Abstract
Percutaneous coronary intervention is the preferred revascularization approach for most patients with coronary artery disease. However, this strategy is limited by renarrowing of the vessel by neointimal hyperplasia within the stent lumen (in-stent restenosis). Vascular smooth muscle cell proliferation is a major component in this healing process. This process is mediated by multiple cytokines and growth factors, which share a common pathway in inducing cell proliferation: the cell cycle. The cell cycle is highly regulated by numerous mechanisms ensuring orderly and coordinated cell division. The present review discusses current concepts related to regulation of the cell cycle and new therapeutic options that target aspects of the cell cycle.
Keywords: Cell cycle, Drug-eluting stent, Gene therapy, Pharmacology, Restenosis
Abstract
L’intervention coronaire percutanée est la démarche de revascularisation de choix pour la plupart des patients atteints d’une coronaropathie. Cependant, cette stratégie est limitée par un nouveau rétrécissement du vaisseau imputable à une hyperplasie néo-intimale dans la lumière de l’endoprothèse (une resténose dans l’endoprothèse). La prolifération des cellules vasculaires des muscles lisses est un élément important de ce processus de guérison. Ce processus est médié par de multiples cytokines et facteurs de croissance, qui partagent une voie commune dans l’induction de la prolifération cellulaire : le cycle cellulaire. Le cycle cellulaire est hautement régularisé par de nombreux mécanismes qui assurent une division cellulaire ordonnée et coordonnée. La présente analyse porte sur les concepts courants reliés à la régulation du cycle cellulaire et sur de nouvelles options thérapeutiques qui ciblent des aspects du cycle cellulaire.
Coronary artery disease is the leading cause of death in the Western population (1). Atherosclerosis is the primary pathological process leading to obstructive coronary artery disease, myocardial infarction and sudden death. Angina due to obstructive coronary disease is most frequently treated by percutaneous coronary interventions (PCIs) such as balloon angioplasty or stents. Lumen renarrowing after initially successful coronary stenting, termed ‘restenosis’, is predominantly due to tissue proliferation along the inner lining of the stent (intimal proliferation). Vein graft disease and transplant vasculopathy also share the similar pathological entity of intimal hyperplasia. A critical aspect of intimal hyperplasia is the activation and subsequent proliferation of vascular smooth muscle cells (VSMCs). Tremendous progress has been made in the past few years in the understanding of in-stent restenosis (ISR) and other vascular proliferative diseases. The cell cycle plays a central role in smooth muscle cell (SMC) proliferation. Therapeutic strategies that target various proteins of the cell cycle or upstream to the cell cycle have been developed. The aim of this paper is to review the current knowledge of cell cycle biology and to provide an update on antiproliferative strategies that target the cell cycle to prevent restenosis.
MECHANISMS OF RESTENOSIS
In the past two decades, extensive research efforts have been directed toward understanding the pathophysiology leading to restenosis after vascular injury that occurs as part of PCIs. Three principle mechanisms of restenosis after balloon angioplasty have been identified: elastic recoil, negative remodelling and neointimal hyperplasia (2–4). Coronary stenting prevents acute elastic recoil and negative remodelling, but does not have any effect against neointimal formation (5). In fact, stenting appears to actually increase cell proliferative components of intimal hyperplasia in restenosis (6–9). Although multiple cell types, cytokines and mediators all play roles in the complex process of neointimal formation, there is a final common pathway: the entry of VSMCs into the cell cycle. Before reviewing the cell cycle, we describe the earlier steps that lead to this pathway.
Vessel wall injury
Endothelial denudation and medial wall injury are the initial effects of balloon or stent injury in the vessel wall. Studies in the porcine coronary artery injury model have shown that the extent of neointimal hyperplasia is proportional to the extent of injury (10). This relationship has also been validated in humans (11). The endothelium normally provides a nonpermeable barrier to protect VSMCs against the effect of circulating growth factors (12). The endothelium also produces nitric oxide, which has several antimitogenic properties (13,14). Nitric oxide has been shown to downregulate cyclin-dependent kinase (CDK) 2 and cyclin A and upregulate p21, resulting in cell cycle arrest (14).
Thrombus formation
Within the first three days after PCI, mural thrombus forms along the injured vessel wall and on the stent struts in the porcine coronary artery model (12,15). The amount of thrombus is proportional to the extent of arterial wall injury (12). Although unproved, a direct relationship may exist between the absolute amount of thrombus and the subsequent neointimal hyperplasia (15). Macrophages actively remove thrombus over a period of several weeks (16). Resolution of thrombus in human arteries after PCI seems to mimic the porcine model (15).
Inflammation
Activated platelets within the thrombus release a number of chemokines essential for initiation of inflammation. This includes P-selectin and integrins such as beta2-integrin Mac-1 (CD11b/CD18) (17). Local production of cytokines (eg, inter-leukin [IL]-1, IL-6 and tumour necrosis factor-alpha [TNF-α]) by activated macrophages, VSMCs and endothelial cells also play a major role in inducing an inflammatory reaction at the site of injury (18–20). Increased neointimal macrophage infiltration correlates with the extent of neointima formation in humans (21). Vessel wall overstretching during PCI may also induce an inflammatory response by stimulating infiltration of inflammatory leukocytes into the adventitia (22). Experimental studies have shown that the degree of inflammation and subsequent neointima formation is proportional to the degree of penetration of the stent struts into the vessel wall (12). Moreover, a foreign body immune response directed against the stent struts has been shown in a porcine model of coronary artery injury (23,24) and in humans (25). Multinucleated giant cells, indicative of a chronic inflammatory response, are occasionally present in the peri-strut area one month after stent insertion in pig coronary arteries (24). Corrosion products of the metallic stent may be of great importance in this process. In some patients, allergic response on contact with certain elements (eg, chrome, molybdenum, nickel) may also promote excessive neointimal growth (26).
EARLY CELL CYCLE PROGRESSION (G0/G1/S PHASE)
VSMCs within the media of adult arteries are normally quiescent, proliferate at low indexes and exist in the G0 phase of the cell cycle. An imbalance between stimulatory growth factors and cytokines (eg, platelet-derived growth factor [PDGF], IL-1, IL-6 and TNF-α) and inhibitory factors (eg, endothelial-derived nitric oxide, heparan sulfate proteoglycan) induces VSMC activation; that is, they change from a quiescent and contractile phenotype into a synthetic phenotype. Once activated, the VSMCs proliferate in the media and then migrate from the media to the intima, where they undergo further cycles of proliferation and extracellular matrix formation. Some groups have proposed that intimal VSMCs may originate from other sites than the medial layer. Adventitial fibroblasts may also migrate to the intima, differentiate into myofibroblasts and contribute to neointima formation (27,28). Moreover, several groups have shown that bone marrow-derived vascular cells with some characteristics of VSMCs accumulate in the neointima of transplant vasculopathy and in a severe vascular injury animal model (29–31).
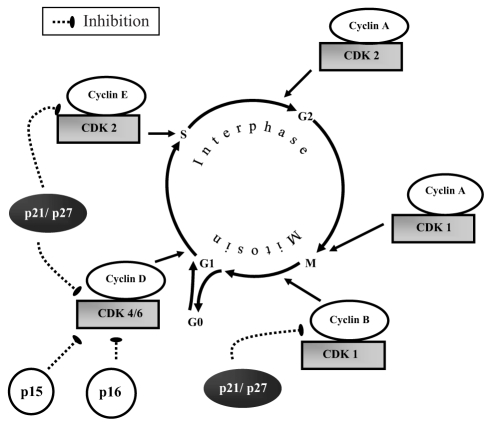
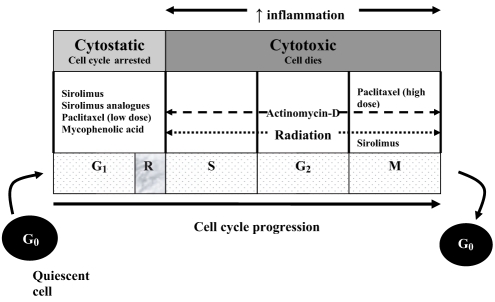
Cell division involves two consecutive processes: inter-phase, ie, the time between two mitosis (M) periods, and the mitosis itself, ie, the process of cell division. The interphase is divided into four phases: G0, G1, S and G2 (Figure 1). Cells in the G0 phase are quiescent and nonproliferative. The G1 phase is the period during which the cell assembles the factors necessary for DNA replication. In the late G1 phase, the activated cell reaches the restriction point (R), defined as the point of no return. Beyond this point, the cell is committed to DNA replication. After the restriction point, cycle progression to the M phase is relatively independent of any growth factor stimulation. The S phase is the period of DNA replication. In the G2 phase, the cell synthesizes proteins that are required for mitosis. In addition, a critical checkpoint occurs during the G2 phase to ensure quality of the DNA.
Figure 1).
Cell cycle steps (G0, G1, S and M) and their associated cyclin-dependent kinase (CDK)-cyclin complex holoenzyme and CDK inhibitors. There are two families of CDK inhibitors: inhibitors of CDL 4 (INK4) (p15, p16) and CDK interacting protein/inhibitory protein (Cip/Kip) (p21, p27)
Tyrosine kinase receptors – Phosphatidylinositol 3-kinase pathway
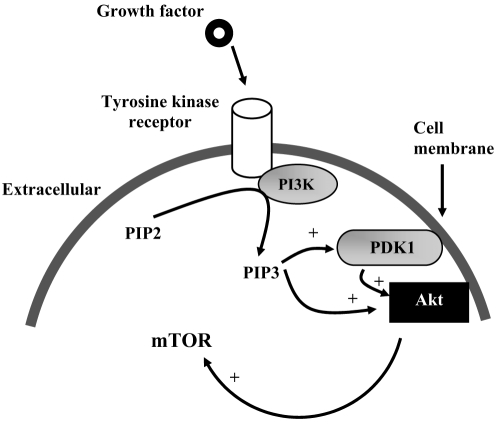
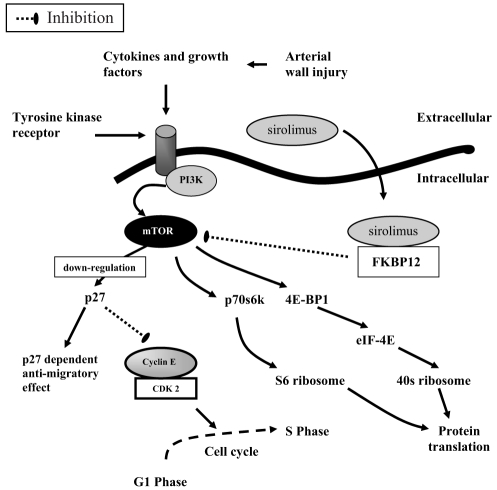
A number of growth factors stimulate vascular cell proliferation. Fibroblast growth factor-2 (FGF-2, previously termed ‘basic FGF’), epidermal growth factor, PDGF and insulin-like growth factor-1 can initiate proliferation of VSMCs by stimulating several classes of tyrosine kinase receptors (TKRs) (32). Although these classes share common properties, the effects of receptor activation are dependent on specific growth factors and the intracellular signalling pathways. One example of intracellular signal transmission through the TKR is the phosphatidylinositol (PI) 3-kinase (PI3K) pathway (Figure 2). PI3K is a lipid kinase that phosphorylates PI. The predominant lipid products generated by PI3K – PI 3,4-bisphosphate (PIP2) and PI 3,4,5-trisphosphate (PIP3) – are potent signalling molecules (33). Signalling through this PI3K pathway is initiated by an interaction between the growth factor ligand with its TKR. This interaction activates the PI3K pathway, resulting in the recruitment of PI3K to the membrane and the generation of PIP3. Once generated, the PIP3 recruits phosphoinositide-dependent kinase-1 and Akt to the plasma membrane. At the plasma membrane, Akt is activated, in part through phosphorylation by phosphoinositide-dependent kinase-1 and PIP3. Akt is then capable of phosphorylating a number of downstream targets, such as mammalian target of rapamycin (mTOR) (33–35).
Figure 2).
Phosphatidylinositol 3-kinase (PI3K) intracellular signalling pathway. + Activation through phosphorylation; mTOR Mammalian target of rapamycin; PDK1 Phosphoinositide-dependent kinase 1; PIP2 Phosphatidylinositol 4,5-bisphosphate; PIP3 Phosphatidylinositol 3,4,5-trisphosphate
Mitogen-activated protein kinase pathway
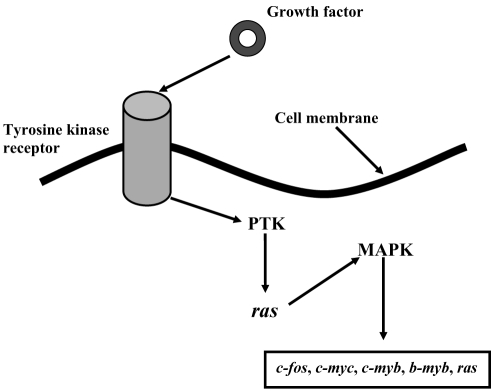
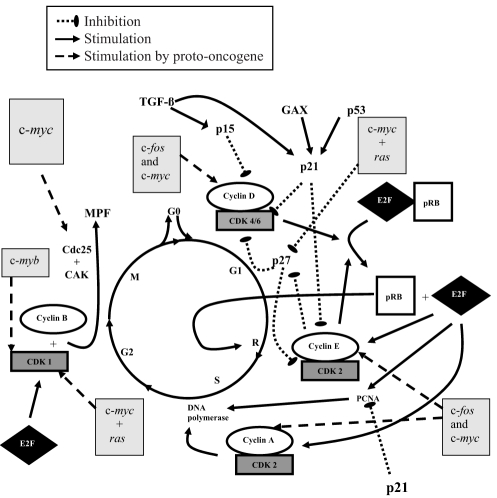
The mitogen-activated protein kinase (MAPK) pathway (Figure 3) is a second intracellular signalling pathway that may be initiated by TKR activation; Ras is a membrane G-protein that transmits the signal received by the TKR at the cell surface to activate the intracellular MAPK pathway. The MAPKs promote transcription of nuclear proto-oncogenes: c-fos, c-myb, b-myb, c-myc and ras (36). These gene products play a major role in the expression of specific regulatory proteins for progression of the cell cycle (37). PDGF and FGF-2 induce transcription of fos and myc early proto-oncogenes that allow cell cycle entry (38,39). The proto-oncogene c-fos leads to an increase in the concentration of cyclin D1, cyclin E and CDK4 mRNA, which are involved in the progression of the G1 phase (Figure 4) (40). Similar to c-fos, c-myc induces G1 phase cyclin accumulation, and increases cyclin D- and cyclin E-associated kinase activities (Figure 4) (41,42). Insulin-like growth factor-1 and epidermal growth factor are two factors that stimulate competent cells to progress toward the S phase by inducing ras (43,44). As mentioned, ras is essential for signal transmission through the MAPK pathway. Moreover, ras interacts with c-myc to decrease the concentration of CDK inhibitor (CKI) p27 that leads to increased activation of E2F-1 (a potent transcription factor) and the cyclin E-CDK2 complex (Figure 4) (45).
Figure 3).
Mitogen-activated protein kinase (MAPK) intracellular signalling pathway. After binding of growth hormone (epidermal growth factor, platelet-derived growth factor, fibroblast growth factor-2, insulin-like growth factor-1) to its tyrosine kinase receptor, the receptor becomes activated as a protein tyrosine kinase (PTK). PTK undergoes autophosphorylation at multiple tyrosine residues, which interacts with signalling proteins, in turn activating Ras, a low molecular weight G-protein. Ras leads to activation of MAPK through protein kinase cascade. Thereafter, MAPK induces the transcription of proto-oncogenes (c-fos, c-myc, c-myb, b-myb, ras)
Figure 4).
Overview of the most important steps of cell cycle regulation. The key regulatory proteins are the cyclin-dependent kinase (CDK) proteins, which are activated at specific time points of the cell cycle. To be active, CDK needs association with cyclin to form a holoenzyme complex. Different cyclins are required at different steps of the cell cycle. CDK activity is also regulated by cell cycle inhibitory proteins called cyclin dependent kinase inhibitors (CKIs), which conteract CDK activity. Also shown are proto-oncogenes: c-fos, c-myb, c-myc and ras. Their gene products play a major role in the expression of specific regulatory proteins for the progression of the cell cycle. CAK CDK-activating kinase; GAX Growth arrest-specific homeobox; MPF Mitosis-promoting factor; PCNA Proliferating cell nuclear antigen; pRB Retinoblastoma gene product; TGF-β Transforming growth factor-beta
Entry into the cell cycle
Entry into G1 is promoted by a decrease in the high concentration of p27 (secondary to the overexpression of ras and c-myc) in the G0 phase (46,47). In uninjured rat carotid arteries, p27 protein concentrations are high and fall rapidly to reach a nadir in the first 48 h after injury (48). Growth factors may also induce transition from G0 to G1 by decreasing the concentration of a transcription factor known as growth arrest-specific homeobox (GAX) (Figure 4) (49,50) that normally induces expression of p21 (51). In uninjured blood vessels, GAX mRNA is detectable, but after balloon injury, the mRNA is rapidly downregulated (49), reflecting the upregulated transcription of c-fos and c-myc.
CDK-cyclin holoenzyme complex
Following entry into the G1 phase, progression through the cell cycle depends on the expression and activation of regulatory proteins. The key regulatory proteins are the CDKs, which are activated at specific time points of the cell cycle. Nine CDKs have been identified, but only five are known to play a role in the cell cycle: CDK4, CDK6 and CDK2 (during G1), CDK2 (during S), CDK1 (during G2 and M). A separate CDK, CDK7, is unique because it binds to cyclin H to act as CDK-activating kinase (CAK) (52). Phosphorylation by CAK is required for complete activation of CDK family proteins. This phosphorylation induces a conformational change that enhances the binding capability to cyclins (53,54). CDK association with a particular cyclin forms a holoenzyme complex that is required for its activity (Figure 1). Different cyclins are required at different steps of the cell cycle. In contrast to CDKs, the cyclin protein concentrations increase and decrease at various stages along the cell cycle and periodically activate CDK (55,56). There are four types of cyclin: D, E, A and B. Cyclin D (D1, D2, D3) binds to CDK4 and CDK6. These complexes are very important for entry into G1 phase (57). Cyclin D is a growth factor sensor and, unlike other cyclins, is expressed constitutively during the period of growth factor stimulation (58). Cyclin E, which forms a complex with CDK2, is essential for the progression from G1 to S phase (59). Cyclin A binds with CDK2, and this complex is important during the S phase (60,61). At the end of the G2 phase, cyclin A forms a complex with CDK1 to initiate the M phase. Mitosis is also regulated by the cyclin B-CDK1 complex (62,63).
CKIs
CDK activity is also regulated by cell cycle inhibitory proteins, called CKIs (Figure 1, Figure 4), which counteract CDK activity by binding to CDK alone or the cyclin-CDK complex. There are two families of CKIs: inhibitors of CDK 4 (INK4) and CDK interacting protein/Kinase inhibitory protein (Cip/Kip) (46). The p15, p16, p18 and p19 proteins belong to the INK4 family, which inactivate G1 CDK (CDK4 and CDK6) by direct binding with CDK, thus preventing association with cyclin (64). The second family of inhibitors (Cip/Kip) includes p21, p27 and p57. These inhibitors bind to the cyclin-CDK complex to interact with both cyclin D-CDK4 and cyclin E-CDK2 complexes in the G1 phase but preferentially inhibit cyclin E-CDK2 activity and, to a lesser extent, the cyclin B-CDK1 complex (65). There are several unique features of p21 regulation of the cyclin-CDK complex (48). Binding of a single p21 protein promotes assembly of the cyclin-CDK complex (66), while binding of multiple p21 proteins inhibits the complex. Concentrations of p21 protein are low at the beginning of the cell cycle (G0 phase), but upregulation occurs late in the G1 phase to counterbalance the increased concentration of the cyclin-CDK complex (48). Transforming growth factor-beta has an inhibitory effect on cell proliferation by inducing expression of p15, p21 and p27, which counterbalances the effects of the cyclin-CDK complex in the G1 phase (67,68).
Cyclin-CDK complexes
Cyclin-CDK complex formation leads to target protein phos-phorylation and progression of the cell cycle. For example, the phosphorylation of the retinoblastoma gene product (pRB) is an important regulator of the cell cycle (Figure 4). At the beginning of the G1 phase, the hypophosphorylated pRB binds to E2F-1 and histone deacetylase protein, inhibiting their actions. Cyclin D-CDK4/6 holoenzymes phosphorylate the pRB, leading to disruption of the complex, which results in the release of E2F-1. pRB behaves like a timer marker, because once it is phosphorylated, cells are committed to DNA replication in the S phase (ie, reach the R point) (69). Once released, the E2F-1 upregulates gene expression of CDK1, cyclin E, cyclin A and proliferating cell nuclear antigen (PCNA), which are required for progression through the cell cycle (S, G2, M phases) (70). The specific role of PCNA is to stimulate the processing ability of DNA polymerase for DNA replication in the S phase (Figure 4) (71). Increased concentrations of cyclin E-CDK2 complex participate in maintaining the pRB in its hyperphosphorylated state, which leads to irreversible progression of the cell through the G1 to S transition. Moreover, the cyclin E-CDK2 complex also induces degradation of p27 (an inhibitor of cell cycle progression) during the transition between G1 and S phases (72). Another effect of cyclin E-CDK2 is to phosphorylate histone H1 that may be important for chromosome condensation during DNA replication. The complex cyclin A-CDK2 activates DNA polymerase alpha-primase, which leads to DNA replication (Figure 4) (73).
Checkpoint controls
Checkpoint controls exist during the cell cycle to ensure an orderly sequence of events during the cell division process (74). DNA checkpoints stop the cell cycle to allow DNA repair and are found before the cell enters the S phase (G1-S checkpoint) and after DNA replication (before cell division, G2-M checkpoint). At the G1-S checkpoint, the cell cycle arrest induced by DNA damage is p53 dependent. Normally, the concentration of p53 (a transcription factor) is very low, but DNA damage leads to induction of p53 (75). p53 modulates the expression of p21 (76), a CKI that results in CDK inhibition and cell cycle arrest in the G1 phase to prevent damaged DNA replication (Figure 4). p21 can also directly block the ability of PCNA to induce DNA polymerase (Figure 4) (77). The cell cycle arrest mediated by p53 is probably the main mechanism of the antiproliferative effect of radiation. Moreover, in case of a severely damaged cell, p53 may induce cell apoptosis, even if the cell has progressed past the restriction point (78,79).
LATE CELL CYCLE PROGRESSION (G2 THROUGH M PHASE)
Some proto-oncogenes are believed to play an important role in the completion of the cell cycle (Figure 4). c-myc increases the protein concentration of cyclins, but also induces Cdc25 phosphatase, which is important for CDK1 activity (80). c-myc also acts in concert with ras to increase the CDK1 protein concentrations (81). The proto-oncogene c-myb is also able to upregulate transcription of CDK1 (82).
As the cell enters the G2 phase after DNA replication, the concentration of cyclin B increases. This increase in protein concentrations of both CDK1 and cyclin B promotes the formation of the cyclin B-CDK1 complex, known as mitosis-promoting factor (MPF). The activation of MPF necessitates both the phosphorylation of CDK1 at a threonine residue (Thr-161) by CAK (ie, complex of CDK7 and cyclin H) and the dephosphorylation of a tyrosine residue (Tyr-15) by Cdc25 phosphatase (62,83). Once activated, MPF can initiate prophase, the first step of mitosis. At the end of the process, CDK1 undergoes dephosphorylation of Thr-161, which terminates the cell cycle and resets the cell into G0 state.
TARGETING THE CELL CYCLE TO INHIBIT VASCULAR PROLIFERATIVE DISEASE
The cell cycle is the final common pathway that leads to intimal hyperplasia after vascular injury. Therapeutic strategies directed upstream of the cell cycle (eg, at the cell surface receptor) have had only limited effectiveness in inhibiting VSMC proliferation. Our improved understanding of the steps regulating VSMC proliferation have led to novel approaches that focus on the regulators of the cell cycle, the final common pathway. There are two different strategies to control and inhibit neointimal hyperplasia after vascular injury. First, cytostatic therapy controls cell cycle regulation by altering the expression of cell cycle promoting or inhibitory proteins. Second, a cytotoxic strategy results in cell death and necrosis, which may induce inflammation and enhance restenosis. Thus, a cytostatic strategy may be a preferable approach to prevent neointimal proliferation.
Therapeutic success is also dependent on achieving adequate delivery of the therapy at the site of interest. Many drugs, which have shown promising results in in vitro and pre-clinical animal trials, have proved to be ineffective for decreasing restenosis rates after PCI in humans, largely due to the requirement for systemic administration (84–93). Discrepancies in efficacy between animal studies and human trials may also be due to species differences in the response to vascular injury or the inability to achieve adequate drug concentrations at the target vascular site in human patients. Local delivery of cell cycle-arresting agents appears to be a logical alternative, because it can achieve high drug concentration in the area of interest, with low systemic side effects. Drug delivery directly into the artery through a catheter-based system has been shown to be partially effective. Major limitations of this approach are the low efficiency of drug delivery and the natural washout from the vessel wall by circulating blood (94). The increasing use of coronary stents to treat coronary artery disease has stimulated intense interest in developing local drug release from a local reservoir, ie, drug-eluting stents (DESs). DESs combine the mechanical scaffolding properties of stents with local, sustained delivery of therapeutic drugs at the site of vascular injury. The drug can be either bonded directly onto the surface of the stent or mixed with a polymer film that is programmed for controlled drug release. However, the polymer can also induce inflammatory reactions, which may lead to increased intimal hyperplasia (15).
PHARMACOLOGICAL APPROACH
The sites of action of antiproliferative drugs in the cell cycle are illustrated in Figure 5. A summary of the completed DES trials is shown in Table 1.
Figure 5).
Principal sites of action of antiproliferative drugs
TABLE 1.
Summary of clinical trials of antiproliferative and gene therapy agents
| Study (reference) | Design | n | In-stent late loss (mm), Drug-eluting stent versus bare metal stent* | In-stent binary restenosis (%) Drug-eluting stent versus bare metal stent* |
|---|---|---|---|---|
| Sirolimus | ||||
| RAVEL (195) | Multicentre, PRDBC study | 238 | –0.01 versus 0.80, P<0.001 | 0.0 versus 26.6, P<0.001 |
| SIRIUS (196) | Multicentre, PRDBC study | 1101 | 0.17 versus 1.00, P<0.001 | 3.2 versus 35.4, P<0.001 |
| E-SIRIUS (197) | Multicentre, PRDBC study | 350 | 0.20 versus 1.05, P<0.0001 | 3.9 versus 41.7, P<0.0001 |
| C-SIRIUS (198) | Multicentre, PRDBC study | 102 | 0.12 versus 1.02, P<0.001 | 0.0 versus 45.5, P<0.001 |
| Paclitaxel | ||||
| DELIVER I (133) | Multicentre, PRSBC study | 1043 | 0.81 versus 0.98, P=0.0025 | 14.9 versus 20.6, P=0.076 |
| TAXUS II (134) | Multicentre, PRDBC study; Slow-release cohort | 267 | 0.31 versus 0.79, P<0.001 | 2.3 versus 17.9, P<0.001 |
| TAXUS II (134) | Multicentre, PRDBC study; Moderate-release cohort | 269 | 0.30 versus 0.77, P<0.001 | 4.7 versus 20.2, P<0.001 |
| TAXUS IV (135) | Multicentre, PRDBC study; Slow-release formulation | 1314 | 0.39 versus 0.92, P<0.0001 | 5.5 versus 24.4, P<0.0001 |
| Everolimus | ||||
| FUTURE I (199) | Single centre, PRSBC study | 42 | 0.11 versus 0.85, P<0.0001 | 0.0 versus 9.1, P=NS |
| FUTURE II (200) | Multicentre, PRSBC study | 64 | 0.12 versus 0.85, P<0.0001 | 0.0 versus 19.4, P=0.039 |
| SPIRIT FIRST (201) | Multicentre, PRDBC study | 60 | 0.10 versus 0.84, P<0.0001 | 0.0 versus 26.9, P=0.01 |
| Tacrolimus | ||||
| PRESENT I (128) | Single centre, registry | 22 | 0.81±0.39 | 19.0 |
| 17-beta-estradiol | ||||
| EASTER (144) | Single centre, registry | 30 | 0.54±0.44 | 6.7 |
| Actinomycin D | ||||
| ACTION (137) | Multicentre, PRSBC study; High-dose group | 360 | 0.93 versus 0.76, P=0.03 | 17.0 versus 11.0, P=0.38 |
| Antisense oligodeoxynucleotides | ||||
| ITALICS (137) | Single centre, PRDBC study | 85 | 1.2 versus 1.3, P=0.98 | 34.2 versus 38.5, P=0.81 |
Angiographic follow-up performed between six and nine months. ACTION ACTinomycin eluting stent Improves Outcomes by reducing Neointimal hyperplasia; C Canada; E Europe; EASTER ESTrogen and Stents to Eliminate Restenosis; FUTURE First Use To Underscore restenosis Reduction with Everolimus; ITALICS Investigation by the Thoraxcenter on Antisense DNA using Local delivery and IVUS after Coronary Stenting; NS Not significant; PRDBC Prospective, randomized, double-blind, controlled; PRESENT PREliminary Safety Evaluation of Nanoporous Tacrolimus-eluting stents; PRSBC Prospective, randomized, single-blind, controlled;RAVEL RAndomized study with the sirolimus-eluting VElocity balloon-expandable stent in the treatment of patients with de novo native coronary artery Lesions; SIRIUS SIRolImUS-eluting stent in de novo native coronary lesions
Sirolimus
The immunosuppressant sirolimus (rapamycin) is a natural macrocyclic lactone produced by Streptomyces hygroscopicus that was initially discovered in the soil of Easter Island, Chile. The chemical structure is similar to the immunosuppressant FK506 (tacrolimus).
Inhibition of VSMC proliferation:
The intracellular receptor for sirolimus is the immunophilin FK506 binding protein, 12 kDa (FKBP12), which is elevated in patients with symptomatic ISR (95). The rapamycin-FKBP12 complex binds to and inhibits the activation of mTOR. mTOR is a protein kinase enzyme located in the cytoplasm that plays an important role in connecting extracellular signals with intracellular pathways that modulate the cell cycle (96,97). Stent- or balloon-induced vascular injury activates mTOR, which in turn leads to downregulation of p27 (98,99), and phosphorylation of p70s6k and eukaryotic initiation factor-4E binding protein 1 (Figure 6) (100,101). The inhibition of mTOR impedes downregulation of p27, which binds and inactivates the cyclin E-CDK2 holoenzyme complex, blocking cell cycle progression at the G1/S transition. Moreover, inhibition of phosphorylation of p70s6k and 4E binding protein 1 blocks activation of the S6 and 40S ribosomes, respectively, which are involved in the protein translation required for cell growth. The reduction in protein translation through these two pathways may contribute to the antiproliferative and/or migratory effects of mTOR (102).
Figure 6).
Antiproliferative effects of sirolimus. 4E-BP1 Eukaryotic initiation factor 4E binding protein; CDK Cyclin-dependent kinase; eIF-4E Eukaryotic initiation factor 4E; FKBP12 FK506 binding protein, 12 kDa; mTOR Mammalian target of rapamycin; PI3K Phosphatidylinositol 3-kinase
Inhibition of VSMC migration:
Mitogenic stimulation of VSMCs initiates the cell cycle and cell migration. Cell migration is not possible during the late S or G2/M phase of the cell cycle (103). In the G1 to G1/S transition, VSMCs are able to migrate in response to mitogenic stimuli. Sirolimus has been shown to inhibit rat, porcine and human VSMC migration. Sun et al (104) studied the effect of rapamycin on the migration of mouse VSMCs in in vitro and in vivo models. In mouse VSMCs containing p27 (p27+/+ and p27+/−) or lacking p27 (p27−/−), cell migration was inhibited in a dose-dependent manner. At low doses of rapamycin, the inhibition of cell migration was p27 dependent. However, at higher doses, inhibition of cell migration was observed, even in mice lacking p27, indicating a p27-independent mechanism as well. This study also showed that both p27-dependent and p27-independent antimigratory properties of rapamycin are mediated through FKBP12.
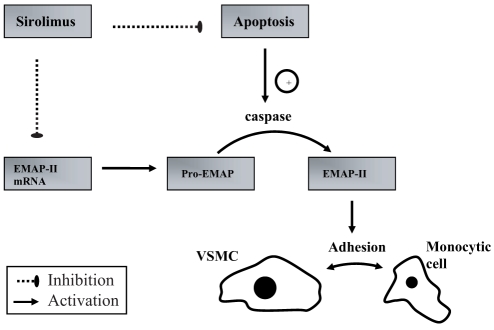
Anti-inflammatory properties:
Preclinical studies have shown a significant reduction in stent strut-associated inflammation after deployment of sirolimus-eluting stents (SESs). This reduction of inflammation may be explained by rapamycin-induced downregulation of endothelial monocyte-activating polypeptide-II (EMAP-II) gene expression and apoptosis (Figure 7) (105). EMAP-II is a proinflammatory cytokine and a chemoattractant for monocytes. Decreased gene expression of EMAP-II seems to be involved in reduction of monocyte cell adhesion to VSMCs (105). The reduction of apoptosis mediated by rapamycin may also contribute to its anti-inflammatory effect (105). The family of caspase enzymes released by apoptotic cells can mediate cleavage of pro-EMAP (106). Active EMAP-II resulting from cleavage of pro-EMAP has been identified as the linking molecule between apoptosis and inflammation (107).
Figure 7).
Anti-inflammatory effect of sirolimus. EMAP Endothelial monocyte-activating polypeptide; mRNA Messenger RNA; VSMC Vascular smooth muscle cell
Preclinical and clinical studies:
Initially, systemic administration of sirolimus was shown to effectively inhibit intimal proliferation after PCI in a porcine model (108). Systemic administration in humans has also been reported in small numbers of patients with variable effects on restenosis and high rates of side effects (109–112). Advances in biopolymer chemistry have led to the development of SESs. The results of multiple randomized clinical trials have consistently shown marked efficacy of SESs in reducing ISR rates to less than 10% (Table 1). The effectiveness of SESs in unselected patients treated in the real world, daily practice was unknown until the results of the Rapamycin-Eluting Stent Evaluated at Rotterdam Cardiology Hospital (RESEARCH) registry were published (113). Five hundred eight consecutive patients with de novo lesions were treated exclusively with Cypher (J&J Cordis, USA) SESs over a six-month period. The control group (450 patients) consisted of all patients treated with PCIs in the six-month period preceding the study and having received a bare metal stent (BMS). Patients in the SES group had more multivessel disease, had more complex (type C) lesions, received more stents and had more bifurcation stenting. Despite these high-risk features, the one-year cumulative risk of major adverse cardiac events (MACE) was significantly reduced in the group receiving SESs compared with those receiving BMSs (9.7% versus 14.8%, respectively; P=0.008), mainly due to a decrease in the need for clinically driven repeat interventions (3.7% versus 10.9%, respectively; P<0.001).
Sirolimus analogues
With the remarkable reduction of restenosis with the Cypher SESs, several competing device companies have pursued sirolimus analogues, including everolimus, tacrolimus and ABT-578. All three compounds have been effective in decreasing neointimal formation in animal models (114–118).
Everolimus-eluting stents:
Everolimus has been developed to overcome the formulation problems of sirolimus while keeping its main pharmacological properties. Everolimus differs from sirolimus by substitution of a stable 2-hydroxyethyl chain in position 40 on the sirolimus chemical structure. Everolimus has increased solubility in organic solvents compared with sirolimus and shares a similar ability to inhibit SMC proliferation despite a twofold to threefold lower affinity for FKBP12 (119). Everolimus is more lipophilic, leading to more rapid absorption into the arterial wall. This property may decrease drug accumulation in the endothelium and improve endothelial healing (120). Everolimus, similar to sirolimus, inhibits mTOR through FKBP12 binding, which blocks the cell cycle in the G/S phase transition. Excellent safety and efficacy results of everolimus-eluting stents in preclinical studies (116,117) led to the First Use To Underscore restenosis Reduction with Everolimus (FUTURE) clinical trial program. The FUTURE I and FUTURE II trials were designed to determine the safety and feasibility of everolimus-eluting stents in focal, de novo coronary lesions. These feasibility studies established an acceptable safety profile, with a 7.7% and 4.8% MACE rate at six months post-PCI, respectively, in the FUTURE I and II trials. Moreover, these studies showed an important reduction of in-stent neointimal hyperplasia in the everolimus group (Table 1). Guidant Corporation (USA) is also continuing development of their cobalt chromium MULTI-LINK VISION everolimus-eluting stent; it was first implanted in a human in December 2003 and is being assessed in the SPIRIT FIRST trial. The six-month angiographic follow-up data presented at the 2004 Transcatheter Cardiovascular Therapeutics Annual Scientific Symposia (Washington, USA) are shown in Table 1.
Tacrolimus:
Mechanism of action:
Tacrolimus or FK506 is a potent immunosuppressive and antiproliferative agent structurally similar to sirolimus. Tacrolimus binds to the same intracellular binding protein, FKPB12, but unlike sirolimus, does not inhibit activation of mTOR. Compared with sirolimus, tacrolimus may allow earlier endothelial regeneration (121), but its inhibitory activity on human VSMC cells is about 100 times lower (121,122). As mentioned previously, inflammation is one of the major determinants of neointima formation after stent implantation. Tacrolimus exerts profound inhibitory effects on T lymphocyte activation by binding specifically to FKBP12. The tacrolimus-FKBP12 complex inhibits cytoplasmic phosphatase calcineurin (CaN). As a result, CaN fails to dephosphorylate the cytoplasmic component of the nuclear factor of activated T cells, and thereby the transport of nuclear factor of activated T cells to the nucleus, where they act as a transcription factor for several cytokine genes involved in the immune response and in inflammation (123). By its inhibitory effect on CaN, tacrolimus decreases the expression of IL-2, IL-3, IL-4, IL-5, interferon-gamma and granulocyte macrophage colony-stimulating factor, leading to a reduced inflammatory response (124). Studies in FKBP12−/−knockout mice have shown that the FKBP12-FK506 complex is also affecting the expression of cell cycle proteins. An increase in expression of p21 in FKBP12−/− knockout mice led to G1 cell cycle arrest (125). This mechanism may account for the antiproliferative effect observed in VSMCs (122). Tacrolimus also possesses activity against apoptosis (126) and increases the expression of transforming growth factor-beta (127), which has an inhibitory effect on cell proliferation by inducing expression of p15, p21 and p27.
Preclinical and clinical studies:
Preclinical studies of tacrolimus-eluting stents have shown a significant reduction of neointima proliferation compared with BMSs (114,115). Jomed, a Swedish company, has performed two studies with tacrolimus-eluting stents. First, tacrolimus-eluting (325 mg), expanded polytetrafluoroethylene-covered Jomed stents were implanted in de novo saphenous vein graft lesions (EndoVascular Investigation Determining the safety of a new tacrolimus-Eluting steNT graft [EVIDENT] study). The six-month MACE rate was 36.4% and the binary restenosis rate at six months was disappointing at 27%, which is similar to standard coronary stent grafts (128). On the basis of these results, this approach was not further investigated. A second study examined the Jomed FlexMaster tacrolimus- (60 μg and 230 μg) eluting stent, which used a nanoporous ceramic layer of aluminium oxide as the delivery platform. The PREliminary Safety Evaluation of Nanoporous Tacrolimus-eluting stents (PRESENT) I (low dose – 60 μg) study was stopped after enrolment of 22 patients in the treatment group; two cases of target vessel revascularization were reported (Table 1) (128). The PRESENT II registry study showed a MACE rate at six months of 32%, despite a higher dose of tacrolimus (230 μg) (128). Given these results and concerns about the ceramic coating (115), this stent program was stopped.
The failure of these tacroliumus DESs illustrates the impact of stent designs, especially the drug carrier properties. Two tacrolimus DES studies are ongoing that use different drug delivery techniques. In the PRESET study, tacrolimus is directly applied onto electro-polished stent surface without coating. In the JUPITER-I study, the Janus (Italy) Carbostent is characterized by deep sculpturing on the outer strut surface and integral Carbofilm (Janus) coating. The sculptures provide a deep housing for tacrolimus, which is released only toward the vessel wall, and the Carbofilm coating creates a persistently thromboresis-tant surface toward the blood.
ABT-578:
ABT-578 is a new synthetic analogue of sirolimus. The chemical structure of the drug is different from sirolimus through the substitution of a tetrazole ring at the 42 position (118). Like sirolimus, ABT-578 binds to FKBP12 and inhibits SMC proliferation by blocking mTOR. The Endeavor clinical program (Medtronic, USA) is designed to examine the safety and efficacy of ABT-578 released from a phosphotidylcholine delivery matrix on the cobalt-based alloy Driver stent (Medtronic). The 12-month follow-up data presented at EuroPCR 2004 (Paris, France) (129) showed a favourable MACE rate of 2% but an in-stent late loss of 0.58 mm, which is higher than published trials with the sirolimus DES. Several studies of this particular stent are ongoing.
Paclitaxel
Paclitaxel is an antineoplastic agent originally isolated from the bark of the Pacific Yew tree, Taxus brevifolia. Microtubules are polymers assembled from tubulin dimers. The assembly and disassembly of microtubules are maintained in a dynamic equilibrium that is regulated according to cell cycle phase. In vitro, paclitaxel enhances the polymerization of tubulin to stable microtubules and interacts directly with microtubules, stabilizing them against depolymerization. Paclitaxel prevents reorganization of the microtubule network, which is vital for numerous cell activities such as cell division and migration, signal transduction and secretory processes. Paclitaxel acts in a dose-dependent manner (130). At high doses, paclitaxel is cytotoxic. It arrests the cell in the G2/M phase of the cell cycle and induces apoptosis. At lower concentrations, recent data suggest that the cells are predominantly inhibited in a premitotic phase of the cell cycle (G0/G1 and G1/G2) (131). For that reason, the antiproliferative and antimigratory effects of paclitaxel are not associated with cell necrosis and apoptosis (132).
Two types of paclitaxel elution have been evaluated in human studies: elution of paclitaxel directly onto a BMS without any polymer; and elution of paclitaxel in a polymer with programmed release kinetics in the vascular wall. As showed in the DELIVER I study (Cook Incorporated, USA; Guidant Corporation), high-dose paclitaxel delivery through nonpolymer-based stents resulted in a significant inhibition of neointima proliferation; however, this reduction did not translate into a sufficient decrease of targeted vessel failure and ISR (Table 1) (133). The TAXUS program (Boston Scientific, USA) employs a polymer coating that provides biphasic drug release, with an initial burst of paclitaxel release in the first two days, followed by low-level release lasting for the next 10 days. The TAXUS stent platform has been shown to be safe and markedly effective in reducing clinical and angio-graphic restenosis (Table 1) (134,135).
Actinomycin D
Actinomycin D is a chemotherapeutic agent produced by Streptomyces antibioticus with antibiotic and antineoplasic properties. This drug acts by binding to double-stranded DNA, thereby interfering with the action of enzymes such as RNA polymerase, which are engaged in replication and transcription (136). At low concentrations, actinomycin D inhibits DNA-dependent RNA synthesis, and at higher concentrations, DNA synthesis is also inhibited. All types of RNA are affected, but ribosomal RNA is more sensitive. Actinomycin D prevents cell division and protein synthesis. It behaves like a cell cycle nonspecific agent that stops cell proliferation in all phases of the cycle. Preclinical studies showed that high doses of actinomycin D (40 μg/cm2 and 70 μg/cm2) on a DES in miniature swine was associated with incomplete healing and positive remodelling. However, lower doses (2.5 μg/cm2 and 10 μg/cm2) led to a significant decrease in restenosis rate compared with control groups, as well as complete re-endothelialization (136). The ACTinomycin eluting stent Improves Outcomes by reducing Neointimal hyperplasia (ACTION) trial was designed to evaluate the safety and efficacy of actinomycin D DES in 360 patients (137). Patients were randomly assigned to three groups: high-dose (10 μg/cm2) coated stent; low-dose (2.5 μg/cm2) coated stent; and noncoated stent. The six-month angiographic late loss and restenosis rates were higher in both DES groups than in the control group (Table 1). On the basis of these discouraging results, Guidant suspended all further development of actinomycin D-eluting stents.
Mycophenolic acid
Mycophenolic acid (MPA) is the active compound formed after the administration of the prodrug mycophenolate mofetil. MPA is a cytostatic immunosuppressant that inhibits de novo purine synthesis by its reversible inhibition of inosine monophosphate dehydrogenase. In the de novo purine synthesis pathway, inosine monophosphate dehydrogenase is responsible for the conversion of inosine monophosphate to GMP. By its activity, MPA decreases initiation of RNA and DNA synthesis and stops cell proliferation in the G1/S phase of the cell cycle. Moreover, it may increase the expression of p21, which decreases the activity of CDK and arrests the cell cycle in the G0/G1 phase. A preclinical porcine study showed a significant 45% decrease in area stenosis in MPA-coated stents compared with BMSs (138). Human studies are underway in the Inhibition with MPA of Coronary restenosis Trial (IMPACT), a double-blind, randomized study designed to assess the safety and neointimal inhibitory effects of an MPA-eluting Duraflex stent (Avantec Vascular Corporation, USA).
Estradiol
Estradiol possesses multiple vascular protective properties, as shown in preclinical studies (139–141). Estradiol may improve vascular healing, decrease proliferation and migration of SMCs and induce local angiogenesis (12). Beside its beneficial impact on the lipid profile, estrogen stimulates nitric oxide production by endothelial cells (139,140). Nitric oxide has an antiproliferative effect on SMCs and it downregulates the expression of the proto-oncogene c-myc, which is involved in SMC proliferation (142). Moreover, estradiol promotes vascular healing by stimulation of the estrogen receptor alpha, which improves re-endothelization. Conversely, inhibition of SMC proliferation and migration is mediated by the beta estrogen receptor (143). In a porcine model, 17-beta-estradiol-eluting stents were associated with a significant decrease in neointimal hyperplasia (141). The Estrogen And Stent To Eliminate Restenosis (EASTER) pilot trial evaluated 17-beta-estradiol loaded on BiodivYsio (Biocompatibles Cardiovascular Inc, USA) phosphorylcholine-coated stents in 60 patients. Late loss after six months of follow-up was quite high at 0.54±0.44 mm (Table 1) (144). A multicentre, second-phase EASTER study has been completed, and results are pending. Moreover, the 17-beta-ESTRAdiol to CUre REstenosis (ESTRACURE) clinical trial has just completed the recruitment of 300 patients. This clinical trial has evaluated the effect of local 17-beta-estradiol delivery through the Scimed Remedy (Boston Scientific) catheter during PCIs to reduce restenosis.
The synthetic CKI
Blockade of the ATP binding site of CDKs is a promising pharmacological target because the ATP binding site in CDKs is structurally different from other kinases (145). The most attractive drug in this family is flavopiridol. This molecule effectively blocks CDKs 1, 2, 4 and 7, while exerting negligible effects on other kinases. In vitro, flavopiridol stops the progression of the cell cycle at the G1/S and G2/M transitions, inducing a potent, dose-dependent, antiproliferative effect on human coronary artery SMCs (HCASMCs) (146). Moreover, flavopiridol effectively inhibits mitogen-induced HCASMC migration (146). In addition to the effect of blocking CDK, flavopiridol is able to induce upregulation of the protein expression of p21, p27 and p53, and down-regulation of cyclin A and D in HCASMCs (146). Also, no detectable cytotoxic and proapoptotic effects have been described in either HCASMCs or coronary artery endothelial cells treated with flavopiridol (146). Systemic administration of flavopiridol inhibits the vasculoproliferative reaction after balloon injury to the rat carotid artery model (147). Flavopiridol-eluting, phosphorylcholine-coated DESs (BiodivYsio) led to potent inhibition of neointimal formation in rat carotid arteries compared with BMSs (146). However, no clinical studies have yet been initiated.
Perlecan
In the absence of vascular trauma, VSMCs are quiescent and resistant to stimulation by most mitogens, suggesting the presence of growth suppression mechanisms. One potential growth suppressor is perlecan, a large heparan sulfate proteoglycan that is expressed in most extracellular matrixes and basement membranes (148). Perlecan has been shown to inhibit in vitro SMC proliferation (149,150), adhesion (151) and migration (152). In vivo, perlecan has been shown to inhibit thrombosis and intimal hyperplasia after arterial injury (153). Several studies have shown that endothelial-derived perlecan is a potent inhibitor of SMC proliferation in vitro (149,154,155). Perlecan binds to heparin-binding mitogens such as FGF-2, leading to inactivation of FGF-2, a potent stimulatory growth factor, due to its heparin-binding property. Perlecan-induced suppression of SMC proliferation may also be mediated through increased activity of a tumour suppressor – phosphatase and tensin homologue deleted on chromosome 10 (156) – which functions as a protein phosphatase. This phosphatase and tensin homologue hydrolyzes PIP2 and PIP3 (lipid products of PI3K), which decrease signalling through protein kinase B-1 and Akt (33). This effect results in decreased activation of mTOR, cell cycle arrest, and decreased cell migration and apoptosis. Our group is evaluating several perlecan-inducing compounds on coated stents in preclinical animal models.
TKR inhibitors
PDGF expressed by platelets, VSMCs and macrophages play an important role in VSMC proliferation and migration. The degree of neointimal formation has been shown to depend substantially on both PDGF-β receptor overexpression and activation by PDGF (157–160). PDGF acts as a ligand for the PDGF-β receptor, which belongs to the family of TKRs. Stimulation of this TKR activates the MAPK pathway and promotes transcription of proto-oncogenes. Tyrphostins are low molecular weight, synthetic compounds that are inhibitors of several TKRs (161). With their low molecular weight and hydrophobic characteristics, tyrphostins can more easily gain access to receptor sites deep within tissues such as the arterial media (162). In vitro experiments have shown that the tyrphostin AGL-2043, as well as the related tyrphostin AG-1295, selectively inhibit PDGF receptors and selectively inhibit SMC proliferation in culture (163). Local delivery of AG-1295 by a perivascular polymeric matrix or intraluminally with nanoparticules inhibited intimal hyperplasia in a rat carotid injury model (164,165). AGL-2043 DESs with a biodegradable, polymeric coating (162) led to a reduction of in-stent neointima formation in a pig coronary injury model. A poly-L-lactic acid biodegradable stent coated with TKR inhibitor (ST 638) also inhibited in-stent hyperplasia (166). We are not aware of any ongoing human clinical evaluations of TKR inhibitors.
GENE THERAPY APPROACH
Gene therapy can target either the activation or suppression of key regulatory genes involved in the cell cycle. Viral or plasmid technology can be used to overexpress endogenous cell cycle inhibitory proteins or novel cell cycle inhibitory proteins. It has been shown that transduction of cells with a gene encoding for a nonphosphorylatable, constitutively active form of the pRb gene product significantly inhibited neointimal hyperplasia (167). Overexpression of cell cycle inhibitors, such as p21 and p27 by an adenoviral technology or p53 by a naked plasmid vector, significantly reduced neointimal hyperplasia in several animal models (168–172). In addition, increased expression of the homeobox gene GAX reduced the vasculo-proliferative response after arterial wall injury in both rat and rabbit models (173–175). As well, overexpression of nitric oxide synthase gene has reduced neointimal proliferation in an animal model (51). Recently, this approach was tested in humans, although the results are not yet published (176).
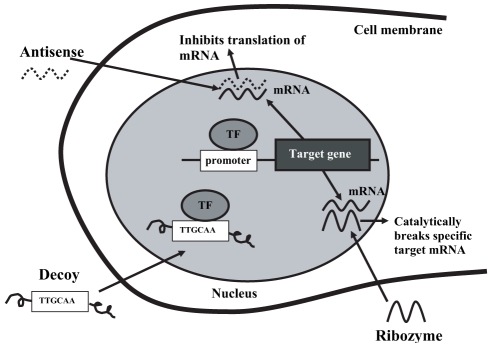
Endogenous gene expression can be downregulated by anti-sense oligodeoxynucleotides (ODNs), ribozyme RNA and decoy ODN technologies (Figure 8). Antisense ODNs are short chains of DNA that block the expression of a specific gene without any interference with other genes. This molecule has a base sequence that is complementary to a specific segment of target mRNA. Binding of antisense ODNs with mRNA leads to inhibition of mRNA translation and increases its degradation. In animal models, antisense ODNs directed against mRNA of c-myc, c-myb, PCNA, cyclin B, CDK1 and CDK2 genes have reduced neointimal hyperplasia (177–183). Transfection of anti-sense ODNs against CDK1 also led to a significant reduction of VSMC proliferation in a preclinical model of a transplanted heart (184). Only one human trial of antisense ODNs directed against c-myc has been reported. By using a local delivery balloon catheter, the neointimal volume obstruction and the angiographic restenosis rate wre not reduced (Table 1) (185). The clinical application of antisense ODN technology remains limited by a relative lack of specificity, slow uptake across the cell membrane and fast intracellular destruction of the ODN (186). It may be necessary to target more than one gene. Combination antisense ODNs directed against more than one cell cycle regulatory gene, such as CDK1 with PCNA or CDK1 with cyclin B, were more effective than a single target approach in a rat model of carotid artery injury (177,178) or experimental vein graft models (187).
Figure 8).
Mechanisms of action of antisense oligodeoxynucleotides (ODN), ribozyme and decoy ODN gene therapy. mRNA Messenger RNA; TF Transcription factor. Adapted from reference 176
An advanced six-ring morpholino backbone c-myc antisense (AVI-4126 [Resten NG], AVI BioPharma, USA) with increased specificity was shown to inhibit c-myc expression and intimal hyperplasia after local catheter delivery in a porcine stent restenosis model (186). This new molecular configuration increased intracellular uptake, resisted enzymatic degradation and enhanced specificity to its target mRNA (186). Recently, the results of the AVAIL study, a phase II clinical trial evaluating intramural delivery of AVI-4126 in patients with focal de novo stenosis or ISR, were presented (188). Patients treated with the highest dose (group B, 10 mg) of AVI-4126 presented less late loss (0.74±0.16 mm versus 1.26±0.19 mm; P=0.025) and less binary restenosis (8.3% versus 33.3%) at six months than the control group. AVI-4126 has also been placed on phos-phorylcholine-coated stents in a porcine coronary model, and this has led to a significant inhibition of neointimal hyperplasia and allowed complete re-endothelialization (189).
Gene activity can also be regulated negatively by ribozymes, which are RNA molecules designed to cleave the target mRNA of a specific gene. The degradation of mRNA leads to inhibition of gene expression. Preclinical studies in both balloon rat carotid artery and stent porcine coronary artery models have shown reduction of intimal hyperplasia after local delivery of ribozymes against c-myb, PCNA and CDK1 (190,191). No human clinical trials are underway.
Finally, the expression of a specific gene can be blocked by decoy ODN transfection. Decoy ODN is a synthetic, double-stranded DNA with high affinity to a specific transcription factor. To allow gene transcription, a transcription factor must bind a specific sequence found in the promoter, which then induces mRNA production. Decoy ODN shares the same base sequence as the promoter. Decoy ODN works by binding with the transcription factor before it binds to its promoter, therefore preventing mRNA formation. As mentioned previously, the E2F-1 transcription factor positively regulates expression of specific genes that are required for progression through the cell cycle, such as CDK 1, cyclin E, cyclin A and PCNA. The introduction of a sufficient quantity of decoy ODN targeting E2F-1 would effectively bind to it and prevent its interaction with its specific promoter region, leading to a decrease in gene expression of important cell cycle proteins and thereby inhibition of VSMC proliferation. A preclinical study of E2F-1 decoy in a carotid rat model and coronary porcine model of balloon-induced injury showed inhibition of neointimal proliferation (192). A clinical trial of hydrogel catheter delivery of E2F decoy to treat restenosis after angioplasty was initiated in 2000, but no results have been published (176).
This E2F-1 decoy ODN approach has also been used to successfully inhibit neointimal hyperplasia in a rabbit vein graft model (193). Recently, studies have been initiated in human peripheral bypass grafting. The PRoject in Ex-vivo Vein graft ENgineering via Transfection (PREVENT) I trial (193) showed the safety, feasibility and biological efficacy of intraop-erative transfection of human vein graft with E2F-1 decoy ODN. Vein grafts treated with E2F-1 decoy ODN showed a significant reduction in gene expression of PCNA and c-myc, as well as inhibition of vascular cell proliferation. Although the study was not designed to assess clinical outcome, grafts treated with E2F-1 decoy ODN were associated with a reduction in graft failure. PREVENT II is a randomized, double-blind, placebo controlled trial investigating the effects of E2F-1 decoy ODN in coronary bypass vein grafts. Preliminary results have suggested a 30% relative reduction in critical lesions (75% or greater diameter stenosis) in the treated group (P=0.03) and a statistically significant diminution (32%) in neointimal volume compared with placebo (194). To determine the clinical benefit of E2F-1 decoy ODN in the treatment of coronary and peripheral diseases, a large phase III clinical trial has been recently completed.
CONCLUSIONS
An improved understanding of the complexity of cell cycle regulation is mandatory to develop new therapeutic approaches against VSMC proliferation in several clinical conditions such as restenosis, transplant arteriopathy and vein graft disease. Preclinical models are important and useful for testing new drugs or gene therapy, but positive results in the animal model do not necessarily predict favourable results in humans. Current challenges include developing efficacious antiproliferative agents that can be delivered at adequate concentrations at the target vascular site. Drug delivery strategies also require additional developments. Recently, tremendous advances have been made with the rampamycin and paclitaxel DESs. Future work is required to improve stent platforms and drug release polymers, which are still not ideal. In addition, stents treat only relatively focal disease, while the vasculoproliferative disorders are typified by much more widespread involvement.
REFERENCES
- 1.Tunstall-Pedoe H, Kuulasmaa K, Mahonen M, Tolonen H, Ruokokoski E, Amouyel P. Contribution of trends in survival and coronary-event rates to changes in coronary heart disease mortality: 10-year results from 37 WHO MONICA project populations. Monitoring trends and determinants in cardiovascular disease. Lancet. 1999;353:1547–57. doi: 10.1016/s0140-6736(99)04021-0. [DOI] [PubMed] [Google Scholar]
- 2.Mintz GS, Popma JJ, Pichard AD, et al. Arterial remodeling after coronary angioplasty: A serial intravascular ultrasound study. Circulation. 1996;94:35–43. doi: 10.1161/01.cir.94.1.35. [DOI] [PubMed] [Google Scholar]
- 3.Hoffmann R, Mintz GS, Dussaillant GR, et al. Patterns and mechanisms of in-stent restenosis. A serial intravascular ultrasound study. Circulation. 1996;94:1247–54. doi: 10.1161/01.cir.94.6.1247. [DOI] [PubMed] [Google Scholar]
- 4.Wilensky RL, March KL, Gradus-Pizlo I, Sandusky G, Fineberg N, Hathaway DR. Vascular injury, repair, and restenosis after percutaneous transluminal angioplasty in the atherosclerotic rabbit. Circulation. 1995;92:2995–3005. doi: 10.1161/01.cir.92.10.2995. [DOI] [PubMed] [Google Scholar]
- 5.Wong A, Chan C. Drug-eluting stents: The end of restenosis? Ann Acad Med Singapore. 2004;33:423–31. [PubMed] [Google Scholar]
- 6.Topol EJ, Serruys PW. Frontiers in interventional cardiology. Circulation. 1998;98:1802–20. doi: 10.1161/01.cir.98.17.1802. [DOI] [PubMed] [Google Scholar]
- 7.El-Omar MM, Dangas G, Iakovou I, Mehran R. Update on in-stent restenosis. Curr Interv Cardiol Rep. 2001;3:296–305. [PubMed] [Google Scholar]
- 8.Mintz GS, Popma JJ, Hong MK, et al. Intravascular ultrasound to discern device-specific effects and mechanisms of restenosis. Am J Cardiol. 1996;78:18–22. doi: 10.1016/s0002-9149(96)00493-6. [DOI] [PubMed] [Google Scholar]
- 9.Edelman ER, Rogers C. Pathobiologic responses to stenting. Am J Cardiol. 1998;81:4E–6E. doi: 10.1016/s0002-9149(98)00189-1. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz RS, Huber KC, Murphy JG, et al. Restenosis and the proportional neointimal response to coronary artery injury: Results in a porcine model. J Am Coll Cardiol. 1992;19:267–74. doi: 10.1016/0735-1097(92)90476-4. [DOI] [PubMed] [Google Scholar]
- 11.Colombo A, De Scheerder I, Isner JM, Campbell R, Strauss BH. Restenosis: Multiple Strategies for Stent Drug Delivery. London: ReMEDICA; 2001. pp. 33–54. [Google Scholar]
- 12.Kipshidze N, Dangas G, Tsapenko M, et al. Role of the endothelium in modulating neointimal formation: Vasculoprotective approaches to attenuate restenosis after percutaneous coronary interventions. J Am Coll Cardiol. 2004;44:733–9. doi: 10.1016/j.jacc.2004.04.048. [DOI] [PubMed] [Google Scholar]
- 13.Garg UC, Hassid A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest. 1989;83:1774–7. doi: 10.1172/JCI114081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo K, Andres V, Walsh K. Nitric oxide-induced downregulation of Cdk2 activity and cyclin A gene transcription in vascular smooth muscle cells. Circulation. 1998;97:2066–72. doi: 10.1161/01.cir.97.20.2066. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz RS, Chronos NA, Virmani R. Preclinical restenosis models and drug-eluting stents: Still important, still much to learn. J Am Coll Cardiol. 2004;44:1373–85. doi: 10.1016/j.jacc.2004.04.060. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz RS. Characteristics of an ideal stent based upon restenosis pathology. J Invasive Cardiol. 1996;8:386–7. [PubMed] [Google Scholar]
- 17.Rogers C, Edelman ER, Simon DI. A mAb to the beta2-leukocyte integrin Mac-1 (CD11b/CD18) reduces intimal thickening after angioplasty or stent implantation in rabbits. Proc Natl Acad Sci USA. 1998;95:10134–9. doi: 10.1073/pnas.95.17.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rectenwald JE, Moldawer LL, Huber TS, Seeger JM, Ozaki CK. Direct evidence for cytokine involvement in neointimal hyperplasia. Circulation. 2000;102:1697–702. doi: 10.1161/01.cir.102.14.1697. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Romanic AM, Yue TL, Feuerstein GZ, Ohlstein EH. Expression of interleukin-1beta, interleukin-1 receptor, and interleukin-1 receptor antagonist mRNA in rat carotid artery after balloon angioplasty. Biochem Biophys Res Commun. 2000;271:138–43. doi: 10.1006/bbrc.2000.2588. [DOI] [PubMed] [Google Scholar]
- 20.Hojo Y, Ikeda U, Katsuki T, et al. Interleukin 6 expression in coronary circulation after coronary angioplasty as a risk factor for restenosis. Heart. 2000;84:83–7. doi: 10.1136/heart.84.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farb A, Weber DK, Kolodgie FD, Burke AP, Virmani R. Morphological predictors of restenosis after coronary stenting in humans. Circulation. 2002;105:2974–80. doi: 10.1161/01.cir.0000019071.72887.bd. [DOI] [PubMed] [Google Scholar]
- 22.Colombo A, De Scheerder I, Isner JM, Campbell R, Strauss BH. Restenosis: Multiple Strategies for Stent Drug Delivery. London: ReMEDICA; 2001. p. 14. [Google Scholar]
- 23.Karas SP, Gravanis MB, Santoian EC, Robinson KA, Anderberg KA, King SB., III Coronary intimal proliferation after balloon injury and stenting in swine: An animal model of restenosis. J Am Coll Cardiol. 1992;20:467–74. doi: 10.1016/0735-1097(92)90119-8. [DOI] [PubMed] [Google Scholar]
- 24.Kornowski R, Hong MK, Tio FO, Bramwell O, Wu H, Leon MB. In-stent restenosis: Contributions of inflammatory responses and arterial injury to neointimal hyperplasia. J Am Coll Cardiol. 1998;31:224–30. doi: 10.1016/s0735-1097(97)00450-6. [DOI] [PubMed] [Google Scholar]
- 25.Yutani C, Ishibashi-Ueda H, Suzuki T, Kojima A. Histologic evidence of foreign body granulation tissue and de novo lesions in patients with coronary stent restenosis. Cardiology. 1999;92:171–7. doi: 10.1159/000006967. [DOI] [PubMed] [Google Scholar]
- 26.Koster R, Vieluf D, Kiehn M, et al. Nickel and molybdenum contact allergies in patients with coronary in-stent restenosis. Lancet. 2000;356:1895–7. doi: 10.1016/S0140-6736(00)03262-1. [DOI] [PubMed] [Google Scholar]
- 27.Shi Y, O’Brien JE, Fard A, Mannion JD, Wang D, Zalewski A. Adventitial myofibroblasts contribute to neointimal formation in injured porcine coronary arteries. Circulation. 1996;94:1655–64. doi: 10.1161/01.cir.94.7.1655. [DOI] [PubMed] [Google Scholar]
- 28.Scott NA, Cipolla GD, Ross CE, et al. Identification of a potential role for the adventitia in vascular lesion formation after balloon overstretch injury of porcine coronary arteries. Circulation. 1996;93:2178–87. doi: 10.1161/01.cir.93.12.2178. [DOI] [PubMed] [Google Scholar]
- 29.Han CI, Campbell GR, Campbell JH. Circulating bone marrow cells can contribute to neointimal formation. J Vasc Res. 2001;38:113–9. doi: 10.1159/000051038. [DOI] [PubMed] [Google Scholar]
- 30.Saiura A, Sata M, Hirata Y, Nagai R, Makuuchi M. Circulating smooth muscle progenitor cells contribute to atherosclerosis. Nat Med. 2001;7:382–3. doi: 10.1038/86394. [DOI] [PubMed] [Google Scholar]
- 31.Shimizu K, Sugiyama S, Aikawa M, et al. Host bone-marrow cells are a source of donor intimal smooth-muscle-like cells in murine aortic transplant arteriopathy. Nat Med. 2001;7:738–41. doi: 10.1038/89121. [DOI] [PubMed] [Google Scholar]
- 32.Graves JD, Krebs EG. Protein phosphorylation and signal transduction. Pharmacol Ther. 1999;82:111–21. doi: 10.1016/s0163-7258(98)00056-4. [DOI] [PubMed] [Google Scholar]
- 33.Huang J, Kontos CD. Inhibition of vascular smooth muscle cell proliferation, migration, and survival by the tumor suppressor protein PTEN. Arterioscler Thromb Vasc Biol. 2002;22:745–51. doi: 10.1161/01.atv.0000016358.05294.8d. [DOI] [PubMed] [Google Scholar]
- 34.Carter AJ. TOR of the cell cycle: Are there important implications for diabetics in the era of the drug-eluting stent? Catheter Cardiovasc Interv. 2004;61:233–6. doi: 10.1002/ccd.10764. [DOI] [PubMed] [Google Scholar]
- 35.Braun-Dullaeus RC, Mann MJ, Seay U, et al. Cell cycle protein expression in vascular smooth muscle cells in vitro and in vivo is regulated through phosphatidylinositol 3-kinase and mammalian target of rapamycin. Arterioscler Thromb Vasc Biol. 2001;21:1152–8. doi: 10.1161/hq0701.092104. [DOI] [PubMed] [Google Scholar]
- 36.Colombo A, De Scheerder I, Isner JM, Campbell R, Strauss BH. Restenosis: Multiple Strategies for Stent Drug Delivery. London: ReMEDICA; 2001. p. 38. [Google Scholar]
- 37.Hunter T. Oncoprotein networks. Cell. 1997;88:333–46. doi: 10.1016/s0092-8674(00)81872-3. [DOI] [PubMed] [Google Scholar]
- 38.Kelly K, Cochran BH, Stiles CD, Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983;35:603–10. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- 39.Hanson KD, Shichiri M, Follansbee MR, Sedivy JM. Effects of c-myc expression on cell cycle progression. Mol Cell Biol. 1994;14:5748–55. doi: 10.1128/mcb.14.9.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phuchareon J, Tokuhisa T. Deregulated c-Fos/AP-1 modulates expression of the cyclin and the cdk gene in splenic B cells stimulated with lipopolysaccharide. Cancer Lett. 1995;92:203–8. doi: 10.1016/0304-3835(95)03780-z. [DOI] [PubMed] [Google Scholar]
- 41.Daksis JI, Lu RY, Facchini LM, Marhin WW, Penn LJ. Myc induces cyclin D1 expression in the absence of de novo protein synthesis and links mitogen-stimulated signal transduction to the cell cycle. Oncogene. 1994;9:3635–45. [PubMed] [Google Scholar]
- 42.Steiner P, Philipp A, Lukas J, et al. Identification of a Myc-dependent step during the formation of active G1 cyclin-cdk complexes. EMBO J. 1995;14:4814–26. doi: 10.1002/j.1460-2075.1995.tb00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu JJ, Chao JR, Jiang MC, Ng SY, Yen JJ, Yang-Yen HF. Ras transformation results in an elevated level of cyclin D1 and acceleration of G1 progression in NIH 3T3 cells. Mol Cell Biol. 1995;15:3654–63. doi: 10.1128/mcb.15.7.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dobrowolski S, Harter M, Stacey DW. Cellular ras activity is required for passage through multiple points of the G0/G1 phase in BALB/c 3T3 cells. Mol Cell Biol. 1994;14:5441–9. doi: 10.1128/mcb.14.8.5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leone G, DeGregori J, Sears R, Jakoi L, Nevins JR. Myc and Ras collaborate in inducing accumulation of active cyclin E/Cdk2 and E2F. Nature. 1997;387:422–6. doi: 10.1038/387422a0. [DOI] [PubMed] [Google Scholar]
- 46.Sherr CJ, Roberts JM. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–63. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 47.Colombo A, De Scheerder I, Isner JM, Campbell R, Strauss BH. Restenosis: Multiple Strategies for Stent Drug Delivery. London: ReMEDICA; 2001. p. 35. [Google Scholar]
- 48.Braun-Dullaeus RC, Mann MJ, Dzau VJ. Cell cycle progression: New therapeutic target for vascular proliferative disease. Circulation. 1998;98:82–9. doi: 10.1161/01.cir.98.1.82. [DOI] [PubMed] [Google Scholar]
- 49.Weir L, Chen D, Pastore C, Isner JM, Walsh K. Expression of gax, a growth arrest homeobox gene, is rapidly down-regulated in the rat carotid artery during the proliferative response to balloon injury. J Biol Chem. 1995;270:5457–61. doi: 10.1074/jbc.270.10.5457. [DOI] [PubMed] [Google Scholar]
- 50.Gorski DH, LePage DF, Patel CV, Copeland NG, Jenkins NA, Walsh K. Molecular cloning of a diverged homeobox gene that is rapidly down-regulated during the G0/G1 transition in vascular smooth muscle cells. Mol Cell Biol. 1993;13:3722–33. doi: 10.1128/mcb.13.6.3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sriram V, Patterson C. Cell cycle in vasculoproliferative diseases: Potential interventions and routes of delivery. Circulation. 2001;103:2414–9. doi: 10.1161/01.cir.103.19.2414. [DOI] [PubMed] [Google Scholar]
- 52.Fisher RP, Morgan DO. A novel cyclin associates with MO15/CDK7 to form the CDK-activating kinase. Cell. 1994;78:713–24. doi: 10.1016/0092-8674(94)90535-5. [DOI] [PubMed] [Google Scholar]
- 53.Jeffrey PD, Russo AA, Polyak K, et al. Mechanism of CDK activation revealed by the structure of a cyclinA-CDK2 complex. Nature. 1995;376:313–20. doi: 10.1038/376313a0. [DOI] [PubMed] [Google Scholar]
- 54.Paulovich AG, Hartwell LH. A checkpoint regulates the rate of progression through S phase in S. cerevisiae in response to DNA damage. Cell. 1995;82:841–7. doi: 10.1016/0092-8674(95)90481-6. [DOI] [PubMed] [Google Scholar]
- 55.Evans T, Rosenthal ET, Youngblom J, Distel D, Hunt T. Cyclin: A protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell. 1983;33:389–96. doi: 10.1016/0092-8674(83)90420-8. [DOI] [PubMed] [Google Scholar]
- 56.Pines J. Cyclins: Wheels within wheels. Cell Growth Differ. 1991;2:305–10. [PubMed] [Google Scholar]
- 57.Sherr CJ. G1 phase progression: Cycling on cue. Cell. 1994;79:551–5. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- 58.Vermeulen K, Van Bockstaele DR, Berneman ZN. The cell cycle: A review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003;36:131–49. doi: 10.1046/j.1365-2184.2003.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ohtsubo M, Theodoras AM, Schumacher J, Roberts JM, Pagano M. Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Mol Cell Biol. 1995;15:2612–24. doi: 10.1128/mcb.15.5.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Girard F, Strausfeld U, Fernandez A, Lamb NJ. Cyclin A is required for the onset of DNA replication in mammalian fibroblasts. Cell. 1991;67:1169–79. doi: 10.1016/0092-8674(91)90293-8. [DOI] [PubMed] [Google Scholar]
- 61.Walker DH, Maller JL. Role for cyclin A in the dependence of mitosis on completion of DNA replication. Nature. 1991;354:314–7. doi: 10.1038/354314a0. [DOI] [PubMed] [Google Scholar]
- 62.King RW, Jackson PK, Kirschner MW. Mitosis in transition. Cell. 1994;79:563–71. doi: 10.1016/0092-8674(94)90542-8. [DOI] [PubMed] [Google Scholar]
- 63.Arellano M, Moreno S. Regulation of CDK/cyclin complexes during the cell cycle. Int J Biochem Cell Biol. 1997;29:559–73. doi: 10.1016/s1357-2725(96)00178-1. [DOI] [PubMed] [Google Scholar]
- 64.Carnero A, Hannon GJ. The INK4 family of CDK inhibitors. Curr Top Microbiol Immunol. 1998;227:43–55. doi: 10.1007/978-3-642-71941-7_3. [DOI] [PubMed] [Google Scholar]
- 65.Hengst L, Reed SI. Inhibitors of the Cip/Kip family. Curr Top Microbiol Immunol. 1998;227:25–41. doi: 10.1007/978-3-642-71941-7_2. [DOI] [PubMed] [Google Scholar]
- 66.Swanton C. Cell-cycle targeted therapies. Lancet Oncol. 2004;5:27–36. doi: 10.1016/s1470-2045(03)01321-4. [DOI] [PubMed] [Google Scholar]
- 67.Hannon GJ, Beach D. p15INK4B is a potential effector of TGF-beta-induced cell cycle arrest. Nature. 1994;371:257–61. doi: 10.1038/371257a0. [DOI] [PubMed] [Google Scholar]
- 68.Reynisdottir I, Polyak K, Iavarone A, Massague J. Kip/Cip and Ink4 Cdk inhibitors cooperate to induce cell cycle arrest in response to TGF-beta. Genes Dev. 1995;9:1831–45. doi: 10.1101/gad.9.15.1831. [DOI] [PubMed] [Google Scholar]
- 69.Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–30. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 70.DeGregori J, Kowalik T, Nevins JR. Cellular targets for activation by the E2F1 transcription factor include DNA synthesis- and G1/S-regulatory genes. Mol Cell Biol. 1995;15:4215–24. doi: 10.1128/mcb.15.8.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stillman B. Smart machines at the DNA replication fork. Cell. 1994;78:725–8. doi: 10.1016/s0092-8674(94)90362-x. [DOI] [PubMed] [Google Scholar]
- 72.Montagnoli A, Fiore F, Eytan E, et al. Ubiquitination of p27 is regulated by Cdk-dependent phosphorylation and trimeric complex formation. Genes Dev. 1999;13:1181–9. doi: 10.1101/gad.13.9.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Voitenleitner C, Fanning E, Nasheuer HP. Phosphorylation of DNA polymerase alpha-primase by cyclin A-dependent kinases regulates initiation of DNA replication in vitro. Oncogene. 1997;14:1611–5. doi: 10.1038/sj.onc.1200975. [DOI] [PubMed] [Google Scholar]
- 74.Hartwell LH, Weinert TA. Checkpoints: Controls that ensure the order of cell cycle events. Science. 1989;246:629–34. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- 75.Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–31. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 76.Agarwal ML, Taylor WR, Chernov MV, Chernova OB, Stark GR. The p53 network. J Biol Chem. 1998;273:1–4. doi: 10.1074/jbc.273.1.1. [DOI] [PubMed] [Google Scholar]
- 77.Waga S, Hannon GJ, Beach D, Stillman B. The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature. 1994;369:574–8. doi: 10.1038/369574a0. [DOI] [PubMed] [Google Scholar]
- 78.Gottlieb TM, Oren M. p53 and apoptosis. Semin Cancer Biol. 1998;8:359–68. doi: 10.1006/scbi.1998.0098. [DOI] [PubMed] [Google Scholar]
- 79.Lowe SW, Schmitt EM, Smith SW, Osborne BA, Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993;362:847–9. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- 80.Galaktionov K, Chen X, Beach D. Cdc25 cell-cycle phosphatase as a target of c-myc. Nature. 1996;382:511–7. doi: 10.1038/382511a0. [DOI] [PubMed] [Google Scholar]
- 81.Born TL, Frost JA, Schonthal A, Prendergast GC, Feramisco JR. c-Myc cooperates with activated Ras to induce the cdc2 promoter. Mol Cell Biol. 1994;14:5710–8. doi: 10.1128/mcb.14.9.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ku DH, Wen SC, Engelhard A, et al. c-myb transactivates cdc2 expression via Myb binding sites in the 5’-flanking region of the human cdc2 gene. J Biol Chem. 1993;268:2255–9. [PubMed] [Google Scholar]
- 83.Lew DJ, Kornbluth S. Regulatory roles of cyclin dependent kinase phosphorylation in cell cycle control. Curr Opin Cell Biol. 1996;8:795–804. doi: 10.1016/s0955-0674(96)80080-9. [DOI] [PubMed] [Google Scholar]
- 84.Serruys PW, Foley DP, Jackson G, et al. A randomized, placebo controlled trial of fluvastatin for prevention of restenosis after successful coronary balloon angioplasty; final results of the fluvastatin angiographic restenosis (FLARE) trial. Eur Heart J. 1999;20:58–69. doi: 10.1053/euhj.1998.1150. [DOI] [PubMed] [Google Scholar]
- 85.Serruys PW, de Feyter P, Macaya C, et al. Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention: A randomized controlled trial. JAMA. 2002;287:3215–22. doi: 10.1001/jama.287.24.3215. [DOI] [PubMed] [Google Scholar]
- 86.Serruys PW, Rutsch W, Heyndrickx GR, et al. Prevention of restenosis after percutaneous transluminal coronary angioplasty with thromboxane A2-receptor blockade. A randomized, double-blind, placebo-controlled trial. Coronary Artery Restenosis Prevention on Repeated Thromboxane-Antagonism Study (CARPORT) Circulation. 1991;84:1568–80. doi: 10.1161/01.cir.84.4.1568. [DOI] [PubMed] [Google Scholar]
- 87.Faxon DP. Effect of high dose angiotensin-converting enzyme inhibition on restenosis: Final results of the MARCATOR Study, a multicenter, double-blind, placebo-controlled trial of cilazapril. The Multicenter American Research Trial With Cilazapril After Angioplasty to Prevent Transluminal Coronary Obstruction and Restenosis (MARCATOR) Study Group. J Am Coll Cardiol. 1995;25:362–9. doi: 10.1016/0735-1097(94)00368-z. [DOI] [PubMed] [Google Scholar]
- 88.Meurice T, Bauters C, Hermant X, et al. Effect of ACE inhibitors on angiographic restenosis after coronary stenting (PARIS): A randomised, double-blind, placebo-controlled trial. Lancet. 2001;357:1321–4. doi: 10.1016/s0140-6736(00)04518-9. [DOI] [PubMed] [Google Scholar]
- 89.Emanuelsson H, Beatt KJ, Bagger JP, et al. Long-term effects of angiopeptin treatment in coronary angioplasty. Reduction of clinical events but not angiographic restenosis. European Angiopeptin Study Group. Circulation. 1995;91:1689–96. doi: 10.1161/01.cir.91.6.1689. [DOI] [PubMed] [Google Scholar]
- 90.Galassi AR, Tamburino C, Nicosia A, et al. A randomized comparison of trapidil (triazolopyrimidine), a platelet-derived growth factor antagonist, versus aspirin in prevention of angiographic restenosis after coronary artery Palmaz-Schatz stent implantation. Catheter Cardiovasc Interv. 1999;46:162–8. doi: 10.1002/(SICI)1522-726X(199902)46:2<162::AID-CCD10>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 91.Serruys PW, Foley DP, Pieper M, Kleijne JA, de Feyter PJ. The TRAPIST Study. A multicentre randomized placebo controlled clinical trial of trapidil for prevention of restenosis after coronary stenting, measured by 3-D intravascular ultrasound. Eur Heart J. 2001;22:1938–47. doi: 10.1053/euhj.2001.2627. [DOI] [PubMed] [Google Scholar]
- 92.Holmes DR, Jr, Savage M, LaBlanche JM, et al. Results of Prevention of REStenosis with Tranilast and its Outcomes (PRESTO) trial. Circulation. 2002;106:1243–50. doi: 10.1161/01.cir.0000028335.31300.da. [DOI] [PubMed] [Google Scholar]
- 93.Lee CW, Chae JK, Lim HY, et al. Prospective randomized trial of corticosteroids for the prevention of restenosis after intracoronary stent implantation. Am Heart J. 1999;138:60–3. doi: 10.1016/s0002-8703(99)70247-4. [DOI] [PubMed] [Google Scholar]
- 94.Bailey S. Local drug delivery during percutaneous coronary intervention. Curr Interv Cardiol Rep. 2000;2:349–57. [PubMed] [Google Scholar]
- 95.Zohlnhofer D, Klein CA, Richter T, et al. Gene expression profiling of human stent-induced neointima by cDNA array analysis of microscopic specimens retrieved by helix cutter atherectomy: Detection of FK506-binding protein 12 upregulation. Circulation. 2001;103:1396–402. doi: 10.1161/01.cir.103.10.1396. [DOI] [PubMed] [Google Scholar]
- 96.Abraham RT. Mammalian target of rapamycin: Immunosuppressive drugs uncover a novel pathway of cytokine receptor signaling. Curr Opin Immunol. 1998;10:330–6. doi: 10.1016/s0952-7915(98)80172-6. [DOI] [PubMed] [Google Scholar]
- 97.Dennis PB, Fumagalli S, Thomas G. Target of rapamycin (TOR): Balancing the opposing forces of protein synthesis and degradation. Curr Opin Genet Dev. 1999;9:49–54. doi: 10.1016/s0959-437x(99)80007-0. [DOI] [PubMed] [Google Scholar]
- 98.Marx SO, Jayaraman T, Go LO, Marks AR. Rapamycin-FKBP inhibits cell cycle regulators of proliferation in vascular smooth muscle cells. Circ Res. 1995;76:412–7. doi: 10.1161/01.res.76.3.412. [DOI] [PubMed] [Google Scholar]
- 99.Luo Y, Marx SO, Kiyokawa H, Koff A, Massague J, Marks AR. Rapamycin resistance tied to defective regulation of p27Kip1. Mol Cell Biol. 1996;16:6744–51. doi: 10.1128/mcb.16.12.6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brunn GJ, Hudson CC, Sekulic A, et al. Phosphorylation of the translational repressor PHAS-I by the mammalian target of rapamycin. Science. 1997;277:99–101. doi: 10.1126/science.277.5322.99. [DOI] [PubMed] [Google Scholar]
- 101.Burnett PE, Barrow RK, Cohen NA, Snyder SH, Sabatini DM. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc Natl Acad Sci USA. 1998;95:1432–7. doi: 10.1073/pnas.95.4.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Woods TC, Marks AR. Drug-eluting stents. Annu Rev Med. 2004;55:169–78. doi: 10.1146/annurev.med.55.091902.105243. [DOI] [PubMed] [Google Scholar]
- 103.Bonneton C, Sibarita JB, Thiery JP. Relationship between cell migration and cell cycle during the initiation of epithelial to fibroblastoid transition. Cell Motil Cytoskeleton. 1999;43:288–95. doi: 10.1002/(SICI)1097-0169(1999)43:4<288::AID-CM2>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 104.Sun J, Marx SO, Chen HJ, Poon M, Marks AR, Rabbani LE. Role for p27(Kip1) in vascular smooth muscle cell migration. Circulation. 2001;103:2967–72. doi: 10.1161/01.cir.103.24.2967. [DOI] [PubMed] [Google Scholar]
- 105.Zohlnhofer D, Nuhrenberg TG, Neumann FJ, et al. Rapamycin effects transcriptional programs in smooth muscle cells controlling proliferative and inflammatory properties. Mol Pharmacol. 2004;65:880–9. doi: 10.1124/mol.65.4.880. [DOI] [PubMed] [Google Scholar]
- 106.Knies UE, Behrensdorf HA, Mitchell CA, et al. Regulation of endothelial monocyte-activating polypeptide II release by apoptosis. Proc Natl Acad Sci USA. 1998;95:12322–7. doi: 10.1073/pnas.95.21.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Daemen MA, van’t Veer C, Denecker G, et al. Inhibition of apoptosis induced by ischemia-reperfusion prevents inflammation. J Clin Invest. 1999;104:541–9. doi: 10.1172/JCI6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gallo R, Padurean A, Jayaraman T, et al. Inhibition of intimal thickening after balloon angioplasty in porcine coronary arteries by targeting regulators of the cell cycle. Circulation. 1999;99:2164–70. doi: 10.1161/01.cir.99.16.2164. [DOI] [PubMed] [Google Scholar]
- 109.Rodriguez AE, Alemparte MR, Vigo CF, et al. Pilot study of oral rapamycin to prevent restenosis in patients undergoing coronary stent therapy: Argentina Single-Center Study (ORAR Trial) J Invasive Cardiol. 2003;15:581–4. [PubMed] [Google Scholar]
- 110.Waksman R, Ajani AE, Pichard AD, et al. Oral rapamycin to inhibit restenosis after stenting of de novo coronary lesions: The Oral Rapamune to Inhibit Restenosis (ORBIT) study. J Am Coll Cardiol. 2004;44:1386–92. doi: 10.1016/j.jacc.2004.06.069. [DOI] [PubMed] [Google Scholar]
- 111.Brara PS, Moussavian M, Grise MA, et al. Pilot trial of oral rapamycin for recalcitrant restenosis. Circulation. 2003;107:1722–4. doi: 10.1161/01.CIR.0000066282.05411.17. [DOI] [PubMed] [Google Scholar]
- 112.Hausleiter J, Kastrati A, Mehilli J, et al. Randomized, double-blind, placebo-controlled trial of oral sirolimus for restenosis prevention in patients with in-stent restenosis: The Oral Sirolimus to Inhibit Recurrent In-stent Stenosis (OSIRIS) trial. Circulation. 2004;110:790–5. doi: 10.1161/01.CIR.0000138935.17503.35. [DOI] [PubMed] [Google Scholar]
- 113.Lemos PA, Serruys PW, van Domburg RT, et al. Unrestricted utilization of sirolimus-eluting stents compared with conventional bare stent implantation in the “real world”: The Rapamycin-Eluting Stent Evaluated At Rotterdam Cardiology Hospital (RESEARCH) registry. Circulation. 2004;109:190–5. doi: 10.1161/01.CIR.0000109138.84579.FA. [DOI] [PubMed] [Google Scholar]
- 114.Wieneke H, Dirsch O, Sawitowski T, et al. Synergistic effects of a novel nanoporous stent coating and tacrolimus on intima proliferation in rabbits. Catheter Cardiovasc Interv. 2003;60:399–407. doi: 10.1002/ccd.10664. [DOI] [PubMed] [Google Scholar]
- 115.Grube E, Buellesfeld L. Rapamycin analogs for stent-based local drug delivery. Everolimus- and tacrolimus-eluting stents. Herz. 2004;29:162–6. doi: 10.1007/s00059-004-2556-6. [DOI] [PubMed] [Google Scholar]
- 116.Honda H, Kar S, Honda T, et al. Everolimus eluting stents significantly inhibit neointimal hyperplasia in an experimental pig coronary model Am J Cardiol 200290Suppl 6A73H12088788 [Google Scholar]
- 117.Honda T, Kar S, Honda H, et al. Stent-based delivery of everolimus leads to complete vessel wall healing without toxicity in a 90-day porcine model. Am J Cardiol. 2002;90(Suppl 6A):80H. [Google Scholar]
- 118.Buellesfeld L, Grube E. ABT-578-eluting stents. The promising successor of sirolimus- and paclitaxel-eluting stent concepts? Herz. 2004;29:167–70. doi: 10.1007/s00059-004-2557-5. [DOI] [PubMed] [Google Scholar]
- 119.Schuler W, Sedrani R, Cottens S, et al. SDZ RAD, a new rapamycin derivative: Pharmacological properties in vitro and in vivo. Transplantation. 1997;64:36–42. doi: 10.1097/00007890-199707150-00008. [DOI] [PubMed] [Google Scholar]
- 120.Grube E, Buellesfeld L. Everolimus for stent-based intracoronary applications. Rev Cardiovasc Med. 2004;5(Suppl 2):S3–8. [PubMed] [Google Scholar]
- 121.Matter MC, Wnendt S, Kurz DJ, et al. Tacrolimus, but not sirolimus targets human vascular smooth muscle cells, but spares endothelial cells – implications for drug-eluting stents Eur Heart J 20024Suppl143(Abst) [Google Scholar]
- 122.Mohacsi PJ, Tuller D, Hulliger B, Wijngaard PL. Different inhibitory effects of immunosuppressive drugs on human and rat aortic smooth muscle and endothelial cell proliferation stimulated by platelet-derived growth factor or endothelial cell growth factor. J Heart Lung Transplant. 1997;16:484–92. [PubMed] [Google Scholar]
- 123.Morris RE. Mechanisms of action of new immunosuppressive drugs. Kidney Int Suppl. 1996;53:S26–38. [PubMed] [Google Scholar]
- 124.Sakuma S, Higashi Y, Sato N, et al. Tacrolimus suppressed the production of cytokines involved in atopic dermatitis by direct stimulation of human PBMC system. (Comparison with steroids) Int Immunopharmacol. 2001;1:1219–26. doi: 10.1016/s1567-5769(01)00059-5. [DOI] [PubMed] [Google Scholar]
- 125.Aghdasi B, Ye K, Resnick A, et al. FKBP12, the 12-kDa FK506-binding protein, is a physiologic regulator of the cell cycle. Proc Natl Acad Sci USA. 2001;98:2425–30. doi: 10.1073/pnas.041614198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Brunner T, Yoo NJ, LaFace D, Ware CF, Green DR. Activation-induced cell death in murine T cell hybridomas. Differential regulation of Fas (CD95) versus Fas ligand expression by cyclosporin A and FK506. Int Immunol. 1996;8:1017–26. doi: 10.1093/intimm/8.7.1017. [DOI] [PubMed] [Google Scholar]
- 127.Khanna A, Cairns V, Hosenpud JD. Tacrolimus induces increased expression of transforming growth factor-beta1 in mammalian lymphoid as well as nonlymphoid cells. Transplantation. 1999;67:614–9. doi: 10.1097/00007890-199902270-00021. [DOI] [PubMed] [Google Scholar]
- 128.Grube E. Final tacrolimus outcomes in native coronaries and saphenous vein grafts: PRESENT & EVIDENT. American College of Cardiology Meeting; Chicago. March 30 to April 2, 2003. [Google Scholar]
- 129.Meredith IT.ENDEAVOR I: Safety and efficacy of the ABT-578-coated Endeavor stent – 12-month follow-up EuroPCRParis, May25 to 282004
- 130.Jordan MA, Toso RJ, Thrower D, Wilson L. Mechanism of mitotic block and inhibition of cell proliferation by taxol at low concentrations. Proc Natl Acad Sci USA. 1993;90:9552–6. doi: 10.1073/pnas.90.20.9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Giannakakou P, Robey R, Fojo T, Blagosklonny MV. Low concentrations of paclitaxel induce cell type-dependent p53, p21 and G1/G2 arrest instead of mitotic arrest: Molecular determinants of paclitaxel-induced cytotoxicity. Oncogene. 2001;20:3806–13. doi: 10.1038/sj.onc.1204487. [DOI] [PubMed] [Google Scholar]
- 132.Axel DI, Kunert W, Goggelmann C, et al. Paclitaxel inhibits arterial smooth muscle cell proliferation and migration in vitro and in vivo using local drug delivery. Circulation. 1997;96:636–45. doi: 10.1161/01.cir.96.2.636. [DOI] [PubMed] [Google Scholar]
- 133.Lansky AJ, Costa RA, Mintz GS, et al. Non-polymer-based paclitaxel-coated coronary stents for the treatment of patients with de novo coronary lesions: Angiographic follow-up of the DELIVER clinical trial. Circulation. 2004;109:1948–54. doi: 10.1161/01.CIR.0000127129.94129.6F. [DOI] [PubMed] [Google Scholar]
- 134.Colombo A, Drzewiecki J, Banning A, et al. Randomized study to assess the effectiveness of slow- and moderate-release polymer-based paclitaxel-eluting stents for coronary artery lesions. Circulation. 2003;108:788–94. doi: 10.1161/01.CIR.0000086926.62288.A6. [DOI] [PubMed] [Google Scholar]
- 135.Stone GW, Ellis SG, Cox DA, et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. 2004;350:221–31. doi: 10.1056/NEJMoa032441. [DOI] [PubMed] [Google Scholar]
- 136.Moses JW, Kipshidze N, Leon MB. Perspectives of drug-eluting stents: The next revolution. Am J Cardiovasc Drugs. 2002;2:163–72. doi: 10.2165/00129784-200202030-00004. [DOI] [PubMed] [Google Scholar]
- 137.Serruys PW, Ormiston JA, Sianos G, et al. Actinomycin-eluting stent for coronary revascularization: A randomized feasibility and safety study: The ACTION trial. J Am Coll Cardiol. 2004;44:1363–7. doi: 10.1016/j.jacc.2004.03.084. [DOI] [PubMed] [Google Scholar]
- 138.Abizaid AC. Novel studies with drug-eluting stents: New drug-eluting stent trials: EASTER, IMPACT, PISCES, BioRest, and more. Transcatheter Cardiovascular Therapeutics Annual Scientific Symposia; Washington. September 15 to 19, 2003. [Google Scholar]
- 139.Sudhir K, Chou TM, Mullen WL, et al. Mechanisms of estrogen-induced vasodilation: In vivo studies in canine coronary conductance and resistance arteries. J Am Coll Cardiol. 1995;26:807–14. doi: 10.1016/0735-1097(95)00248-3. [DOI] [PubMed] [Google Scholar]
- 140.White CR, Shelton J, Chen SJ, et al. Estrogen restores endothelial cell function in an experimental model of vascular injury. Circulation. 1997;96:1624–30. doi: 10.1161/01.cir.96.5.1624. [DOI] [PubMed] [Google Scholar]
- 141.New G, Moses JW, Roubin GS, et al. Estrogen-eluting, phosphorylcholine-coated stent implantation is associated with reduced neointimal formation but no delay in vascular repair in a porcine coronary model. Catheter Cardiovasc Interv. 2002;57:266–71. doi: 10.1002/ccd.10339. [DOI] [PubMed] [Google Scholar]
- 142.Chen SJ, Li H, Durand J, Oparil S, Chen YF. Estrogen reduces myointimal proliferation after balloon injury of rat carotid artery. Circulation. 1996;93:577–84. doi: 10.1161/01.cir.93.3.577. [DOI] [PubMed] [Google Scholar]
- 143.Geraldes P, Sirois MG, Tanguay JF. Specific contribution of estrogen receptors on mitogen-activated protein kinase pathways and vascular cell activation. Circ Res. 2003;93:399–405. doi: 10.1161/01.RES.0000088640.18462.42. [DOI] [PubMed] [Google Scholar]
- 144.Abizaid A, Albertal M, Costa MA, et al. First human experience with the 17-beta-estradiol-eluting stent: The Estrogen And Stents To Eliminate Restenosis (EASTER) trial. J Am Coll Cardiol. 2004;43:1118–21. doi: 10.1016/j.jacc.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 145.Gray N, Detivaud L, Doerig C, Meijer L. ATP-site directed inhibitors of cyclin-dependent kinases. Curr Med Chem. 1999;6:859–75. [PubMed] [Google Scholar]
- 146.Jaschke B, Milz S, Vogeser M, et al. Local cyclin-dependent kinase inhibition by flavopiridol inhibits coronary artery smooth muscle cell proliferation and migration: Implications for the applicability on drug-eluting stents to prevent neointima formation following vascular injury. FASEB J. 2004;18:1285–7. doi: 10.1096/fj.04-1646fje. [DOI] [PubMed] [Google Scholar]
- 147.Ruef J, Meshel AS, Hu Z, et al. Flavopiridol inhibits smooth muscle cell proliferation in vitro and neointimal formation In vivo after carotid injury in the rat. Circulation. 1999;100:659–65. doi: 10.1161/01.cir.100.6.659. [DOI] [PubMed] [Google Scholar]
- 148.Iozzo RV, Cohen IR, Grassel S, Murdoch AD. The biology of perlecan: The multifaceted heparan sulphate proteoglycan of basement membranes and pericellular matrices. Biochem J. 1994;302:625–39. doi: 10.1042/bj3020625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Forsten KE, Courant NA, Nugent MA. Endothelial proteoglycans inhibit bFGF binding and mitogenesis. J Cell Physiol. 1997;172:209–20. doi: 10.1002/(SICI)1097-4652(199708)172:2<209::AID-JCP8>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 150.Paka L, Goldberg IJ, Obunike JC, et al. Perlecan mediates the antiproliferative effect of apolipoprotein E on smooth muscle cells. An underlying mechanism for the modulation of smooth muscle cell growth? J Biol Chem. 1999;274:36403–8. doi: 10.1074/jbc.274.51.36403. [DOI] [PubMed] [Google Scholar]
- 151.Lundmark K, Tran PK, Kinsella MG, Clowes AW, Wight TN, Hedin U. Perlecan inhibits smooth muscle cell adhesion to fibronectin: Role of heparan sulfate. J Cell Physiol. 2001;188:67–74. doi: 10.1002/jcp.1094. [DOI] [PubMed] [Google Scholar]
- 152.Koyama N, Kinsella MG, Wight TN, Hedin U, Clowes AW. Heparan sulfate proteoglycans mediate a potent inhibitory signal for migration of vascular smooth muscle cells. Circ Res. 1998;83:305–13. doi: 10.1161/01.res.83.3.305. [DOI] [PubMed] [Google Scholar]
- 153.Nugent MA, Nugent HM, Iozzo RV, Sanchack K, Edelman ER. Perlecan is required to inhibit thrombosis after deep vascular injury and contributes to endothelial cell-mediated inhibition of intimal hyperplasia. Proc Natl Acad Sci USA. 2000;97:6722–7. doi: 10.1073/pnas.97.12.6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kojima T, Leone CW, Marchildon GA, Marcum JA, Rosenberg RD. Isolation and characterization of heparan sulfate proteoglycans produced by cloned rat microvascular endothelial cells. J Biol Chem. 1992;267:4859–69. [PubMed] [Google Scholar]
- 155.Benitz WE, Kelley RT, Anderson CM, Lorant DE, Bernfield M. Endothelial heparan sulfate proteoglycan. I. Inhibitory effects on smooth muscle cell proliferation. Am J Respir Cell Mol Biol. 1990;2:13–24. doi: 10.1165/ajrcmb/2.1.13. [DOI] [PubMed] [Google Scholar]
- 156.Garl PJ, Wenzlau JM, Walker HA, Whitelock JM, Costell M, Weiser-Evans MC. Perlecan-induced suppression of smooth muscle cell proliferation is mediated through increased activity of the tumor suppressor PTEN. Circ Res. 2004;94:175–83. doi: 10.1161/01.RES.0000109791.69181.B6. [DOI] [PubMed] [Google Scholar]
- 157.Sirois MG, Simons M, Edelman ER. Antisense oligonucleotide inhibition of PDGFR-beta receptor subunit expression directs suppression of intimal thickening. Circulation. 1997;95:669–76. doi: 10.1161/01.cir.95.3.669. [DOI] [PubMed] [Google Scholar]
- 158.Desfaits AC, Raymond J, Muizelaar JP. Growth factors stimulate neointimal cells in vitro and increase the thickness of the neointima formed at the neck of porcine aneurysms treated by embolization. Stroke. 2000;31:498–507. doi: 10.1161/01.str.31.2.498. [DOI] [PubMed] [Google Scholar]
- 159.Ding H, Wang R, Marcel R, Fisher DZ. Adenovirus-mediated expression of a truncated PDGFbeta receptor inhibits thrombosis and neointima formation in an avian arterial injury model. Thromb Haemost. 2001;86:914–22. [PubMed] [Google Scholar]
- 160.Buetow BS, Tappan KA, Crosby JR, Seifert RA, Bowen-Pope DF. Chimera analysis supports a predominant role of PDGFRbeta in promoting smooth-muscle cell chemotaxis after arterial injury. Am J Pathol. 2003;163:979–84. doi: 10.1016/s0002-9440(10)63457-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gazit A, Yee K, Uecker A, et al. Tricyclic quinoxalines as potent kinase inhibitors of PDGFR kinase, Flt3 and Kit. Bioorg Med Chem. 2003;11:2007–18. doi: 10.1016/s0968-0896(03)00048-8. [DOI] [PubMed] [Google Scholar]
- 162.Banai S, Gertz SD, Gavish L, et al. Tyrphostin AGL-2043 eluting stent reduces neointima formation in porcine coronary arteries. Cardiovasc Res. 2004;64:165–71. doi: 10.1016/j.cardiores.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 163.Banai S, Wolf Y, Golomb G, et al. PDGF-receptor tyrosine kinase blocker AG1295 selectively attenuates smooth muscle cell growth in vitro and reduces neointimal formation after balloon angioplasty in swine. Circulation. 1998;97:1960–9. doi: 10.1161/01.cir.97.19.1960. [DOI] [PubMed] [Google Scholar]
- 164.Fishbein I, Waltenberger J, Banai S, et al. Local delivery of platelet-derived growth factor receptor-specific tyrphostin inhibits neointimal formation in rats. Arterioscler Thromb Vasc Biol. 2000;20:667–76. doi: 10.1161/01.atv.20.3.667. [DOI] [PubMed] [Google Scholar]
- 165.Fishbein I, Chorny M, Banai S, et al. Formulation and delivery mode affect disposition and activity of tyrphostin-loaded nanoparticles in the rat carotid model. Arterioscler Thromb Vasc Biol. 2001;21:1434–9. doi: 10.1161/hq0901.095567. [DOI] [PubMed] [Google Scholar]
- 166.Yamawaki T, Shimokawa H, Kozai T, et al. Intramural delivery of a specific tyrosine kinase inhibitor with biodegradable stent suppresses the restenotic changes of the coronary artery in pigs in vivo. J Am Coll Cardiol. 1998;32:780–6. doi: 10.1016/s0735-1097(98)00312-x. [DOI] [PubMed] [Google Scholar]
- 167.Chang MW, Barr E, Seltzer J, et al. Cytostatic gene therapy for vascular proliferative disorders with a constitutively active form of the retinoblastoma gene product. Science. 1995;267:518–22. doi: 10.1126/science.7824950. [DOI] [PubMed] [Google Scholar]
- 168.Chang MW, Barr E, Lu MM, Barton K, Leiden JM. Adenovirus-mediated over-expression of the cyclin/cyclin-dependent kinase inhibitor, p21 inhibits vascular smooth muscle cell proliferation and neointima formation in the rat carotid artery model of balloon angioplasty. J Clin Invest. 1995;96:2260–8. doi: 10.1172/JCI118281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chen D, Krasinski K, Sylvester A, Chen J, Nisen PD, Andres V. Downregulation of cyclin-dependent kinase 2 activity and cyclin A promoter activity in vascular smooth muscle cells by p27(KIP1), an inhibitor of neointima formation in the rat carotid artery. J Clin Invest. 1997;99:2334–41. doi: 10.1172/JCI119414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yang ZY, Simari RD, Perkins ND, et al. Role of the p21 cyclin-dependent kinase inhibitor in limiting intimal cell proliferation in response to arterial injury. Proc Natl Acad Sci USA. 1996;93:7905–10. doi: 10.1073/pnas.93.15.7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yonemitsu Y, Kaneda Y, Tanaka S, et al. Transfer of wild-type p53 gene effectively inhibits vascular smooth muscle cell proliferation in vitro and in vivo. Circ Res. 1998;82:147–56. doi: 10.1161/01.res.82.2.147. [DOI] [PubMed] [Google Scholar]
- 172.Yonemitsu Y, Kaneda Y, Hata Y, Nakashima Y, Sueishi K. Wild-type p53 gene transfer: A novel therapeutic strategy for neointimal hyperplasia after arterial injury. Ann N Y Acad Sci. 1997;811:395–400. doi: 10.1111/j.1749-6632.1997.tb52019.x. [DOI] [PubMed] [Google Scholar]
- 173.Maillard L, Van Belle E, Smith RC, et al. Percutaneous delivery of the gax gene inhibits vessel stenosis in a rabbit model of balloon angioplasty. Cardiovasc Res. 1997;35:536–46. doi: 10.1016/s0008-6363(97)00147-8. [DOI] [PubMed] [Google Scholar]
- 174.Perlman H, Luo Z, Krasinski K, et al. Adenovirus-mediated delivery of the Gax transcription factor to rat carotid arteries inhibits smooth muscle proliferation and induces apoptosis. Gene Ther. 1999;6:758–63. doi: 10.1038/sj.gt.3300893. [DOI] [PubMed] [Google Scholar]
- 175.Maillard L, Van Belle E, Tio FO, et al. Effect of percutaneous adenovirus-mediated Gax gene delivery to the arterial wall in double-injured atheromatous stented rabbit iliac arteries. Gene Ther. 2000;7:1353–61. doi: 10.1038/sj.gt.3301255. [DOI] [PubMed] [Google Scholar]
- 176.Morishita R. Perspective in progress of cardiovascular gene therapy. J Pharmacol Sci. 2004;95:1–8. doi: 10.1254/jphs.95.1. [DOI] [PubMed] [Google Scholar]
- 177.Morishita R, Gibbons GH, Ellison KE, et al. Single intraluminal delivery of antisense cdc2 kinase and proliferating-cell nuclear antigen oligonucleotides results in chronic inhibition of neointimal hyperplasia. Proc Natl Acad Sci USA. 1993;90:8474–8. doi: 10.1073/pnas.90.18.8474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Morishita R, Gibbons GH, Ellison KE, et al. Intimal hyperplasia after vascular injury is inhibited by antisense cdk 2 kinase oligonucleotides. J Clin Invest. 1994;93:1458–64. doi: 10.1172/JCI117123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Morishita R, Gibbons GH, Kaneda Y, Ogihara T, Dzau VJ. Pharmacokinetics of antisense oligodeoxyribonucleotides (cyclin B1 and CDC 2 kinase) in the vessel wall in vivo: Enhanced therapeutic utility for restenosis by HVJ-liposome delivery. Gene. 1994;149:13–9. doi: 10.1016/0378-1119(94)90406-5. [DOI] [PubMed] [Google Scholar]
- 180.Abe J, Zhou W, Taguchi J, et al. Suppression of neointimal smooth muscle cell accumulation in vivo by antisense cdc2 and cdk2 oligonucleotides in rat carotid artery. Biochem Biophys Res Commun. 1994;198:16–24. doi: 10.1006/bbrc.1994.1003. [DOI] [PubMed] [Google Scholar]
- 181.Simons M, Edelman ER, DeKeyser JL, Langer R, Rosenberg RD. Antisense c-myb oligonucleotides inhibit intimal arterial smooth muscle cell accumulation in vivo. Nature. 1992;359:67–70. doi: 10.1038/359067a0. [DOI] [PubMed] [Google Scholar]
- 182.Simons M, Edelman ER, Rosenberg RD. Antisense proliferating cell nuclear antigen oligonucleotides inhibit intimal hyperplasia in a rat carotid artery injury model. J Clin Invest. 1994;93:2351–6. doi: 10.1172/JCI117240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Shi Y, Fard A, Galeo A, et al. Transcatheter delivery of c-myc antisense oligomers reduces neointimal formation in a porcine model of coronary artery balloon injury. Circulation. 1994;90:944–51. doi: 10.1161/01.cir.90.2.944. [DOI] [PubMed] [Google Scholar]
- 184.Suzuki J, Isobe M, Morishita R, et al. Prevention of graft coronary arteriosclerosis by antisense cdk2 kinase oligonucleotide. Nat Med. 1997;3:900–3. doi: 10.1038/nm0897-900. [DOI] [PubMed] [Google Scholar]
- 185.Kutryk MJ, Foley DP, van den Brand M, et al. Local intracoronary administration of antisense oligonucleotide against c-myc for the prevention of in-stent restenosis: Results of the randomized investigation by the Thoraxcenter of antisense DNA using local delivery and IVUS after coronary stenting (ITALICS) trial. J Am Coll Cardiol. 2002;39:281–7. doi: 10.1016/s0735-1097(01)01741-7. [DOI] [PubMed] [Google Scholar]
- 186.Kipshidze NN, Kim HS, Iversen P, et al. Intramural coronary delivery of advanced antisense oligonucleotides reduces neointimal formation in the porcine stent restenosis model. J Am Coll Cardiol. 2002;39:1686–91. doi: 10.1016/s0735-1097(02)01830-2. [DOI] [PubMed] [Google Scholar]
- 187.Mann MJ, Gibbons GH, Kernoff RS, et al. Genetic engineering of vein grafts resistant to atherosclerosis. Proc Natl Acad Sci USA. 1995;92:4502–6. doi: 10.1073/pnas.92.10.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kipshidze N, Overlie P, Dunlap T, et al. First human experience with local delivery of novel antisense AVI-4126 with infiltrator catheter in de novo native and restenotic coronary arteries: Six-month clinical and angiographic follow-up from AVAIL study Circulation 2004110Suppl 3757(Abst) [DOI] [PubMed] [Google Scholar]
- 189.Kipshidze NN, Iversen P, Kim HS, et al. Advanced c-myc antisense (AVI-4126)-eluting phosphorylcholine-coated stent implantation is associated with complete vascular healing and reduced neointimal formation in the porcine coronary restenosis model. Catheter Cardiovasc Interv. 2004;61:518–27. doi: 10.1002/ccd.20007. [DOI] [PubMed] [Google Scholar]
- 190.Frimerman A, Welch PJ, Jin X, et al. Chimeric DNA-RNA hammerhead ribozyme to proliferating cell nuclear antigen reduces stent-induced stenosis in a porcine coronary model. Circulation. 1999;99:697–703. doi: 10.1161/01.cir.99.5.697. [DOI] [PubMed] [Google Scholar]
- 191.Macejak DG, Lin H, Webb S, et al. Adenovirus-mediated expression of a ribozyme to c-myb mRNA inhibits smooth muscle cell proliferation and neointima formation in vivo. J Virol. 1999;73:7745–51. doi: 10.1128/jvi.73.9.7745-7751.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Morishita R, Gibbons GH, Horiuchi M, et al. A gene therapy strategy using a transcription factor decoy of the E2F binding site inhibits smooth muscle proliferation in vivo. Proc Natl Acad Sci USA. 1995;92:5855–9. doi: 10.1073/pnas.92.13.5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mann MJ, Whittemore AD, Donaldson MC, et al. Ex-vivo gene therapy of human vascular bypass grafts with E2F decoy: The PREVENT single-centre, randomised, controlled trial. Lancet. 1999;354:1493–8. doi: 10.1016/S0140-6736(99)09405-2. [DOI] [PubMed] [Google Scholar]
- 194.Mann MJ, Conte MS. Transcription factor decoys for the prevention of vein bypass graft failure. Am J Cardiovasc Drugs. 2003;3:79–85. doi: 10.2165/00129784-200303020-00001. [DOI] [PubMed] [Google Scholar]
- 195.Morice MC, Serruys PW, Sousa JE, et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002;346:1773–80. doi: 10.1056/NEJMoa012843. [DOI] [PubMed] [Google Scholar]
- 196.Moses JW, Leon MB, Popma JJ, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349:1315–23. doi: 10.1056/NEJMoa035071. [DOI] [PubMed] [Google Scholar]
- 197.Schofer J, Schluter M, Gershlick AH, et al. Sirolimus-eluting stents for treatment of patients with long atherosclerotic lesions in small coronary arteries: Double-blind, randomised controlled trial (E-SIRIUS) Lancet. 2003;362:1093–9. doi: 10.1016/S0140-6736(03)14462-5. [DOI] [PubMed] [Google Scholar]
- 198.Schampaert E, Cohen EA, Schluter M, et al. The Canadian study of the sirolimus-eluting stent in the treatment of patients with long de novo lesions in small native coronary arteries (C-SIRIUS) J Am Coll Cardiol. 2004;43:1110–5. doi: 10.1016/j.jacc.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 199.Grube E, Sonoda S, Ikeno F, et al. Six- and twelve-month results from first human experience using everolimus-eluting stents with bioabsorbable polymer. Circulation. 2004;109:2168–71. doi: 10.1161/01.CIR.0000128850.84227.FD. [DOI] [PubMed] [Google Scholar]
- 200.Grube E, Costa R, Mehran R. Everolimus-eluting stents for the prevention of restenosis: Results of the FUTURE II trial. J Am Coll Cardiol. 2004;43(Suppl A):86A. [Google Scholar]
- 201.Serruys PW. SPIRIT FIRST: Everolimus-eluting stent looks promising at six months. Transcatheter Cardiovascular Therapeutics Annual Scientific Symposia; Washington. September 27 to October 1, 2004. [Google Scholar]