Abstract
Compound C is commonly used as an inhibitor of AMP-activated protein kinase (AMPK), which serves as a key energy sensor in cells. In this study, we found that Compound C treatment of MCF7 cells led to Bax redistribution from the cytoplasm to mitochondria and cell death. However, this effect does not involve AMPK. In addition, we found that treatment with this compound leads to an enhanced ceramide production. Analyses by quantitative PCR and ceramide synthase activity assay suggest that ceramide synthase 5 (LASS/CerS 5) is involved in Compound C-induced ceramide upregulation. Downregulation of LASS/CerS 5 was found to attenuate Compound C-mediated ceramide production, Bax redistribution, and cell death.
Keywords: AMP-activated protein kinase signaling pathway, sphingolipid, cell death
AMP-activated protein kinase (AMPK) serves as a key sensor in monitoring energy supply in cells, and it is activated by an increase in the ratio of AMP/ATP. Compounds including aminoimidazole-4-carboxamide riboside (AICAR) and phenformin have been widely used to activate AMPK. AICAR is an adenosine analog that is easily taken up by cells. It is then rapidly phosphorylated to form 5-aminoimidazole-4-carboxamide-1-d-ribofuranosyl-5′-monophosphate, which mimics the activating effects of AMP on AMPK (1). In contrast, the mechanism by which phenformin activates AMPK is still unclear (1). Once activated, AMPK plays a diverse role in cells by switching off energy-utilizing pathways and switching on energy-generating pathways (2, 3). Thus, AMPK promotes catabolic metabolism to enhance ATP synthesis and reduces anabolism to decrease ATP utilization. AMPK is also important in the normal development and functioning of the heart (4, 5). Recently it has been reported that activated AMPK is involved in attenuating stress-induced cell death (6).
The activity of AMPK could be inhibited by (6-[4-(2- piperidin-1-yl-ethoxy)-phenyl)]3-pyridin-4-yl-pyrazolo[1,5-a] pyrimidine, which is also known as Compound C (7). It was shown that this compound could antagonize AICAR by blocking the uptake of AICAR into cells (8). Moreover, Compound C was shown to prevent the inactivation of acetyl CoA carboxylase (ACC) following incubation with either AICAR or metformin (1).
The role of AMPK in apoptosis induction is unclear. It has been reported that cotreatment of AICAR with tert-butylhydroxyquinone (t-BHQ) resulted in the induction of apoptosis in H4IIE hepatocytes, as evidenced by changes in mitochondrial cytochrome c content, poly(ADP-ribose) polymerase cleavage, and caspase-3 activation (9). Application of Compound C has been shown to attenuate apoptosis, implying a role of AMPK in apoptosis induction (9). In contrast, PC12 cells treatment with Compound C resulted in enhanced susceptibility to cell death upon exposure to low glucose medium, serum withdrawal, or DNA replication inhibitors (6, 10).
Members of the Bcl-2 family play an important role in apoptosis regulation. Bax is a pro-apoptotic member of this family (11). It normally resides in the cytoplasm, but upon apoptosis induction, it redistributes to mitochondria (12), oligomerizes (13), and leads to mitochondrial outer membrane permeabilization and apoptosis (14, 15). A previous report showed that AICAR stimulated Bax translocation in isolated rat neonatal cardiomyocytes, suggesting that AMPK was involved in Bax redistribution to mitochondria (16).
Currently, the molecular mechanisms that regulate Bax translocation to mitochondria are unclear. It has been reported that elevation in the levels of ceramide as a result of apoptotic insults could trigger Bax redistribution from the cytoplasm to mitochondria (17–19). Ceramide is a bioactive lipid that plays an important role in a wide range of biological functions (20). It could be produced via the de novo pathway from palmitate and serine or from the salvage pathway via the acylation of sphingosine or the hydrolysis of sphingomyelin (21). Ceramide synthase [longevity assurance homolog (LASS/CerS)], participates in both pathways by converting sphinganine and fatty acyl-CoA into dihydroceramide and by converting sphingosine into ceramide. Currently, six isoforms of LASS/CerS have been identified in mammalian cells. Each isoform appears to be selective for the production of ceramide with different chain lengths: C18-ceramide for LASS/CerS 1; C22:0, C24:0, and C26:0-ceramide for LASS/CerS 2; C18:0 and C24:0-ceramide for LASS/CerS 3; C22:0 and C24:0-ceramide for LASS/CerS 4; C12:0, C14:0, C16:0, and C18:0-ceramide for LASS/CerS 5; and C12:0, C14:0, C16:0, and C18:0-ceramide for LASS/CerS 6 (22, 23).
In this study, we have identified a new cellular signaling pathway of Compound C. We show that this compound could trigger cell death and promote Bax redistribution to mitochondria in MCF7 breast carcinoma cells, independently of the AMPK pathway. In addition, we show that this compound mediates Bax activation by elevating ceramide production.
MATERIALS AND METHODS
Materials
MCF7 human breast carcinoma cells were obtained from ATCC. FBS, DMEM, tissue culture supplements, and propidium iodide were from Invitrogen. AICAR and Compound C were purchased from Calbiochem. Pan-caspase inhibitor zVAD-fmk was purchased from Axxora. Antibodies against AMPKα, ACC, phospho-AMPKα (pT172), and phospho-acetyl CoA carboxylase (pS79) were obtained from Cell Signaling. HRP-conjugated sheep anti-rabbit or mouse immunoglobulins and the ECL Western blot detection kit were from GE Healthcare. Human AMPKα1/2 small interfering RNA (siRNA; sc-45312) and control scrambled siRNA oligonucleotides were from Santa Cruz Biotechnology. LASS/CerS 5 and LASS/CerS 6 sequence-specific siRNA oligonucleotides, Hiperfect transfection reagent, and minikits for mRNA extraction were from Qiagen. The reverse transcriptase kit was from Promega. The iQ SYBR Green PCR kit was purchased from Bio-Rad. 17C-sphingosine was from Avanti Polar Lipids. All other chemicals were from either Fisher Scientific or Sigma-Aldrich.
Development of MCF7 cells stably expressing green fluorescent protein-Bax
MCF7 cells were cultured in DMEM supplemented with 2 mM glutamine and 10% FBS and maintained at 37°C in the presence of 5% CO2. To develop stable clones of MCF7 cells expressing green fluorescent protein (GFP)-Bax, MCF7 cells were seeded onto 10 cm plates and cultured in DMEM supplemented with 10% FBS. The cells were transfected with 12 μg pcDNA 3/EGFP-Bax with the FuGENE transfection reagent. The day after transfection, the cells were selected with 0.5 mg/ml G418. After a week of selection, the surviving cells were then trypsinized, serially diluted, and plated onto 96-well plates. Fluorescence microscopy was used for the screening of GFP-Bax stable clones. GFP-Bax stable MCF7 cells were maintained in the DMEM culture medium in the presence of 0.2 mg/ml G418. To generate GFP-Bax/Ds-red-mito stable cells, GFP-Bax stable MCF7 cells were transfected with the Ds-red-mito plasmid. After 2 weeks of growth, the cells were sorted by flow cytometry (carried out by the MUSC Flow Cytometry Facility) to select for GFP-Bax and Ds-red-mito stable cells.
MTT assay
Cell viability was determined using an in vitro toxicology assay kit (MTT-based; Sigma-Aldrich) according to the manufacturer's instructions. Briefly, MCF7 cells were seeded onto 6-well plates at a density of 6 × 105 cells/ml. After 24 h, the cells were incubated with different concentrations of Compound C for 24, 48, or 72 h. The IC50 of Compound C was determined from cell growth plots (24).
Apoptosis detection with Annexin V and Hoechst staining
MCF7 cells were seeded onto 6-well plates at a density of 1.2 × 106 cells/well. After treatment with different concentrations of Compound C for specified time periods, cells were trypsinized and washed twice with ice-cold PBS. The cells (1 × 106) were then labeled with Annexin V and propidium iodide as described by the manufacturer. The labeled cells were analyzed with flow cytometry using a FACStarplus flow cytometer (BD Biosciences) in the Flow Cytometry Facility at the Medical University of South Carolina.
To visualize apoptotic nuclei, GFP-Bax stable MCF7 cells were treated with Compound C for 48 h. The cells were then stained with Hoechst nuclear stain (10 µg/ml) and examined with an Olympus IX-70 fluorescence microscope by using an LCPlanFI ×20 objective lens. The images were captured with an Optronics DEI-750D digital imaging camera.
Bax translocation analysis
GFP-Bax stable MCF7 cells were plated onto 6-well plates. The cells were then treated with different concentrations of Compound C for specified times. The percentages of GFP-Bax punctate cells were determined by fluorescence microscopy as previously described (25).
Downregulation of AMPKα or LASS/CerS by siRNA oligonucleotides
Knockdown of human AMPKα, LASS/CerS 5, and LASS/CerS 6 mRNA levels was performed essentially as previously described (26). Briefly, GFP-Bax stable MCF7 cells were plated onto either 6-well (for Bax localization analysis) or 10 cm [for Western blotting, quantitative PCR (qPCR), or lipid analysis] plates. The cells were then transfected with control scrambled siRNA oligonucleotides or siRNA oligonucleotides against human AMPKα (10 nM) or LASS 5/LASS 6 (80 nM) using the Hiperfect transfection reagent. At 48–72 h posttransfection, the efficacy of gene silencing was assessed by Western blotting for AMPKα, and qPCR and lipid analyses for LASS/CerS 5 and LASS/CerS 6.
SDS-PAGE and Western blotting
GFP-Bax stable MCF7 cells transfected with siRNA oligonucleotides or treated with various compounds were collected and lysed in the lysis buffer (PBS, 1% Triton X-100, 0.1% SDS, and 25 μg/ml PMSF, 1:1,000 protease inhibitor cocktail, 5 mM sodium fluoride, and 1 mM sodium orthovanadate). Cell lysates were normalized to protein concentration using the Bio-Rad protein assay kit. SDS-PAGE (8–10%) and Western blotting analyses were performed as described previously (27). For immunoblotting analysis, the blots were probed with antibodies against AMPKα, β-actin, ACC, phospho-AMPKα, and phospho-acetyl CoA carboxylase. The ECL detection system was used to visualize labeled protein bands as described by the manufacturer.
Analyses of endogenous sphingolipids
Ceramide contents in MCF7 cells were determined as described previously (28, 29). Briefly, GFP-Bax stable MCF7 cells subjected to siRNA oligonucleotide transfection or Compound C treatment were collected, fortified with internal standards, and subjected to lipid extraction. Simultaneous ESI/MS/MS analyses of ceramides were performed with a Thermo Finnigan TSQ 7000 triple quadrupole mass spectrometer operating in a multiple reaction monitoring positive ionization mode. Results were normalized to total phospholipid contents and expressed as pmol ceramide/nmol phosphate or as a percentage of the control.
Ceramide synthase activity assay
GFP-Bax stable MCF7 cells were grown to 70–80% confluency and treated with Compound C. At indicated time points, cells were treated with 1 µM 17C-sphingosine for 30 min prior to harvest. Ceramide synthase activity was determined by measuring the accumulation of 17Cn-ceramide using a Thermo Finnigan TSQ 7000 triple quadrupole mass spectrometer operating in a multiple reaction monitoring positive ionization mode (30). Ceramide synthase activity was normalized to total phospholipid levels and expressed as pmol 17C-ceramide/nmol phosphate or as a percentage of the control.
Real-time qPCR analysis
The mRNA levels of LASSes/CerSes were measured by real-time qPCR as described previously (31, 32). Briefly, RNAs extracted from untransfected, siRNA oligonucleotide transfected, and Compound C-treated GFP-Bax stable MCF7 cells were used to produce cDNAs. The cDNA preparations were then used for qPCR analyses using the iQ SYBR Green PCR kit and an ABI 7300 Q-PCR system. The results were expressed either as a percentage of the control or as mean normalized expression (MNE), which represents the ratio of the target gene to β-actin reference gene.
Statistical analysis
All data represent the mean ± SEM. Experiments were repeated three times. Statistical analyses were performed using the Student's t-test or ANOVA for multiple comparisons. A P value of 0.05 or less is considered as statistically significant and marked with an asterisk.
RESULTS
Compound C inhibits cell growth and leads to apoptosis in MCF7 cells
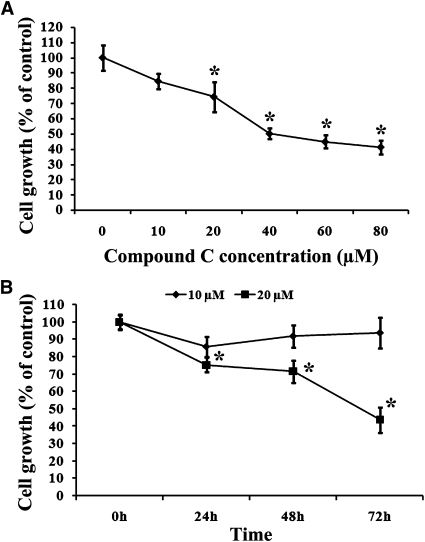
To assess the effect of Compound C on the growth of MCF7 breast cancer cells, these cells were subjected to different concentrations of Compound C (from 10–80 µM). At 24 h after treatment, the cells were analyzed by the MTT assay. As shown in Fig. 1A, increasing concentrations of Compound C enhanced the inhibition of cell growth. The IC50 of this compound was found to be 40 µM (Fig. 1A). We also monitored cell growth inhibition over time. As showed in Fig. 1B, cell growth inhibition was >50% after 72 h treatment with 20 µM Compound C.
Fig. 1.
Compound C inhibited MCF7 breast carcinoma cell growth in a dose- and time-dependent manner. MCF7 cells were plated onto 6-well plates and treated with different concentrations of Compound C (from 10–80 µM) for 24 h (A) or with 10 and 20 µM Compound C for various time periods (B). At given time points, MTT assays were performed. Values are means ± SEM. * P < 0.05, n = 3.
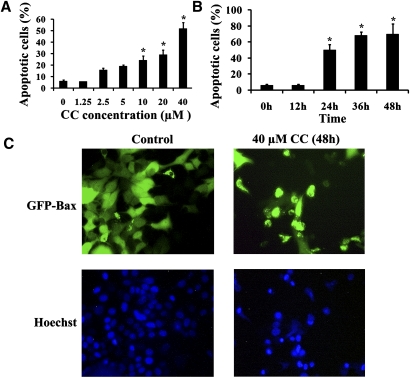
The treatment of MCF7 cells with Compound C led to the rounding up of a number of cells, indicative of cell death. To evaluate the Compound C-induced cell death, MCF7 cells were treated with different concentrations of the drug for 24 h and then stained with Annexin V. As shown in Fig. 2A, after 24 h of Compound C incubation (from 1.25–40 µM), the extent of cell death was dependent on the dose of Compound C, and it ranged from 4.53% to 47.8% (Fig. 2A). The level of cell death also increased over time (Fig. 2B). In addition, the treated cells were stained with the Hoechst dye and examined by fluorescence microscopy. As shown in Fig. 2C, whereas untreated cells maintained normal nuclear morphology, cells treated with 40 µM Compound C (48 h) had either condensed or fragmented nuclei, which is a common feature associated with apoptosis.
Fig. 2.
Compound C induced apoptosis in MCF7 cells. MCF7 cells were exposed to different concentrations of Compound C for 24 h (A) or 40 µM Compound C for different time periods (B). The cells were then labeled with Annexin V and propidium iodide and subjected to flow cytometry analysis. The percentages of apoptotic cells were determined. Data are means ± SEM. * P < 0.05, n = 3. C: GFP-Bax stable MCF7 cells were treated with 40 µM Compound C (CC) for 48 h. The cells were then stained with Hoechst nuclear stain (10 µg/ml) and analyzed by fluorescence microscopy to detect apoptotic nuclei.
Compound C induces Bax translocation to mitochondria
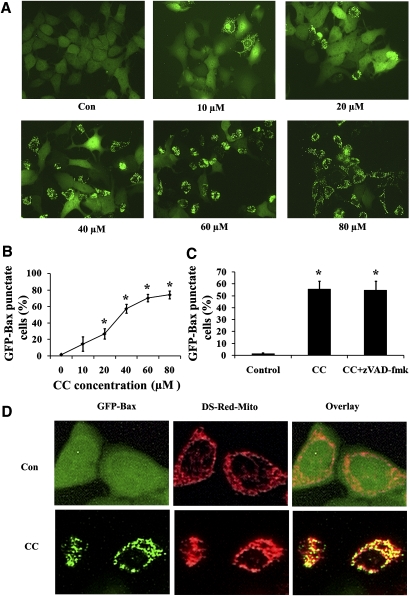
Since Bax translocation from the cytoplasm to mitochondria represents an important step in apoptosis, we examined the effect of Compound C on the subcellular distribution of this protein. We have generated an MCF7 cell line stably expressing GFP-Bax for a rapid assessment of Bax subcellular localization. Using this system, we have shown that apoptosis by a variety of apoptotic stimuli would trigger the redistribution of GFP-Bax in H9c2 and NT-2 cells (25, 31). In untreated MCF7 cells, almost all the cells display a diffuse, cytoplasmic GFP-Bax localization pattern (Fig. 3A). When these cells were subjected to Compound C treatment, there was a shift in the fluorescence pattern of GFP-Bax from a diffuse cytoplasmic state to a punctate membrane-bound state (Fig. 3A). The puctate Bax colocalizes with Ds-red-mito-tag-labeled mitochondria (Fig. 3D), confirming its mitochondrial localization. Compound C induced Bax redistribution in a concentration-dependent manner, as increased concentrations of this compound enhanced the percentages of cells having Bax localized to mitochondria (Fig. 3B). Interestingly, this translocation induced by Compound C was not blocked by the addition of the pan-caspase inhibitor zVAD-fmk (Fig. 3C), indicating that this translocation process was caspase independent.
Fig. 3.
Compound C induced Bax translocation from the cytoplasm to mitochondria in a caspase-independent pathway. A: GFP-Bax stable MCF7 cells were treated with different concentrations of Compound C for 24 h and then visualized by fluorescence microscopy. B: The percentages of GFP-Bax punctate cells were quantitated from four separate visual fields. Values are means ± SEM. * P < 0.05, n = 3. C: GFP-Bax stable MCF7 cells were treated with 40 µM Compound C for 24 h either in the absence or presence of 50 μM zVAD-fmk. The percentages of GFP-Bax punctate cells were visualized by fluorescence microscopy and quantitated from four separate visual fields. Values are means ± SEM. * P < 0.05, n = 3. D: GFP-Bax and Ds-red-mito stable MCF7 cells were treated either with or without 40 µM Compound C for 24 h. The cells were then visualized by fluorescence microscopy.
AMPK pathway is not involved in Compound C-induced Bax translocation
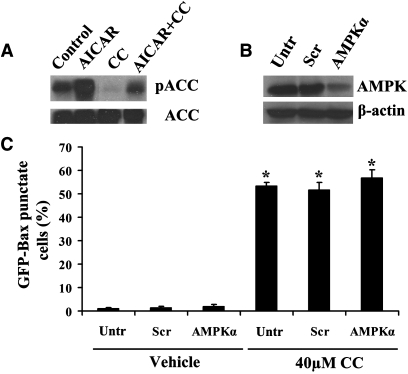
Compound C was originally identified as an inhibitor of AMPK. Thus, it is important to determine whether this enzyme is involved in Compound C-induced Bax translocation. In agreement with previous findings, we confirmed that Compound C could inhibit the basal phosphorylation and AICAR-enhanced phosphorylation levels (Ser-79) of ACC (Fig. 4A), a direct target of AMPK (33). This suggests that Compound C could directly inhibit the AMPK signaling pathway. Mammalian AMPK is a heterotrimeric complex comprising a catalytic α-subunit and regulatory β- and γ-subunits. To examine the role of AMPK in Compound C-induced Bax redistribution, GFP-Bax stable MCF7 cells were transfected with siRNA oligonucleotides against the α-subunit of AMPK to mimic the inhibition of this enzyme by Compound C. The downregulation of AMPKα was confirmed by Western blotting (Fig. 4B). As shown in Fig. 4C, downregulation of AMPKα alone had no effect on the cytoplasmic localization of GFP-Bax. Similarly, there was no significant change in percentages of cells (both control and AMPKα knockdown) having Bax localized to mitochondria upon Compound C addition. This result suggests that AMPK is not involved in Compound C-induced Bax redistribution and that other cellular signaling mechanisms may be involved.
Fig. 4.
AMPK is not involved in Compound C-induced Bax translocation. A: Cell lysates from GFP-Bax stable MCF7 cells treated with 1 mM AICAR, 40 µM compound C, or both for 24 h were subjected to Western blotting analysis with antibodies against ACC or phospho-acetyl CoA carboxylase (pSer79, pACC). B: GFP-Bax stable MCF7 cell lysates from untransfected (control) and scrambled control (Scr) or human AMPKα siRNA transfected cells were subjected to Western blotting analysis with anti-AMPKα and β-actin antibodies. C: GFP-Bax stable MCF7 cells were transfected with scrambled control (Scr) or siRNA oligonucleotides against human AMPKα. At 48 h after transfection, the cells were treated with 40 µM Compound C for 24 h. The percentages of GFP-Bax punctate cells were determined by fluorescence microscopy and quantitated from four separate visual fields. Values are means ± SEM. * P < 0.05, n = 3.
Compound C upregulates the level of ceramide in MCF7 cells
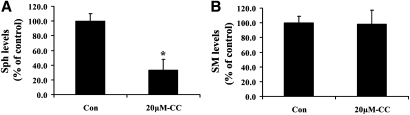
Physiological upregulation of ceramide has been implicated in cellular apoptosis (34–37). In order to determine whether ceramide is involved in Compound C-induced Bax translocation, the ceramide contents of the treated cells were determined. As shown in Table 1, treatment with 20 and 40 μM Compound C for 24 h significantly elevated the levels of C16-ceramide. On the other hand, exposure to 1 mM AICAR for 24 h had no effect on the ceramide levels (data not shown). In addition, we found that Compound C treatment led not only to ceramide elevation but also to a decrease in sphingosine (Fig. 5A). On the other hand, a change in the level of sphingomyelin was not observed (Fig. 5B), suggesting that sphingomyelin hydrolysis was unlikely to be involved in Compound C-induced ceramide elevation. As sphingosine is used by ceramide synthases (LASSes/CerSes) to produce ceramide, it implicates a possible involvement of these enzymes in Compound C-induced ceramide elevation.
TABLE 1.
Compound C elevates the level of ceramide in MCF7 cells
| Con | 20 µM CC | 40 µM CC | |
|---|---|---|---|
| 17C14:0 | 248 ± 5 | 228 ± 8 | 306 ± 17 |
| 17C16:0 | 6,100 ± 563 | 25,600 ± 1,890* | 34,300 ± 2,770* |
| 17C18:0 | 707 ± 71 | 2,270 ± 200* | 3,040 ± 247* |
| 17C18:1 | 72 ± 2 | 74 ± 7 | 99 ± 8 |
| 17C20:0 | 491 ± 22 | 485 ± 47 | 550 ± 54 |
| 17C20:1 | 231 ± 4 | 281 ± 15 | 277 ± 24 |
| 17C22:0 | 285 ± 20 | 213 ± 17 | 285 ± 24 |
| 17C22:1 | 838 ± 18 | 870 ± 63 | 965 ± 6 |
| 17C24:0 | 445 ± 48 | 400 ± 42 | 536 ± 49 |
| 17C24:1 | 1,590 ± 161 | 1,660 ± 90 | 1,930 ± 155 |
| 17C26:0 | 331 ± 30 | 360 ± 28 | 382 ± 37 |
| 17C26:1 | 1,090 ± 62 | 1,230 ± 116 | 1,350 ± 119 |
| Total | 12,400 ± 445 | 33,700 ± 2,400* | 44,100 ± 3,260* |
GFP-Bax stable MCF7 cells were treated with 20 or 40 µM Compound C for 24 h. The extracted lipids from collected cells were subjected to HPLC and tandem MS analyses to determine total ceramide and ceramide species content (pmol/µmol Pi). Values are means ± SEM; n = 3. *P < 0.05.
Fig. 5.
Compound C affects the level of sphingolipids in MCF7 cells. GFP-Bax stable MCF7 cells were treated with 20 µM Compound C for 24 h. The extracted lipids from collected cells were subjected to HPLC and tandem MS analyses to determine sphingosine (A) and sphingomyelin (B) levels. Treatment with Compound C lowered the level of sphingosine (from 108.3 ± 9.9 to 36.3 ± 4.8 pmol/µmol Pi), but there was no significant change in the level of total sphingomyelin (17744.7 ± 876.5 pmol/µmol Pi for untreated versus 17478.5 ± 947.2 pmol/µmol Pi for treated). The results are expressed as a percentage of the control untreated cells. Data are means ± SEM. * P < 0.05, n = 3.
LASS/CerS 5 is involved in Compound C-induced ceramide upregulation and Bax redistribution
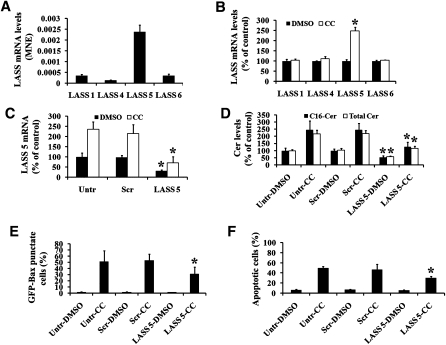
To determine the contributory role of ceramide synthase in Compound C-induced ceramide upregulation, ceramide synthase activity was first examined. GFP-Bax stable MCF7 cells were treated with Compound C for 24 h and then labeled with synthetic 17C-sphingosine. The incorporation of 17C-sphingosine into ceramide was determined by MS analysis. As shown in Table 2, Compound C treatment led to an increase in 17C-ceramide production, in particular 17C16-ceramide, followed by 17C18-ceramide. Analyses by qPCR indicated that LASS/CerS 5 was found in the greatest abundance in MCF7 cells, followed by LASS/CerS 6, 1, and 4 (Fig. 6A). Importantly, the mRNA level of LASS/CerS 5 was significantly elevated upon Compound C treatment, while those of LASS/CerS 1, LASS/CerS 4, and LASS/CerS 6 remained relatively the same (Fig. 6B). This is in agreement with the ceramide synthase activity assay result, as C16- and C18-ceramides are preferentially formed by LASS/CerS 5.
TABLE 2.
Compound C triggers the upregulation of ceramide synthase activity in MCF7 cells
| Con | 20 µM CC | 40 µM CC | |
|---|---|---|---|
| C14:0 | 18 ± 2 | 17 ± 2 | 20 ± 3 |
| C16:0 | 774 ± 53 | 1,270 ± 112* | 1,910 ± 107* |
| C18:0 | 4 ± 0.5 | 22 ± 0.8* | 34 ± 3* |
| C18:1 | 19 ± 1 | 19 ± 2 | 23 ± 2 |
| C20:0 | 9 ± 0.6 | 10 ± 0.8 | 13 ± 1 |
| C20:1 | 2 ± 0.1 | 2 ± 0.1 | 2 ± 0.2 |
| C22:0 | 24 ± 2 | 23 ± 3 | 25 ± 3 |
| C22:1 | 12 ± 0.7 | 12 ± 1 | 14 ± 0.5 |
| C24:0 | 298 ± 28 | 287 ± 21 | 325 ± 31 |
| C24:1 | 223 ± 1 | 307 ± 22 | 448 ± 34* |
| C26:0 | 7 ± 0.3 | 7 ± 0.6 | 8 ± 0.9 |
| C26:1 | 20 ± 0.7 | 21 ± 3 | 24 ± 3 |
| Total | 1,410 ± 66 | 1,990 ± 94* | 2,840 ± 143* |
GFP-Bax stable MCF7 cells were treated with 20 or 40 µM Compound C (CC) for 24 h. The cells were then labeled with 1 µM 17C-sphingosine, and ceramide synthase activities (pmol 17C-ceramide/µmol phosphate) of the collected cells were determined by tandem MS. Values are means ± SEM; n = 3. *P < 0.05.
Fig. 6.
LASS/CerS 5 is involved in Compound C-mediated ceramide accumulation and Bax translocation. A: The mRNA expression profiles of various LASSes/CerSes in MCF7 cells were determined by qPCR. B: GFP-Bax stable MCF7 cells were treated with 40 µM Compound C for 24 h. The mRNA expression profiles of various LASSes/CerSes in MCF7 cells were determined by qPCR and expressed as a percentage of the untreated cells. C: GFP-Bax stable MCF7 cells were transfected with scrambled (Scr) or LASS/CerS 5 siRNA oligonucleotides. Untransfected (Untr) and transfected cells were then treated with either DMSO carrier or 40 µM Compound C for 24 h (same conditions for D–F). Subsequently, LASS/CerS 5 mRNA expression levels in these cells were determined by qPCR and expressed as a percentage of the control untransfected and untreated cells. D: The levels of C16-ceramide and total ceramide in cells were measured by tandem MS and expressed as a percentage of the untransfected and untreated cells. E: The percentages of GFP-Bax punctate cells were quantitated from four separate visual fields. F: The percentages of apoptotic cells were determined from untransfected or siRNA oligonucleotide transfected cells treated either with DMSO carrier or 40 µM Compound C for 24 h. Data are means ± SEM. * P < 0.05, n = 3.
In order to study the role of LASS/CerS 5 in Compound C-mediated ceramide upregulation and Bax redistribution, siRNA oligonucleotides specific for LASS/CerS 5 were transfected into MCF7 cells. As shown in Fig. 6C, LASS/CerS 5 siRNA transfected cells displayed a lower basal LASS/CerS 5 mRNA level compared with untransfected and scrambled siRNA oligonucleotide transfected cells. Similarly, in LASS/CerS 5 siRNA oligonucleotide transfected cells, the Compound C-induced elevation of LASS/CerS 5 mRNA level was attenuated. In addition, LASS/CerS 5 knockdown decreased Compound C-induced C16 and total ceramide accumulation (Fig. 6D). Finally, LASS/CerS 5 knockdown had no effect on the subcellular distribution of Bax in untreated cells. However, Compound C-mediated Bax redistribution to mitochondria was partially attenuated in LASS/CerS 5 siRNA transfected cells (Fig. 6E). Similarly, the level of apoptosis induced by Compound C was reduced by LASS/CerS 5 knockdown (Fig. 6F). These results suggest that LASS/CerS 5 plays a role in mediating Compound C-induced ceramide upregulation, Bax translocation to mitochondria, and cell death. The partial inhibition of Bax redistribution and cell death by the knockdown of LASS/CerS 5 suggests that additional signaling mechanisms may be involved in Compound C-mediated cytotoxicity.
DISCUSSION
Compound C has been used primarily as an inhibitor of AMPK in a number of cellular systems (38–40). It has also been shown to inhibit ACC inactivation by AICAR in cultured rat hepatocytes (7). Here, we have identified a new cellular effect of Compound C. We show that Compound C could lead to ceramide accumulation and apoptotic cell death in MCF7 breast carcinoma cells. In addition, at concentrations generally used for AMPK inhibition, Compound C was shown to upregulate ceramide synthase LASS/CerS 5 activity. Moreover, this compound could trigger the translocation of the pro-apoptotic protein Bax from the cytoplasm to mitochondria. Lastly, we show that LASS/CerS 5 plays a role in Compound C-mediated cytotoxicity, as downregulation of LASS/CerS 5 would decrease Compound C-induced ceramide upregulation and attenuate Bax translocation to mitochondria and cell death.
Some earlier reports have shown that inhibition of AMPK by Compound C could reverse AICAR- or metformin/t-BHQ-induced apoptosis (9, 41, 42). On the other hand, another report suggested that Compound C could enhance apoptosis and cell death induced by some apoptotic stimuli (6, 10). In this study, we show that Compound C alone could induce cell death via upregulating ceramide production and inducing Bax redistribution to mitochondria.
Ceramide is a signaling molecule that is involved in cellular growth, differentiation, and apoptosis (43). Ceramide can be generated from both the salvage and de novo synthetic pathways. The salvage pathway involves either the hydrolysis of sphingomyelin by sphingomyelinases or acylation of sphingosine via ceramide synthase. In our study, we found that in MCF7 cells, Compound C led to a decrease in the sphingosine content without affecting the sphingomyelin level. This suggests that ceramide synthase, rather than sphingomyelinase, is involved in Compound C-mediated ceramide upregulation. Consistently, addition of Fumonisin B1, an inhibitor of ceramide synthase, would attenuate Compound C-induced ceramide elevation (data not shown). However, the de novo synthesis of ceramide does not appear to play a role in ceramide elevation, as addition of myriocin, an inhibitor of serine palmitoyl transferase, could not block this ceramide elevation (data not shown).
Currently, six mammalian LASS/CerS homologs have been identified, with each displaying a unique substrate specificity for fatty acyl CoAs of particular chain lengths and/or saturation (22). In this study, ceramide subspecies analysis revealed that C16-ceramide was increased the most. Similarly, LASS/CerS activity assay indicated that 17C16-ceramide was the predominant ceramide species synthesized in MCF7 cells exposed to Compound C. Analysis by qPCR indicated that LASS/CerS 5 was the major ceramide synthase isoform in MCF7 cell. Upon Compound C treatment, it was observed that LASS/CerS 5 mRNA level was increased significantly. This elevated level of LASS/CerS 5 mRNA likely results in the enhanced production of the enzyme and could account for the observed increase in ceramide synthase activity. Consistently, downregulation of LASS/CerS 5 by siRNA oligonucleotide transfection reduced the Compound C-induced ceramide accumulation, especially C16-ceramide. Even though LASS/CerS 6 shared similar substrate specificity as LASS/CerS 5, LASS/CerS 6 mRNA was present in a significantly lower level than LASS/CerS 5, and inhibition of LASS/CerS 6 by siRNA oligonucleotides had no effect on Compound C-mediated ceramide elevation (data not shown).
The cytoplasmic protein Bax plays a key role in apoptosis by translocating from the cytoplasm to mitochondria and releasing apoptogenic factors from the mitochondrial intermembrane space (16). The AMPK pathway has been implicated in Bax activation in isolated rat neonatal cardiomyocytes, as AMPK activator, AICAR, could induce Bax translocation to mitochondria (16). This translocation was shown to be dependent on p38 mitogen-activated protein kinase (MAPK), as SB203580, an inhibitor of p38MAPK, was reported to inhibit Bax redistribution (16). In MCF7 cells, we have confirmed that AICAR could induce Bax redistribution (data not shown). But to our surprise, we found that Compound C could also promote Bax translocation and apoptosis, independently of AMPK. In contrast to AICAR, p38MAPK was not involved in this process since SB203580 could not inhibit the effect of Compound C (data not shown). In support of the pleiotropic cellular effect of Compound C is a recent report that showed that Compound C inhibited hypoxia-induced activation of hypoxia inducible factor-1, independently of AMPK (44). Thus, as Compound C is used as a pharmacologic inhibitor for AMPK, care should be taken when evaluating the involvement of AMPK in a particular cellular process.
Ceramide elevation resulted from apoptotic insults have been shown to promote Bax redistribution from the cytoplasm to mitochondria. In HL-60 cells, addition of exogenous C2- and C6-ceramide was reported to enhance Bax localization to mitochondria (17). Acid sphingomyelinase has been implicated in UV irradiation-induced ceramide upregulation and Bax redistribution (18, 45). More recently, we have shown that acid sphingomyelinase and LASS/CerS 5 are involved in hypoxia/reoxygenation-induced ceramide accumulation, Bax redistribution, and cell death in NT-2 neuronal precursor cells (31). In this report, we further showed that LASS/CerS 5 plays an important role in Compound C-induced ceramide upregulation, Bax activation, and cell death, as downregulation of LASS/CerS 5 would attenuate these processes.
In conclusion, we have identified a new physiological function of Compound C. We have shown that Compound C would lead to ceramide accumulation through LASS/CerS 5 of the salvage pathway. This in effect leads to Bax redistribution to mitochondria and cell death. In the future, it would be interesting to determine how Compound C treatment leads to the transcriptional activation of LASS/CerS 5.
Footnotes
Abbreviations:
- ACC
- acetyl CoA carboxylase
- AICAR
- aminoimidazole-4-carboxamide riboside
- AMPK
- AMP-activated protein kinase
- GFP
- green fluorescent protein
- LASS/CerS
- longevity assurance homologue/ceramide synthase
- MAPK
- mitogen-activated protein kinase
- qPCR
- quantitative polymerase chain reaction
- siRNA
- small interfering RNA
- t-BHQ
- tert-butylhydroxyquinone
This research was supported in part by National Institutes of Health Grant NS40932 (to Y-T.H.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. Special acknowledgment is given for the National Institutes of Health Grant NCRR-C06RR018823 in providing laboratory space for lipidomics shared resource in the Children's Research Institute.
REFERENCES
- 1.King T. D., Song L., Jope R. S. 2006. AMP-activated protein kinase (AMPK) activating agents cause dephosphorylation of Akt and glycogen synthase kinase-3. Biochem. Pharmacol. 71: 1637–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hardie D. G., Carling D., Carlson M. 1998. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu. Rev. Biochem. 67: 821–855 [DOI] [PubMed] [Google Scholar]
- 3.Kemp B. E., Mitchelhill K. I., Stapleton D., Michell B. J., Chen Z. P., Witters L. A. 1999. Dealing with energy demand: the AMP-activated protein kinase. Trends Biochem. Sci. 24: 22–25 [DOI] [PubMed] [Google Scholar]
- 4.Blair E., Redwood C., Ashrafian H., Oliveira M., Broxholme J., Kerr B., Salmon A., Ostman-Smith I., Watkins H. 2001. Mutations in the gamma (2) subunit of AMP-activated protein kinase cause familial hypertrophic cardiomyopathy: evidence for the central role of energy compromise in disease pathogenesis. Hum. Mol. Genet. 10: 1215–1220 [DOI] [PubMed] [Google Scholar]
- 5.Gollob M. H., Green M. S., Tang A. S., Gollob T., Karibe A., Ali Hassan A. S., Ahmad F., Lozado R., Shah G., Fananapazir L., et al. 2001. Identification of a gene responsible for familial Wolff-Parkinson-White syndrome. N. Engl. J. Med. 344: 1823–1831 [DOI] [PubMed] [Google Scholar]
- 6.Shaw M. M., Gurr W. K., McCrimmon R. J., Schorderet D. F., Sherwin R. S. 2007. 5′AMP-activated protein kinase alpha deficiency enhances stress-induced apoptosis in BHK and PC12 cells. J. Cell. Mol. Med. 11: 286–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou G., Myers R., Li Y., Chen Y., Shen X., Fenyk-Melody J., Wu M., Ventre J., Doebber T., Fujii N., et al. 2001. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 108: 1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fryer L. G., Parbu-Patel A., Carling D. 2002. Protein kinase inhibitors block the stimulation of the AMP-activated protein kinase by 5-amino-4-imidazolecarboxamide riboside. FEBS Lett. 531: 189–192 [DOI] [PubMed] [Google Scholar]
- 9.Bae E. J., Cho M. J., Kim S. G. 2007. Metformin prevents an adaptive increase in GSH and induces apoptosis under the conditions of GSH deficiency in H4IIE cells. J. Toxicol. Environ. Health A. 70: 1371–1380 [DOI] [PubMed] [Google Scholar]
- 10.Niesler C. U., Myburgh K. H., Moore F. 2007. The changing AMPK expression profile in differentiating mouse skeletal muscle myoblast cells helps confer increasing resistance to apoptosis. Exp. Physiol. 92: 207–217 [DOI] [PubMed] [Google Scholar]
- 11.Adams J. M., Cory S. 1998. The Bcl-2 protein family: arbiters of cell survival. Science. 281: 1322–1326 [DOI] [PubMed] [Google Scholar]
- 12.Hsu Y. T., Wolter K. G., Youle R. J. 1997. Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proc. Natl. Acad. Sci. USA. 94: 3668–3672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eskes R., Desagher S., Antonsson B., Martinou J. C. 2000. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol. Cell. Biol. 20: 929–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finucane D. M., Bossy-Wetzel E., Waterhouse N. J., Cotter T. G., Green D. R. 1999. Bax-induced caspase activation and apoptosis via cytochrome c release from mitochondria is inhibitable by Bcl-xL. J. Biol. Chem. 274: 2225–2233 [DOI] [PubMed] [Google Scholar]
- 15.Smaili S. S., Hsu Y. T., Sanders K. M., Russell J. T., Youle R. J. 2001. Bax translocation to mitochondria subsequent to a rapid loss of mitochondrial membrane potential. Cell Death Differ. 8: 909–920 [DOI] [PubMed] [Google Scholar]
- 16.Capano M., Crompton M. 2006. Bax translocates to mitochondria of heart cells during simulated ischaemia: involvement of AMP-activated and p38 mitogen-activated protein kinases. Biochem. J. 395: 57–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim H. J., Mun J. Y., Chun Y. J., Choi K. H., Kim M. Y. 2001. Bax-dependent apoptosis induced by ceramide in HL-60 cells. FEBS Lett. 505: 264–268 [DOI] [PubMed] [Google Scholar]
- 18.Kashkar H., Wiegmann K., Yazdanpanah B., Haubert D., Kronke M. 2005. Acid sphingomyelinase is indispensable for UV light-induced Bax conformational change at the mitochondrial membrane. J. Biol. Chem. 280: 20804–20813 [DOI] [PubMed] [Google Scholar]
- 19.Birbes H., Luberto C., Hsu Y. T., El Bawab S., Hannun Y. A., Obeid L. M. 2005. A mitochondrial pool of sphingomyelin is involved in TNFalpha-induced Bax translocation to mitochondria. Biochem. J. 386: 445–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hannun Y. A., Obeid L. M. 2008. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 9: 139–150 [DOI] [PubMed] [Google Scholar]
- 21.Kitatani K., Idkowiak-Baldys J., Hannun Y. A. 2008. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell. Signal. 20: 1010–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mizutani Y., Kihara A., Igarashi Y. 2005. Mammalian Lass6 and its related family members regulate synthesis of specific ceramides. Biochem. J. 390: 263–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spassieva S., Seo J. G., Jiang J. C., Bielawski J., Alvarez-Vasquez F., Jazwinski S. M., Hannun Y. A., Obeid L. M. 2006. Necessary role for the Lag1p motif in (dihydro)ceramide synthase activity. J. Biol. Chem. 281: 33931–33938 [DOI] [PubMed] [Google Scholar]
- 24.Rossi M. J., Sundararaj K., Koybasi S., Phillips M. S., Szulc Z. M., Bielawska A., Day T. A., Obeid L. M., Hannun Y. A., Ogretmen B. 2005. Inhibition of growth and telomerase activity by novel cationic ceramide analogs with high solubility in human head and neck squamous cell carcinoma cells. Otolaryngol. Head Neck Surg. 132: 55–62 [DOI] [PubMed] [Google Scholar]
- 25.Hou Q., Hsu Y. T. 2005. Bax translocates from cytosol to mitochondria in cardiac cells during apoptosis: development of a GFP-Bax-stable H9c2 cell line for apoptosis analysis. Am. J. Physiol. Heart Circ. Physiol. 289: H477–H487 [DOI] [PubMed] [Google Scholar]
- 26.Zeidan Y. H., Pettus B. J., Elojeimy S., Taha T., Obeid L. M., Kawamori T., Norris J. S., Hannun Y. A. 2006. Acid ceramidase but not acid sphingomyelinase is required for tumor necrosis factor-{alpha}-induced PGE2 production. J. Biol. Chem. 281: 24695–24703 [DOI] [PubMed] [Google Scholar]
- 27.Hsu Y. T., Youle R. J. 1997. Nonionic detergents induce dimerization among members of the Bcl-2 family. J. Biol. Chem. 272: 13829–13834 [DOI] [PubMed] [Google Scholar]
- 28.Yu J., Novgorodov S. A., Chudakova D., Zhu H., Bielawska A., Bielawski J., Obeid L. M., Kindy M. S., Gudz T. I. 2007. JNK3 signaling pathway activates ceramide synthase leading to mitochondrial dysfunction. J. Biol. Chem. 282: 25940–25949 [DOI] [PubMed] [Google Scholar]
- 29.Bielawski J., Szulc Z. M., Hannun Y. A., Bielawska A. 2006. Simultaneous quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods. 39: 82–91 [DOI] [PubMed] [Google Scholar]
- 30.Schulz A., Mousallem T., Venkataramani M., Persaud-Sawin D. A., Zucker A., Luberto C., Bielawska A., Bielawski J., Holthuis J. C., Jazwinski S. M., et al. 2006. The CLN9 protein, a regulator of dihydroceramide synthase. J. Biol. Chem. 281: 2784–2794 [DOI] [PubMed] [Google Scholar]
- 31.Jin J., Hou Q., Mullen T. D., Zeidan Y. H., Bielawski J., Kraveka J. M., Bielawska A., Obeid L. M., Hannun Y. A., Hsu Y. T. 2008. Ceramide generated by sphingomyelin hydrolysis and the salvage pathway is involved in hypoxia/reoxygenation-induced Bax redistribution to mitochondria in NT-2 cells. J. Biol. Chem. 283: 26509–26517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu R., Jin J., Hu W., Sun W., Bielawski J., Szulc Z., Taha T., Obeid L. M., Mao C. 2006. Golgi alkaline ceramidase regulates cell proliferation and survival by controlling levels of sphingosine and S1P. FASEB J. 20: 1813–1825 [DOI] [PubMed] [Google Scholar]
- 33.Ha J., Daniel S., Kong I. S., Park C. K., Tae H. J., Kim K. H. 1994. Cloning of human acetyl-CoA carboxylase cDNA. Eur. J. Biochem. 219: 297–306 [DOI] [PubMed] [Google Scholar]
- 34.Garzotto M., White-Jones M., Jiang Y., Ehleiter D., Liao W. C., Haimovitz-Friedman A., Fuks Z., Kolesnick R. 1998. 12-O-tetradecanoylphorbol-13-acetate-induced apoptosis in LNCaP cells is mediated through ceramide synthase. Cancer Res. 58: 2260–2264 [PubMed] [Google Scholar]
- 35.Pastorino J. G., Tafani M., Rothman R. J., Marcinkeviciute A., Hoek J. B., Farber J. L. 1999. Functional consequences of the sustained or transient activation by Bax of the mitochondrial permeability transition pore. J. Biol. Chem. 274: 31734–31739 [DOI] [PubMed] [Google Scholar]
- 36.Von Haefen C., Wieder T., Gillissen B., Starck L., Graupner V., Dorken B., Daniel P. T. 2002. Ceramide induces mitochondrial activation and apoptosis via a Bax-dependent pathway in human carcinoma cells. Oncogene. 21: 4009–4019 [DOI] [PubMed] [Google Scholar]
- 37.Kolesnick R., Fuks Z. 2003. Radiation and ceramide-induced apoptosis. Oncogene. 22: 5897–5906 [DOI] [PubMed] [Google Scholar]
- 38.Lee Y. M., Lee J. O., Jung J. H., Kim J. H., Park S. H., Park J. M., Kim E. K., Suh P. G., Kim H. S. 2008. Retinoic acid leads to cytoskeletal rearrangement through AMPK-Rac1 and stimulates glucose uptake through AMPK-p38 MAPK in skeletal muscle cells. J. Biol. Chem. 283: 33969–33974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Egawa M., Kudo Y., Kamata H., Kushiyama A., Sakoda H., Fujishiro M., Horike N., Yoneda M., Nakatsu Y., Ying G., et al. 2008. Long-term Forskolin stimulation induces AMPK activation and thereby enhances tight junction formation in human placental trophoblast BeWo cells. Placenta. 29: 1003–1008 [DOI] [PubMed] [Google Scholar]
- 40.Kayampilly P. P., Menon K. M. 2009. Follicle stimulating hormone inhibits AMPK activation and promotes cell proliferation of primary granulosa cells in culture through an Akt dependent pathway. Endocrinology. 150: 929–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakatsu Y., Kotake Y., Hino A., Ohta S. 2008. Activation of AMP-activated protein kinase by tributyltin induces neuronal cell death. Toxicol. Appl. Pharmacol. 230: 358–363 [DOI] [PubMed] [Google Scholar]
- 42.Kim J. E., Ahn M. W., Baek S. H., Lee I. K., Kim Y. W., Kim J. Y., Dan J. M., Park S. Y. 2008. AMPK activator, AICAR, inhibits palmitate-induced apoptosis in osteoblast. Bone. 43: 394–404 [DOI] [PubMed] [Google Scholar]
- 43.Ruvolo P. P.2003. Intracellular signal transduction pathways activated by ceramide and its metabolites. Pharmacol. Res. 47: 383–392 [DOI] [PubMed] [Google Scholar]
- 44.Emerling B. M., Viollet B., Tormos K. V., Chandel N. S. 2007. Compound C inhibits hypoxic activation of HIF-1 independent of AMPK. FEBS Lett. 581: 5727–5731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeidan Y. H., Wu B. X., Jenkins R. W., Obeid L. M., Hannun Y. A. 2008. A novel role for protein kinase Cdelta-mediated phosphorylation of acid sphingomyelinase in UV light-induced mitochondrial injury. FASEB J. 22: 183–193 [DOI] [PubMed] [Google Scholar]