Abstract
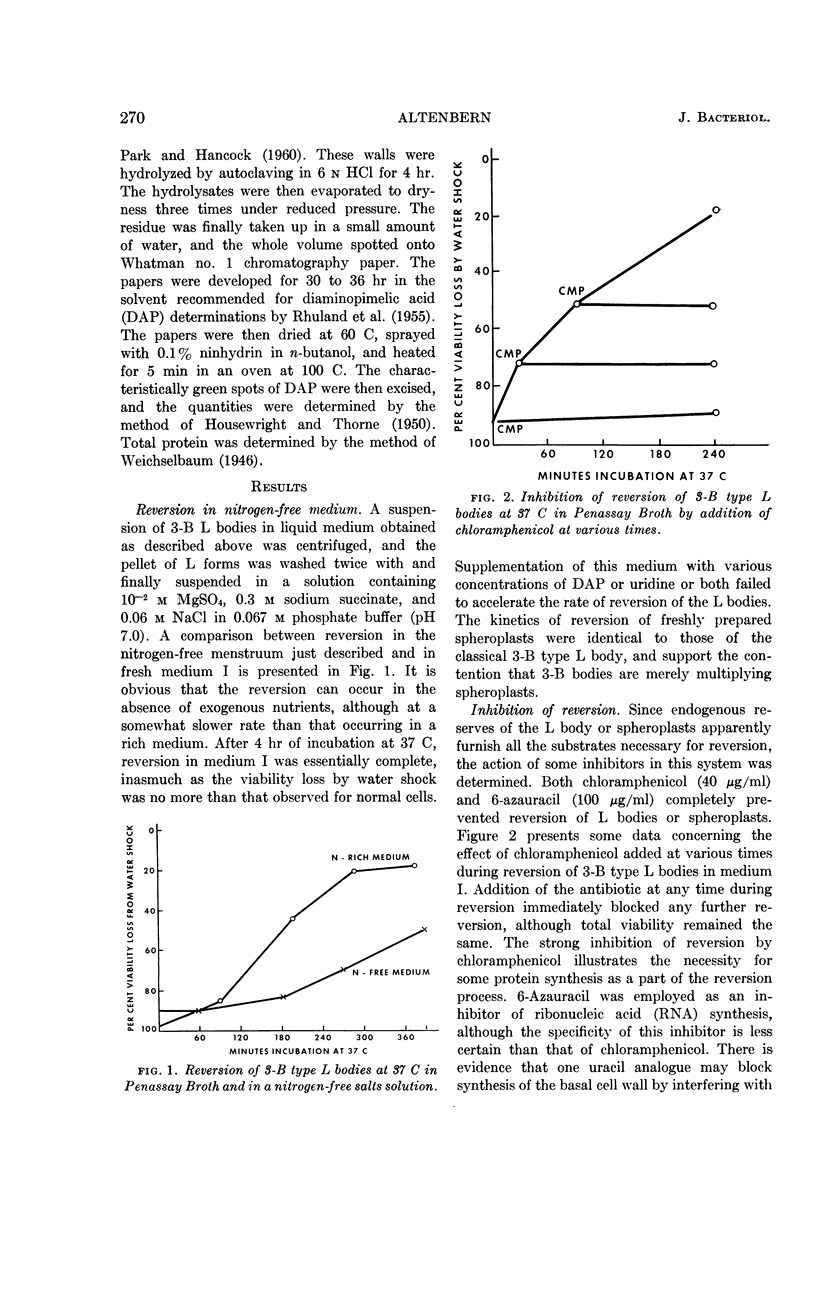
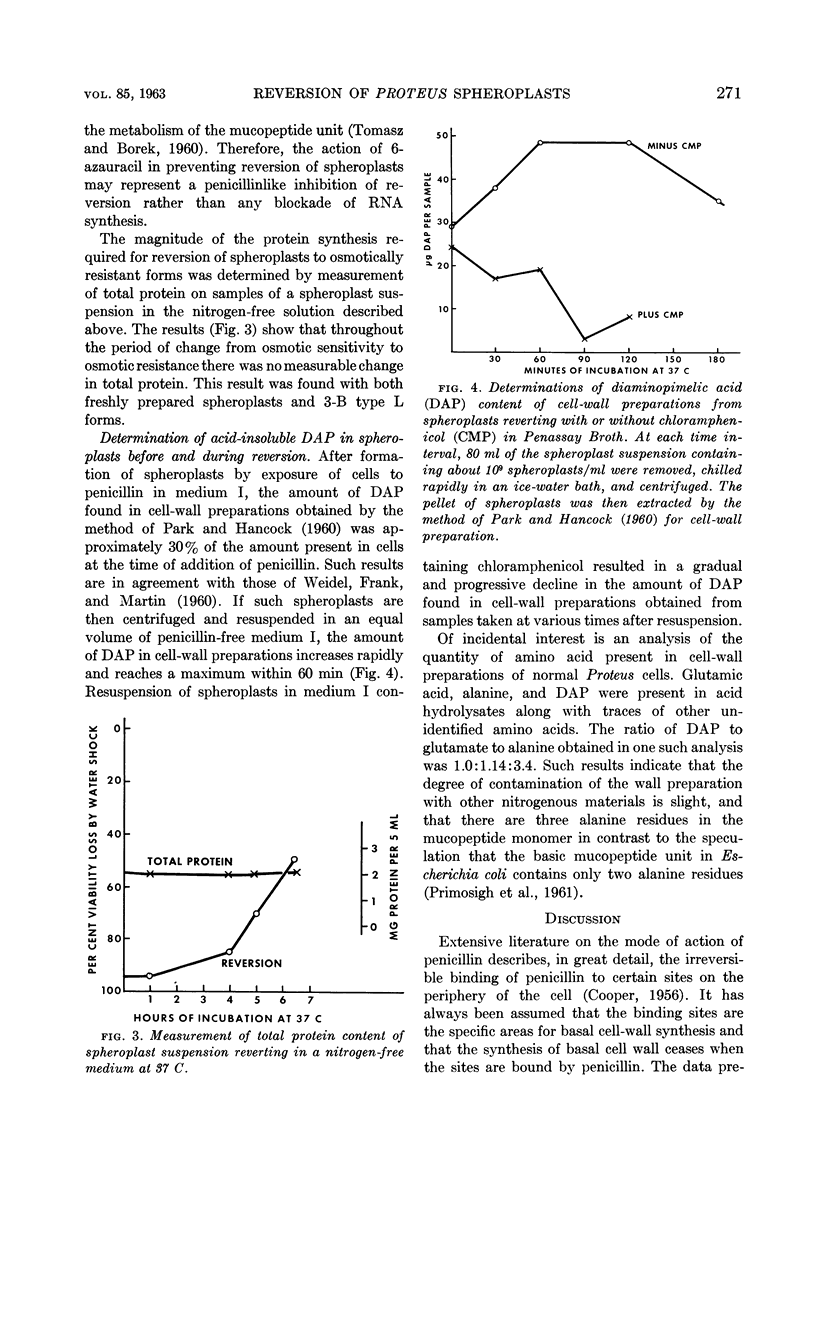
Altenbern, Robert A. (U.S. Army Chemical Corps, Fort Detrick, Frederick, Md.). Reversion of L forms and spheroplasts of Proteus mirabilis. J. Bacteriol. 85:269–272. 1963.—Spheroplasts or 3-B type L forms of Proteus mirabilis formed by growth in the presence of penicillin are able to revert to the bacillary form, as judged by regain of resistance to osmotic shock. Reversion takes place in the absence of exogenous nutrients, although at a lesser rate than that observed in a nitrogen-rich medium. Either chloramphenicol or 6-azauracil can completely inhibit reversion of spheroplasts in a penicillin-free medium. By direct measurement, there was no detectable change in total protein content throughout the period of reversion of a spheroplast suspension. Assay for acid-insoluble diaminopimelic acid (DAP) revealed that a large part of the DAP of the cell wall of normal cells was lost when the cells were converted to spheroplasts by exposure to penicillin. Upon transfer to penicillin-free medium, there was a rapid increase in acid-insoluble DAP in the wall of the reverting spheroplast. When spheroplasts were transferred to penicillin-free medium containing chloramphenicol, the acid-insoluble DAP in the wall of the spheroplast continued to decrease. The significance of these results in regard to sites of activity of the antibiotics involved is discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALTENBERN R. A., LANDMAN O. E. Growth of L-forms of Proteus mirabilis in liquid media. J Bacteriol. 1960 Apr;79:510–518. doi: 10.1128/jb.79.4.510-518.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALTENBERN R. A. Reversion of 3A type L forms of Proteus mirabilis. J Bacteriol. 1961 May;81:762–767. doi: 10.1128/jb.81.5.762-767.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOPER P. D. Site of action of radiopenicillin. Bacteriol Rev. 1956 Mar;20(1):28–48. doi: 10.1128/br.20.1.28-48.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIENES L., WEINBERGER H. J. The L forms of bacteria. Bacteriol Rev. 1951 Dec;15(4):245–288. doi: 10.1128/br.15.4.245-288.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANCOCK R., PARK J. T. Cell-wall synthesis by Staphylococcus aureus in the presence of chloramphenicol. Nature. 1958 Apr 12;181(4615):1050–1052. doi: 10.1038/1811050a0. [DOI] [PubMed] [Google Scholar]
- HOUSEWRIGHT R. D., THORNE C. B. Synthesis of glutamic acid and glutamyl polypeptide by Bacillus anthracis. I. Formation of glutamic acid by transamination. J Bacteriol. 1950 Jul;60(1):89–100. doi: 10.1128/jb.60.1.89-100.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEDERBERG J., ST CLAIR J. Protoplasts and L-type growth of Escherichia coli. J Bacteriol. 1958 Feb;75(2):143–160. doi: 10.1128/jb.75.2.143-160.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NATHENSON S. G., STROMINGER J. L. Effects of penicillin on the biosynthesis of the cell walls of Escherichia coli and Staphylococcus aureus. J Pharmacol Exp Ther. 1961 Jan;131:1–6. [PubMed] [Google Scholar]
- PARK J. T., HANCOCK R. A fractionation procedure for studies of the synthesis of cell-wall mucopeptide and of other polymers in cells of Staphylococcus aureus. J Gen Microbiol. 1960 Feb;22:249–258. doi: 10.1099/00221287-22-1-249. [DOI] [PubMed] [Google Scholar]
- PRIMOSIGH J., PELZER H., MAASS D., WEIDEL W. Chemical characterization of mucopeptides released from the E. coli B cell wall by enzymic action. Biochim Biophys Acta. 1961 Jan 1;46:68–80. doi: 10.1016/0006-3002(61)90647-3. [DOI] [PubMed] [Google Scholar]
- Tomasz A., Borek E. THE MECHANISM OF BACTERIAL FRAGILITY PRODUCED BY 5-FLUOROURACIL: THE ACCUMULATION OF CELL WALL PRECURSORS. Proc Natl Acad Sci U S A. 1960 Mar;46(3):324–327. doi: 10.1073/pnas.46.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDEL W., FRANK H., MARTIN H. H. The rigid layer of the cell wall of Escherichia coli strain B. J Gen Microbiol. 1960 Feb;22:158–166. doi: 10.1099/00221287-22-1-158. [DOI] [PubMed] [Google Scholar]
- Zinder N. D., Arndt W. F. PRODUCTION OF PROTOPLASTS OF ESCHERICHIA COLI BY LYSOZYME TREATMENT. Proc Natl Acad Sci U S A. 1956 Sep;42(9):586–590. doi: 10.1073/pnas.42.9.586. [DOI] [PMC free article] [PubMed] [Google Scholar]


