijc.23218. [DOI] [PubMed] [Google Scholar]
- 41.Hattori R, Otani H, Maulik N, Das DK. Pharmacological preconditioning with resveratrol: Role of nitric oxide. Am J Physiol Heart Circ Physiol. 2002;282:H1988–H1995. doi: 10.1152/ajpheart.01012.2001. [DOI] [PubMed] [Google Scholar]
- 42.Athar M, et al. Photoprotective effects of sulindac against ultraviolet B-induced phototoxicity in the skin of SKH-1 hairless mice. Toxicol Appl Pharmacol. 2004;195:370–378. doi: 10.1016/j.taap.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 43.Mesches MH, et al. Sulindac improves memory and increases NMDA receptor subunits in aged Fischer 344 rats. Neurobiol Aging. 2004;25:315–324. doi: 10.1016/S0197-4580(03)00116-7. [DOI] [PubMed] [Google Scholar]
- 44.Dairam A, Müller AC, Daya S. Nonsteroidal anti-inflammatory agents, tolmetin, and sulindac attenuate quinolinic acid (QA)-induced oxidative stress in primary hippocampal neurons and reduce QA-induced spatial reference memory deficits in male Wistar rats. Life Sci. 2007;80:1431–1438. doi: 10.1016/j.lfs.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 45.Charles HH, Steven B. Cyclooxygenase-2 inhibitors and most traditional nonsteroidal anti-inflammatory drugs cause similar moderately increased risks of cardiovascular disease. J Cardiovasc Pharmacol Ther. 2008;13:41–50. doi: 10.1177/1074248407312990. [DOI] [PubMed] [Google Scholar]
- 46.Webster KA, et al. Hypoxia-activated apoptosis of cardiac myocytes requires reoxygenation or a pH shift and is independent of p53. J Clin Invest. 1999;104:239–252. doi: 10.1172/JCI5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Skrzypiec-Spring M, Grotthus B, Szelag A, Schulz R. Isolated heart perfusion according to Langendorff- Still viable in the new millennium. J Pharmacol Toxicol Methods. 2007;55:113–126. doi: 10.1016/j.vascn.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 48.Yoshida T, Maulik N, Engelman RM, Ho YS, Das DK. Targeted disruption of the mouse SODI gene makes the heart vulnerable to ischemic reperfusion injury. Circ Res. 2000;86:264–269. doi: 10.1161/01.res.86.3.264. [DOI] [PubMed] [Google Scholar]
- 49.Shinmura K, et al. Aldose reductase is an obligatory mediator of the late phase of ischemic preconditioning. Circ Res. 2002;91:240–246. doi: 10.1161/01.res.0000029970.97247.57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.