Abstract
OBJECTIVE
Cross-sectional studies found less microalbuminuria in type 2 diabetic patients with the Ala12 allele of the peroxisome proliferator–activated receptor-γ2 (PPAR-γ2) Pro12Ala polymorphism. We prospectively evaluated the association between Pro12Ala polymorphism (rs1801282) and new-onset microalbuminuria.
RESEARCH DESIGN AND METHODS
Pro12Ala polymorphism was genotyped by TaqMan-based assay in genomic DNA of 1,119 consenting patients from BErgamo NEphrologic DIabetic Complications Trial (BENEDICT)—a prospective, randomized trial evaluating ACE inhibition effect on new-onset microalbuminuria (albuminuria 20–200 μg/min in at least two of three consecutive overnight urine collections in two consecutive visits) in hypertensive type 2 diabetes with albuminuria <20 μg/min at inclusion.
RESULTS
Baseline characteristics of Ala (Ala/Ala or Ala/Pro) carriers and Pro/Pro homozygotes were similar, with a nonsignificant trend to lower albuminuria (P = 0.1107) in the 177 Ala carriers. Over a median (interquartile range) of 44.0 (17.1–51.9) months, 7 (4%) Ala carriers and 86 (9.1%) Pro/Pro homozygotes developed microalbuminuria (hazard ratio [HR] 0.45 [95% CI 0.21–0.97]; P = 0.042). Final albuminuria was significantly lower in Ala carriers than Pro/Pro homozygotes (7.3 ± 9.1 vs. 10.5 ± 24.9 μg/min, respectively), even after adjustment for baseline albuminuria (P = 0.048). Baseline and follow-up blood pressure and metabolic control were similar in both groups. Incidence of microalbuminuria was significantly decreased by ACE versus non-ACE inhibitor therapy in Pro/Pro homozygotes (6.3 vs. 11.9%, respectively, HR 0.46 [0.29–0.72]; P < 0.001).
CONCLUSIONS
In type 2 diabetes, the Ala allele protects from worsening albuminuria and new-onset microalbuminuria, and ACE inhibition blunts the excess risk of microalbuminuria associated with the Pro/Pro genotype. Evaluating Pro12Ala polymorphism may help identifying patients at risk who may benefit the most from early renoprotective therapy.
Since the early 1980s, microalbuminuria—defined as a urinary albumin excretion rate (AER) between 20 and 200 μg/min (or 30 and 300 mg/24 h) in overnight (or daily) urine collections—has been identified as a new recognized renal and cardiovascular risk factor in both diabetic and nondiabetic subjects (1–3). Although findings of several randomized clinical trials and community-based cohort studies subsequently converged to indicate that incremental increases in albuminuria even within the normal range already carry higher risk of nephropathy or cardiovascular events (4–6), which led to consider the possibility to abandon this term (7,8), microalbuminuria continued to be used as a clear-cut baseline or outcome variable for observational and intervention studies (9,10). In particular, studies in type 2 diabetes used microalbuminuria as a surrogate end point for diabetic nephropathy and related cardiovascular morbidity and mortality (11–13).
This approach was justified by evidence that microalbuminuria is an independent predictor of progression to overt proteinuria and eventual renal function loss, as well as of cardiovascular morbidity and mortality in people with diabetes (14). Thus, early identification of subjects expected to develop microalbuminuria is instrumental to implement intervention strategies aimed at limiting the excess renal and cardiovascular risk in this population (13,15).
Microalbuminuria is often associated with metabolic syndrome, a syndrome of insulin resistance, obesity, dyslipidemia, and increased renal and cardiovascular morbidity. Data suggest that insulin resistance precedes and probably contributes to the development of microalbuminuria in type 1 diabetic patients (16) and in nondiabetic subjects (17). A cross-sectional study showed that in type 2 diabetic men, an increase in the homeostasis model assessment index, a surrogate of insulin resistance, was significantly associated with an increased spot urine albumin-to-creatinine ratio, taken as an indicator of microalbuminuria (18). Consistently, a case-control study showed that the total-body glucose disposal rate, the gold-standard marker of insulin sensitivity quantified by means of a euglycemic-hyperinsulinemic clamp technique, was significantly lower in type 2 diabetic patients with microalbuminuria than in those with “normal” urinary albumin excretion and demonstrated that more severe insulin resistance was independently associated with an excess of microalbuminuria in this population (19).
Although largely sustained by acquired factors such as decreased physical activity and obesity, familial clustering of insulin resistance suggests that genetic factors may also contribute to reduced insulin sensitivity (20,21) and to the associated excess risk of renal involvement. Indeed, both insulin resistance (20,22–26) and the risk of developing microalbuminuria (27) or macroalbuminuria (27,28) have been linked, at least in part, to the peroxisome proliferator–activated receptor-γ (PPAR-γ) gene. PPAR-γ is a nuclear hormone receptor that exists in four isoforms. The PPAR-γ2 isoform is a widely expressed ligand-activated transcription factor and a member of the steroid receptor superfamily (29), which is involved in lipid and glucose metabolism, fatty acid transport, and cell differentiation (30). Of interest, in several studies the Ala12 variant of the Pro12Ala polymorphism (rs1801282) of the PPAR-γ2 isoform has been associated with less insulin resistance and a reduced risk of both type 2 diabetes (20,24–26) and microalbuminuria (27,28). All these studies, however, were cross-sectional in nature, and the confounding effect of a survival bias could not be definitely excluded. This bias may be particularly relevant when, as in the above studies, populations at increased mortality rate (such as those with type 2 diabetes or metabolic syndrome) are considered. This might also explain why the above findings were apparently conflicting with data from another cross-sectional study failing to detect any significant association between Ala12 variant and reduced risk of microalbuminuria in a Japanese series of type 2 diabetic patients (31).
To address this issue, we took advantage of a large cohort of patients included in the BErgamo NEphrologic DIabetic Complications Trial (BENEDICT) and followed them prospectively in the setting of a randomized, double-blind, placebo-controlled design to assess whether an ACE inhibitor (either alone or in combination with a nondihydropyridine calcium-channel blocker) may prevent microalbuminuria in subjects with type 2 diabetes and hypertension but no evidence of renal involvement at inclusion (13). This trial offered the unique opportunity to test the role of the PPAR-γ2 Pro12Ala polymorphism and of its interaction, if any, with different intervention strategies on the risk of developing microalbuminuria in this patient population.
RESEARCH DESIGN AND METHODS
BENEDICT was a double-blind, randomized, placebo-controlled study aimed to assess whether ACE inhibitors and nondihydropyridine calcium-channel blockers, alone or in combination, prevent microalbuminuria in subjects with type 2 diabetes, hypertension, and normal urinary albumin excretion at baseline (13). Patients (n = 1,204) were randomly allocated to at least 3-year treatment with the ACE inhibitor trandolapril, the nondihydropyridine calcium-channel blocker verapamil, the combination of verapamil plus trandolapril, or placebo, added on background non–ACE-inhibiting therapy targeting systolic/diastolic blood pressure <120/80 mmHg (13). Primary end point was new-onset persistent microalbuminuria (urinary albumin excretion ≥20 μg and <200 μg/min in at least two of three consecutive overnight urine collections on two consecutive visits 2 months apart). Urinary albumin excretion was measured on fresh urines at the coordinating center by nephelometry (Beckman Array System; Beckman Coulter) at randomization and every 6 months thereafter.
Trandolapril plus verapamil and trandolapril alone significantly delayed the onset of microalbuminuria by factors of 2.6 and 2.1, respectively, compared with placebo (P < 0.01 for both). The incidence of microalbuminuria was reduced to a similar extent by both treatments, whereas verapamil had an effect on the incidence of microalbuminuria that was similar to that of placebo (12,13). Thus, patients were pooled in two cohorts according to their original allocation to ACE or non-ACE inhibitor therapy regardless of concomitant therapy with verapamil or placebo. Gene-by-treatment interactions were tested according to ACE inhibitor therapy (yes or no).
The study protocol was approved by the local ethics committees, and only samples from patients who provided written informed consent according to the Helsinki Declaration guidelines were considered for genetic analyses. All data were handled in respect of patient confidentiality and anonymity.
Genotyping.
Genomic DNA was extracted from peripheral blood leukocytes by standard methods. Genotyping for Pro12Ala single nucleotide polymorphism (SNP; rs1801282) was performed using TaqMan Pre-Designed SNP genotyping assay from Applied Biosystems (assay C_1129864_10; ABI, Foster City, CA). TaqMan genotyping reactions were performed on ABI 7900HT genetic analyzer following manufacturer's instructions. Genotypes were scored by analyzing data on both real-time as well as allele discrimination assay platforms using SDS software provided by ABI. Genotyping success rate was >99%. As a quality-control procedure, we double-genotyped a subset of 200 individuals (18% of the whole sample) using a different genotyping method on the LightCycler 480 System (Roche Diagnostics, http://www.roche-applied-science.com). This was a melting curve-based genotyping method using fluorescence-labeled hybridization probes (32). Primers and probes for genotyping Pro12Ala SNP on LightCycler 480 System were designed, synthesized, and validated by TIB Molbiol (Genoa, Italy, http://www.tib-molbiol.com) using the genomic sequence available from NCBI dbSNP for the rs1801282. Reactions were performed using 1 × LightCycler 480 Genotyping Master (catalog no. 04 707 524 001; Roche Applied Science), 10 pmol of each primer (forward 5′gCC CAg TCC TTT CTg TgT T 3′ and reverse 5′ gTC CCC AAT AgC CgT ATC Tg 3′, melting temperature 55°C), 4 pmol of each probe (Sensor HybProbe LC Red 640-CCT TCA CTg ATA CAC TgT CTg CAA ACA TAT CAC-phosphate and Anchor HybProbe TCT CCT ATT gAC CCA gAA AgC gAT-fluorescein, melting temperature 60°C and 64°C, respectively), and 50 ng of genomic DNA in a final volume of 20 μl according to manufacturer's instructions. The concordance rate between the two genotyping methods was 100%.
Statistical analyses.
Patients' baseline characteristics according to PPAR-γ2 Pro12Ala polymorphism are reported as means ± SD or frequencies and percentages. P values refer to Mann-Whitney U test and Pearson χ2 for continuous and categorical variables, respectively. Time to onset of microalbuminuria was considered as the main end point. For patients who did not reach the main end point, time was censored at the last follow-up visit. Cox proportional hazards regression models were used, and results are expressed as hazard ratios (HRs) and 95% CIs.
To test the effect of Pro12Ala polymorphism on outcome, three different statistical models have been designed: the first was a model adjusted only for ACE inhibitor therapy (i.e., yes or no = 0 or 1); in the second model, variables that predicted microalbuminuria onset in the whole cohort, such as sex, smoking status, and A1C, were added to ACE inhibitor therapy; in the third model, baseline AER levels, which were slightly lower at study entry in Ala carriers, were taken into account in addition to ACE inhibitor therapy and baseline covariates. Between-groups comparison of AER at final visit was carried out by ANCOVA, adjusting for baseline measurement. Nonnormally distributed covariates (namely, AER and triglycerides) were log transformed before analysis. P values < 0.05 were considered significant. All the analyses were performed using SAS Statistical Package Release 9.1 (SAS Institute, Cary, NC).
RESULTS
Patient characteristics.
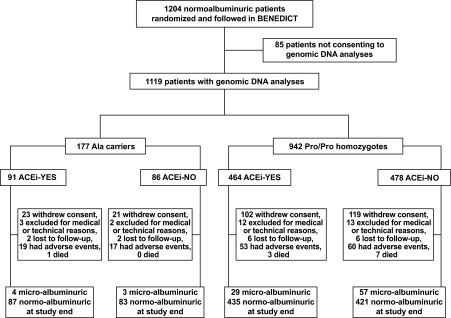
Of the 1,204 BENEDICT patients, 1,119 consented to genomic DNA analyses. Of these patients, 7 (0.6%) and 170 (15.2%) were homozygous and heterozygous for the Ala12 allele, respectively, from now Ala carriers, and 942 (84.2%) were homozygous for the Pro12 allele, from now Pro/Pro homozygotes (Fig. 1). Frequency of Ala carriers was comparable with that previously reported in other white populations (25). The proportion of the Pro12Ala genotypes did not significantly deviate from Hardy-Weinberg equilibrium (χ2 = 0.05; P = 0.82). Data from homozygous and heterozygous Ala carriers were pooled and considered together for comparative analyses with Pro/Pro homozygotes. Baseline characteristics of the two groups were similar (Tables 1 and 2), with a trend to lower albuminuria at inclusion in Ala carriers (Table 1), as well as the proportion of patients allocated to ACE inhibitor therapy (yes or no) within each group (Table 1). Baseline characteristics of patients who had been randomized to ACE inhibitor therapy (yes or no) within the two genotype groups were similar (Table 1).
FIG. 1.
Schematic diagram of the study. ACEi, ACE inhibitor therapy.
TABLE 1.
Baseline clinical features of patients with type 2 diabetes
| Ala carriers |
Pro/Pro homozygotes |
|||||
|---|---|---|---|---|---|---|
| Overall | ACEi yes | ACEi no | Overall | ACEi yes | ACEi no | |
| n | 177 | 91 | 86 | 942 | 464 | 478 |
| Age (years) | 62.6 ± 8.3 | 61.8 ± 8.0 | 63.4 ± 8.6 | 62.3 ± 8.0 | 62.1 ± 8.0 | 62.4 ± 8.1 |
| Male subjects | 86 (48.6) | 46 (50.6) | 40 (46.5) | 503 (53.4) | 253 (54.5) | 250 (52.3) |
| BMI (kg/m2) | 29.4 ± 4.5 | 29.4 ± 4.8 | 29.3 ± 4.2 | 29.0 ± 4.7 | 29.1 ± 5.0 | 29.0 ± 4.5 |
| Diabetes duration (years) | 7.7 ± 7.2 | 7.7 ± 7.1 | 7.8 ± 7.5 | 7.8 ± 6.5 | 7.6 ± 6.6 | 8.1 ± 6.5 |
| Smokers | 67 (37.9) | 40 (44.0) | 27 (31.4) | 399 (42.4) | 214 (46.1) | 185 (38.7) |
| Current | 13 (7.3) | 9 (9.9) | 4 (4.7) | 121 (12.9) | 64 (13.8) | 57 (11.9) |
| Never | 110 (62.2) | 51 (56.0) | 59 (68.6) | 543 (57.6) | 250 (53.9) | 293 (61.3) |
| Former | 54 (30.5) | 31 (34.1) | 23 (26.7) | 278 (29.5) | 150 (32.3) | 128 (26.8) |
| A1C (%) | 5.9 ± 1.5 | 6.0 ± 1.6 | 5.9 ± 1.4 | 5.8 ± 1.3 | 5.7 ± 1.4 | 5.8 ± 1.3 |
| SBP (mmHg) | 151.2 ± 13.3 | 151.4 ± 12.9 | 151.1 ± 13.8 | 150.6 ± 14.5 | 150.3 ± 14.3 | 150.9 ± 14.6 |
| DBP (mmHg) | 87.5 ± 7.6 | 88.0 ± 7.7 | 87.1 ± 7.5 | 87.4 ± 7.6 | 87.2 ± 7.9 | 87.6 ± 7.3 |
| Serum creatinine (mg/dl) | 0.91 ± 0.16 | 0.91 ± 0.17 | 0.90 ± 0.16 | 0.91 ± 0.16 | 0.90 ± 0.15 | 0.91 ± 0.17 |
| Triglycerides (mg/dl) | 130.8 (120.8–141.7) | 127.2 (113.4–142.7) | 134.7 (120.4–150.7) | 127.4 (123.4–131.5) | 128.6 (123.1–134.4) | 126.2 (120.6–132.1) |
| Total cholesterol (mg/dl) | 211.3 ± 37.5 | 206.3 ± 36.1 | 216.4 ± 38.6 | 209.3 ± 36.3 | 206.5 ± 35.1 | 211.9 ± 37.3 |
| HDL cholesterol (mg/dl) | 47.0 ± 12.3 | 47.4 ± 11.7 | 46.5 ± 13.0 | 46.9 ± 12.0 | 46.9 ± 12.4 | 47.0 ± 11.6 |
| AER (μg/min) | 5.2 (4.8–5.7) | 5.1 (4.5–5.8) | 5.4 (4.7–6.2) | 5.7 (5.5–5.9) | 5.6 (5.2–5.9) | 5.8 (5.5–6.2) |
Data are means ±SD, n (%), or geometric means (95% CIs). Differences between Ala carriers and Pro/Pro homozygotes (overall) as well as between ACEi yes and ACEi no (within each genotype group) were not significant. ACEi, ACE inhibitor therapy; DBP, diastolic blood pressure; and SBP, systolic blood pressure.
TABLE 2.
Concomitant treatments in patients with type 2 diabetes at baseline and on follow-up*
| Treatment | Baseline |
Follow-up |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ala carriers |
Pro/Pro homozygotes |
Ala carriers |
Pro/Pro homozygotes |
|||||||||
| Overall | ACEi yes | ACEi no | Overall | ACEi yes | ACEi no | Overall | ACEi yes | ACEi no | Overall | ACEi yes | ACEi no | |
| Concomitant medication | 177 | 91 | 86 | 942 | 464 | 478 | 177 | 91 | 86 | 942 | 464 | 478 |
| Glucose-lowering regimen | ||||||||||||
| Diet alone | 59 (33.3) | 31 (34.1) | 28 (32.6) | 273 (29.0) | 130 (28.0) | 143 (29.9) | 41 (23.2) | 21 (23.1) | 20 (23.3) | 190 (20.2) | 92 (19.8) | 98 (20.5) |
| Oral hypoglycemic agent alone | 97 (54.8) | 49 (53.8) | 48 (55.8) | 555 (58.9) | 284 (61.2) | 271 (56.7) | 101 (57.1) | 51 (56.0) | 50 (58.1) | 586 (62.2) | 294 (63.4) | 292 (61.1) |
| Insulin and oral hypoglycemic agent | 14 (7.9) | 8 (8.8) | 6 (7.0) | 58 (6.2) | 24 (5.2) | 34 (7.1) | 30 (17.0) | 15 (16.5) | 15 (17.4) | 112 (11.9) | 50 (10.8) | 62 (13.0) |
| Insulin alone | 7 (4.0) | 3 (3.3) | 4 (4.7) | 56 (5.9) | 26 (5.6) | 30 (6.3) | 5 (2.8) | 4 (4.4) | 1 (1.2) | 54 (5.7) | 28 (6.0) | 26 (5.4) |
| Antihypertensive agents | ||||||||||||
| Any | 97 (54.8) | 48 (52.8) | 49 (57.0) | 519 (55.1) | 258 (55.6) | 261 (54.6) | 105 (59.3) | 53 (58.2) | 52 (60.5) | 586 (62.2) | 277 (59.7) | 309 (64.6) |
| Diuretic | 41 (23.2) | 19 (20.9) | 22 (25.6) | 202 (21.4) | 95 (20.5) | 107 (22.4) | 26 (14.7) | 14 (15.4) | 12 (14.0) | 186 (19.8) | 78 (16.8) | 108 (22.6) |
| β-blocker | 14 (7.9) | 10 (11.0) | 4 (4.7) | 76 (8.1) | 37 (8.0) | 39 (8.2) | 16 (9.0) | 11 (12.1) | 5 (5.8) | 81 (8.6) | 39 (8.4) | 42 (8.8) |
| Calcium-channel blocker (dihydropyridine) | 40 (22.6) | 16 (17.6) | 24 (27.9) | 274 (29.1) | 130 (28.0) | 144 (30.1) | 47 (26.6) | 21 (23.1) | 26 (30.2) | 262 (27.8) | 122 (26.3) | 140 (29.3) |
| Sympatholytic agent | 41 (23.2) | 16 (17.6) | 25 (29.1) | 195 (20.7) | 107 (23.1) | 88 (18.4) | 75 (42.4) | 34 (37.4) | 41 (47.7) | 442 (46.9) | 193 (41.6) | 249 (52.1)† |
| Lipid-lowering agents | ||||||||||||
| Any | 19 (10.7) | 9 (9.9) | 10 (11.6) | 110 (11.7) | 58 (12.5) | 52 (10.9) | 38 (21.5) | 16 (17.6) | 22 (25.6) | 201 (21.3) | 99 (21.3) | 102 (21.3) |
| Statin alone | 11 (6.2) | 4 (4.4) | 7 (8.1) | 70 (7.4) | 43 (9.3) | 27 (5.6) | 23 (13.0) | 8 (8.8) | 15 (17.4) | 141 (15.0) | 72 (15.5) | 69 (14.4) |
| Fibrate alone | 5 (2.8) | 2 (2.2) | 3 (3.5) | 35 (3.7) | 14 (3.0) | 21 (4.4) | 5 (2.8) | 3 (3.3) | 2 (2.3) | 39 (4.1) | 17 (3.7) | 22 (4.6) |
| Statin and fibrate | 0 | 0 | 0 | 3 (0.3) | 1 (0.2) | 2 (0.4) | 6 (3.4) | 2 (2.2) | 4 (4.7) | 14 (1.5) | 8 (1.7) | 6 (1.3) |
| Antiplatelet agent | 5 (2.8) | 4 (4.4) | 1 (1.2) | 22 (2.3) | 10 (2.2) | 12 (2.5) | 18 (10.2) | 12 (13.2) | 6 (7.0) | 70 (7.4) | 37 (8.0) | 33 (6.9) |
Data are n or n (%).
*All differences between the two genotype groups (Ala vs. Pro/Pro) were not significant.
†P < 0.05 vs. ACEi yes. ACEi, ACE inhibitor therapy.
Outcomes according to Pro12Ala genotypes.
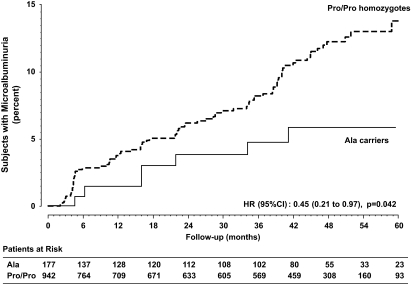
Over a median (interquartile range) of 44 (17.1–51.9) months of follow-up, 7 Ala carriers (4%) compared with 86 Pro/Pro homozygotes (9.1%) developed persistent microalbuminuria (χ2 = 4.35; P = 0.037). The unadjusted HR for microalbuminuria in Ala carriers compared with Pro/Pro homozygotes was 0.45 (95% CI 0.21–0.97) (P = 0.042) (Fig. 2).
FIG. 2.
Kaplan-Meier curves for the percentages of Ala carriers or Pro/Pro homozygotes in subjects who developed persistent microalbuminuria throughout the study period.
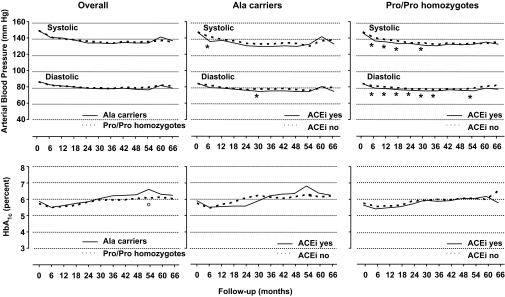
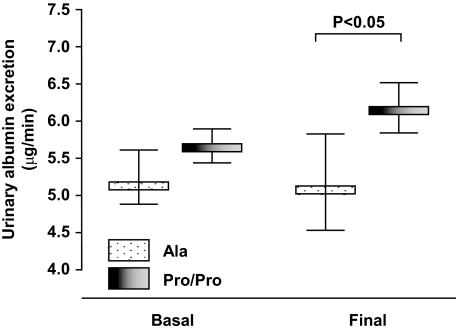
Blood pressure and A1C levels at baseline as well as throughout the whole study period were similar in the two genotype groups (Table 1 and Fig. 3, overall: upper and lower panel, respectively). Conversely, in Ala carriers compared with Pro/Pro homozygotes urinary AER increased less during the observation period and was significantly lower at final visit even after adjustment for baseline albuminuria (ANCOVA, P = 0.048) (Fig. 4).
FIG. 3.
Upper panels: Trough systolic and diastolic blood pressure throughout the study period in Ala carriers or Pro/Pro homozygotes (left), in Ala carriers according to ACE inhibitor therapy (ACEi) (yes or no) (middle), and in Pro/Pro homozygotes according to ACE inhibitor therapy (yes or no). *P < 0.05 (ACE inhibitor therapy yes vs. ACE inhibitor therapy no, without Bonferroni correction for multiple comparisons). Lower panels: A1C throughout the study period in Ala carriers or Pro/Pro homozygotes (left), in Ala carriers according to ACE inhibitor therapy (yes or no) (middle), and in Pro/Pro homozygotes according to ACE inhibitor therapy (yes or no). °P < 0.05 (Pro/Pro homozygotes vs. Ala carriers, without Bonferroni correction for multiple comparisons).
FIG. 4.
Urinary AER (geometric mean and 95% CI) at basal and final visits in Ala carriers or Pro/Pro homozygotes. The difference in urinary AER between the two genotype groups at final visit was significant after adjustment for baseline albumin excretion.
In multivariable analyses, sex, smoking status, baseline albuminuria, baseline and follow-up A1C, and follow-up mean arterial pressure significantly predicted incident microalbuminuria in the whole BENEDICT sample (P < 0.001 for all considered covariates). Ala carriers compared with Pro/Pro homozygotes showed a trend with lower incidence of microalbuminuria even after adjusting for sex, smoking status, and baseline A1C (HR 0.48 [95% CI 0.22–1.05]; P = 0.065), but not after adjusting also for baseline albuminuria (HR 0.64 [0.29–1.40]; P = 0.263). The difference in the cumulative incidence of events between the two genotype groups was constant throughout the whole observation period, and new cases of microalbuminuria were approximately 50% less in Ala compared with Pro/Pro homozygotes at each considered time point of the follow-up (proportionality test, P = 0.85).
To assess whether the difference in the development of microalbuminuria by Pro12Ala genotype was due to difference in blood pressure or glucose control, we evaluated interactions between baseline/follow-up mean arterial pressure or A1C and genotype on incidence of microalbuminuria. There were no significant interactions in the overall population. Furthermore, the distribution of concomitant treatments with glucose-, blood pressure–, and lipid-lowering agents as well as of antiplatelet agents at baseline and on follow-up was similar in the two genotype groups (Table 2). The proportion of patients on two or more antihypertensive medications was also similar in the two groups. However, there were numerically more patients on diet alone and fewer patients on insulin alone among Ala carriers than among Pro/Pro homozygotes at baseline as well as throughout the follow-up period (Table 2). Patients prematurely leaving the study were comparable in the two genotype groups (Fig. 1).
Patient outcomes according to Pro12Ala genotypes and ACE inhibitor therapy.
Among Ala carriers, 4 of the 91 patients on ACE inhibitor compared with 3 of the 86 on non-ACE inhibitor therapy developed microalbuminuria. Thus, in the two treatment groups the incidence of microalbuminuria (4.4 vs. 3.5%, respectively) was similar (HR 1.21 [95% CI 0.27–5.40]; P = 0.805). Concomitant medications, systolic/diastolic blood pressure, and A1C at baseline and on follow-up were similar in the two treatment groups (Table 1 and Fig. 3, Ala carriers: upper and lower panel, respectively).
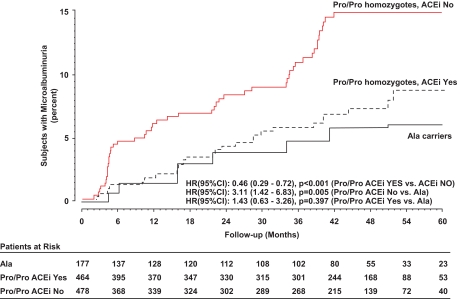
Among Pro/Pro homozygotes, 29 of the 464 patients on ACE inhibitor and 57 of the 478 on non-ACE inhibitor therapy developed microalbuminuria (χ2 = 9.14; P = 0.003). Thus, the incidence of microalbuminuria (6.3 vs. 11.9%, respectively) was significantly lower in those on ACE than in those on non-ACE inhibitor therapy (unadjusted HR 0.46 [95% CI 0.29–0.72], P < 0.001; adjusted for baseline characteristics including or not including albuminuria 0.44 [0.28–0.69], P < 0.001). A1C was similar in the two groups at baseline and follow-up. Systolic/diastolic blood pressure was similar at baseline, but tended to be lower in patients on ACE inhibitor therapy in the follow-up period (Table 1 and Fig. 3, Pro/Pro homozygotes: upper panel). On follow-up, a similar proportion of patients was on concomitant treatment with antidiabetic, lipid-lowering, and antiplatelet agents and blood pressure–lowering medications, with the exception of sympatholytic agents that were more frequently prescribed in the non-ACE inhibitor treatment arm (Table 2). The incidence of microalbuminuria was significantly lower in Ala carriers than in Pro/Pro homozygotes among subjects on non-ACE inhibitor therapy (unadjusted HR 0.30 [0.09–0.95]; P = 0.041). The difference was marginally significant after adjusting for sex, smoking status, and baseline A1C (HR 0.33 [0.10–1.05]; P = 0.061) but failed to achieve the statistical significance after adjusting also for baseline albuminuria (HR 0.56 [0.17–1.82]; P = 0.333). Among subjects on ACE inhibitor therapy, the incidence of microalbuminuria was similar in the two genotype groups (unadjusted HR 0.75 [0.26–2.12]; P = 0.582). Altogether, the rate of microalbuminuria in ACE inhibitor–treated Pro/Pro homozygotes was similar to that of Ala carriers considered as a whole regardless of therapy (Fig. 5).
FIG. 5.
Kaplan-Meier curves for the percentages of Pro/Pro homozygotes considered according to concomitant ACE inhibitor therapy (ACEi) (yes or no) and of Ala carriers considered as a whole in subjects who developed persistent microalbuminuria throughout the study period.
The interaction between baseline A1C and genotype on development of microalbuminuria was significant (P = 0.035) in Pro/Pro homozygotes without ACE inhibitor therapy, but not in those on ACE inhibition (P = 0.64). Baseline A1C was higher in Pro/Pro homozygotes progressing to microalbuminuria compared with those with stable urinary albumin excretion among patients without ACE inhibitor therapy (P < 0.0001), but not in those on ACE inhibition. No significant interactions were observed in Ala carriers.
There was no significant interaction between follow-up A1C or mean blood pressure and genotype on development of microalbuminuria in any considered group.
DISCUSSION
The main finding of our present study is that in a large and homogeneous cohort of hypertensive patients with type 2 diabetes and normal AER prospectively followed in the setting of a randomized clinical trial, those who carried the Ala12 allele of the Pro12Ala PPAR-γ2 polymorphism had a significantly lower incidence of persistent microalbuminuria compared with carriers of the wild-type Pro/Pro genotype. Consistently, Ala carriers compared with Pro/Pro homozygotes showed a slower increase in albuminuria over time that eventually translated into a significantly lower urinary albumin excretion at final visit. The two genotype groups were very similar for all considered parameters, including prevalence of well-recognized risk factors for microalbuminuria such as male sex and smoking habit. Even more important, A1C and blood pressure control were similar at inclusion as well as throughout the whole study period, ruling out any confounding effect of different metabolic or blood pressure control on considered outcomes. The comparable distribution to ACE or non-ACE inhibitor therapy within each group also excluded the possibility that time-dependent changes in albuminuria could be affected by an unbalanced inhibition of the renin-angiotensin system. Altogether, the above findings can be taken to suggest that the Ala12 allele is associated with an inborn protective effect against time-associated worsening of albuminuria that explained the trend to less albuminuria at inclusion as well as the significantly reduced incidence of microalbuminuria observed throughout the approximately 4 years of follow-up. Consistently, the cumulative incidence of microalbuminuria was ∼50% lower in Ala carriers than in Pro/Pro homozygotes at any considered time point of the follow-up, and the difference between the two genotype groups was not appreciably affected by censoring all events observed in the first 6 months after inclusion. These findings provide consistent evidence that the reduced incidence of microalbuminuria in Ala carriers was only partially explained by less albuminuria at baseline and rather reflected a genuine protective effect of the Ala allele against progression to microalbuminuria that was sustained throughout the whole observation period.
To the best of our knowledge, this is the first longitudinal evidence of a (conceivably) causal association between the PPAR-γ2 Pro12Ala polymorphism and microalbuminuria. All previous studies exploring such association were cross-sectional in nature (27,28,31) and could not describe time-dependent changes in albuminuria according to different genotypes. Among the strengths of our present study was the rigorous definition of the entry criteria (including the diagnosis of normoalbuminuria that was established on the basis of a urinary AER <20 μg/min in at least two of three consecutive overnight urine collections), which allowed to identify a homogeneous population of patients with type 2 diabetes, and the standardized monitoring and treatment of included patients that was based on the guidelines of the BENEDICT study protocol (13). In particular, the diagnosis of new-onset persistent microalbuminuria was established on the basis of a urinary AER between 20 and 200 μg/min in at least two of three consecutive overnight urine collections confirmed in two consecutive visits 2 months apart, which allowed to avoid the confounding effect of random fluctuations of albuminuria and intermittent microalbuminuria. The reliability of urine collections was evaluated by measuring urinary creatinine excretion, and when the creatinine excretion was out of the expected ranges the sample was discarded and the patient was invited to repeat the collection.
Addressing the mechanisms explaining the reduced risk of microalbuminuria in carriers of the PPARγ2 Ala12 allele was beyond the purposes of the present study. However, more insulin sensitivity in Ala carriers might explain why, at comparable metabolic control, these patients tended to need less hypoglycemic agents or insulin than Pro/Pro homozygotes at baseline as well as throughout the whole observation period. Whether less insulin resistance might have a role in the reduced risk of developing microalbuminuria in this population is a matter of speculation (23). Of note, failure to detect a significant interaction of follow-up A1C (or mean blood pressure) and genotype on microalbuminuria was likely explained by the optimized metabolic and blood pressure control achieved during the study in all considered groups. Conceivably, this leveled out the independent effect of blood glucose and arterial pressure on progression to the end point. This increased the study power to detect a specific predictive value for the Pro12Ala polymorphism in this population.
An additional finding of our present study was that ACE compared with non-ACE inhibitor therapy halved the incidence of microalbuminuria in Pro/Pro homozygotes that had their rate of microalbuminuria decreased to the rates observed in Ala12 carriers considered as a whole. On the other hand, the incidence of events was so low in Ala carriers that the analyses were not sufficiently powered to test the treatment effect in this population. The significant interaction we found between baseline A1C and risk of microalbuminuria in Pro/Pro homozygotes without ACE inhibitor therapy might indicate that less effective metabolic control might increase the rate of progression to microalbuminuria in those subjects who are at increased risk because of their genetic background. This interaction was lost in Pro/Pro homozygotes on ACE inhibitor therapy most likely because treatment reduced the incidence of microalbuminuria in particular in those at increased risk because of higher A1C at inclusion. Thus, the Pro/Pro genotype might be associated with a genetic predisposition to microalbuminuria that manifests in those with less effective metabolic control and is prevented by ACE inhibitor therapy. Conversely, Ala carriers would be intrinsically protected.
Of note, different outcomes in considered groups are not likely explained by differences in metabolic or blood pressure control on follow-up because multivariable analyses failed to detect any significant interaction between follow-up A1C (or mean blood pressure) and treatment arm on microalbuminuria. More frequent use of sympatholytic agents in patients on non-ACE inhibitor treatment did not likely affect progression to microalbuminuria in this population because these agents have not been reported to worsen insulin sensitivity or albuminuria in people with diabetes (33).
Whether amelioration of insulin resistance through inhibited angiotensin II production might have contributed to limit microalbuminuria in Pro/Pro homozygotes on ACE inhibitor therapy independently of glycemia (34) is worth investigating.
Limitations.
Our present data on treatment effect according to different genotypes cannot be taken to conclude that ACE inhibitor therapy is not effective in Ala carriers and that response to treatment is different in the two genotype groups. Indeed, the genotype-by-treatment interaction failed to achieve statistical significance (P = 0.222)—a finding most likely explained by the small number of events in Ala carriers that limited the power of comparative analyses between patients on ACE or non-ACE inhibitor therapy in this subgroup. Moreover, data should be taken with caution because they were generated from exploratory analyses of a study that was not originally powered to test the hypotheses under evaluation here. On the other hand, the protective effect of ACE inhibitor therapy against the development of microalbuminuria in Pro/Pro homozygotes was clinically relevant and statistically significant. These data were obtained from carefully monitored patients in the setting of a prospective, controlled clinical trial and were in harmony with previous findings from cross-sectional studies in similar patient populations (27,28). Thus, they appear to be sufficiently robust and reliable even if they were not verified in an independent external test set. Of note, baseline characteristics of study patients were similar to those of patients referred to the Diabetology Units of the BENEDICT network who had been screened for study participation but had not been included in the trial. This provides the evidence that our findings can be generalized to the average population of patients with type 2 diabetes, in particular to those with arterial hypertension but still no evidence of renal involvement.
In conclusion, our prospective study found that among normoalbuminuric hypertensive patients with type 2 diabetes, PPAR-γ2 Ala carriers had a reduced rate of persistent microalbuminuria. Pro/Pro homozygotes were at increased risk of developing microalbuminuria but their risk was appreciably reduced by ACE inhibitor therapy. Thus, screening for the Pro12Ala PPAR-γ2 polymorphism may help to identify patients at increased risk who may benefit the most from early ACE inhibitor therapy. Further studies are needed to unravel whether protection from microalbuminuria may translate into a reduced long-term risk of renal and cardiovascular events in this population.
Acknowledgments
The study was partially supported by a grant from Fondazione CARIPLO, Milan, Italy (Rif 2002.2307/10.4963), by the European Commission within the EuReGene project (LSHG-CT-2004-005085), the GENECURE project (LSHM-CT-2006-037697), and by grants from the Italian Ministry of Health (RC 2008 and 2009) (to S.P.).
No potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in abstract form at the 69th Scientific Sessions of the American Diabetes Association, New Orleans, Louisiana, 5–9 June 2009.
The authors thank Annalisa Perna for supervising the statistical analyses, Laura Gallizioli for contributing to data handling, Paola Bettinaglio for DNA extraction, and Esteban Porrini and Marina Noris for critically revising the article. Manuela Passera helped to prepare the article.
APPENDIX
BENEDICT Study Organization: members of the BENEDICT Study Organization were as follows (all in Italy unless otherwise noted): principal investigator: G. Remuzzi (Bergamo); study coordinator: P. Ruggenenti (Bergamo); coordinating center: Mario Negri Institute for Pharmacological Research, Clinical Research Center for Rare Diseases Aldo e Cele Daccò, Villa Camozzi, Ranica (Bergamo); participating centers: G. Nastasi, A. Ongaro, F. Querci, A. Anabaya (Alzano Lombardo); R. Trevisan, A.R. Dodesini, G. Lepore, I. Nosari, C.A. Aros Espinoza, A. Fassi (Bergamo); A. Belviso, A. Parvanova, I.P. Iliev (Ponte San Pietro, Villa d'Almè); C. Chiurchiu, F. Arnoldi, L. Mosconi, M. Monducci (Ranica); A. Bossi, A. Parvanova, I.P. Iliev, M. Facchetti, V. Brusegan (Romano di Lombardia,Treviglio); F. Inversi, V. Bertone, R. Mangili, S. Bruno (Seriate); ophthalmologists: M. Filipponi, I.P. Iliev, S. Tadini (Bergamo); monitoring and drug distribution (Mario Negri Institute): N. Rubis, G. Gherardi, W. Calini, O. Diadei, M. Lesti, G. Noris, K. Pagani, D. Rossoni, D. Villa (Ranica); carriers (Mario Negri Institute): G. Gaspari, S. Gelmi, G. Gervasoni (Ranica); database and data validation (Mario Negri Institute): A. Remuzzi, B. Ene-Iordache, V. Gambara (Ranica); data analysis (Mario Negri Institute): A. Perna, N. Motterlini, M. Ganeva, J. Zamora, B.D. Dimitrov (Ranica); laboratory measurements (Mario Negri Institute): F. Gaspari, F. Carrara, E. Centemeri, S. Ferrari, M. Pellegrino, N. Stucchi, C. Petrò, C. Locatelli, A. Cannata, E. Savoldelli (Ranica); regulatory affairs (Mario Negri Institute): P. Boccardo (Ranica); steering committee: L. Minetti (Bergamo), G. Remuzzi (Bergamo), U.F. Legler (Ludwigshafen, Germany), B. Kalsch (Ludwigshafen, Germany), D. Nehrdich (Ludwigshafen, Germany), A. Nicolucci (S. Maria Imbaro), A. Perna (Bergamo), P. Ruggenenti (Bergamo); safety committee: G.L. Bakris (Chicago, IL), R. Kay (Sheffield, U.K.), G.C. Viberti (London, U.K.).
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Viberti GC, Hill RD, Jarrett RJ, Argyropoulos A, Mahmud U, Keen H: Microalbuminuria as a predictor of clinical nephropathy in insulin-dependent diabetes mellitus. Lancet 1982; 1: 1430– 1432 [DOI] [PubMed] [Google Scholar]
- 2.Borch-Johnsen K, Feldt-Rasmussen B, Strandgaard S, Schroll M, Jensen JS: Urinary albumin excretion: an independent predictor of ischemic heart disease. Arterioscler Thromb Vasc Biol 1999; 19: 1992– 1997 [DOI] [PubMed] [Google Scholar]
- 3.Hillege HL, Fidler V, Diercks GF, van Gilst WH, de Zeeuw D, van Veldhuisen DJ, Gans RO, Janssen WM, Grobbee DE, de Jong PE: Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation 2002; 106: 1777– 1782 [DOI] [PubMed] [Google Scholar]
- 4.Klausen K, Borch-Johnsen K, Feldt-Rasmussen B, Jensen G, Clausen P, Scharling H, Appleyard M, Jensen JS: Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes. Circulation 2004; 110: 32– 35 [DOI] [PubMed] [Google Scholar]
- 5.Arnlov J, Evans JC, Meigs JB, Wang TJ, Fox CS, Levy D, Benjamin EJ, D'Agostino RB, Vasan RS: Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation 2005; 112: 969– 975 [DOI] [PubMed] [Google Scholar]
- 6.Wang TJ, Evans JC, Meigs JB, Rifai N, Fox CS, D'Agostino RB, Levy D, Vasan RS: Low-grade albuminuria and the risks of hypertension and blood pressure progression. Circulation 2005; 111: 1370– 1376 [DOI] [PubMed] [Google Scholar]
- 7.Ruggenenti P, Remuzzi G: Time to abandon microalbuminuria? Kidney Int 2006; 70: 1214– 1222 [DOI] [PubMed] [Google Scholar]
- 8.Forman JP, Brenner BM: ‘Hypertension’ and ‘microalbuminuria’: the bell tolls for thee. Kidney Int 2006; 69: 22– 28 [DOI] [PubMed] [Google Scholar]
- 9.Gansevoort RT, Verhave JC, Hillege HL, Burgerhof JG, Bakker SJ, de Zeeuw D, de Jong PE: The validity of screening based on spot morning urine samples to detect subjects with microalbuminuria in the general population. Kidney Int Suppl2005; S28– S35 [DOI] [PubMed] [Google Scholar]
- 10.Asselbergs FW, Diercks GF, Hillege HL, van Boven AJ, Janssen WM, Voors AA, de Zeeuw D, de Jong PE, van Veldhuisen DJ, van Gilst WH: Effects of fosinopril and pravastatin on cardiovascular events in subjects with microalbuminuria. Circulation 2004; 110: 2809– 2816 [DOI] [PubMed] [Google Scholar]
- 11.Parving HH, Persson F, Lewis JB, Lewis EJ, Hollenberg NK: Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med 2008; 358: 2433– 2446 [DOI] [PubMed] [Google Scholar]
- 12.Ruggenenti P, Perna A, Ganeva M, Ene-Iordache B, Remuzzi G: Impact of blood pressure control and angiotensin-converting enzyme inhibitor therapy on new-onset microalbuminuria in type 2 diabetes: a post hoc analysis of the BENEDICT trial. J Am Soc Nephrol 2006; 17: 3472– 3481 [DOI] [PubMed] [Google Scholar]
- 13.Ruggenenti P, Fassi A, Ilieva AP, Bruno S, Iliev IP, Brusegan V, Rubis N, Gherardi G, Arnoldi F, Ganeva M, Ene-Iordache B, Gaspari F, Perna A, Bossi A, Trevisan R, Dodesini AR, Remuzzi G: Preventing microalbuminuria in type 2 diabetes. N Engl J Med 2004; 351: 1941– 1951 [DOI] [PubMed] [Google Scholar]
- 14.Parving H-H, Mauer M, Ritz E: Diabetic nephropathy. In Brenner & Rector's The Kidney 7th ed.Brenner BM. Ed. Philadelphia, Saunders, 2004, p. 1777– 1818 [Google Scholar]
- 15.Eurich DT, Majumdar SR, Tsuyuki RT, Johnson JA: Reduced mortality associated with the use of ACE inhibitors in patients with type 2 diabetes. Diabetes Care 2004; 27: 1330– 1334 [DOI] [PubMed] [Google Scholar]
- 16.Orchard TJ, Chang YF, Ferrell RE, Petro N, Ellis DE: Nephropathy in type 1 diabetes: a manifestation of insulin resistance and multiple genetic susceptibilities? Further evidence from the Pittsburgh Epidemiology of Diabetes Complication Study. Kidney Int 2002; 62: 963– 970 [DOI] [PubMed] [Google Scholar]
- 17.Mykkanen L, Zaccaro DJ, Wagenknecht LE, Robbins DC, Gabriel M, Haffner SM: Microalbuminuria is associated with insulin resistance in nondiabetic subjects: the insulin resistance atherosclerosis study. Diabetes 1998; 47: 793– 800 [DOI] [PubMed] [Google Scholar]
- 18.De Cosmo S, Minenna A, Ludovico O, Mastroianno S, Di Giorgio A, Pirro L, Trischitta V: Increased urinary albumin excretion, insulin resistance, and related cardiovascular risk factors in patients with type 2 diabetes: evidence of a sex-specific association. Diabetes Care 2005; 28: 910– 915 [DOI] [PubMed] [Google Scholar]
- 19.Parvanova AI, Trevisan R, Iliev IP, Dimitrov BD, Vedovato M, Tiengo A, Remuzzi G, Ruggenenti P: Insulin resistance and microalbuminuria: a cross-sectional, case-control study of 158 patients with type 2 diabetes and different degrees of urinary albumin excretion. Diabetes 2006; 55: 1456– 1462 [DOI] [PubMed] [Google Scholar]
- 20.Altshuler D, Hirschhorn JN, Klannemark M, Lindgren CM, Vohl MC, Nemesh J, Lane CR, Schaffner SF, Bolk S, Brewer C, Tuomi T, Gaudet D, Hudson TJ, Daly M, Groop L, Lander ES: The common PPARgamma Pro12Ala polymorphism is associated with decreased risk of type 2 diabetes. Nat Genet 2000; 26: 76– 80 [DOI] [PubMed] [Google Scholar]
- 21.Almind K, Doria A, Kahn CR: Putting the genes for type II diabetes on the map. Nat Med 2001; 7: 277– 279 [DOI] [PubMed] [Google Scholar]
- 22.Deeb SS, Fajas L, Nemoto M, Pihlajamaki J, Mykkanen L, Kuusisto J, Laakso M, Fujimoto W, Auwerx J: A Pro12Ala substitution in PPARgamma2 associated with decreased receptor activity, lower body mass index and improved insulin sensitivity. Nat Genet 1998; 20: 284– 287 [DOI] [PubMed] [Google Scholar]
- 23.Poulsen P, Andersen G, Fenger M, Hansen T, Echwald SM, Volund A, Beck-Nielsen H, Pedersen O, Vaag A: Impact of two common polymorphisms in the PPARgamma gene on glucose tolerance and plasma insulin profiles in monozygotic and dizygotic twins: thrifty genotype, thrifty phenotype, or both? Diabetes 2003; 52: 194– 198 [DOI] [PubMed] [Google Scholar]
- 24.Tonjes A, Scholz M, Loeffler M, Stumvoll M: Association of Pro12Ala polymorphism in peroxisome proliferator-activated receptor gamma with pre-diabetic phenotypes: meta-analysis of 57 studies on nondiabetic individuals. Diabetes Care 2006; 29: 2489– 2497 [DOI] [PubMed] [Google Scholar]
- 25.Ludovico O, Pellegrini F, Di Paola R, Minenna A, Mastroianno S, Cardellini M, Marini MA, Andreozzi F, Vaccaro O, Sesti G, Trischitta V: Heterogeneous effect of peroxisome proliferator-activated receptor gamma2 Ala12 variant on type 2 diabetes risk. Obesity (Silver Spring) 2007; 15: 1076– 1081 [DOI] [PubMed] [Google Scholar]
- 26.Morini E, Tassi V, Capponi D, Ludovico O, Dallapiccola B, Trischitta V, Prudente S: Interaction between PPARgamma2 variants and gender on the modulation of body weight. Obesity (Silver Spring) 2008; 16: 1467– 1470 [DOI] [PubMed] [Google Scholar]
- 27.Herrmann SM, Ringel J, Wang JG, Staessen JA, Brand E: Peroxisome proliferator-activated receptor-gamma2 polymorphism Pro12Ala is associated with nephropathy in type 2 diabetes: The Berlin Diabetes Mellitus (BeDiaM) Study. Diabetes 2002; 51: 2653– 2657 [DOI] [PubMed] [Google Scholar]
- 28.Caramori ML, Canani LH, Costa LA, Gross JL: The human peroxisome proliferator-activated receptor gamma2 (PPARgamma2) Pro12Ala polymorphism is associated with decreased risk of diabetic nephropathy in patients with type 2 diabetes. Diabetes 2003; 52: 3010– 3013 [DOI] [PubMed] [Google Scholar]
- 29.Issemann I, Green S: Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature 1990; 347: 645– 650 [DOI] [PubMed] [Google Scholar]
- 30.Willson TM, Cobb JE, Cowan DJ, Wiethe RW, Correa ID, Prakash SR, Beck KD, Moore LB, Kliewer SA, Lehmann JM: The structure-activity relationship between peroxisome proliferator-activated receptor gamma agonism and the antihyperglycemic activity of thiazolidinediones. J Med Chem 1996; 39: 665– 668 [DOI] [PubMed] [Google Scholar]
- 31.Mori H, Ikegami H, Kawaguchi Y, Seino S, Yokoi N, Takeda J, Inoue I, Seino Y, Yasuda K, Hanafusa T, Yamagata K, Awata T, Kadowaki T, Hara K, Yamada N, Gotoda T, Iwasaki N, Iwamoto Y, Sanke T, Nanjo K, Oka Y, Matsutani A, Maeda E, Kasuga M: The Pro12→Ala substitution in PPAR-gamma is associated with resistance to development of diabetes in the general population: possible involvement in impairment of insulin secretion in individuals with type 2 diabetes. Diabetes 2001; 50: 891– 894 [DOI] [PubMed] [Google Scholar]
- 32.Bennett CD, Campbell MN, Cook CJ, Eyre DJ, Nay LM, Nielsen DR, Rasmussen RP, Bernard PS: The LightTyper: high-throughput genotyping using fluorescent melting curve analysis. Biotechniques 2003; 34: 1288– 1292, 1294–1295 [DOI] [PubMed] [Google Scholar]
- 33.Dell'Omo G, Penno G, Del Prato S, Pedrinelli R: Doxazosin in metabolically complicated hypertension. Expert Rev Cardiovasc Ther 2007; 5: 1027– 1035 [DOI] [PubMed] [Google Scholar]
- 34.Bakris GL, Ruilope LM, McMorn SO, Weston WM, Heise MA, Freed MI, Porter LE: Rosiglitazone reduces microalbuminuria and blood pressure independently of glycemia in type 2 diabetes patients with microalbuminuria. J Hypertens 2006; 24: 2047– 2055 [DOI] [PubMed] [Google Scholar]