Abstract
OBJECTIVE
Alteration in endoplasmic reticulum (ER) stress in diabetic hearts and its effect on cytoprotective signaling are unclear. Here, we examine the hypothesis that ER stress in diabetic hearts impairs phospho–glycogen synthase kinase (GSK)-3β–mediated suppression of mitochondrial permeability transition pore (mPTP) opening, compromising myocardial response to cytoprotective signaling.
RESEARCH DESIGN AND METHODS
A rat model of type 2 diabetes (OLETF) and its control (LETO) were treated with tauroursodeoxycholic acid (TUDCA) (100 mg · kg−1 · day−1 for 7 days), an ER stress modulator. Infarction was induced by 20-min coronary occlusion and 2-h reperfusion.
RESULTS
Levels of ER chaperones (GRP78 and GRP94) in the myocardium and level of nonphoshopho–GSK-3β in the mitochondria were significantly higher in OLETF than in LETO rats. TUDCA normalized levels of GRP78 and GRP94 and mitochondrial GSK-3β in OLETF rats. Administration of erythropoietin (EPO) induced phosphorylation of Akt and GSK-3β and reduced infarct size (% risk area) from 47.4 ± 5.2% to 23.9 ± 3.5% in LETO hearts. However, neither phosphorylation of Akt and GSK-3β nor infarct size limitation was induced by EPO in OLETF rats. The threshold for mPTP opening was significantly lower in mitochondria from EPO-treated OLETF rats than in those from EPO-treated LETO rats. TUDCA restored responses of GSK-3β, mPTP opening threshold, and infarct size to EPO receptor activation in OLETF rats. There was a significant correlation between mPTP opening threshold and phospho–GSK-3β–to–total GSK-3β ratio in the mitochondrial fraction.
CONCLUSIONS
Disruption of protective signals leading to GSK-3β phosphorylation and increase in mitochondrial GSK-3β are dual mechanisms by which increased ER stress inhibits EPO-induced suppression of mPTP opening and cardioprotection in diabetic hearts.
Despite recent progress in coronary intervention strategies, diabetes is associated with higher mortality after acute myocardial infarction due to more extensive atherosclerotic lesions and hypertrophied and dysfunctional left ventricle (1–3). Therefore, diabetic patients with coronary artery diseases are patients who most require novel protective strategies against myocardial ischemia reperfusion injury. However, diabetes is known to impair responses of the myocardium to protective interventions. Protection afforded by preinfarct angina is lost in diabetic patients (4). In animal models, ischemic preconditioning (IPC) and some pharmacological agents failed to reduce infarct size in diabetic hearts (5–8). Recently, Gross et al. (7) have reported that responses of Akt, extracellular signal–related kinase (ERK), and glycogen synthase kinase (GSK)-3β to opioid receptor stimulation were blunted in streptozotocin-induced diabetes. GSK-3β has been shown to regulate a variety of cellular functions (9,10), and recent studies (10–14) have indicated that inactivation of GSK-3β by phosphorylation at Ser9 enhances myocardial tolerance against ischemia reperfusion injury. Furthermore, accumulating evidence indicates that phospho–GSK-3β–mediated cytoprotection is achieved by elevation of the threshold for opening of the mitochondrial permeability transition pore (mPTP), a probable final common step in stress-induced cell necrosis (11,15–17). However, derangements in GSK-3β regulation and its downstream targets in type 2 diabetes have not yet been clarified.
The endoplasmic reticulum (ER) has received much attention recently for its role in signal transduction relevant to cell survival and death. Various pathophysiological conditions induce Ca2+ overload and/or accumulation of unfolding or misfolding proteins within the ER, a condition referred to as ER stress (18). ER stress induces multiple responses, including adaptive changes in translation, protein folding, secretion, and degradation. Prolonged ER stress can trigger apoptosis by induction of C/EBP homologous protein (CHOP), activation of c-JUN NH2-terminal kinase (JNK), or caspase 12–dependent pathways. ER stress has been reported to be involved in the pathogenesis of diabetes (18–20), neurodegenerative disease, immune response, atherosclerosis, ischemia reperfusion injury, and heart failure (18,21–25).
We hypothesized that ER stress is increased in the diabetic myocardium and that increased ER stress in diabetic hearts impairs phospho–GSK-3β–mediated suppression of mPTP opening, leading to loss of myocardial response to cytoprotective signaling. The rationale for this hypothesis is threefold. First, increased ER stress has been observed in epididymal fat tissue in obese diabetic mice (26). Second, an increase in GSK-3β activity induced by ER stress through dephosphorylation of phospho-Ser9 has been reported in noncardiac cells (27). Finally, elevated levels of GSK-3 protein and activity were observed in skeletal muscle of type 2 diabetic patients (28). To test our hypothesis, we investigated changes in anti-infarct tolerance, myocardial ER stress, cytoprotective signaling, and mPTP opening threshold in a rat model of type 2 diabetes. ER stress modulators, sodium tauroursodeoxycholic acid (TUDCA), and 4-phenylbutyric acid (4-PBA) (29) were used to suppress ER stress. Erythropoietin (EPO) was used to induce GSK-3β phosphorylation in this study, since we have characterized signaling pathways from the EPO receptor leading to myocardial protection and modification of the pathways by concurrent pathological conditions (13,30,31).
RESEARCH DESIGN AND METHODS
This study was conducted in accordance with The Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH) (NIH publication no. 85-23, revised 1996) and approved by the animal use committee of Sapporo Medical University.
Animal model and surgical preparation.
Male Otsuka Long-Evans-Tokushima fatty rats (OLETF) (32), a rat model of obese type 2 diabetes, and their controls (Long-Evans-Tokushima-Otsuka rats [LETO]), both at ages between 25 and 30 weeks, were used in this study. In OLETF and LETO rats, TUDCA (100 mg · kg−1 · day−1) or the same volume of a vehicle (saline) was administered intraperitoneally for 7 days before the experiments. In a post hoc series of experiments, we examined whether 4-PBA (40 mg · kg−1 · day−1 i.p. for 7 days) could mimic effects of TUDCA and whether Goto-Kakizaki (GK) rats, a model of nonobese type 2 diabetes (33), respond to EPO similarly to OLETF rats. Rats were prepared for induction of myocardial infarction as in our previous studies (13) (see expanded methods section in the online appendix [available at http://diabetes.diabetesjournals.org/cgi/content/full/db09-0158/DC1]).
Infarct size experiments.
After a 20-min stabilization period, blood samples were collected from the jugular vein, and the left coronary artery was occluded for 20 min and then reperfused for 2 h to induce infarction. EPO (5,000 units/kg) was administered 15 min before ischemia, and SB216763 (1.2 mg/kg), a GSK-3 inhibitor, and PD98059 (2.5 mg/kg), a mitogen-activated protein kinase kinase inhibitor, were injected at 10 and 25 min before ischemia, respectively. After 2-h reperfusion, hearts were excised, and infarcts and areas at risk were visualized by tetrazolium staining and by fluorescent microspheres, respectively, as previously reported (13,30,31). Since pretreatment with TUDCA did not modify infarct size in LETO rats with or without EPO administration (see results section), a group of TUDCA-treated LETO rats were not included in the following series of experiments (i.e., immunoblot experiments and Ca2+-induced mPTP opening experiments).
Immunoblot experiments
Tissue sampling protocols.
In protocol 1, hearts were isolated and perfused with Krebs-Henseleit buffer at 75 mmHg, as previously reported (13,30,31). Biopsy samples (0.2–0.3 g) were taken from the left ventricle at baseline (after stabilization) or at 15 min after EPO infusion (10 units/ml). In a post hoc series of experiments, we injected a JNK inhibitor, SP600125 (0.1 mg/kg), 15 min before isolation of hearts in OLETF rats (34) or infused thapsigargin (0.1 or 1 μmol/l) for 20 min before EPO treatment in LETO rat hearts (25). In protocol 2, rats were surgically prepared as in infarct size experiments in vivo and received no agent (control) or EPO (5,000 units/kg) before coronary occlusion. In the TUDCA-treated OLETF rats, some rats were treated with PD98059 (2.5 mg/kg) and EPO before ischemia. At 5 min after reperfusion following a 20-min coronary occlusion, hearts were isolated and perfused with ice-cold saline, and the tissue in the risk region was sampled and snap-frozen in liquid nitrogen.
Samples for electrophoresis were prepared from total tissue homogenate or cytosolic and mitochondrial fractions as in our previous studies (16,30), and proteins of interest were immunoblotted and quantified by image analysis as detailed in the expanded methods section (online appendix).
Ca2+-induced mitochondrial permeability transition.
Opening of the mPTP was assessed following in vitro Ca2+ overload using isolated mitochondria according to a method by Argaud et al. (35) with some modification, as detailed in the expanded methods section (online appendix).
Statistics.
All data are presented as means ± SE. Differences between treatment groups were tested by one-way or two-way ANOVA, and the Student-Newman-Keuls post hoc test was used to test for multiple comparisons when ANOVA indicated significant differences. The difference was considered significant if the P value was <0.05.
RESULTS
Body weight and plasma glucose level.
As shown in Table 1, body weight was larger in OLETF than in LETO rats. Blood glucose and plasma insulin levels were higher in OLETF than in LETO rats, confirming phenotypes of type 2 diabetes in OLETF rats. In LETO rats, treatment with TUDCA did not modify body weight, blood glucose, and plasma insulin level. However, treatment with TUDCA for 7 days significantly reduced blood glucose and plasma insulin levels in OLETF rats, suggesting an improvement in insulin sensitivity. We measured blood glucose levels at baseline, before ischemia (i.e., after treatment), and 60 min after reperfusion, and none of treatments, including administration of SB216763, significantly affected the time courses of blood glucose levels in each study group (data not shown).
TABLE 1.
Body weight and plasma glucose levels
| Body weight (g) | Glucose (mg/dl) | Total cholesterol (mg/dl) | Triglycerides (mg/dl) | Insulin (ng/ml) | |
|---|---|---|---|---|---|
| LETO | 527.3 ± 5.4 | 116.0 ± 3.8 | 89.2 ± 1.2 | 11.8 ± 3.0 | 6.8 ± 0.8 |
| L + TUD | 510.8 ± 8.0 | 114.5 ± 6.6 | 97.3 ± 3.2 | 19.9 ± 4.9 | 5.1 ± 0.8 |
| OLETF | 604.7 ± 9.8* | 227.1 ± 13.4* | 79.2 ± 2.6* | 58.9 ± 10.2* | 9.1 ± 0.8* |
| O + TUD | 596.7 ± 7.7* | 184.1 ± 11.9*† | 75.7 ± 4.1* | 33.0 ± 5.8*† | 3.2 ± 0.5*† |
Data are means ± SE.
*P < 0.05 vs. LETO;
†P < 0.05 vs. OLETF. L + TUD, LETO pretreated with TUDCA; O + TUD, OLETF pretreated with TUDCA.
Infarct size data.
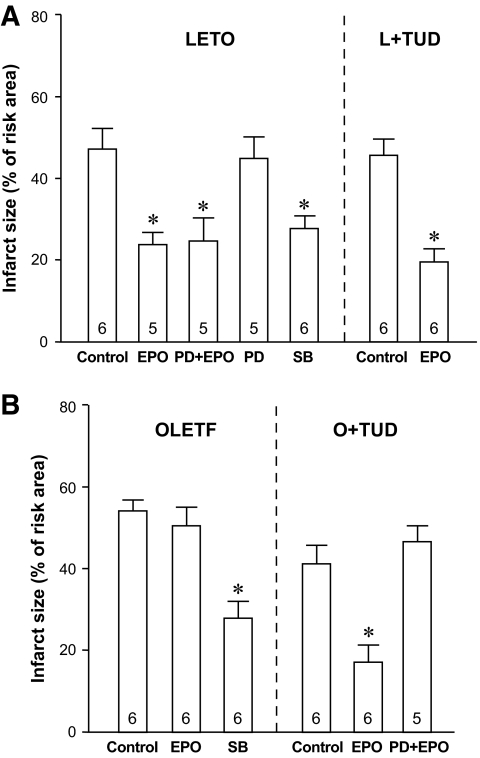
Heart rate and mean blood pressure levels were comparable in all study groups under baseline conditions, during ischemia, and after reperfusion, except for slightly lower heart rate in LETO rats after SB216763 administration (see the online appendix Table). In LETO rats, pretreatment with EPO significantly reduced infarct size as a percentage of risk area (%I/R) from 47.4 ± 5.2% in controls to 23.9 ± 3.5% (Fig. 1A). This EPO-induced protection was not blocked by PD98059, a mitogen-activated protein kinase kinase inhibitor, and this blocker by itself had no effect on infarct size. Treatment with SB216763, a GSK-3 inhibitor, mimicked the infarct size–limiting effect of EPO. In TUDCA-treated LETO rats, infarct sizes with and without EPO pretreatment (%I/R = 19.2 ± 4.1 and 45.7 ± 4.0%, respectively) were similar to those in LETO rats.
FIG. 1.
Infarct size expressed as a percentage of risk area. Number at each bar indicates n values for each group. *P < 0.05 vs. control in each group. L+TUD, LETO treated with TUDCA; O+TUD, OLETF treated with TUDCA; PD, PD98059; SB, SB216763.
In OLETF controls, infarct size was 14% larger than that in LETO rats, but this difference was not statistically significant due to the small number of hearts used in the experiments. Thus, we performed post hoc experiments in which four hearts were added to each of the OLETF control and LETO control groups. The pooled data (n = 10 in each group) showed a statistically significant difference between %I/Rs in the OLETF control group and LETO control group (57.4 ± 3.0 vs. 47.7 ± 3.1%, P < 0.05). In contrast to its effect in LETO rats, EPO failed to limit infarct size in OLETF rats, though SB216763 afforded significant protection in OLETF rats as well (Fig. 1B). Comparison of %I/Rs in LETO control, TUDCA-treated LETO control, OLETF control, and TUDCA-treated OLETF control groups by one-way ANOVA indicated that TUDCA alone reduced infarct size in OLETF but not in LETO rats. More importantly, TUDCA prevented loss of myocardial response to EPO in OLETF rats, %I/R being reduced by EPO to 17.0 ± 5.0%, and this EPO-induced protection was sensitive to PD98059. Failure of EPO to limit infarct size was observed also in GK rats (supplemental data in the online appendix), suggesting that this is a common feature in diabetic animals.
To confirm that loss of myocardial protection by EPO in OLETF rats is derived from lack of response in Jak2-mediated protective mechanisms, we further assessed effects of [d-Ala2, d-Leu5]-enkephalin acetate (DADLE), a δ-opioid receptor agonist, on infarct size (online appendix Fig. 1). Pretreatment with DADLE (1 mg/kg) significantly reduced infarct size in LETO but not in OLETF rats. Treatment with TUDCA restored myocardial response to DADLE also in OLETF rats.
Immunoblot experiments
Protocol 1: ER stress markers and response of protein kinases to EPO receptor activation.
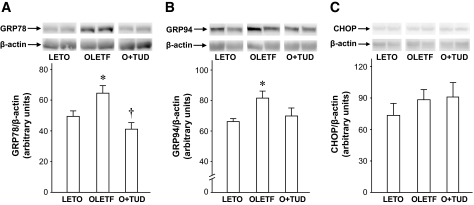
Protein levels of GRP78 and GRP94, ER stress chaperone proteins, were significantly elevated in OLETF rats (Fig. 2A and B), indicating that ER stress was enhanced in the diabetic myocardium. One-week treatment with TUDCA reduced the elevation of myocardial GRP78 and GRP94 levels in OLETF rats. This effect of TUDCA was mimicked by 4-PBA, a structurally different ER stress modulator (29,36) (data not shown). Levels of CHOP protein, an ER-mediated proapoptotic transcription factor (37), in the myocardium were similar in OLETF and LETO rats (Fig. 2C).
FIG. 2.
Myocardial levels of ER chaperones in LETO and OLETF rats. A: GRP78. B: GRP94. C: CHOP. Representative Western blots and summarized data are presented for each group. Values are expressed as arbitrary units and as means ± SE. Blots for β-actin are loading controls. *P < 0.05 vs. LETO. †P < 0.05 vs. OLETF. n = 4–6. O+TUD, OLETF treated with TUDCA.
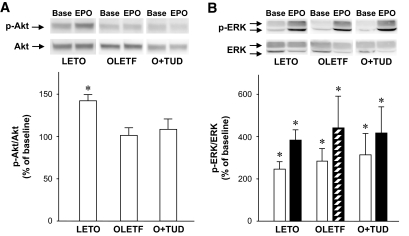
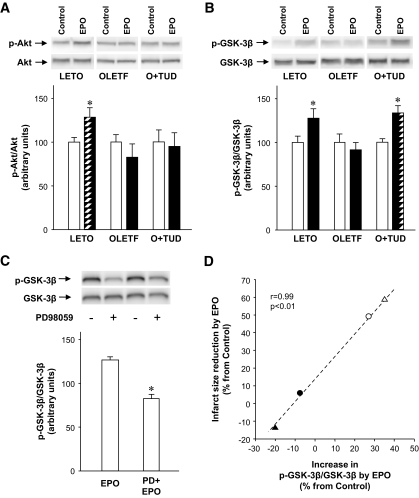
EPO receptor protein levels in the myocardium were similar in LETO and OLETF rats (data not shown). As shown in Fig. 3A, administration of EPO significantly increased the level of phospho-Akt in LETO hearts. However, such phosphorylation of Akt by EPO was not observed in OLETF rats, and pretreatment with TUDCA did not restore Akt response in OLETF rats (Fig. 3A). In contrast, EPO-induced ERK1 and ERK2 phosphorylation occurred similarly in LETO and OLETF rats (Fig. 3B). Effects of 4PBA on EPO-induced Akt and ERK phosphorylation were similar to those of TUDCA (online appendix Fig. 2A and B). A JNK inhibitor, SP600125, had no effects on responses of Akt and ERK to EPO receptor activation in OLETF rats (online appendix Fig. 2A and B).
FIG. 3.
Effects of EPO infusion on phosphorylation of Akt and ERK1/2 in the myocardium. A: Akt. B: ERK1/2. Representative Western blots of samples from baseline (Base) and after EPO treatment (EPO) and summarized data are presented for each treatment group. Values are expressed as ratios of phospho-Akt to total Akt protein or phospho-ERK to total ERK protein and as means ± SE. □, ERK1; ▨, ERK2. *P < 0.05 vs. baseline. n = 4. O+TUD, OLETF treated with TUDCA.
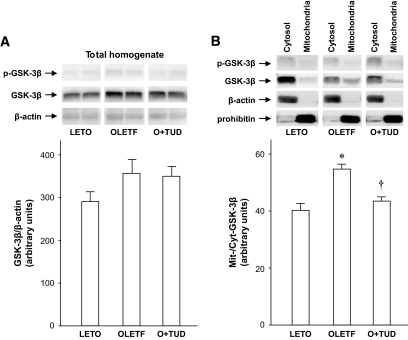
GSK-3β level in total myocardial homogenates in OLETF rats was 25% higher than that in LETO rats, though this difference did not reach statistical significance (Fig. 4A). However, a significant difference between the two groups was observed for mitochondrial GSK-3β. As shown in Fig. 4B, GSK-3β level in the mitochondrial fraction was significantly higher in OLETF than in LETO rats, while phospho–Ser9–GSK-3β level in the mitochondrial fraction was comparable between LETO and OLETF rats. The upregulation of non–Ser9–phosphorylated GSK-3β in the mitochondria was attenuated by TUDCA pretreatment.
FIG. 4.
Phospho–GSK-3β and total GSK-3β in total homogenates and subcellular fractions of the myocardium. A: Total tissue homogenate. B: Cytosol and mitochondrial fractions. Blots for β-actin and prohibitin are cytosolic and mitochondrial marker proteins, respectively, and also serve as loading controls. *P < 0.05 vs. LETO. †P < 0.05 vs. OLETF. n = 4–6. O+TUD, OLETF treated with TUDCA.
Infusion of 1 μmol/l thapsigargin for 20 min increased tissue GRP78 level by 70% and prevented phosphorylation of Akt and GSK-3β in response to EPO infusion in LETO hearts (online appendix). However, these effects of thapsigargin were associated with 40% reduction in left ventricular–developed pressure.
Protocol 2: phosphorylation of protein kinases after ischemia reperfusion in vivo.
EPO administration before ischemia significantly increased levels of phospho-Akt and phospho–GSK-3β upon reperfusion in LETO rats (Fig. 5A and B). However, such phosphorylation of Akt and GSK-3β by EPO was not observed in OLETF rats. Treatment of OLETF rats with TUDCA restored GSK-3β phosphorylation response to EPO (Fig. 5B), whereas Akt phosphorylation by EPO was not recovered by TUDCA (Fig. 5A). These effects of TUDCA in OLETF rats were mimicked by 4-PBA but not by SP600125 (online appendix Fig. 2). Phospho-ERK levels upon reperfusion were not different in LETO, OLETF, and TUDCA-treated OLETF rats (data not shown). Interestingly, restoration by TUDCA of EPO-induced GSK-3β phosphorylation was abrogated by PD98059 (Fig. 5C), as was infarct size limitation by EPO after TUDCA pretreatment (Fig. 1B). Infarct size limitation was correlated with phospho–GSK-3β–to–total GSK-3β ratio (Fig. 5D), which is consistent with our previous findings in Sprague-Dawley rats (13).
FIG. 5.
Effects of EPO on phosphorylation of Akt and GSK-3β at reperfusion. A: Akt. B: GSK-3β. □, Control (i.e., 20-min ischemia/5-min reperfusion); ▨, EPO (5,000 units/kg i.v., plus 20-min ischemia/5-min reperfusion). *P < 0.05 vs. control. n = 4–7. C: Effects of PD98059 on EPO-induced phosphorylation of GSK-3β at reperfusion in OLETF rats treated with TUDCA (O+TUD). *P < 0.05 vs. EPO. n = 3. D: Relationship between increases in phospho–GSK-3β levels by EPO from control and mean infarct size reduction by EPO in each treatment group. See data of infarct size experiments (Fig. 1) and immunoblot experiments (Fig. 5B and C). ○, LETO; ●, OLETF; △, OLETF+TUDCA; ▲, EPO+PD98059 treatment in OLETF and TUDCA rats.
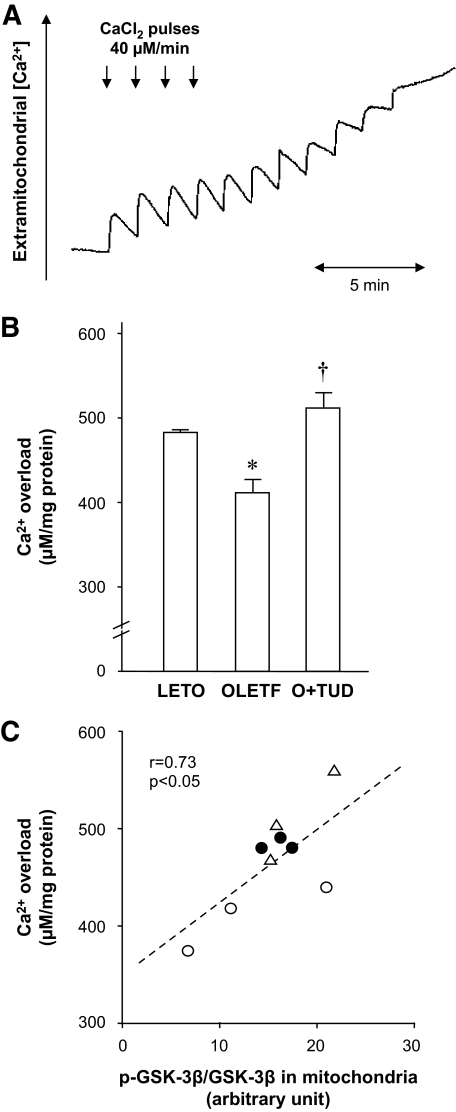
Ca2+-induced mitochondrial permeability transition.
The amount of Ca2+ required to open the mPTP in the mitochondria isolated after EPO treatment was 483.3 ± 3.3 μmol/mg mitochondrial protein in LETO rats, but it was significantly decreased to 411.1 ± 19.8 μmol/mg mitochondrial protein in OLETF rats (Fig. 6B). In TUDCA-treated OLETF rats, a significantly larger amount of Ca2+ was required to induce mPTP opening (510.0 ± 26.5 μmol/mg) than in untreated OLETF rats. To confirm that mPTP opening was accurately monitored by the present method, we assessed the effect of cyclosporine A, an inhibitor of mPTP opening. Mitochondria isolated from LETO rats were treated with cyclosporin A (0.3 μmol/l) for 5 min before Ca2+ challenge. The amount of Ca2+ required to induce mPTP opening in the cyclosporine A–treated mitochondria was much larger (740.0 ± 90.2 μmol/mg, n = 3) than that in untreated mitochondria. Using some of the mitochondria isolated for mPTP experiments, we determined levels of phospho– and total GSK-3β by immunoblotting. As shown in Fig. 6C, phospho–GSK-3β–to–total GSK-3β ratio in the mitochondria was significantly correlated with the amounts of Ca2+ required to open the mPTP. These results suggest that level of mitochondrial GSK-3β phosphorylation at reperfusion is a determinant of the threshold for mPTP opening.
FIG. 6.
Ca2+-induced mPTP opening. A: Typical recording of mPTP opening in a sample of isolated mitochondria. B: Summarized data of Ca2+ load required to induce mPTP opening are presented for each group. *P < 0.05 vs. LETO; †P < 0.05 vs. OLETF. n = 3. C: Relationship between the amounts of Ca2+ required for mPTP opening and phosphorylation levels of GSK-3β in mitochondria at reperfusion. ●, LETO; ○, OLETF; △, OLETF+TUDCA. O+TUD, OLETF treated with TUDCA.
DISCUSSION
The present study showed for the first time that ER stress marker proteins, GRP78 and GRP94, were significantly elevated in the myocardium of a model of type 2 diabetes (Fig. 2), while CHOP protein level was not elevated unlike that in a model of type 1 diabetes. To assess the contribution of increased ER stress to cell signaling and mPTP regulation, we utilized TUDCA and 4-PBA to reduce ER stress in the present study. TUDCA, a derivative of an endogenous bile acid (38), and 4-PBA, a low–molecular weight fatty acid (36), have been demonstrated to suppress ER stress elevated in the liver and fat in ob/ob mice (29). Efficacy of TUDCA and 4-PBA in the OLETF myocardium was confirmed by findings that these agents normalized protein levels of both GRP78 and GRP94.
Phosphatidylinositol-3 kinase (PI3K)-Akt–GSK-3β signaling is crucial in cytoprotection afforded by EPO and other Jak2-activating ligands (13,30,39). In the present study, not only EPO but also DADLE failed to protect the myocardium of OLETF rats from infarction, suggesting that Jak2-mediated protection is dysfunctional. In fact, EPO failed to activate PI3K-Akt signaling in OLETF rats (Fig. 3), whereas activation of ERK was spared. This pattern of modification in cytoprotective signaling is apparently similar to the pattern that we observed in postinfarct remodeled hearts (30,31). However, EPO could limit infarct size in an ERK-dependent manner in the remodeled hearts (31) in contrast with its failure in OLETF hearts (Fig. 1B). These findings suggest that there is a pathological factor in the OLETF myocardium that prevents phosphorylated ERK from compensating the role of Akt in myocardial protection. Interestingly, pretreatment with TUDCA or 4-PBA restored the response of GSK-3β to EPO receptor activation (Fig. 5B) (online appendix Fig. 2C), without having an effect on Akt phosphorylation, and restored the myocardial response to EPO-induced protection. The GSK-3β phosphorylation by EPO in TUDCA-treated OLETF rats was inhibited by PD98059 (Fig. 5C), as was the infarct size–limiting effect of EPO in the same treatment group (Fig. 1B). Taken together, these results suggest that increased ER stress in OLETF rats inhibits the compensatory function of ERK to phosphorylate GSK-3β in the presence of a defect in PI3K-Akt signaling from the EPO receptor.
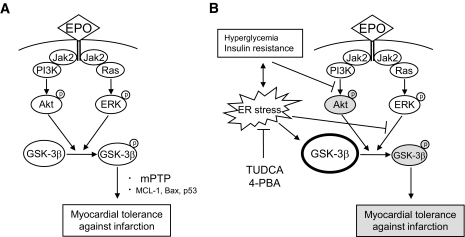
The lack of Akt signaling from activated EPO receptors and disruption of ERK–GSK-3β signaling were not the only modifications of the GSK-3β–linked signal pathway in OLETF rats. GSK-3β level in the mitochondria was 45% higher in OLETF than in LETO rats under baseline conditions, whereas phospho–GSK-3β levels were similar in the two groups, indicating an increase in nonphospho–GSK-3β in OLETF mitochondria. The increased nonphospho–GSK-3β in the mitochondria may be responsible for slight enlargement of infarct size in OLETF rats compared with that in LETO rats. The nonphosphorylated form of GSK-3β is constitutively active, forms a complex with two major subunits of mPTP (adenine nucleotide translocase [ANT] and voltage-dependent anion channel [VDAC]) after ischemia/reperfusion (16), and reduces the threshold for mPTP opening (10,14). Phosphorylation of GSK-3β at Ser9 reduces its activity and elevates the mPTP opening threshold (10,14). Thus, the ratio of phospho–GSK-3β to total GSK-3β can be used as a surrogate index of threshold for mPTP opening. Since critical timing of mPTP opening is within several minutes after reperfusion, we focused on phospho–GSK-3β–to–total GSK-3β ratio at 5 min after reperfusion. There was a correlation between phospho–GSK-3β–to–total GSK-3β ratio and threshold for mPTP opening in mitochondria isolated after ischemia/reperfusion, and the ratio was lower in OLETF than in LETO rats (Fig. 6). Suppression of ER stress by TUDCA reduced mitochondrial GSK-3β level (Fig. 4) and infarct size (Fig. 1) and increased the threshold for mPTP opening (Fig. 6) to their levels in LETO controls. Taken together, these results suggest that preischemic elevation of nonphospho–GSK-3β in OLETF rats contributes to the low phospho–GSK-3β–to–total GSK-3β ratio upon reperfusion, facilitating mPTP opening and cell necrosis. Our current hypothesis based on the present observations is schematically presented in Fig. 7.
FIG. 7.
Schematic presentation of alteration in EPO-provoked signaling in diabetic myocardium. A: In the normal myocardium (i.e., LETO), EPO phosphorylated both Akt and ERK, but phosphorylation of Akt is sufficient to phosphorylate GSK-3β, resulting in infarct size limitation. B: In diabetic rats (i.e., OLETF rats), Akt phosphorylation by EPO and phosphorylation of GSK-3β by ERK were impaired. Constitutively active GSK-3β is increased, particularly in the mitochondria, which blunts the effect of intracellular signaling leading to GSK-3β phosphorylation.
Ischemia/reperfusion induces ATP depletion, Ca2+ overloading, and production of reactive oxygen species (ROS) in cardiomyocytes, and these events are known to promote mPTP opening and also to induce ER stress (10,18,23,40). As recently reviewed by Lamarca and Scorrano (41), cross-talks have been proposed between ER-derived and mitochondria-derived cell death/survival pathways. The results of the present study suggest that ER stress associated with diabetes modulates the mPTP in mitochondria via modification of GSK-3β phosphorylation signaling (Fig. 5) and mitochondrial translocation of GSK-3β (Fig. 4). However, it remains unclear whether ischemia/reperfusion-induced ER stress in the myocardium differs between LETO and OLETF rats.
A link between change in the threshold for mPTP opening by GSK-3β phosphorylation and change in the extent of myocardial necrosis in situ is difficult to directly demonstrate. However, several lines of circumstantial evidence indicate that elevation of the threshold for mPTP opening results in limitation of infarct size. First, multiple agents that induce phosphorylation of GSK-3β have been shown to induce both infarct size limitation and elevation of the threshold for mPTP opening in response to ROS (11,15). Second, level of phospho–GSK-3β upon reperfusion was shown to be inversely correlated with infarct size in a previous study (13), and such a correlation was confirmed in the present study as well (Fig. 5D). Third, pharmacological inhibitors of GSK-3β, which mimics the effect of phosphorylation of GSK-3β at Ser9, significantly limited infarct size in earlier studies (12,13) and in the present experiments. It is notable that SB216763 limited infarct size to similar extents in LETO and OLETF rats, suggesting that the protective mechanism downstream of GSK-3β remains intact in the diabetic myocardium. Thus, GSK-β inhibitors are potentially useful agents for myocardial salvage in patients with acute myocardial infarction and diabetes.
It is unclear how phospho–GSK-3β elevates the threshold for opening of the mPTP in response to oxygen radicals and calcium overload at the time of reperfusion (10,11,14,15). Recently, we found that phosphorylation of GSK-3β at Ser9 by IPC and EPO treatment induced physical interaction of phospho–GSK-3β with ANT, and this phospho–GSK-3β–ANT interaction inhibited binding of ANT to cyclophilin D (10,16). Since cyclophilin D increases sensitivity of ANT to Ca2+, inhibition of ANT-cyclophilin D interaction should suppress mPTP opening. In addition to this possible mechanism, suppression of hexokinase release from the mitochondria and reduction in ATP hydrolysis during ischemia have been suggested to be involved in inhibition of mPTP opening by phospho–GSK-3β (14).
Alterations in signal transduction and ER stress in the myocardium of OLETF rats, a model of type 2 diabetes, are unlikely to be extrapolated to type 1 diabetes. Disruption in PI3K-Akt signaling and elevation of GRP78 protein level were observed in the myocardium of streptozotocin-induced diabetes as well (7,42). However, in this model of type 1 diabetes, protein levels of CHOP and JNK were significantly elevated, and ERK phosphorylation was also impaired in the myocardium (7,42). In contrast, CHOP protein was not significantly elevated and ERK phosphorylation was intact in OLETF rats (Figs. 2 and 3). Involvement of JNK in impaired Akt and GSK-3β response to EPO was not supported by the findings that SP600125 did not restore phosphorylation of Akt or GSK-3β after EPO receptor activation (online appendix Fig. 2). Furthermore, focal myocardial apoptosis and fibrosis reported for the myocardium of type 1 diabetic rats (42) were scarcely observed in OLETF rats aged 25–30 weeks (data not shown). There are a few possible explanations for the differences in modification of protective signaling between type 2 diabetic rats and streptozotocin-induced diabetic rats. First, the level of hyperglycemia is modest in OLETF rats and GK rats and severe in streptozotocin-induced diabetes (227 ± 13 and 193 ± 9 mg/dl vs. >500 mg/dl) (7,42). Earlier studies have shown that hyperglycemia, per se, disturbs cardioprotective mechanisms of IPC and pharmacological agents against ischemia reperfusion (43–46). Interestingly, this untoward effect of hyperglycemia depends on the level of hyperglycemia; it could be overcome by augmentation of protective stimuli, such as repetition of IPC (6) and increasing doses of protective agents (44,46) when blood glucose level was ∼300 mg/dl but not when it was ∼600 mg/dl. Second, time courses of hyperglycemia in the two diabetes models are different. Adaptive response in cellular signaling would be different depending on whether hyperglycemia is rapidly induced by streptozotocin or gradually developed in type 2 diabetes as in the case of OLETF and GK rats. Duration of hyperglycemia may also affect the phenotype of modified signaling, as inhibition of ischemia-induced ERK phosphorylation by hyperglycemia has been shown to depend on the duration of hyperglycemia (47). Third, presence or absence of insulin resistance might contribute to differences in modifications of cell signaling between type 1 and type 2 diabetes.
There are limitations in the present study. First, we cannot exclude the possibility of the presence of targets of phospho–GSK-3β for cytoprotection besides mPTP, such as Bax, p53, and myeloid cell leukemia sequence-1 (MCL-1) (9). Second, the specific ER stress pathway that inhibited activated ERK to phosphorylate GSK-3β in OLETF (Figs. 3 and 5) has not been identified, and the relationship between duration of hyperglycemia and impairment of signaling remains unclear. Since ER stress activates multiple signal pathways via inositol-requiring enzyme 1 (IRE1), PKR-like eukaryotic initiation factor-2α kinase (PERK), and activating transcription factor 6 (ATF6) (18), the possibility that these proteins influence the accessibility of activated ERK to mitochondrial GSK-3β might warrant investigations. Third, it remains unclear whether increased ER stress alone is sufficient for disruption of ERK–GSK-3β signaling and elevation of GSK-3β level in mitochondria. Elevation of GRP78 by 1 μmol/l thapisigargin was associated with lack of Akt and GSK-3β phosphorylation in response to EPO receptor activation in isolated LETO hearts. However, this dose of thapsigargin severely compromised ventricular contraction, and the possibility of involvement of ER stress–independent factors in the derangement in Akt–GSK-3β signaling cannot be excluded.
In conclusion, the present study showed for the first time that cytoprotective regulation of the mPTP and myocyte salvage are compromised in the type 2 diabetic heart by a defect in cell signaling upstream of GSK-3β phosphorylation and increase in antisurvival form of GSK-3β in mitochondria. Therapy to reduce ER stress may provide a benefit in prevention of defects of cardioprotective mechanisms in diabetic hearts.
Supplementary Material
Acknowledgments
This study was supported by a grant for scientific research (no. 20590870) from the Japan Society for Promotion of Science, Tokyo, Japan, and a grant from Chugai Pharmaceutical, Tokyo, Japan. Human recombinant erythropoietin was provided by Chugai Pharmaceutical. OLETF and LETO rats were gifts from the Tokushima Research Institute of Otsuka Pharmaceutical, Tokushima, Japan.
No potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in abstract form at the 80th Scientific Sessions of the American Heart Association, 4–7 November 2007, Orlando, Florida.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Flaherty JD, Davidson CJ: Diabetes and coronary revascularization. JAMA 2005; 293: 1501– 1508 [DOI] [PubMed] [Google Scholar]
- 2.Bhadriraju S, Ray KK, DeFranco AC, Barber K, Bhadriraju P, Murphy SA, Morrow DA, McCabe CH, Gibson CM, Cannon CP, Braunwald E: Association between blood glucose and long-term mortality in patients with acute coronary syndromes in the OPUS-TIMI 16 trial. Am J Cardiol 2006; 97: 1573– 1577 [DOI] [PubMed] [Google Scholar]
- 3.Kirtane AJ, Ellis SG, Dawkins KD, Colombo A, Grube E, Popma JJ, Fahy M, Leon MB, Moses JW, Mehran R, Stone GW: Paclitaxel-eluting coronary stents in patients with diabetes mellitus: pooled analysis from 5 randomized trials. J Am Coll Cardiol 2008; 51: 708– 715 [DOI] [PubMed] [Google Scholar]
- 4.Ishihara M, Inoue I, Kawagoe T, Shimatani Y, Kurisu S, Nishioka K, Kouno Y, Umemura T, Nakamura S, Sato H: Diabetes mellitus prevents ischemic preconditioning in patients with a first acute anterior wall myocardial infarction. J Am Coll Cardiol 2001; 38: 1007– 1011 [DOI] [PubMed] [Google Scholar]
- 5.Kersten JR, Toller WG, Gross ER, Pagel PS, Warltier DC: Diabetes abolishes ischemic preconditioning: role of glucose, insulin, and osmolality. Am J Physiol Heart Circ Physiol 2000; 278: H1218– H1224 [DOI] [PubMed] [Google Scholar]
- 6.Tsang A, Hausenloy DJ, Mocanu MM, Carr RD, Yellon DM: Preconditioning the diabetic heart: the importance of Akt phosphorylation. Diabetes 2005; 54: 2360– 2364 [DOI] [PubMed] [Google Scholar]
- 7.Gross ER, Hsu AK, Gross GJ: Diabetes abolishes morphine-induced cardioprotection via multiple pathways upstream of glycogen synthase kinase-3β. Diabetes 2007; 56: 127– 136 [DOI] [PubMed] [Google Scholar]
- 8.Katakam PV, Jordan JE, Snipes JA, Tulbert CD, Miller AW, Busija DW: Myocardial preconditioning against ischemia-reperfusion injury is abolished in Zucker obese rats with insulin resistance. Am J Physiol Regul Integr Comp Physiol 2007; 292: R920– R926 [DOI] [PubMed] [Google Scholar]
- 9.Beurel E, Jope RS: The paradoxical pro- and anti-apoptotic actions of GSK3 in the intrinsic and extrinsic apoptosis signaling pathways. Prog Neurobiol 2006; 79: 173– 189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miura T, Miki T: GSK-3β, a therapeutic target for cardiomyocyte protection. Circ J 2009; 73: 1184– 1192 [DOI] [PubMed] [Google Scholar]
- 11.Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, Ziman BD, Wang S, Ytrehus K, Antos CL, Olson EN, Sollott SJ: Glycogen synthase kinase-3β mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest 2004; 113: 1535– 1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gross ER, Hsu AK, Gross GJ: Opioid-induced cardioprotection occurs via glycogen synthase kinase β inhibition during reperfusion in intact rat hearts. Circ Res 2004; 94: 960– 966 [DOI] [PubMed] [Google Scholar]
- 13.Nishihara M, Miura T, Miki T, Sakamoto J, Tanno M, Kobayashi H, Ikeda Y, Ohori K, Takahashi A, Shimamoto K: Erythropoietin affords additional cardioprotection to preconditioned hearts by enhanced phosphorylation of glycogen synthase kinase-3β. Am J Physiol Heart Circ Physiol 2006; 291: H748– H755 [DOI] [PubMed] [Google Scholar]
- 14.Juhaszova M, Zorov DB, Yaniv Y, Nuss HB, Wang S, Sollott SJ: Role of glycogen synthase kinase-3β in cardioprotection. Circ Res 2009; 104: 1240– 1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halestrap AP, Clarke SJ, Javadov SA: Mitochondrial permeability transition pore opening during myocardial reperfusion: a target for cardioprotection. Cardiovasc Res 2004; 61: 372– 385 [DOI] [PubMed] [Google Scholar]
- 16.Nishihara M, Miura T, Miki T, Tanno M, Yano T, Naitoh K, Ohori K, Hotta H, Terashima Y, Shimamoto K: Modulation of the mitochondrial permeability transition pore complex in GSK-3β-mediated myocardial protection. J Mol Cell Cardiol 2007; 43: 564– 570 [DOI] [PubMed] [Google Scholar]
- 17.Obame FN, Plin-Mercier C, Assaly R, Zini R, Dubois-Rande JL, Berdeaux A, Morin D: Cardioprotective effect of morphine and a blocker of glycogen synthase kinase 3β, SB216763 [3-(2,4-dichlorophenyl)-4(1-methyl-1H-indol-3-yl)-1H-pyrrole-2,5-dione], via inhibition of the mitochondrial permeability transition pore. J Pharmacol Exp Ther 2008; 326: 252– 258 [DOI] [PubMed] [Google Scholar]
- 18.Marciniak SJ, Ron D: Endoplasmic reticulum stress signaling in disease. Physiol Rev 2006; 86: 1133– 1149 [DOI] [PubMed] [Google Scholar]
- 19.Eizirik DL, Cardozo AK, Cnop M: The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev 2008; 29: 42– 61 [DOI] [PubMed] [Google Scholar]
- 20.Laybutt DR, Preston AM, Akerfeldt MC, Kench JG, Busch AK, Biankin AV, Biden TJ: Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia 2007; 50: 752– 763 [DOI] [PubMed] [Google Scholar]
- 21.Myoishi M, Hao H, Minamino T, Watanabe K, Nishihira K, Hatakeyama K, Asada Y, Okada K, Ishibashi-Ueda H, Gabbiani G, Bochaton-Piallat ML, Mochizuki N, Kitakaze M: Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation 2007; 116: 1226– 1233 [DOI] [PubMed] [Google Scholar]
- 22.Tabas I: Apoptosis and plaque destabilization in atherosclerosis: the role of macrophage apoptosis induced by cholesterol. Cell Death Differ 2004; 11: S12– S16 [DOI] [PubMed] [Google Scholar]
- 23.Martindale JJ, Fernandez R, Thuerauf D, Whittaker R, Gude N, Sussman MA, Glembotski CC: Endoplasmic reticulum stress gene induction and protection from ischemia/reperfusion injury in the hearts of transgenic mice with a tamoxifen-regulated form of ATF6. Circ Res 2006; 98: 1186– 1193 [DOI] [PubMed] [Google Scholar]
- 24.Thuerauf DJ, Marcinko M, Gude N, Rubio M, Sussman MA, Glembotski CC: Activation of the unfolded protein response in infarcted mouse heart and hypoxic cultured cardiac myocytes. Circ Res 2006; 99: 275– 282 [DOI] [PubMed] [Google Scholar]
- 25.Okada K, Minamino T, Tsukamoto Y, Liao Y, Tsukamoto O, Takashima S, Hirata A, Fujita M, Nagamachi Y, Nakatani T, Yutani C, Ozawa K, Ogawa S, Tomoike H, Hori M, Kitakaze M: Prolonged endoplasmic reticulum stress in hypertrophic and failing heart after aortic constriction: possible contribution of endoplasmic reticulum stress to cardiac myocyte apoptosis. Circulation 2004; 110: 705– 712 [DOI] [PubMed] [Google Scholar]
- 26.Eldar-Finkelman H, Schreyer SA, Shinohara MM, LeBoeuf RC, Krebs EG: Increased glycogen synthase kinase-3 activity in diabetes- and obesity-prone C57BL/6J mice. Diabetes 1999; 48: 1662– 1666 [DOI] [PubMed] [Google Scholar]
- 27.Song L, De Sarno P, Jope RS: Central role of glycogen synthase kinase-3β in endoplasmic reticulum stress-induced caspase-3 activation. J Biol Chem 2002; 277: 44701– 44708 [DOI] [PubMed] [Google Scholar]
- 28.Nikoulina SE, Ciaraldi TP, Mudaliar S, Mohideen P, Carter L, Henry RR: Potential role of glycogen synthase kinase-3 in skeletal muscle insulin resistance of type 2 diabetes. Diabetes 2000; 49: 263– 271 [DOI] [PubMed] [Google Scholar]
- 29.Özcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Görgün CZ, Hotamisligil GS: Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 2006; 313: 1137– 1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miki T, Miura T, Yano T, Takahashi A, Sakamoto J, Tanno M, Kobayashi H, Ikeda Y, Nishihara M, Naitoh K, Ohori K, Shimamoto K: Alteration in erythropoietin-induced cardioprotective signaling by postinfarct ventricular remodeling. J Pharmacol Exp Ther 2006; 317: 68– 75 [DOI] [PubMed] [Google Scholar]
- 31.Miki T, Miura T, Tanno M, Nishihara M, Naitoh K, Sato T, Takahashi A, Shimamoto K: Impairment of cardioprotective PI3K-Akt signaling by post-infarct ventricular remodeling is compensated by an ERK-mediated pathway. Basic Res Cardiol 2007; 102: 163– 170 [DOI] [PubMed] [Google Scholar]
- 32.Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T: Spontaneous long-term hyperglycemic rat with diabetic complications: Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes 1992; 41: 1422– 1428 [DOI] [PubMed] [Google Scholar]
- 33.Goto Y, Kakizaki M, Masaki N: Spontaneous diabetes produced by selective breeding of normal Wister rats. Proc Japan Acad 1975; 51: 80– 85 [Google Scholar]
- 34.Heidbreder M, Naumann A, Tempel K, Dominiak P, Dendorfer A: Remote vs. ischaemic preconditioning: the differential role of mitogen-activated protein kinase pathways. Cardiovasc Res 2008; 78: 108– 115 [DOI] [PubMed] [Google Scholar]
- 35.Argaud L, Gateau-Roesch O, Muntean D, Chalabreysse L, Loufouat J, Robert D, Ovize M: Specific inhibition of the mitochondrial permeability transition prevents lethal reperfusion injury. J Mol Cell Cardiol 2005; 38: 367– 374 [DOI] [PubMed] [Google Scholar]
- 36.Kubota K, Niinuma Y, Kaneko M, Okuma Y, Sugai M, Omura T, Uesugi M, Uehara T, Hosoi T, Nomura Y: Suppressive effects of 4-phenylbutyrate on the aggregation of Pael receptors and endoplasmic reticulum stress. J Neurochem 2006; 97: 1259– 1268 [DOI] [PubMed] [Google Scholar]
- 37.Oyadomari S, Mori M: Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ 2004; 11: 381– 389 [DOI] [PubMed] [Google Scholar]
- 38.Poupon RE, Bonnand AM, Chrétien Y, Poupon R: Ten-year survival in ursodeoxycholic acid-treated patients with primary biliary cirrhosis: the UDCA-PBC study group. Hepatology 1999; 29: 1668– 1671 [DOI] [PubMed] [Google Scholar]
- 39.Cai Z, Semenza GL: Phosphatidylinositol-3-kinase signaling is required for erythropoietin-mediated acute protection against myocardial ischemia/reperfusion injury. Circulation 2004; 109: 2050– 2053 [DOI] [PubMed] [Google Scholar]
- 40.Piper HM, Abdallah Y, Schäfer C: The first minutes of reperfusion: a window of opportunity for cardioprotection. Cardiovasc Res 2004; 61: 365– 371 [DOI] [PubMed] [Google Scholar]
- 41.Lamarca V, Scorrano L: When separation means death: killing through the mitochondria, but starting from the endoplasmic reticulum. EMBO J 2009; 28: 1681– 1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Z, Zhang T, Dai H, Liu G, Wang H, Sun Y, Zhang Y, Ge Z: Endoplasmic reticulum stress is involved in myocardial apoptosis of streptozotocin-induced diabetic rats. J Endocrinol 2008; 196: 565– 572 [DOI] [PubMed] [Google Scholar]
- 43.Kersten JR, Schmeling TJ, Orth KG, Pagel PS, Warltier DC: Acute hyperglycemia abolishes ischemic preconditioning in vivo. Am J Physiol Heart Circ Physiol 1998; 275: H721– H725 [DOI] [PubMed] [Google Scholar]
- 44.Kersten JR, Montgomery MW, Ghassemi T, Gross ER, Toller WG, Pagel PS, Warltier DC: Diabetes and hyperglycemia impair activation of mitochondrial KATP channels. Am J Physiol Heart Circ Physiol 2001; 275: H721– H725 [DOI] [PubMed] [Google Scholar]
- 45.Huhn R, Heinen A, Weber NC, Hollmann MW, Schlack W, Preckel B: Hyperglycaemia blocks sevoflurane-induced postconditioning in the rat heart in vivo: cardioprotection can be restored by blocking the mitochondrial permeability transition pore. Br J Anaesth 2008; 100: 465– 471 [DOI] [PubMed] [Google Scholar]
- 46.Kehl F, Krolikowski JG, Mraovic B, Pagel PS, Warltier DC, Kersten JR: Hyperglycemia prevents isoflurane-induced preconditioning against myocardial infarction. Anesthesiology 2002; 96: 183– 188 [DOI] [PubMed] [Google Scholar]
- 47.Xu G, Takashi E, Kudo M, Ishiwata T, Naito Z: Contradictory effects of short- and long-term hyperglycemias on ischemic injury of myocardium via intracellular signaling pathway. Exp Mol Pathol 2004; 76: 57– 65 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.