Abstract
Pregnenolone (PREG) and dehydroepiandrosterone (DHEA), and their respective sulfated forms PREGS and DHEAS, were among the first steroids to be identified in rodent brain. However, unreliable steroid isolation and solvolysis procedures resulted in errors, particularly in the case of brain steroid sulfates analyzed by radioimmunology or GC-MS of liberated free steroids. By using a solid-phase extraction recycling/elution procedure, allowing the strict separation of sulfated, free, and fatty acid esters of PREG and DHEA, PREGS and DHEAS, unlike free PREG, were not detected in rat and mouse brain and plasma. Conversely, considerable amounts of PREG and DHEA were released from unknown precursor(s) present in the lipoidal fraction, distinct from fatty acid ester conjugates. Chromatographic and mass spectrometric studies of the nature of the precursor(s) showed that autoxidation of brain cholesterol (CHOL) was responsible for the release of PREG and DHEA from the lipoidal fraction. When inappropriate protocols were used, CHOL was also the precursor of PREG and DHEA obtained from the fraction assumed to contain sulfated steroids. In contrast, free PREG was definitely confirmed as an endogenous steroid in rat brain. Our study shows that an early removal of CHOL from brain extracts coupled to well-validated extraction and fractionation procedures are prerequisites for reliable measurements of free and conjugated PREG and DHEA by GC-MS or other indirect methods.
Keywords: sample preparation, gas chromatography-mass spectrometry, conjugated steroids, analytical artifacts
The availability of reliable methods for analysis of steroids is essential for gaining an understanding of the local modulation of neurosteroidogenesis in pathophysiological contexts and in neurodegenerative diseases. We have established a method based on solid-phase extraction (SPE), HPLC, derivatization, and GC-MS for screening of unconjugated neurosteroids and/or neuroactive steroids and their precursors and metabolites in small regions of the central nervous system and plasma (1, 2). Our method has been applied to studies of the steroidogenic profile in Alzheimer's and nondemented patients (3), the modulation of steroid biosynthesis after spinal cord and traumatic brain injury in rats (4, 5), and steroid profiles of the nematode Caenorhabditis elegans (6).
While the majority of studies concerning identification and quantification of free steroids in the central nervous system have given consistent results in terms of reproducibility, accuracy, and reliability, the situation is less clear for conjugated steroids, such as sulfated pregnenolone (PREGS) and dehydroepiandrosterone (DHEAS) (7). These steroids were among the first to be determined in rodent brain (8, 9), and their presence did not seem to depend on steroidogenic gland secretion. Many articles have described neuromodulatory and neuropharmacological effects of the two steroid sulfates (10–12), in general qualifying them as excitatory steroids. They have mainly promnesic (13) and neuroprotective effects (14) but can be harmful under particular pathophysiological conditions (15). However, the presence and the local synthesis of steroid sulfates was called into question when HPLC/tandem mass spectrometry and immunoassays showed that the levels of PREGS and DHEAS (analyzed as intact conjugates) in rodent brain were close to or below the detection limit (<0.3 ng/g) (16–18). Using a newly developed SPE procedure (2), we were also unable to detect PREGS and DHEAS in rat and mouse brain and plasma. Surprisingly, considerable amounts of pregnenolone (PREG) and dehydroepiandrosterone (DHEA) were released by treating the brain and plasma SPE lipoidal fractions with heptafluorobutyric anhydride (HFBA) and triethylamine (TEA). Preliminary data indicated that PREG and DHEA were not released from fatty acid esters or sulfolipid conjugates. Lieberman's group had previously suggested that sterol peroxides and/or hydroperoxides, named neurosteroid precursors (19), were a source of PREG and DHEA, and this hypothesis could not be discarded. Indeed, the yields of released PREG and DHEA were heat and light dependent, and the precursor(s) was less polar than free PREG and DHEA. We also speculated about noncovalent associations between the steroids and lipoproteins, ion pairs of steroid sulfates with nonpolar cationic lipids, and nonpolar groups covalently bound to the steroids at C-3, C-17, or C-20.
The aim of this study was to determine the nature of the precursor(s) of PREG and DHEA in the lipoidal fraction and to explain the inconsistencies in analyses of PREGS and DHEAS. The study pinpoints cholesterol (CHOL) as the source of both the lipoidal and the sulfated forms of the steroids.
EXPERIMENTAL PROCEDURES
Chemicals
Radioactive steroids, 3H-PREG ([7-3H]PREG, 25 Ci/mmol) and 3H-CHOL ([1α, 2α(n)-3H]CHOL) were supplied by Perkin-Elmer (Boston, MA) and Amersham (UK), respectively, and 3H-PREGS ([7-3H]PREGS, 25 Ci/mmol) was prepared in our laboratory from 3H-PREG using pyridine/sulfur trioxide. PREG, DHEA, and androst-5-ene-3β,17β-diol (ADIOL) were obtained from Roussel-Uclaf (Romainville, France) and oxysterols (hydroxycholesterols) from Steraloids (Newport, RI). Epietiocholanolone (3β-hydroxy-5β-androstan-17-one) was purchased from Sigma-Aldrich (St. Louis, MO). HFBA and N-methyl-N-trimethylsilyltrifluoroacetamide (MSTFA) were from Pierce (Rockford, IL) and TEA from Sigma-Aldrich. All other reagents and solvents were of analytical grade.
Steroid extraction
All the experiments were carried out with young adult (about 3 months old) male Sprague-Dawley rats (Centre d'élevage R. Janvier, Le Genest St-Isle). Animal care was in accordance with the European Communities Council Directive of November 24, 1986 (86/609/EEC). Animals were euthanized by decapitation, and their entire brains were removed and weighed. The extraction protocol using methanol and methanol/CHCl3 (1/1) has been described previously (2) (solvent mixtures are given as volume proportions throughout the text). The supernatants were combined, and aliquots corresponding to 100 mg rat brain were processed in the sample treatment procedure.
FeSO4 treatments of the brain extracts
The effect of FeSO4 on the release of PREG and DHEA was tested with 1 mg CHOL or extract of 100 mg rat brain. Five nanograms of epietiocholanolone was added for quantifying released free PREG and DHEA. The dried extracts were treated with FeSO4 100 mM in methanol/water (7/3) for 2 h at 60°C. The recycling C18 SPE procedure was then applied, and the free steroid fraction (eluted with methanol/water; 9/1) was collected and derivatized with HFBA at 20°C before GC-MS analysis. Control experiments were carried out in the same way with omission of FeSO4.
Purification and fractionation methodologies
C18 SPE with recycling and fractionation.
Unless otherwise stated, the first step in all the experiments was a SPE using C18 cartridges (500 mg, 6 ml; International Sorbent Technology, Mid Glamorgan, UK). The simplified recycling/elution protocol described in our previous article (2) was used to separate sulfated, free, and fatty acid esters of steroids without cross-contamination. Brain extracts were dissolved in 1 ml methanol and applied to the C18 cartridge followed by 5 ml of methanol/water (85/15). The flow-through, containing the free and sulfated forms of PREG and DHEA, was collected and dried. After a previous reconditioning of the same cartridge with 5 ml water, the dried samples were dissolved in methanol/water (2/8) (the residue was first dissolved in methanol and 4 vols of water was then added) and reapplied. The cartridge was then washed with 5 ml water and sulfated, free, and lipoidal steroids were eluted with 5 ml methanol/water (1/1), 5 ml methanol/water (9/1), and 5 ml methanol/CHCl3 (1/1), respectively. An additional 5 ml of hexane was applied to increase the recovery of potentially esterified PREG and DHEA in the lipoidal fraction (the combined methanol/CHCl3 and hexane eluates). Ten nanograms of epietiocholanolone were added as internal standard to the lipoidal fraction for quantification of PREG and DHEA released from 100 mg of rat brain or from 1 mg of CHOL. An alternative C18 SPE protocol was used in some experiments to remove CHOL directly after the steroid extraction from rat brain: extracts were dissolved in 1 ml CH3CN/isopropanol (1/1) and deposited on C18 cartridges previously conditioned with 3 ml CH3CN and 5 ml of CH3CN/isopropanol/water (55/25/20). Free steroids and oxysterols were eluted with 12 ml of CH3CN/isopropanol/water (55/25/20) for further purification (with the C18 SPE recycling procedure), whereas most of the CHOL was retained.
The unreliable C18 SPE procedure used for isolation of steroid sulfates, published in 2000 (1) and later invalidated (2), was also studied further. Briefly, an extract of 100 mg rat brain was dissolved in 10 ml of methanol/water (4/6) and applied to the C18 cartridge previously conditioned with 5 ml methanol, 5 ml water, and 5 ml methanol/water (4/6). Sulfated, free, and lipoidal steroids were eluted with 5 ml methanol/water (4/6), 5 ml methanol/water (9/1), and 5 ml methanol/CHCl3 (1/1), respectively.
Silicic acid chromatography and molecular sieving on Sephadex LH-60
The rat brain lipoidal fraction was subjected to adsorption chromatography on silicic acid (Unisil, activated silicic acid, 200–325 mesh; Clarkson Chromatography Products, South Williamsport, PA). Columns (500 mg, 0.5 cm inner diameter) were prepared in CH2Cl2/hexane (8/2) and the sample applied in 2 ml of this solvent. Two fractions corresponding to nonpolar lipids and free steroids were successively eluted with 40 ml CH2Cl2/hexane (8/2) and 10 ml ethyl acetate, respectively. In one experiment, these fractions were directly derivatized with TEA/HFBA (1/1) in order to analyze the elution profile of PREG and DHEA precursor(s). The fraction containing nonpolar lipids was then subjected to gel-exclusion chromatography on Sephadex LH-60 (15 × 2 cm column) using CH2Cl2/hexane/methanol (7/2/1) as the solvent. Ten fractions (5 ml) were collected and analyzed by GC-MS after previous derivatization with TEA/HFBA.
SPE fractionation on aminopropyl bonded silica
A simplified method was used to separate the different lipid classes in the rat brain (20). The C18 SPE lipoidal fraction was dissolved in 1 ml of hexane and deposited on aminopropyl cartridges (500 mg, 6 ml; International Sorbent Technology) previously conditioned with 0.6 ml acetone/water (7/1) and 2 × 1 ml hexane. Cholesteryl esters (CEs) were eluted with 5 ml of hexane, triglycerides with 6 ml of hexane/CHCl3/ethyl acetate (100/5/5), and monoglycerides and diglycerides with 5 ml of CHCl3/isopropanol (2/1). FFAs and phospholipids were finally eluted with 6 ml of CHCl3/methanol/acetic acid (100/2/2) and 6 ml of methanol/CHCl3/water (10/5/4), respectively. All the fractions were derivatized with TEA/HFBA (1/1), repurified, and analyzed by GC-MS.
Another protocol using aminopropyl SPE was employed in an attempt to improve the fractionation of PREG and DHEA precursor(s). The lipoidal fraction was dissolved in 1 ml of hexane/CH2Cl2 (95/5). CEs were first eluted with 5 ml of hexane, and the cartridge was stepwise eluted with 5 ml of mixtures of hexane/CH2Cl2 (95/5), (9/1), (8/2), (6/4), (4/6), (2/8), and CH2Cl2. Finally, elution was done with 5 ml of isopropanol/CH2Cl2 (1/2). All fractions were derivatized with TEA/HFBA (1/1) and analyzed by GC-MS.
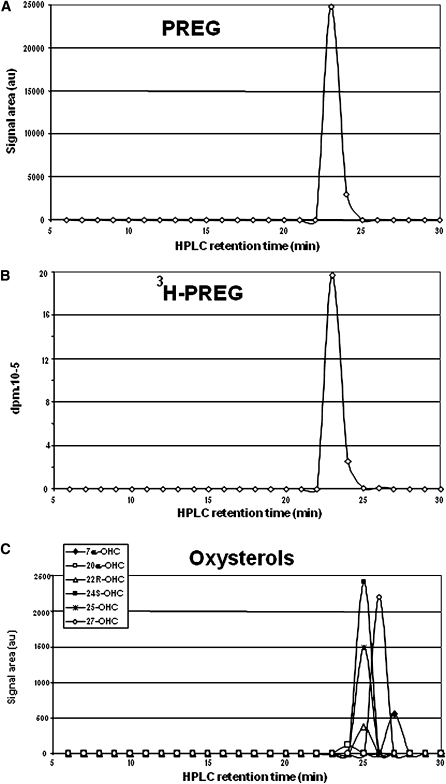
HPLC fractionation
Brain extracts were fractionated by the recycling C18 SPE method. The lipoidal fraction was collected and separated by the aminopropyl SPE. After elution of CE, the lipoidal steroids were eluted with 5 ml of CH2Cl2 and filtered. Samples were dissolved in hexane/isopropanol (9/1) for HPLC fractionation. The HPLC system from Thermo Fisher Scientific (San Jose, CA) consisted of a P1000XR thermo separation product quaternary pump and an AS 100XR thermo separation product autoinjector. A 202 model Gilson fraction collector was used. A Lichrosorb Diol column (25 cm × 4.6 mm, 5 μm) was used in a thermostated block at 30°C and with a mobile phase flow of 1 ml/min. The analytical column was first equilibrated in a solvent system of pure hexane and mixture A (9/1), the latter being composed of hexane/isopropanol (85/15). The elution was first performed with hexane and mixture A (9/1) for 15 min and then with a linear gradient to 100% mixture A in 5 min. This mobile phase was kept constant for 15 min, after which the column was rinsed with pure methanol. HPLC fractions of 1 ml were collected between 1 and 40 min, derivatized with TEA/HFBA, and analyzed by GC-MS. This HPLC procedure was also used for the fractionation of the free steroids, previously isolated by the C18 SPE recycling method from extracts of 400 mg rat brain. This protocol was also applied to establish the elution profile of tritiated PREG and oxysterols. The steroid sulfate fraction obtained with the unreliable C18 SPE of 100 mg brain (1) (see above) was also fractionated by HPLC. Tritiated PREGS was located in this system. One milliliter fractions were collected between 1 and 60 min, derivatized with HFBA at 20°C, and analyzed by GC-MS.
A similar experiment was performed with another straight-phase HPLC system using a Silica (Genesis, Jones Chromatography) column (25 cm × 4.6 mm, 4 µm) with a 0.5 ml/min flow of hexane/ethyl acetate (9/1). The rat brain lipoidal fraction from recycling C18 SPE was separated and 12 fractions of 5 ml were collected. Fraction 6 (containing most of CHOL) was again purified in the same HPLC system, and 1 ml fractions were collected, derivatized with TEA/HFBA, and analyzed by GC-MS. An aliquot of each fraction was derivatized with MSTFA as described below for analysis of CHOL by GC-MS.
Derivatization reactions
Samples coming from the SPE lipoidal fraction of 100 mg brain or 1 mg CHOL were derivatized, unless otherwise stated, with 20 µl TEA and 20 µl HFBA in 100 µl anhydrous acetone in a heating block at 70°C for 30 min. The reaction mixture was partitioned between hexane and water (1/1) (0.5 ml of each), and the organic phase containing the steroid heptafluorobutyrates (HFB) was dried and deposited in 1 ml CH3CN/water (1/1) on a C18 SPE cartridge (500 mg, 6 ml) previously conditioned with 5 ml CH3CN, 5 ml water, and 5 ml CH3CN/water (1/1). The HFBs were eluted with 6 ml CH3CN after a washing step with 4 ml CH3CN/water (1/1).
In order to test the yields of PREG and DHEA under different conditions, CHOL (and in one case oxysterols) was derivatized with either TEA/HFBA, as described above, or with HFBA alone at 20°C and 70°C, or with 50 µl MSTFA for 30 min at 70°C. Epietiocholanolone was added (5 ng) for quantification purposes.
The derivatized standards and biological samples were always dried under a gentle stream of nitrogen and dissolved in hexane before the subsequent GC-MS analysis.
GC-MS analysis
GC-MS analyses were carried out using an Automass Solo mass spectrometer (Thermo Fisher Scientific, San Diego, CA) interfaced with a TraceGC (Carlo Erba, Milan, Italy) gas chromatograph. Samples were injected with an AS 2000 autosampler (Carlo Erba) to the injection chamber maintained at 250°C in the splitless mode. The analytical capillary column was BPX35 (35% phenyl, 65% dimethyl polysiloxane) (SGE, Victoria, Australia), 30 m long with an inner diameter of 0.25 mm, and a 0.25 µm film thickness. The initial oven temperature was kept at 50°C for 1 min and was raised to 350°C at 20°C/min. The transfer line and ionization chamber temperatures were maintained at 300°C and 180°C, respectively. The flow rate of helium carrier gas was kept constant at 1 ml/min. The mass spectrometer was operated in the electron impact mode with an ionization energy and an emission current of 70 eV and 800 µA, respectively.
Identification of heptafluorobutyrate derivatives of PREG (PREG-HFB), DHEA (DHEA-HFB), androst-5-ene-3β,17β-diol (ADIOL-HFB2), CHOL (CHOL-HFB), and epietiocholanolone (internal standard) was achieved in the full scan mode (m/z range 50–550). Quantification was carried out in the selected ion monitoring mode on the major diagnostic ions m/z 298, 270, 468, 368, and 486, respectively. Quantification of trimethylsilyl ether derivatives of PREG (PREG-TMS), DHEA (DHEA-TMS), and epietiocholanolone was achieved with the m/z 388, 360, and 347 diagnostic ions, respectively.
Radioactivity measurements
Tritiated PREG, PREGS, and CHOL were used as tracers in the SPE and HPLC methodologies. Dried radioactive samples were dissolved in 5 ml of Picofluor 15 scintillation liquid and counted in a Packard Tricarb liquid scintillation spectrometer model 4660, equipped with quench correction (Packard Instruments, Downers Grove, IL).
Statistics
Statistical analysis was performed with unpaired Student's t-test on Graphpad Prism 3.0 (San Diego, CA) for the FeSO4 experiment.
RESULTS
Fractionation of the brain lipoidal fraction by molecular sieving and SPE
The extract of 100 mg of rat brain was submitted to the recycling C18 SPE procedure, and the lipoidal fraction was collected. This fraction contained the fatty acid esters (if present) of PREG and DHEA together with free CHOL and other lipids. When separated by silicic acid chromatography, PREG and DHEA precursor(s) were only found in the nonpolar fraction. When fractionated by molecular sieving chromatography on Sephadex LH-60, the elution profiles of PREG and DHEA precursor(s) and 3H-PREG and 14C-CHOL (data not shown) were the same, indicating that the precursor(s) had molecular weight(s) in the range of free steroids.
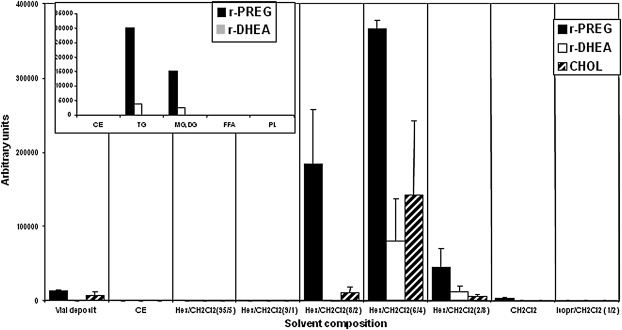
Lipid classes in the brain lipoidal fraction were separated on aminopropyl cartridges (20), and the collected fractions were derivatized with TEA/HFBA and analyzed by GC-MS. As shown in Fig. 1 (inset), PREG and DHEA were released from fractions containing triglyceride and diglyceride + monoglyceride and were completely separated from CEs, FFAs, and phospholipids (certainly also containing gangliosides, cerebrosides, and sulfatides), indicating that the precursor(s) was a neutral entity. Attempts were made to achieve a better resolution of PREG and DHEA precursor(s) by stepwise elution with mixtures of hexane/CH2Cl2.The elution profile of the precursor(s) was compared with that of 3H-CHOL. As shown in Fig. 1, PREG and DHEA were released from fractions where 3H-CHOL was also eluted.
Fig. 1.
Distribution of released PREG (r-PREG) and DHEA (r-DHEA) and mobility of CHOL by aminopropyl SPE stepwise elution of the lipoidal fraction from 100 mg adult male rat brain. Each fraction was analyzed by GC-MS following treatment with TEA/HFBA. Data presented are means with the range of values (n = 2). Inset: distribution of released PREG and DHEA by aminopropyl SPE according to Ågren, Julkunen, and Penttila (20) for separation of lipid classes. CE, cholesteryl esters TG, triglycerides; DG, diglycerides; MG, monoglycerides; PL, phospholipids.
The lipoidal fraction was also subjected to chromatography on lipophilic ion exchangers: anion exchanger DEAP-Lipidex in hydroxide form and cation exchanger SP-LH-20 in hydrogen form (21). In both cases, PREG and DHEA were only released by derivatization of the neutral fractions (data not shown), showing that the precursor(s) is not charged and does not consist of ion pairs between steroid sulfate and nonpolar cations.
HPLC fractionation of the brain lipoidal fraction
The rat brain PREG and DHEA precursor(s) and reference fatty acid esters of PREG and DHEA are eluted in the same fraction in the C18 SPE recycling/elution procedure. However, when reference PREG palmitate or DHEA stearate were subjected to the TEA/HFBA derivatization conditions, there was no transesterification into HFB esters. This shows that fatty acid esters of PREG and DHEA are not the unknown precursor(s) (2).
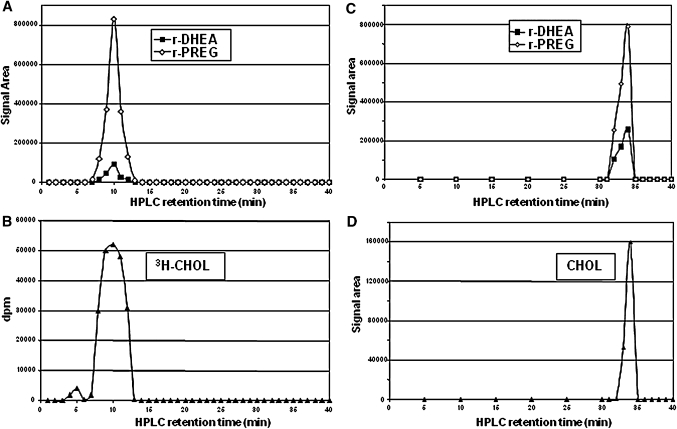
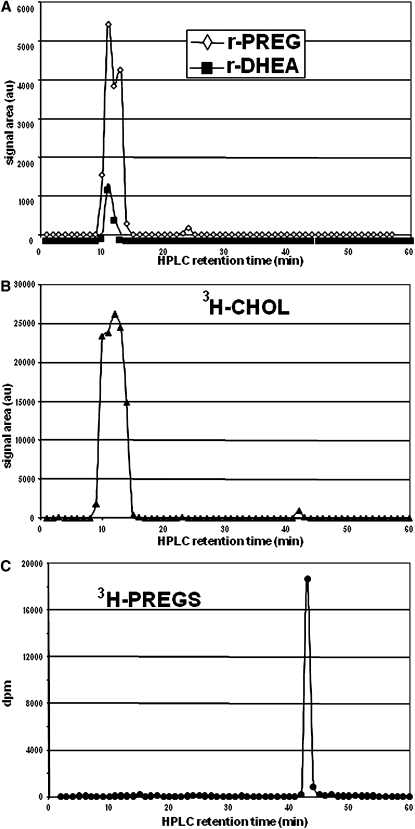
The rat brain lipoidal fraction was then fractionated in a straight-phase HPLC system (4, 5, 22), and the fractions were derivatized with TEA/HFBA and analyzed by GC-MS. The results are illustrated in Fig. 2A. PREG and DHEA were released from the same fractions, and the HPLC profile of the precursor(s) corresponded to that of 3H-CHOL (Fig. 2B). Thus, the polarity seemed to be similar for the precursor(s) of PREG and DHEA, and the HPLC conditions did not give a separation of the precursor(s) and CHOL. The HPLC retention time of PREG palmitate was 5 min, i.e., different from that of the precursor(s).
Fig. 2.
Release of PREG (r-PREG) and DHEA (r-DHEA) from fractions collected in the straight-phase HPLC separation of a lipoidal fraction from 100 mg adult male rat brain on Lichrosorb Diol (A) and silica columns (C). Each fraction was analyzed by GC-MS following treatment with TEA/HFBA. The mobilities of CHOL on the Lichrosorb Diol column are shown for added tracer 3H-CHOL (B) and on the silica column for brain CHOL after derivatization with MSTFA (D).
A different straight-phase solvent system combined with a silica column was used to increase the HPLC resolution. Again, the precursor(s) of PREG and DHEA had the same elution pattern as CHOL (Fig. 2C, D). Thus, the behavior of the precursor(s) and CHOL seemed to be the same in all the chromatographic systems investigated.
Derivatization of CHOL with TEA/HFBA
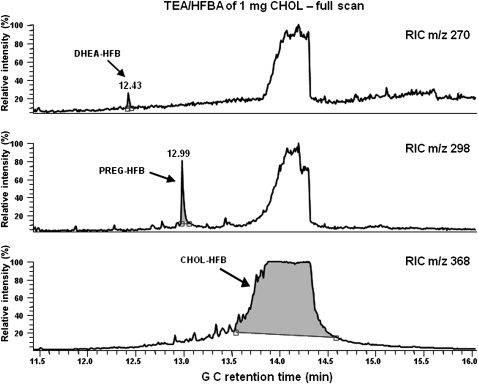
Given the observations described above, we investigated a possible formation of artifacts from CHOL. Indeed, Van Lier and Smith (23) had shown that PREG, and to a lesser extent DHEA, could be minor autoxidation products from CHOL under harsh experimental conditions. We therefore exposed reference CHOL to the TEA/HFBA procedure used to derivatize the brain SPE lipoidal fraction and searched for PREG and DHEA derivatives by GC-MS in full-scan and selected ion monitoring detection modes. To match the large amounts of free CHOL in brain, around 10–20 mg/g (24), we tested the reaction with 1 mg of CHOL, roughly corresponding to the amount found in 100 mg of rat brain tissue. Results of the GC-MS analysis are illustrated in Fig. 3. PREG and DHEA were clearly detected, demonstrating that they can be produced by autoxidation of CHOL. In addition, smaller amounts of ADIOL-HFB2 were detected. This steroid has also been described as a potential minor autoxidation product of CHOL (23).
Fig. 3.
GC-MS analysis of products formed from 1 mg of CHOL treated with TEA/HFBA. The presence of DHEA, PREG, and CHOL derivatives is revealed by the reconstructed ion chromatograms (RIC) of m/z 270, 298, and 368, respectively.
Quantification of PREG and DHEA released from CHOL and brain extract
The yields of PREG and DHEA from CHOL were determined to establish a comparison with the levels found in the rat brain lipoidal fraction. For this purpose, the C18 SPE lipoidal fractions from 100 mg of rat brain and 1 mg of CHOL were collected and treated with TEA/HFBA for quantification of PREG and DHEA. Two additional rat brain extracts were analyzed after insertion of a SPE purification step for removal of CHOL at the beginning of the sample preparation procedure. The results are summarized in Table 1. The yield of PREG from CHOL was about 0.002% (i.e., 20 ng/mg), and about 25 ng of PREG were formed from the lipoidal fraction from 100 mg rat brain. The yields of DHEA and ADIOL from reference CHOL were much lower but significant (around 4 and 0.5 ng/mg, respectively). These amounts were similar to those found from 100 mg of rat brain, suggesting that CHOL was the major precursor of PREG, DHEA, and ADIOL. This was supported by the dramatic decrease of the amounts released by a factor of at least 10 for PREG and DHEA and at least 3 for ADIOL when the main part of CHOL was removed in an initial step.
TABLE 1.
Yields of PREG, DHEA, and ADIOL after derivatization of CHOL and lipoidal fraction of rat brain with TEA/HFBA at 70°C for 30 min.
| PREG | DHEA | ADIOL | |
|---|---|---|---|
| ng | ng | ng | |
| CHOL (1 mg) | 23.8 ± 0.3 | 3.9 ± 0.1 | 0.5 ± 0.03 |
| Lipoidal fraction of rat brain (100 mg) | 25.2 ± 3.4 | 3.3 ± 0.3 | 1.1 ± 0.1 |
| CHOL-depleted lipoidal fraction of rat brain (100 mg)a | 2.1 ± 0.7 | 0.3 ± 0.1 | 0.3 ± 0.2 |
| Yields (%) | |||
| CHOL (1 mg) | 0.002 | 0.0004 | 0.00005 |
Data are presented as means ± SD (n = 2).
Most nonpolar lipids, including CHOL, in the original brain extract were removed by C18 SPE preceding the recycling SPE extraction/fractionation procedure (see Experimental Procedures).
Derivatization of CHOL and oxysterols with HFBA and MSTFA
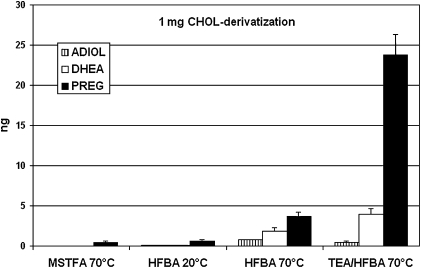
In the light of the data described above, the reliability of analyses of unconjugated PREG and DHEA in rat brain was evaluated. Experiments using tritiated tracer showed that about 1% of CHOL was present in the free steroid fraction of the C18 SPE recycling procedure. Thus, in analyses of 100 mg rat brain, about 10–20 µg of endogenous CHOL could contaminate this fraction. Different derivatization conditions with commonly used reagents such as HFBA, MSTFA, and TEA/HFBA were tested with 1 mg of CHOL. As shown in Fig. 4, derivatives of PREG, DHEA, and ADIOL were also formed in reactions with HFBA at 70°C, although to a lesser extent than with TEA/HFBA, especially for PREG. About 0.5–1.0 ng of PREG derivative was formed with HFBA at 20°C and MSTFA at 70°C, while derivatives of DHEA and ADIOL were not detected under such conditions.
Fig. 4.
Yields of autoxidation products of CHOL under different derivatization conditions. One milligram of CHOL was treated with MSTFA, and the trimethylsilyl ethers of PREG, DHEA, and ADIOL were measured by GC-MS. Heptafluorobutyrates of PREG, DHEA, and ADIOL were measured by GC-MS following treatments with HFBA or TEA/HFBA. Data presented are means ± SEM (n = 4).
Approximately 90% of oxysterols are also eluted in the free steroid fraction of the C18 SPE procedure. Some of these can form PREG by autoxidation (23). Thus, oxysterols, especially 24S-hydroxycholesterol (24S-OHC) that is generally found at 10–20 µg/g in mammalian brain (25), represent a potential source of artifact formation of free PREG and DHEA. Different oxysterols that may be present in rodent brain and CHOL were therefore subjected to different conditions of derivatization, and the formation of derivatives of PREG, DHEA, and ADIOL was measured. This investigation was carried out with 2 µg of CHOL and 24S-OHC and 20 ng of 7α-OHC, 20α-OHC, 22R-OHC, 25-OHC, and 27-OHC. The chosen amounts of oxysterols are in the expected range of values in analyses of 100 mg of rat brain. Formation of derivatives of DHEA and ADIOL from CHOL and the oxysterols was not detected after reaction with HFBA at 20°C. However, a weak but quantifiable signal was observed for PREG after derivatization of 24S-OHC (13.6 ± 3.7 pg), 20α-OHC (7.5 ± 0.7 pg), and 22R-OHC (1.9 ± 0.2 pg).
HPLC fractionation of the brain fraction containing free steroids
An HPLC experiment was performed in order to validate the measurements of unconjugated PREG. The SPE free steroid fraction contained 1% and 90% of brain CHOL and oxysterols, respectively, and it was essential to check that autoxidation of these sterols did not contribute to the measured levels of free PREG. The free steroid fraction collected from 400 mg rat brain by SPE was separated in the Lichrosorb Diol straight-phase HPLC system. One milliliter fractions were collected, derivatized with HFBA under mild conditions (20°C), and analyzed by GC-MS for the presence of HFB derivatives of PREG, DHEA, and ADIOL. These experimental conditions were optimal for the yields in the derivatization of free steroids. As shown in Fig. 5A, one single peak with a retention time of 23 min was obtained. This was exactly superimposable on injected tritiated PREG tracer (Fig. 5B), validating the presence of endogenous-free PREG in the rat brain. Very important was the absence of PREG from CHOL-containing HPLC fractions (8–12 min), meaning that CHOL was not involved in the detection of unconjugated PREG under our experimental conditions. Furthermore, DHEA and ADIOL were not detected, confirming the very small if any contribution of an autoxidation process under these conditions. The HPLC elution profiles of the main endogenous oxysterols were also determined (Fig. 5C). Their retention times were in the range 24–27 min, also excluding their contribution to formation of PREG by autoxidation during the derivatization.
Fig. 5.
A: HPLC of PREG in the free steroid fraction eluted with methanol/water (9/1) in the C18 SPE recycling extraction of 400 mg rat brain. Each HPLC fraction was analyzed by GC-MS following treatment with HFBA at 20°C. DHEA was not detectable in this experiment. B: HPLC of radioactive 3H-PREG. C: HPLC of reference oxysterols analyzed by GC-MS after derivatization with MSTFA. All experiments were performed in duplicate using straight-phase HPLC with the Lichrosorb Diol system.
This result was also supported by analyses of the free PREG content of a rat brain extract from which CHOL had been removed by SPE just after the extraction step. The brain concentration of free PREG was the same, roughly 4 ng/g, whether or not CHOL was removed directly after the extraction step (data not shown). However, these results did not exclude the autoxidative formation of PREG from oxysterols earlier in the procedure and these latter were not removed in the CHOL removal step.
Distribution of CHOL in the unreliable C18 SPE procedure
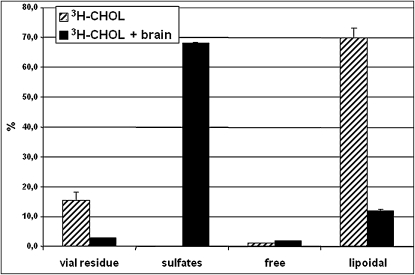
Additional studies were carried out to explain the errors in previous quantifications of the sulfated steroids, PREGS and DHEAS, in rodent brain and plasma. A possible correlation between autoxidation of CHOL and formation of PREG and DHEA from the so-called steroid sulfate fraction was evaluated. 3H-CHOL and extracts of 100 mg rat brain to which 3H-CHOL had been added were subjected to the inaccurate SPE procedure. The different fractions from the SPE were collected, and radioactivity was counted. As shown in Fig. 6, the elution of CHOL is totally disturbed by the presence of a rat brain extract. As expected, in the absence of brain extract, reference 3H-CHOL was predominantly found in the lipoidal fraction (70%) and to a lesser extent remaining in the extraction vial (16%). In the presence of brain extract, most of the radioactivity was found in the sulfate fraction (∼68%) and only 12% in the lipoidal fraction. Thus, the steroid sulfate fraction obtained with this inappropriate SPE procedure is highly contaminated by CHOL.
Fig. 6.
Distribution of 3H-CHOL with or without the presence of an extract of 100 mg rat brain using the unreliable C18 SPE procedure. The residue in the vial and the sulfated, unconjugated, and lipoidal steroid fractions eluted from the cartridge were counted for radioactivity. Duplicate experiments were performed.
Quantification of PREG and DHEA released from the so-called brain steroid sulfate fraction
Since about 1 ng of PREG was formed (Fig. 4) from 1 mg of CHOL under mild derivatization conditions, the presence of endogenous CHOL in the steroid sulfate fraction can account for the detection of PREG at a concentration of about 10 ng/g brain.
We also tested if cholesterol sulfate (CHOLS) could be involved in the formation of PREG and DHEA from the sulfate fraction. However, only about 50 pg of PREG (and no DHEA) was found after derivatization of 1 mg of CHOLS with HFBA at 20°C.
Then, the so-called steroid sulfate fraction from 100 mg rat brain, obtained by the inaccurate SPE procedure, was also subjected to straight-phase HPLC. PREG and DHEA were both released from fractions collected between 10 and 14 min, corresponding to the retention time of CHOL (Figs. 7A, B). A small peak also appeared at 24 min corresponding to free PREG; it is conceivable that a very minor fraction of endogenous free PREG was tailing into the so-called steroid sulfate fraction. 3H-PREGS was also analyzed and was eluted at 42 min (Fig. 7C), corresponding to the very weak signal detected for CHOL (Fig. 7B) from the rat brain tissue. Although the HPLC retention time of CHOLS was not determined, we can suggest that CHOLS was the precursor of this signal, confirming that free CHOL was the only major precursor of PREG and DHEA released from this brain steroid sulfate fraction.
Fig. 7.
Straight-phase HPLC on Lichrosorb Diol of a so-called steroid sulfate fraction from rat brain using the unreliable C18 SPE procedure. Each HPLC fraction was hydrolyzed and derivatized with HFBA at 20°C. The release of PREG (r-PREG) and DHEA (r-DHEA) (A) coincides with the elution of CHOL (B). The elution of 3H-PREGS in the same system is shown for comparison.
Effects of FeSO4 on the release of free PREG and DHEA from CHOL and brain extract
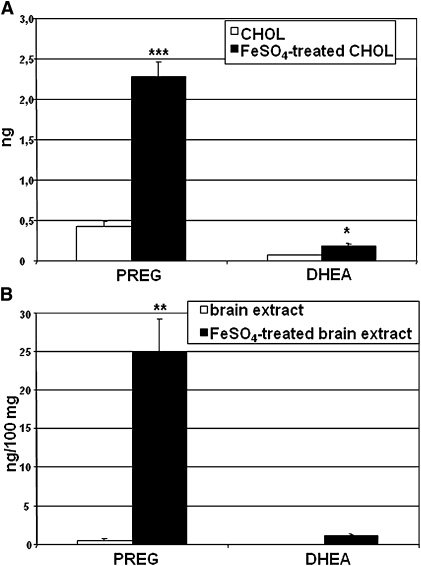
Several studies have shown that PREG and DHEA can be released from brain lipid extracts by treatment with FeSO4 (19, 26). In order to compare our results with those of Lieberman's group, we treated 1 mg of CHOL or the extract from 100 mg of rat brain tissue with FeSO4 and determined the release of free PREG and DHEA using recycling C18 SPE with collection of the free steroid fraction and mild HFBA derivatization. As shown in Fig. 8A, treatment of CHOL with FeSO4 increased the formation of PREG (P < 0.001) and to a lesser extent DHEA (P < 0.05). However, PREG and DHEA were also detected in the absence of FeSO4, meaning that some autoxidation occurred during the incubation and solvent evaporation.
Fig. 8.
Effects of FeSO4 treatment on the formation of PREG and DHEA from 1 mg of CHOL (A) and a methanol extract of 100 mg rat brain (B). Following the treatment, the free steroid fraction obtained by recycling C18 SPE was analyzed by GC-MS of the HFB derivatives. Data presented are means ± SEM (n = 4). Statistical analysis: Student's t-test *, P < 0.05; **, P < 0.01; ***, P < 0.001.
The FeSO4 effect was more pronounced with rat brain tissue than with pure CHOL. The release of rat brain free PREG after the FeSO4 treatment increased to levels up to 250 ng/g (P < 0.01). DHEA was only detected in the FeSO4-treated brain extracts at concentrations around 11.5 ng/g (Fig. 8B). In the absence of FeSO4, autoxidation of CHOL to PREG and DHEA did not occur in the brain extract since no DHEA was detected and the concentration of PREG (∼4 ng/g) corresponded to the levels of endogenous free PREG (see above).
DISCUSSION
This study shows that endogenous rat brain CHOL can be converted into PREG and DHEA, and to a lesser extent ADIOL, during sample processing in analyses of these steroids by GC-MS. By contaminating the sulfate fraction in unsatisfactory SPE and other extraction and partitioning procedures, CHOL can also be the precursor of the PREG and DHEA found in the sulfate fraction. We believe that differences in this artifactual formation of PREG and DHEA during extraction, purification, fractionation, and derivatization prior to the final GC-MS analysis (or other indirect detection methods) are responsible for discrepancies in levels of these steroids encountered in the neurosteroid literature since 30 years.
PREG and DHEA are released from material having the same chromatographic behavior as CHOL
In previous studies using LC-MS and GC-MS methods, we found that, contrary to the prevailing view, PREGS and DHEAS could not be detected in rat and mouse brain and plasma (2, 16). We also found that large amounts of PREG and DHEA (around 1 nmol/g) were released from endogenous precursor(s) present in the SPE lipoidal fraction (2). When this fraction was separated in different chromatographic systems, including silicic acid chromatography, molecular sieving, aminopropyl SPE (27), and ion exchange (21), PREG and DHEA were always released from fractions containing CHOL. The behavior on aminopropyl cartridges and ion exchangers excluded the possibility that the precursor(s) was charged or an ion pair, and the molecular sieving excluded a protein-bound steroid or proteolipid. All these observations are compatible with a formation of PREG and DHEA from CHOL. When HPLC was applied with the aim to separate the great excess of CHOL from the PREG and DHEA precursor(s) to permit the latter to be studied by mass spectrometry, no separation of CHOL and the precursor(s) could be obtained. The released steroids and CHOL were found in the same derivatized HPLC fractions. These experiments strongly supported a formation of PREG and DHEA from CHOL.
Autoxidation of CHOL
CHOL readily undergoes autoxidation by various routes, and a galaxy of autoxidation products have been described (28–30). The ease of autoxidation of CHOL, present at high levels in many biological materials, constitutes a problem, i.e., artifactual generation of oxysterols from CHOL during sample storage, processing, and analysis. Many methods and results have been presented where the control of artifact formation has been insufficient. In those studies, interest has focused on autoxidation products oxygenated in the A/B rings and the side chain (31), and products with a shortened or no side chain have received very little attention in spite of reports of C19 and C21 steroids being autoxidation products of CHOL. Thus, in 1970, Van Lier and Smith reported that PREG, DHEA, ADIOL, and other products could be generated by heating highly purified CHOL in an oven at 100°C for 7 days (23). The initial product, CHOL 20α-hydroperoxide, was a precursor of PREG and ADIOL, which were formed by thermal decomposition of the hydroperoxide (32). With this background, we investigated a possible formation of HFB derivatives of PREG, DHEA, and ADIOL during derivatization of CHOL with TEA/HFBA. Small amounts were formed, the yields being approximately 0.0024%, 0.0004%, and 0.00005%, respectively. Due to the large amounts of CHOL in the brain, (10–20 mg/g) (24), the treatment with TEA/HFBA of rat brain lipoidal fraction released approximately 250 ng/g brain of PREG, 33 ng/g of DHEA, and 10 ng/g of ADIOL. Since the amounts of steroids released were approximately the same when treating 1 mg of CHOL and lipoidal fraction from 100 mg of rat brain (that contained approximately 1 mg of CHOL) with TEA/HFBA, CHOL appears to be the main precursor of PREG and DHEA detected by GC-MS analysis of the rat brain lipoidal fraction. This is supported by the experiments using HPLC to trace the origin of the steroids.
In our previous article (2), maximum yields of PREG and DHEA, now shown to be artifactually formed from CHOL, were obtained by derivatization with HFBA after addition of TEA. TEA has previously been found to induce a release of the two steroids from brain extracts (see below) (19, 33). Probably, the alkaline nature of the reaction mixture increased the formation of PREG and DHEA by autoxidation. In the studies of Mathur et al. (33), treatment of a lipoidal fraction from brain with NaOH resulted in release of PREG similar to that induced by TEA.
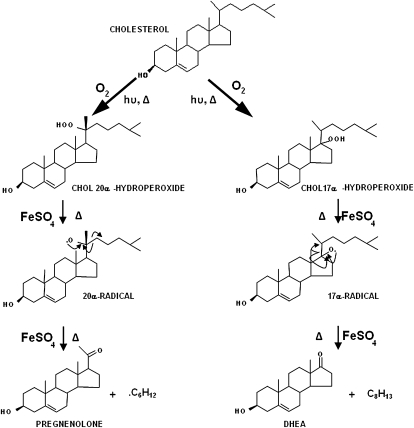
The autoxidation of CHOL by air proceeds via hydroperoxide intermediates, e.g., CHOL 7α-hydroperoxide, CHOL 25α-hydroperoxide, and CHOL 20α-hydroperoxide (23, 28–30). Spontaneous decomposition of CHOL 7α-hydroperoxide gives rise to the common CHOL autoxidation products 7α-OH CHOL, 7β-OH CHOL, and 7-oxo CHOL, whereas the thermal decomposition of CHOL 20α-hydroperoxide gives PREG and ADIOL (32). The pathways of decomposition of these CHOL hydroperoxides involve radical-type reactions leading to alcohols, ketones, and deoxysteroids. The CHOL 20α-hydroperoxide decomposes by homolysis of the oxygen-oxygen bond followed by abstraction of a hydrogen atom from a nearby molecule giving 20α-OH CHOL. Alternatively, oxosteroid products are formed from CHOL 20α-hydroperoxide as a result of a β-scission of carbon-carbon bonds in the initially formed alkoxyl radical formed via homolysis. The β-scission of the C20-C22 bond and expulsion of the isohexyl radical gives PREG as the major product. An alternative β-scission of the C17-C20 bond from the 20α-radical and expulsion of the entire C8H13 side chain gives a C17-radical androst-5-ene. The combination of this latter compound with a hydroxyl radical would yield ADIOL. No DHEA was detected from the CHOL 20α-hydroperoxide in these studies, suggesting that DHEA probably came from a putative CHOL 17α-hydroperoxide. These suggested mechanisms for formation of PREG and DHEA are summarized in Fig. 9.
Fig. 9.
Hypothetical mechanisms for the formation of PREG and DHEA by autoxidation of CHOL via the 20α-hydroperoxide and the 17α-hydroperoxide intermediates.
FeSO4-induced release of PREG and DHEA from CHOL and brain lipoidal fraction
Free PREG and DHEA were formed when CHOL was processed through the analytical procedure in the absence of antioxidant. Thus, PREG and DHEA could be formed during the extraction, SPE, HPLC, and derivatization steps in an analysis of brain. In the presence of FeSO4, the amounts of PREG and DHEA were increased both from a methanol solution of pure CHOL and from a rat brain extract, although not to the same extent. Indeed, about 250 ng/g of PREG and 10 ng/g of DHEA were released from the brain extract, whereas only 2 ng of PREG and 0.2 ng of DHEA were formed by treatment of a solution of 1 mg CHOL with FeSO4. The mechanism behind this difference is not clear but could be related to the high concentration in brain of phospholipids with polyunsaturated fatty acids and the observation that polyunsaturated fatty acids markedly stimulate the rate of CHOL oxidation in liposomes (34). It is also known that the nature of the lipid matrix in which sterols occur greatly influences the rates of autoxidation (28, 35).
Prasad et al. have shown that substantial amounts of PREG and DHEA are released by treating the nonketonic fraction of a rat brain lipid extract with TEA, FeSO4 in water, FeCl3 in water, HCl in benzene/acetone, and lead tetraacetate in benzene (19). The effects of TEA and FeSO4 appeared to be additive (see Table 1 in Ref. 19), and treatment with NaOH gave a similar yield as TEA (33). The authors suggested that endogenous sterols, such as sterol hydroperoxides, may be present in brain and be transformed into PREG and DHEA by these treatments. The amounts of PREG and DHEA released from a nonketonic lipid extract of rat brain (19) were very similar to those found in this study of a rat brain lipoidal fraction. Judging from their experimental conditions, it is conceivable that PREG and DHEA originated from CHOL (a nonketonic lipid). On the other hand, their preliminary data also suggested that PREG and DHEA were produced from a precursor less polar than 20α-OH CHOL and more polar than CHOL. We did not find evidence for such a precursor in our HPLC experiments, but the quantitative importance, if any, of alternative precursors might be indirectly determined by subtraction of the contribution of added CHOL labeled with 14C or 13C in the ring system (36, 37).
Autoxidation of CHOL and the characterization of fatty acid esters of PREG and DHEA in rat brain
We can conclude with certainty that PREG, DHEA, and ADIOL can be formed by autoxidation from brain CHOL during the sample treatment. Similar artifacts can be envisaged in analyses of plasma (2). These results also raise a question about the presence and accuracy in measurements of fatty acid esters of PREG and DHEA by indirect methods. Analyses of these steroid esters by GC-MS (33) or radioimmunology (RIA) (38) after saponification of a lipoidal fraction from a rat brain extract have been reported. However, autoxidation of free or fatty acid esters of CHOL during the saponification step (see above) could result in release of PREG and DHEA before the GC-MS or RIA analysis. We found (data not shown) that exposure at 80°C under N2 of 1 mg of CHOL to 40% KOH in 95% ethanol for 1 h resulted in formation of 0.36 ng PREG but no DHEA or ADIOL (the latter decreased by 0.1 ng compared with control). On the other hand, Shimada and Mukai detected fatty acid esters of PREG and DHEA, especially PREG-3-stearate, in rat brain by LC-MS (39). In this case, the characterizations were performed following derivatization of the 20-oxo group, and the possibility that autoxidation of fatty acid esters of CHOL could generate PREG and DHEA esters during the derivatization reaction needs to be evaluated. An HPLC fractionation procedure, permitting a strict separation of free and esterified CHOL and the potential fatty acid esters of PREG and DHEA, will be required to establish the presence of these steroid conjugates in rat brain.
Autoxidation of CHOL and the characterization of free PREG and DHEA in rat brain
Given the observations described above, we feared that the autoxidation process could interfere also in analyses of free PREG and DHEA (using the method described in Ref. 2 and in this article). Thus, 1% of the free CHOL and 90% of the oxysterols are eluted in the free steroid fraction and constitute a potential risk for formation of PREG and DHEA during evaporation and derivatization. For this reason, we estimated the autoxidative yield of these steroids from CHOL and several oxysterols using mild derivatization conditions, i.e., without heating or addition of catalysts. Free DHEA was not detected in any of these experiments, indicating that autoxidation of CHOL was of minor importance.
By using HPLC to search for potential precursors of PREG in the free steroid fraction, we found that PREG was exclusively present in fractions containing added 3H-PREG and not formed from fractions containing CHOL or oxysterols. Free DHEA was not detected from 400 mg of rat brain (<0.10 ng/g), confirming our previously published data (2, 16). Thus, autoxidation of CHOL or oxysterols did not interfere with the analysis of free 3β-hydroxy-Δ5 steroids by our procedure. However, other analytical protocols will need to be checked for a potential contribution from CHOL or oxysterols to the measured levels of free 3β-hydroxy-Δ5 steroids.
Autoxidation of CHOL and the characterization of PREGS and DHEAS in rat brain
The results of SPE experiments with addition of 3H-CHOL to the brain extract emphasized the solubility problems encountered with the previous, unreliable, SPE methodology (1) and showed that the so-called steroid sulfate fraction was contaminated with brain CHOL. The levels of PREG and DHEA released from the sulfate fraction were in the range 8–10 ng/g and 2–4 ng/g, respectively, (data not shown), which corresponds to the values expected from autoxidation of the amount of CHOL present. The levels of CHOLS in rat brain [about 1.2 μg/g by LC-MS (16) and 15 μg/g by indirect GC-MS (40)] are too low for their autoxidation to contribute to the measured values of PREG and DHEA. In addition, HPLC of the steroid sulfate fraction showed that CHOL was the unique precursor of the released PREG and DHEA (together with a minor contribution of free PREG), whereas no PREG or DHEA were released from fractions corresponding to PREGS and CHOLS. Thus, PREG and DHEA detected in the so-called steroid sulfate fraction obtained with the unreliable extraction and fractionation protocols were generated by autoxidation of CHOL and not by solvolysis of sulfate conjugates as previously assumed.
DHEAS (8) and PREGS (9) were among the first steroids to be identified in rat brain and qualified as neurosteroids. Levels of PREGS (∼5–15 ng/g) and DHEAS (∼1.5–5 ng/g) were initially measured by RIA, and the data obtained in adrenalectomized and castrated animals suggested that these steroids were formed and/or accumulated at least partly independently of peripheral steroidogenic glands. Given that DHEA and DHEAS were below the detection limit in the plasma of most adult mammals except for man and highest nonhuman primates (41, 42), this finding resulted in emergence of the neurosteroid concept. Our results explain why the concentrations of the assumed PREGS and DHEAS did not vary significantly after adrenalectomy and castration (8, 9, 43). Thus, a sulfate fraction free of CHOL is the prerequisite for analysis of PREGS and DHEAS by indirect detection methods. Capillary LC-MS methods for analysis of the intact conjugates (16) now permit validation of methods based on solvolysis and GC-MS and can be used for analysis of the intact conjugates. As mentioned in our previous article (2) and in contrast with the situation in rodents, PREGS and DHEAS are present in human brain. It is also well established that DHEAS and PREGS are quantitatively important steroids in human blood.
The literature contains numerous methods for analysis of steroid sulfates by GC-MS after solvolysis. In several methods, it is possible that CHOL has contaminated the so-called steroid sulfate fraction. The most likely cases are those in which extraction was carried out by homogenization of the brain in isotonic saline (9, 43–45), in buffer (33, 38, 46–50), or in aqueous methanol (51) followed by partitioning between this homogenate and an organic solvent. The abundance of phospholipids and other complex lipids in the brain will result in formation of micelles or aggregates containing CHOL, which will not be adequately transferred into the organic phase. The CHOL remaining in the aqueous so-called steroid sulfate fraction can explain the release of PREG and DHEA in subsequent steps, such as solvolysis and derivatization. Hence, the cited articles report relatively high levels of so-called PREGS and DHEAS in rat and mouse brain (ranges of 3–10 ng/g and 1–5 ng/g, respectively). Another critical point is the SPE fractionation step. Our initial C18 SPE procedure was based on that developed by Bélanger et al. (52) and modified by Wang, Wahlström, and Backström (53). The defect of this procedure was to dissolve the brain extract in solvent mixtures. such as methanol/water (5/95) (53–55) or methanol/water (4/6) (56, 57) unable to solubilize all the brain components, especially lipids, resulting in their inadequate adsorption and separation. CHOL is consequently eluted in more polar fractions than expected supposed to contain sulfated and/or unconjugated steroids. These deficiencies are not revealed by conventional recovery measurements. The unsatisfactory fractionation steps induce falsely high values for PREGS and DHEAS and, depending on the conditions, also for free PREG and DHEA. Interestingly, all the cited articles report levels in rat brain with a constant PREGS/DHEAS ratio (∼3–5) corresponding roughly to that observed for the formation of PREG and DHEA by autoxidation of CHOL. Recently, Ebner et al. published a complete study in terms of screening and rigorous identification of steroids in adult male rat brain (58). They detected DHEAS (but not PREGS) after solvolysis and GC-MS. This result needs to be explained in view of our findings.
The contamination of steroid fractions with CHOL will not influence measurements of steroids lacking the 3β-hydroxy-Δ5 structure, such as hormonal steroids progesterone, 17β-estradiol, testosterone, corticosterone, and cortisol, because they cannot be produced by CHOL autoxidation even under harsh experimental conditions.
CONCLUSION
In summary, our study shows that autoxidation of CHOL constitutes a potential source of error not only in the analysis of oxysterols (31, 36) but also in the analysis of C19 and C21 steroids with a 3β-hydroxy-Δ5 structure. In light of this, the analytical results of many studies of sulfated, free, and fatty acid esters of PREG and DHEA in brain tissue need to be reevaluated. Our data confirm that in rat brain, free PREG is endogenous, but levels of DHEA, PREGS, and DHEAS are below the detection limits of the present method. This study illustrates the importance of suitable extraction and fractionation steps in regard to CHOL partition and in particular its early removal to avoid the generation of autoxidation artifacts. Given the biological relevance of PREG and DHEA in their free and sulfated forms in the nervous system, it is essential to characterize these steroids with reliable methodologies.
Footnotes
Abbreviations:
- ADIOL
- androst-5-ene-3β,17β-diol
- CE
- cholesteryl ester
- CHOL
- cholesterol
- CHOLS
- cholesterol sulfate
- DG
- diglyceride
- DHEA
- dehydroepiandrosterone (3β-hydroxyandrost-5-en-17-one)
- DHEAS
- dehydroepiandrosterone sulfate
- HFB
- heptafluorobutyrate
- HFBA
- heptafluorobutyric anhydride
- MG
- monoglyceride
- MSTFA
- N-methyl-N-trimethylsilyltrifluoroacetamide
- OHC
- hydroxycholesterol
- PL
- phospholipid
- PREG
- pregnenolone (3β-hydroxypregn-5-en-20-one)
- PREGS
- pregnenolone sulfate
- RIA
- radioimmunology analysis
- SPE
- solid-phase extraction
- TEA
- triethylamine
- TG
- triglyceride
REFERENCES
- 1.Liere P., Akwa Y., Weill-Engerer S., Eychenne B., Pianos A., Robel P., Sjövall J., Schumacher M., Baulieu E. E. 2000. Validation of an analytical procedure to measure trace amounts of neurosteroids in brain tissue by gas chromatography-mass spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 739: 301–312 [DOI] [PubMed] [Google Scholar]
- 2.Liere P., Pianos A., Eychenne B., Cambourg A., Liu S., Griffiths W., Schumacher M., Sjövall J., Baulieu E. E. 2004. Novel lipoidal derivatives of pregnenolone and dehydroepiandrosterone and absence of their sulfated counterparts in rodent brain. J. Lipid Res. 45: 2287–2302 [DOI] [PubMed] [Google Scholar]
- 3.Weill-Engerer S., David J. P., Sazdovitch V., Liere P., Eychenne B., Pianos A., Schumacher M., Delacourte A., Baulieu E. E., Akwa Y. 2002. Neurosteroid quantification in human brain regions: comparison between Alzheimer's and nondemented patients. J. Clin. Endocrinol. Metab. 87: 5138–5143 [DOI] [PubMed] [Google Scholar]
- 4.Labombarda F., Pianos A., Liere P., Eychenne B., Gonzalez S., Cambourg A., De Nicola A. F., Schumacher M., Guennoun R. 2006. Injury elicited increase in spinal cord neurosteroid content analyzed by gas chromatography mass spectrometry. Endocrinology. 147: 1847–1859 [DOI] [PubMed] [Google Scholar]
- 5.Meffre D., Pianos A., Liere P., Eychenne B., Cambourg A., Schumacher M., Stein D. G., Guennoun R. 2007. Steroid profiling in brain and plasma of male and pseudopregnant female rats after traumatic brain injury: analysis by gas chromatography/mass spectrometry. Endocrinology. 148: 2505–2517 [DOI] [PubMed] [Google Scholar]
- 6.Broue F., Liere P., Kenyon C., Baulieu E. E. 2007. A steroid hormone that extends the lifespan of Caenorhabditis elegans. Aging Cell. 6: 87–94 [DOI] [PubMed] [Google Scholar]
- 7.Schumacher M., Liere P., Akwa Y., Rajkowski K., Griffiths W., Bodin K., Sjövall J., Baulieu E. E. 2008. Pregnenolone sulfate in the brain: a controversial neurosteroid. Neurochem. Int. 52: 522–540 [DOI] [PubMed] [Google Scholar]
- 8.Corpéchot C., Robel P., Axelson M., Sjövall J., Baulieu E. E. 1981. Characterization and measurement of dehydroepiandrosterone sulfate in rat brain. Proc. Natl. Acad. Sci. USA. 78: 4704–4707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corpéchot C., Synguelakis M., Talha S., Axelson M., Sjövall J., Vihko R., Baulieu E. E., Robel P. 1983. Pregnenolone and its sulfate ester in the rat brain. Brain Res. 270: 119–125 [DOI] [PubMed] [Google Scholar]
- 10.Majewska M. D., Harrison N. L., Schwartz R. D., Barker J. L., Paul S. M. 1986. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 232: 1004–1007 [DOI] [PubMed] [Google Scholar]
- 11.Wu F. S., Gibbs T. T., Farb D. H. 1991. Pregnenolone sulfate - a positive allosteric modulator at the N-methyl-D-aspartate receptor. Mol. Pharmacol. 40: 333–336 [PubMed] [Google Scholar]
- 12.Monnet F. P., Mahe V., Robel P., Baulieu E. E. 1995. Neurosteroids, via sigma-receptors, modulate the [H-3] norepinephrine release evoked by N-methyl-D-aspartate in the rat hippocampus. Proc. Natl. Acad. Sci. USA. 92: 3774–3778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flood J. F., Morley J. E., Roberts E. 1992. Memory-enhancing effects in male-mice of pregnenolone and steroids metabolically derived from it. Proc. Natl. Acad. Sci. USA. 89: 1567–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shirakawa H., Katsuki H., Kume T., Kaneko S., Akaike A. 2005. Pregnenolone sulphate attenuates AMPA cytotoxicity on rat cortical neurons. Eur. J. Neurosci. 21: 2329–2335 [DOI] [PubMed] [Google Scholar]
- 15.Guarneri P., Russo D., Cascio C., De Leo G., Piccoli T., Sciuto V., Piccoli F., Guarneri R. 1998. Pregnenolone sulfate modulates NMDA receptors, inducing and potentiating acute excitotoxicity in isolated retina. J. Neurosci. Res. 54: 787–797 [DOI] [PubMed] [Google Scholar]
- 16.Liu S., Sjövall J., Griffiths W. J. 2003. Neurosteroids in rat brain: extraction, isolation, and analysis by nanoscale liquid chromatography-electrospray mass spectrometry. Anal. Chem. 75: 5835–5846 [DOI] [PubMed] [Google Scholar]
- 17.Higashi T., Daifu Y., Shimada K. 2001. Studies on neurosteroids XIV. Levels of dehydroepiandrosterone sulfate in rat brain and serum determined with newly developed enzyme-linked immunosorbent assay. Steroids. 66: 865–874 [DOI] [PubMed] [Google Scholar]
- 18.Higashi T., Daifu Y., Ikeshima T., Yagi T., Shimada K. 2003. Studies on neurosteroids XIV. Development of enzyme-linked immunosorbent assay for examining whether pregnenolone sulfate is a veritable neurosteroid. J. Pharm. Biomed. Anal. 30: 1907–1917 [DOI] [PubMed] [Google Scholar]
- 19.Prasad V. V. K., Vegesna S. R., Welch M., Lieberman S. 1994. Precursors of the neurosteroids. Proc. Natl. Acad. Sci. USA. 91: 3220–3223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ågren J. J., Julkunen A., Penttila I. 1992. Rapid separation of serum-lipids for fatty-acid analysis by a single aminopropyl column. J. Lipid Res. 33: 1871–1876 [PubMed] [Google Scholar]
- 21.Sjövall J., Axelson M. 1984. Sample work-up by column techniques. J. Pharm. Biomed. Anal. 2: 265–280 [DOI] [PubMed] [Google Scholar]
- 22.Verleye M., Akwa Y., Liere P., Ladurelle N., Pianos A., Eychenne B., Schumacher M., Gillardin J. M. 2005. The anxiolytic etifoxine activates the peripheral benzodiazepine receptor and increases the neurosteroid levels in rat brain. Pharmacol. Biochem. Behav. 82: 712–720 [DOI] [PubMed] [Google Scholar]
- 23.Van Lier J. E., Smith L. L. 1970. Autoxidation of cholesterol via hydroperoxide intermediates. J. Org. Chem. 35: 2627–2632 [DOI] [PubMed] [Google Scholar]
- 24.Xie C., Lund E. G., Turley S. D., Russell D. W., Dietschy J. M. 2003. Quantitation of two pathways for cholesterol excretion from the brain in normal mice and mice with neurodegeneration. J. Lipid Res. 44: 1780–1789 [DOI] [PubMed] [Google Scholar]
- 25.Lütjohann D., Breuer O., Ahlborg G., Nennesmo I., Siden A., Diczfalusy U., Björkhem I. 1996. Cholesterol homeostasis in human brain: evidence for an age-dependent flux of 24S-hydroxycholesterol from the brain into the circulation. Proc. Natl. Acad. Sci. USA. 93: 9799–9804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cascio C., Prasad V. V. K., Lin Y. Y., Lieberman S., Papadopoulos V. 1998. Detection of P450c17-independent pathways for dehydroepiandrosterone (DHEA) biosynthesis in brain glial tumor cells. Proc. Natl. Acad. Sci. USA. 95: 2862–2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christie W. W.1992. Solid phase extraction columns in the analysis of lipids. Advances in Lipid Methodology-One. Christie W. W., editor The Oily Press Ltd., Ayr, Scotland: 1–17 [Google Scholar]
- 28.Smith L. L.1987. Cholesterol Autoxidation 1981–1986. Chem. Phys. Lipids. 44: 87–125 [DOI] [PubMed] [Google Scholar]
- 29.Smith L. L.1981. Cholesterol Autoxidation. Plenum Press, NewYork and London [Google Scholar]
- 30.Smith L. L.1996. Review of progress in sterol oxidations: 1987–1995. Lipids. 31: 453–487 [DOI] [PubMed] [Google Scholar]
- 31.Schroepfer G. J., Jr 2000. Oxysterols: modulators of cholesterol metabolism and other processes. Physiol. Rev. 80: 361–554 [DOI] [PubMed] [Google Scholar]
- 32.Van Lier J. E., Smith L. L. 1970. Sterol metabolism. XI. Thermal decomposition of some cholesterol hydroperoxides. Steroids. 15: 485–503 [DOI] [PubMed] [Google Scholar]
- 33.Mathur C., Prasad V. V. K., Raju V. S., Welch M., Lieberman S. 1993. Steroids and their conjugates in the mammalian brain. Proc. Natl. Acad. Sci. USA. 90: 85–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muto T., Tanaka J., Miura T., Kimura M. 1983. Iron-catalyzed autoxidation of liposomal cholesterol. Chem. Pharm. Bull. (Tokyo). 31: 1561–1566 [Google Scholar]
- 35.Kamal-Eldin A., Lampi A-M. 2008. Oxidation of cholesterol and phytosterols. Lipid Oxidation Pathways, Vol. 2. Kamal-Eldin A., Min D.B., AOCS Press, Urbana, IL: 111–126 [Google Scholar]
- 36.Kudo K., Emmons G. T., Casserly E. W., Via D. P., Smith L. C., St. Pyrek J., Schroepfer G. J., Jr 1989. Inhibitors of sterol synthesis. Chromatography of acetate derivatives of oxygenated sterols. J. Lipid Res. 30: 1097–1111 [PubMed] [Google Scholar]
- 37.Wasilchuk B. A., Le Quesne P. W., Vouros P. 1992. Monitoring cholesterol autoxidation processes using multideuterated cholesterol. Anal. Chem. 64: 1077–1087 [DOI] [PubMed] [Google Scholar]
- 38.Jo D. H., Abdallah M. A., Young J., Baulieu E. E., Robel P. 1989. Pregnenolone, dehydroepiandrosterone, and their sulfate and fatty acid esters in the rat brain. Steroids. 54: 287–297 [DOI] [PubMed] [Google Scholar]
- 39.Shimada K., Mukai Y. 1998. Studies on neurosteroids VII. Determination of pregnenolone and its 3-stearate in rat brains using high-performance liquid chromatography atmospheric pressure chemical ionization mass spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 714: 153–160 [PubMed] [Google Scholar]
- 40.Iwamori M., Moser H. W., Kishimoto Y. 1976. Cholesterol sulfate in rat tissues. Tissue distribution, developmental change and brain subcellular localization. Biochim. Biophys. Acta. 441: 268–279 [DOI] [PubMed] [Google Scholar]
- 41.Cutler G. B., Jr., Glenn M., Bush M., Hodgen G. D., Graham C. E., Loriaux D. L. 1978. Adrenarche: a survey of rodents, domestic animals, and primates. Endocrinology. 103: 2112–2118 [DOI] [PubMed] [Google Scholar]
- 42.Axelson M., Graham C. E., Sjövall J. 1984. Identification and quantitation of steroids in sulphate fractions from plasma of pregnant chimpanzee, orangutan, and Rhesus monkey. Endocrinology. 114: 337–344 [DOI] [PubMed] [Google Scholar]
- 43.Young J., Corpechot C., Perche F., Eychenne B., Haug M., Baulieu E. E., Robel P. 1996. Neurosteroids in the mouse brain: behavioral and pharmacological effects of a 3 beta-hydroxysteroid dehydrogenase inhibitor. Steroids. 61: 144–149 [DOI] [PubMed] [Google Scholar]
- 44.Young J., Corpechot C., Haug M., Gobaille S., Baulieu E. E., Robel P. 1991. Suppressive effects of dehydroepiandrosterone and 3-beta-methyl-androst-5-en-17-one on attack towards lactating female intruders by castrated male-mice. Brain neurosteroids. Biochem. Biophys. Res. Commun. 174: 892–897 [DOI] [PubMed] [Google Scholar]
- 45.Shimada K., Yago K. 2000. Studies on neurosteroids X. Determination of pregnenolone and dehydroepiandrosterone in rat brains using cas chromatography-mass spectrometry-mass spectrometry. J. Chromatogr. Sci. 38: 6–10 [DOI] [PubMed] [Google Scholar]
- 46.Vallee M., Mayo W., Darnaudery M., Corpechot C., Young J., Koehl M., Le Moal M., Baulieu E. E., Robel P., Simon H. 1997. Neurosteroids: deficient cognitive performance in aged rats depends on low pregnenolone sulfate levels in the hippocampus. Proc. Natl. Acad. Sci. USA. 94: 14865–14870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guarneri P., Guarneri R., Cascio C., Pavasant P., Piccoli F., Papadopoulos V. 1994. Neurosteroidogenesis in rat retinas. J. Neurochem. 63: 86–96 [DOI] [PubMed] [Google Scholar]
- 48.Inai Y., Nagai K., Ukena K., Oishi T., Tsutsui K. 2003. Seasonal changes in neurosteroid concentrations in the amphibian brain and environmental factors regulating their changes. Brain Res. 959: 214–225 [DOI] [PubMed] [Google Scholar]
- 49.Kazihnitkova H., Tejkalova H., Benesova O., Bicikova M., Hill M., Hampl R. 2004. Simultaneous determination of dehydroepiandrosterone, its 7-hydroxylated metabolites, and their sulfates in rat brain tissues. Steroids. 69: 667–674 [DOI] [PubMed] [Google Scholar]
- 50.Griffin L. D., Gong W. H., Verot L., Mellon S. H. 2004. Niemann-Pick type C disease involves disrupted neurosteroidogenesis and responds to allopregnanolone. Nat. Med. 10: 704–711 [DOI] [PubMed] [Google Scholar]
- 51.Tagawa N., Sugimoto Y., Yamada J., Kobayashi Y. 2006. Strain differences of neurosteroid levels in mouse brain. Steroids. 71: 776–784 [DOI] [PubMed] [Google Scholar]
- 52.Bélanger A., Couture J., Caron S., Roy R. 1990. Determination of nonconjugated and conjugated steroid levels in plasma and prostate after separation on C-18 columns. Ann. N. Y. Acad. Sci. 595: 251–259 [DOI] [PubMed] [Google Scholar]
- 53.Wang M. D., Wahlström G., Backström T. 1997. The regional brain distribution of the neurosteroids pregnenolone and pregnenolone sulfate following intravenous infusion. J. Steroid Biochem. Mol. Biol. 62: 299–306 [DOI] [PubMed] [Google Scholar]
- 54.Vallee M., Rivera J. D., Koob G. F., Purdy R. H., Fitzgerald R. L. 2000. Quantification of neurosteroids in rat plasma and brain following swim stress and allopregnanolone administration using negative chemical ionization gas chromatography/mass spectrometry. Anal. Biochem. 287: 153–166 [DOI] [PubMed] [Google Scholar]
- 55.O'Dell L. E., Alomary A. A., Vallee M., Koob G. F., Fitzgerald R. L., Purdy R. H. 2004. Ethanol-induced increases in neuroactive steroids in the rat brain and plasma are absent in adrenalectomized and gonadectomized rats. Eur. J. Pharmacol. 484: 241–247 [DOI] [PubMed] [Google Scholar]
- 56.Caldeira J. C., Wu Y., Mameli M., Purdy R. H., Li P. K., Akwa Y., Savage D. D., Engen J. R., Valenzuela C. F. 2004. Fetal alcohol exposure alters neurosteroid levels in the developing rat brain. J. Neurochem. 90: 1530–1539 [DOI] [PubMed] [Google Scholar]
- 57.Leonelli E., Bianchi R., Cavaletti G., Caruso D., Crippa D., Garcia-Segura L. M., Lauria G., Magnaghi V., Roglio I., Melcangi R. C. 2007. Progesterone and its derivatives are neuroprotective agents in experimental diabetic neuropathy: a multimodal analysis. Neuroscience. 144: 1293–1304 [DOI] [PubMed] [Google Scholar]
- 58.Ebner M. J., Corol D. I., Havlikova H., Honour J. W., Fry J. P. 2006. Identification of neuroactive steroids and their precursors and metabolites in adult male rat brain. Endocrinology. 147: 179–190 [DOI] [PubMed] [Google Scholar]