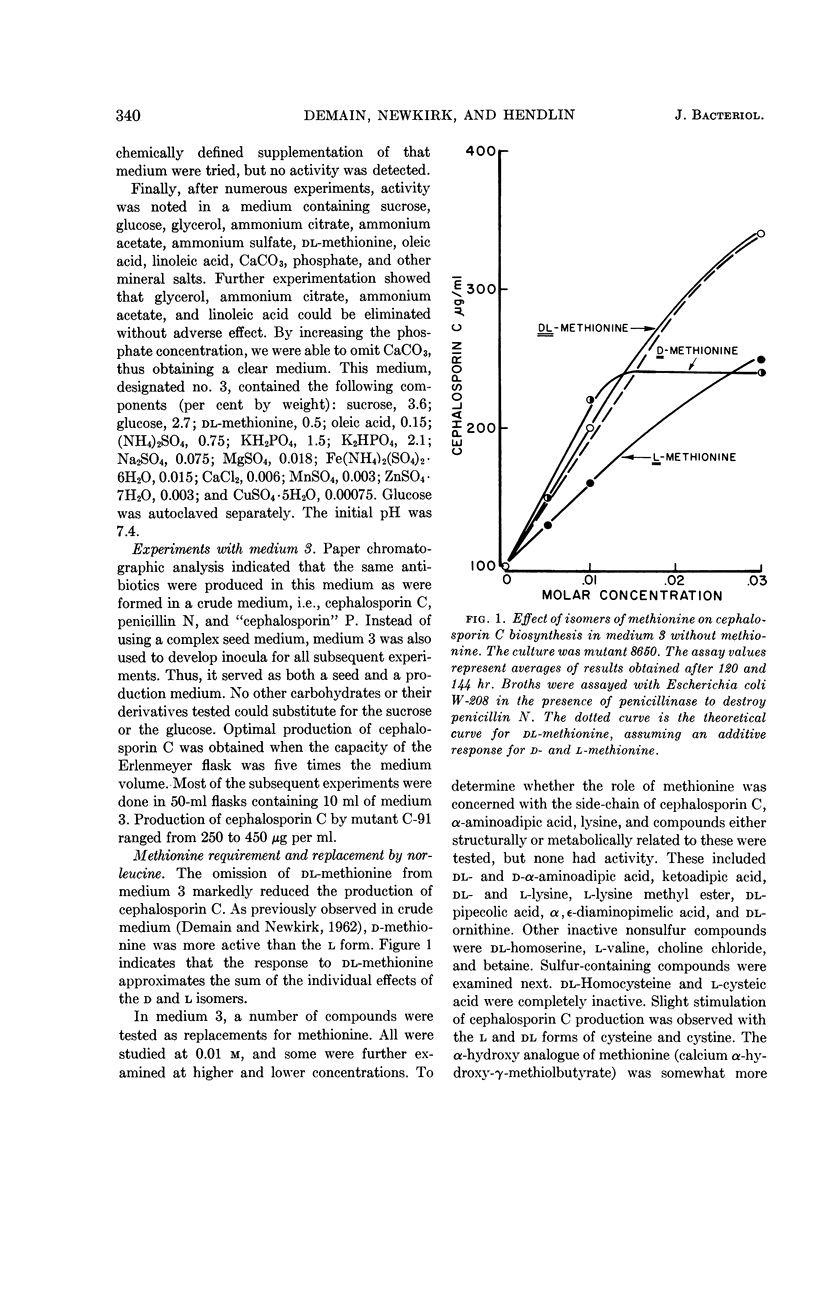
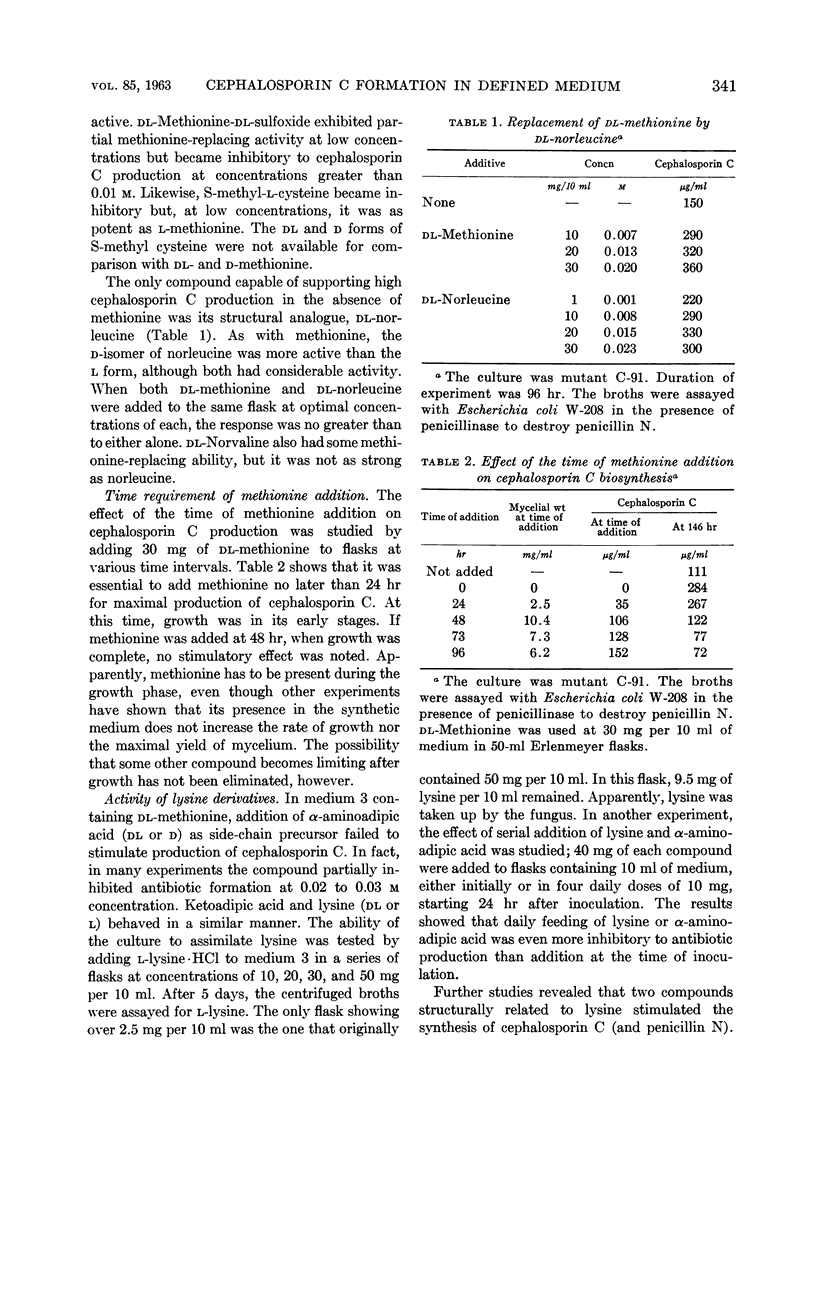
Abstract
Demain, A. L. (Merck Sharp & Dohme Research Laboratories, Rahway, N.J.), Joanne F. Newkirk, and D. Hendlin. Effect of methionine, norleucine, and lysine derivatives on cephalosporin C formation in chemically defined media. J. Bacteriol. 85: 339–344. 1963.—Chemically defined media were developed for production of cephalosporin C by Cephalosporium sp. In such media, the requirement for methionine can be satisfied by norleucine. Further stimulation of antibiotic production was obtained with the lysine derivatives ε-N-acetyl-l-lysine and ε-aminocaproic acid but not with lysine itself. Also inactive were α-aminoadipic and ketoadipic acids. Other lysine derivatives were found to inhibit cephalosporin C production at 0.01 m. The final medium supported the production of approximately 0.5 g of cephalosporin C per liter of medium.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAHAM E. P., NEWTON G. G. New penicillins, cephalosporin C, and penicillinase. Endeavour. 1961 Apr;20:92–100. [PubMed] [Google Scholar]
- BHUYAN B. K., JOHNSON M. J. Chemically defined media for synnematin production. J Bacteriol. 1958 Oct;76(4):376–384. doi: 10.1128/jb.76.4.376-384.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEMAIN A. L., NEWKIRK J. F. Biosynthesis of cephalosporin C. Appl Microbiol. 1962 Jul;10:321–325. doi: 10.1128/am.10.4.321-325.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAVANAGH F., TUNIN D., WILD G. D-methionine and the biosynthesis of cephalosporin N. Arch Biochem Biophys. 1958 Oct;77(2):268–274. doi: 10.1016/0003-9861(58)90075-4. [DOI] [PubMed] [Google Scholar]
- MEISTER A. Enzymatic transamination reactions involving arginine and ornithine. J Biol Chem. 1954 Feb;206(2):587–596. [PubMed] [Google Scholar]
- NARA T., JOHNSON M. J. Production, purification, and characterization of synnematin. J Bacteriol. 1959 Feb;77(2):217–226. doi: 10.1128/jb.77.2.217-226.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuberger A., Sanger F. The metabolism of lysine. Biochem J. 1944;38(1):119–125. doi: 10.1042/bj0380119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAGE E., GINGRAS R., GAUDRY R. L'action anémiante de deux antagonistes de la lysine; l'hexahomosérine et l'acide alpha-aminoadipique. Can J Res. 1949 Dec;27(6 SECT):364–373. [PubMed] [Google Scholar]
- ROWBURY R. J., WOODS D. D. Further studies on the repression of methionine synthesis in Escherichia coli. J Gen Microbiol. 1961 Jan;24:129–144. doi: 10.1099/00221287-24-1-129. [DOI] [PubMed] [Google Scholar]
- SCHAIBERGER G. E., FERRARI A. Automatic enzymatic analysis for L-lysine via decarboxylation. Ann N Y Acad Sci. 1960 Jul 22;87:890–893. doi: 10.1111/j.1749-6632.1960.tb23247.x. [DOI] [PubMed] [Google Scholar]
- WALKER J. B. Canavanine and homoarginine as antimetabolites of arginine and lysine in yeast and algae. J Biol Chem. 1955 Jan;212(1):207–215. [PubMed] [Google Scholar]


