Abstract
Protein acetylation is a widespread modification that is mediated by site-selective acetyltransferases. KATs (lysine Nϵ-acetyltransferases), modify the side chain of specific lysines on histones and other proteins, a central process in regulating gene expression. Nα-terminal acetylation occurs on the ribosome where the α amino group of nascent polypeptides is acetylated by NATs (N-terminal acetyltransferase). In yeast, three different NAT complexes were identified NatA, NatB, and NatC. NatA is composed of two main subunits, the catalytic subunit Naa10p (Ard1p) and Naa15p (Nat1p). Naa50p (Nat5) is physically associated with NatA. In man, hNaa50p was shown to have acetyltransferase activity and to be important for chromosome segregation. In this study, we used purified recombinant hNaa50p and multiple oligopeptide substrates to identify and characterize an Nα-acetyltransferase activity of hNaa50p. As the preferred substrate this activity acetylates oligopeptides with N termini Met-Leu-Xxx-Pro. Furthermore, hNaa50p autoacetylates lysines 34, 37, and 140 in vitro, modulating hNaa50p substrate specificity. In addition, histone 4 was detected as a hNaa50p KAT substrate in vitro. Our findings thus provide the first experimental evidence of an enzyme having both KAT and NAT activities.
Introduction
Protein acetylation is performed by two distinct types of enzymes, histone acetyltransferases/lysine acetyltransferases (KATs)2 and N-terminal acetyltransferases (NATs). It is widely believed that the NAT complexes modify exclusively proteins at the N termini, whereas the KAT enzymes only lysine side chains.
KAT enzymes acetylate the ϵ-amino group of specific lysines on histone proteins as a crucial part of regulating gene expression (1). The KAT family consists of several members, but the p300 enzyme and its paralogue, CREB-binding protein (CBP), have been identified to account for most of the KAT activity (1). Dysregulation of p300/CBP activity has been implicated in several types of cancers and cardiac diseases (2).
The NAT complexes are physically associated with ribosomes and transfer the acetyl group from acetyl-CoA to the N-terminal α-amino group of newly synthesized proteins (3, 4). In yeast and humans, three different NAT complexes were identified, NatA, NatB, and NatC, whose activities were found to account for the majority of Nα-terminal acetylation (5).
The yeast NatA complex is composed of the catalytic subunit Naa10p (earlier known as Ard1p (6)) and the auxiliary subunit Naa15p (earlier known as Nat1p (6, 7)). The yNatA complex has a broad substrate specificity acetylating proteins with Ser, Ala, Gly, Val, Cys, or Thr N termini (8). A third subunit, Naa50p (earlier known as Nat5p (6)), was found to be physically associated with the yNatA complex, but no influence on the NatA activity was detected (4). The presence of a third subunit in the NatA complex has also been demonstrated in the fruit fly, dSan (9), and human, hNaa50p (hNat5p) (10).
Recently, human Naa50p was reported to acetylate itself and purified chromosome pellets in vitro (11). Loss of this protein led to chromatid cohesion defects in both the fruit fly and humans (9, 11). In addition, a recent study of Naa50p in the fruit fly demonstrated its importance in chromosome resolution and segregation (12).
HeLa cells with small interfering RNA-mediated down-regulation of hNAA10 and hNAA15, showed decreased acetylation levels of several proteins matching the predicted NatA specificity (8). In addition, one protein, having an N-terminal sequence that deviated from the NatA substrate pattern, was also less acetylated in hNAA10/hNAA15 knockdown cells. This protein, hnRNP F, has the N-terminal protein sequence MLGPEGG (8). It has been established that knockdown of either hNAA10 or hNAA15 in HeLa cells causes a reduced level of the hNaa50p protein (11). Therefore hnRNP F may represent a potential hNaa50p substrate in vivo.
In this study, the important issue of whether hNaa50p is a bifunctional NAT and KAT enzyme has been addressed. Based on in vitro activity assays and mass spectrometric analyses, it is demonstrated that hNaa50p indeed possesses both NAT and KAT activities. Autoacetylation of hNaa50p was observed in lysine residues 34, 37, and 140, and hNaa50p specifically acetylated histone 4 in vitro. The preferred N-terminal sequence for the NAT activity of hNaa50p matches the sequence of the hypothesized in vivo substrate, hnRNP F.
EXPERIMENTAL PROCEDURES
Xpress-hNaa50p Immunoprecipitation and in Vitro Acetyltransferase Assay
Overexpression and immunoprecipitation of Xpress-hNaa50p were done as described (13). The acetyltransferase assay used to test the Xp-hNaa50p activity and the initial peptide screen were performed as described previously (13). The enzyme was incubated in 250 μl of KAT buffer (50 mm Tris-HCl (pH 8.5), 10% glycerol, 1 mm EDTA) with 5 μl of [1-14C]acetyl-CoA (56 mCi/mmol, GE Healthcare) and 2.5 μl of custom made peptide (2 mm; Biogenes) as substrates. After a 2-h incubation at 37 °C, the peptides were isolated using SP-Sepharose resin (Sigma). Incorporation of acetyl groups was determined by scintillation counting. Because the first residues of the peptide seemed to be most important for enzyme specificity (5), all peptides used in this study vary only within the 7 first N-terminal positions. The next 17 amino acids, indicated by “RRR,” are identical for all peptides and resemble the sequence of adrenocorticotropic hormone (ACTH), except, all Lys residues have been replaced by Arg to minimize the potential interference by Nϵ-acetylation. The positively charged Arg residues facilitate peptide solubility and effective isolation by cation exchange Sepharose beads. See supplemental Table S1 for peptide sequence information.
Plasmid Construction, Protein Expression, and Purification
Prokaryotic expression and purification of recombinant protein was conducted as described (14). The cDNA encoding hNaa50p was cloned into pETM-glutathione S-transferase (GST) (G. Stier, EMBL, Germany) for expression in Escherichia coli. The plasmid encoding Xpress-tagged hNaa50p was previously described (10).
Determination of Steady-state Kinetic Constants by Reverse Phase HPLC
The enzyme activity of hNaa50p was determined by reverse phase HPLC as described (15). Elution times of unmodified peptides were established by injecting pure unmodified peptide on the HPLC system, collecting fractions that corresponded to the absorbance peak of the peptide, and verification of the mass by mass spectrometry (see Fig. 4A). Kinetic parameters were calculated by nonlinear regression analysis using the SigmaPlot Technical Graphing Software (SPSS Inc.).
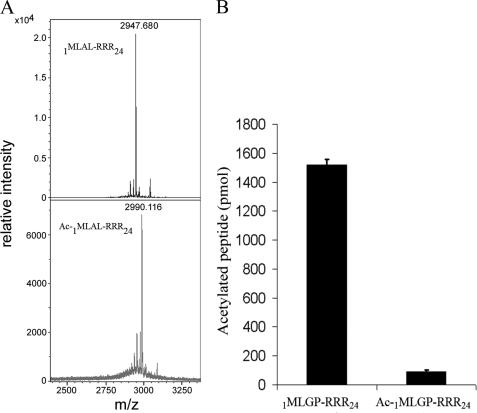
FIGURE 4.
Verification of hNaa50p Nα-acetyltransferase activity. A, MALDI-MS spectra (mass range 2.400–3.350) of non-acetylated (upper panel), and acetylated 1MLAL-RRR24 peptide (lower panel) after in vitro acetylation with GST-hNaa50p. Monoisotopic peaks are labeled with their respective m/z ratios. B, purified GST-hNaa50p was incubated for 2 h at 37 °C with 1MLGP-RRR24 peptide and a chemically N-terminal acetylated version of the 1MLGP-RRR24 peptide (Ac-1MLGP-RRR24). After incubation, the oligopeptides were isolated with SP-Sepharose beads, washed three times in 0.5 m acetic acid, and analyzed with scintillation counting. Error bars (S.D.) are based on three independent experiments.
Mass Spectrometric Verification of Acetylation
Elution times of acetylated peptides were determined by collecting fractions of corresponding absorbance peaks and verifying the molecular mass by MS. The MS data showed increased molecular masses of ∼42 Da, consistent with the acetylation of one residue. Prior to the MS analyses, the samples were diluted 1:1 with a matrix solution consisting of 8 μg/μl alfa-cyano-4-hydroxycinnamic acid, 60% acetonitrile, 15% methanol, and 0.1% trifluoroacetic acid. 1 μl of the sample/matrix mixtures was placed on the target plate (Bruker Daltonics, MTP 384 polished steel). The MALDI-TOF MS analyses were performed with an Ultraflex mass spectrometer (Bruker Daltronics) in a positive-ion mode. Peptide calibration standard (Bruker Daltonics) was mixed 1:1 with matrix solution and placed on the target along with the samples and used for external calibration.
Xpress-hNaa50p and purified GST-hNaa50p separated by SDS-PAGE were excised from gels and washed twice in 50 mm ammonium bicarbonate and 50% acetonitrile. Prior to protease treatment the washed gel pieces were dehydrated by vacuum centrifugation and subsequently treated with dithiothreitol and iodoacetamide for reduction and alkylation of cysteines as described (16). “In gel” digestion with Lys-C endoproteinase was carried out essentially as described by the manufacturer (Roche Applied Science). The digested peptides were purified and concentrated as described (17), and MALDI-TOF MS and MS/MS analyses were performed with an Ultraflex mass spectrometer (Bruker Daltronics) and a matrix solution consisting of 8 μg/μl alfa-cyano-4-hydroxycinnamic acid, 60% acetonitrile, 15% methanol, and 0.1% trifluoroacetic acid.
Generation of GST-hNaa50p Mutants
Mutagenesis was performed as recommended by Stratagene. See supplemental data for primer sequences. The identities of GST-hNaa50p mutants were verified by DNA sequencing.
In Vitro Nϵ-Acetylation Assays
22.5 μl of purified hNaa50p (0.8 mg/ml) was mixed with 37.5 μl of [1-14C]acetyl-CoA (56 mCi/mmol, GE Healthcare) and 262.5 μl of KAT buffer. The mixture was distributed into 2 tubes. One tube was incubated at 37 °C, and aliquots were collected after 0, 30, 60, 90, and 120 min. The other tube was incubated at 4 °C, and an aliquot was collected after 120 min. The enzyme activity was quenched by adding SDS-PAGE sample buffer.
For kinetic analyses of the autoacetylation reaction, 5 μm GST-hNaa50p was incubated with 500 μm acetyl-CoA containing [1-14C]acetyl-CoA and KAT buffer at 37 °C. Aliquots were collected at six different time points and the enzyme reaction stopped by cooling and adding trifluoroacetic acid to a final concentration of 1% (v/v). Autoacetylated GST-hNaa50p was isolated by reverse phase HPLC and analyzed by scintillation counting.
Autoacetylation of GST-hNaa50p WT, or its R84A and Y124F mutants was performed adding 10 μl of the purified GST fusion protein (11 μm) to 30 μl of KAT buffer and 5 μl of non-radioactive acetyl-CoA (5 mm). The samples were incubated at 37 °C for 1 h, and the activity quenched by adding SDS-PAGE sample buffer. Acetylation was detected by Western blotting using an anti-acLys antibody (Upstate). The NAT assay was performed in the presence of 100 μm acetyl-CoA, 30 μm 1MLGP-RRR24 peptide, and 50 nm of each enzyme in KAT buffer. The samples were incubated at 37 °C for 30 min prior to adding trifluoroacetic acid to 1% (v/v). The resulting acetylation was analyzed using reverse phase HPLC as described above.
For autoacetylation assays with GST-hNaa50p WT and the different lysine mutants, 2.4 μm protein was mixed with 500 μm acetyl-CoA in the above mentioned KAT buffer. The samples were incubated at 37 °C for 1 h, and the activity quenched by adding SDS-PAGE sample buffer. Acetylation was detected by Western blotting using an anti-acLys antibody (Upstate).
To explore the effect of autoacetylation on the NAT activity of hNaa50p, 10 μm purified GST-hNaa50p was incubated at 37 °C for 120 h with either 300 μm acetyl-CoA or H2O as control in KAT buffer. An extensive incubation was chosen to ensure a high yield of autoacetylation. To determine Km values for 1MLGP-RRR24 or 1MIGP-RRR24 using these enzyme variants, 30 nm autoacetylated GST-hNaa50p was incubated with either 1MLGP-RRR24 (20–400 μm) or 1MIGP-RRR24 (50–500 μm) at 37 °C for 30 min with saturating levels of acetyl-CoA (300 μm). The activity was quenched by adding trifluoroacetic acid to a final concentration of 1% (v/v).
The KAT activity of hNaa50p toward other proteins was measured using 2 μm pre-autoacetylated hNaa50p, non-preautoacetylated hNaa50p, or bovine serum albumin in the presence of 2 μm of the indicated protein substrates and 80 μm acetyl-CoA. The samples were incubated at 37 °C, and aliquots were collected after 0, 30, and 60 min. The activity was quenched by adding SDS-PAGE sample buffer. Acetylation was detected by Western blotting using an anti-acLys antibody (Upstate).
RESULTS
hNaa50p Exhibits Preferred NAT Activity on Substrates Sharing the N Terminus with hnRNP F
In vitro acetyltransferase assays were performed using immunoprecipitated Xp-hNaa50p from human embryonic kidney 293 cells. Xp-hNaa50p did not extensively acetylate a 1SESS-RRR24 oligopeptide, a known hNatA substrate (8), whereas the 1MLGP-RRR24 oligopeptide was readily acetylated (Fig. 1). This experiment indicates that hNaa50p expressed in cell cultures has Nα-acetyltransferase activity toward an oligopeptide whose N terminus is identical to that of the hnRNP F protein.
FIGURE 1.
In vitro acetyltransferase activity of immunoprecipitated Xpress-hNaa50p. Immunoprecipitated Xpress-hNaa50p from transfected human embryonic kidney 293 cells was incubated with [1-14C]acetyl-CoA and the oligopeptides 1MLGP-RRR24 or 1SESS-RRR24 for 2 h at 37 °C. dH2O used as negative control (−). After incubation, the oligopeptides were isolated with SP-Sepharose beads, washed three times with 0.5 m acetic acid, and analyzed with scintillation counting. Error bars (S.D.) are based on three independent experiments. For details see “Experimental Procedures.”
Recombinant hNaa50p Preferentially Acetylates the N Termini of Met-Leu Oligopeptides
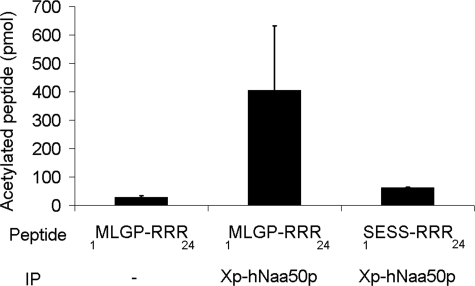
To establish the substrate specificity of hNaa50p in comparison to known NAT complexes, oligopeptides with N termini matching known NatA, NatB, and NatC substrates were tested. The putative hNaa50p substrate 1MLGP-RRR24 and two peptides known to be Nα-acetylated in vivo, but by unknown enzymes (8), were also included. For these experiments, hNaa50p N-terminal fused to GST was expressed in E. coli. The purified enzyme most efficiently acetylated oligopeptides with Met-Leu N termini corresponding to putative substrates of hNatC and hNaa50p (18) (Fig. 2). The NatA substrates, 1SESS-RRR24 and 1SYSM-RRR24, were not acetylated (Fig. 2).
FIGURE 2.
hNaa50p/hSan acetylation of peptides with the N termini Met-Leu or Met-Asp. Purified GST-hNaa50p was incubated with selected oligopeptides and [1-14C]acetyl-CoA for 2 h at 37 °C. After incubation, the oligopeptides were isolated with SP-Sepharose beads, washed three times with 0.5 m acetic acid, and analyzed with scintillation counting. Activity detected in the negative control (dH2O) was subtracted. The NAT complexes that are expected to perform the acetylation in vivo are given. Error bars (S.D.) are based on three independent experiments. For details see “Experimental Procedures.”
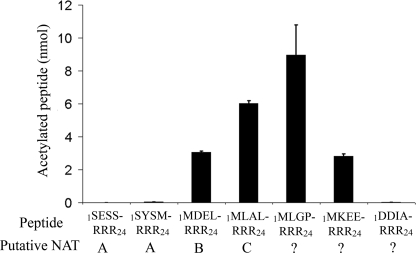
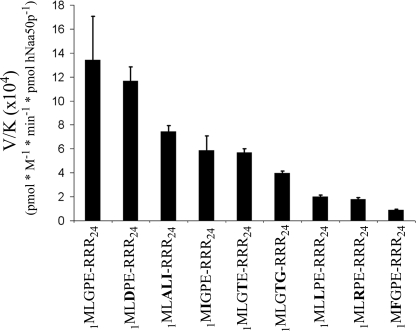
To characterize the enzyme specificity in more detail, several additional oligopeptides with different N termini were tested (Fig. 3 and Tables 1 and 2). The calculating specificity constants (V/K), show that hNaa50p has high specificity toward oligopeptides starting with ML and with a relatively small residue in the third position. Peptides with the N-terminal sequence MD and MK were not acetylated at detectable levels (Table 2). Interestingly, the 1MLGP-RRR24 (representing hnRNP F) and 1MLDP-RRR24 (1MLGP-RRR24 G3D) peptides were the best substrates. Table 1 shows kinetic parameters obtained for acetyl-CoA and the best in vitro oligopeptide substrate found so far, 1MLGP-RRR24. The Km for acetyl-CoA was estimated to be 6.9 μm, whereas the Km for the 1MLGP-RRR24 peptide was determined to be ∼75 μm with a kcat of 7 min−1.
FIGURE 3.
GST-hNaa50p specificity constants (V/K). 70 nm GST-hNaa50p was incubated with selected oligopeptide substrates for 30 min at 37 °C with saturated levels of acetyl-CoA (300 μm). The acetylation kinetics were determined with reverse phase HPLC. V/K is the Vmax/Km (oligopeptides). Error bars indicate the S.D. Experiments were performed in triplicate. For details see “Experimental Procedures.”
TABLE 1.
Preferred substrates of hNaa50p-mediated acetylation
a The kinetic parameters for acetyl-CoA were determined in triplicate in the presence of the 1MLGP-RRR24 peptide (150 μm). The other values were determined in duplicate or triplicate with ± S.D. For experimental details, see “Experimental Procedures” and supplemental data.
b Experiments were performed in duplicate or triplicate. The recorded values are average ± S.D.
TABLE 2.
hNaa50p steady-state kinetic parameters for tested oligopeptide substrates
Experiments were performed in duplicate or triplicate. The recorded values are average ± S.D. The steady-state kinetic constants for the oligopeptides were determined in the presence of 300 μm acetyl-CoA. For more experimental details, see “Experimental Procedures” and supplemental data.
| Peptide substrates (N terminus) | Km | kcat |
|---|---|---|
| μm | min−1 | |
| 1MLGPE-RRR24 | 79 ± 6 | 7.2 ± 0.7 |
| 1MLDPE-RRR24 | 91 ± 12 | 7.2 ± 1.6 |
| 1MIGPE-RRR24 | 185 ± 20 | 10.9 ± 3a |
| 1MLALI-RRR24 | 190 ± 70 | 6.8 ± 0.4 |
| 1MLGTG-RRR24 | 320 ± 20 | 2.3 ± 0.2 |
| 1MLGTE-RRR24 | 416 ± 185 | 5.6 ± 0.1 |
| 1MLRPE-RRR24 | 460 ± 94 | —b |
| 1MLLPE-RRR24 | 478 ± 305 | —b |
| 1MFGPE-RRR24 | 3734 ± 815 | —b |
| 1MKEEV-RRR24 | —c | —c |
| 1MDELF-RRR24 | —c | —c |
a Vmax value (picomole of acetylated peptide min−1 pmol of hNaa50p−1) is given.
b Due to high Km values for these oligopeptide, it was not possible to determine kcat at [peptide] ≫ Km.
c These oligopeptides were tested as substrates, but were not acetylated to a detectable level under our assay conditions.
To verify that the observed modification catalyzed by hNaa50p was indeed the transfer of an acetyl group to the peptide N terminus, modified and unmodified 1MLAL-RRR24 peptides were separated by reverse phase HPLC and analyzed by MALDI-TOF mass spectrometry (Fig. 4A). The resulting MS profiles of non-acetylated 1MLAL-RRR24 peptide (upper panel) revealed a MALDI molecular ion of m/z 2947.68, which is consistent with the theoretical molecular mass of the peptide (2947.7 Da). Acetylation of the 1MLAL-RRR24 peptide (lower panel) should increase its molecular mass by 42 Da. Indeed a MALDI molecular ion of m/z 2990.12 was detected in the MS analyses (Fig. 4A).
Chemically N-terminal-acetylated 1MLGP-RRR24 peptide was then used as substrate in the hNaa50p acetylation assay to test whether additional acetylation could occur. The N-terminal acetylated 1MLGP-RRR24 (Ac-1MLGP-RRR24) peptide was not further acetylated as shown in Fig. 4B. These data further suggest that hNaa50p is an authentic NAT acetylating proteins at their N termini.
Autoacetylation of hNaa50p at Lysine 34, 37, and 140 in Vitro and Detection of Acetylated Lysine 34 and 140 in Vivo
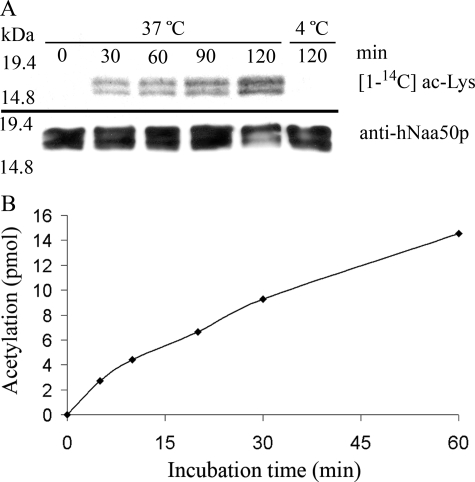
hNaa50p was earlier reported to perform autoacetylation, indicative of an Nϵ-acetyltransferase activity (11). To study if hNaa50p is acetylated in vivo, Xp-hNaa50p expressed in HeLa cells was isolated and analyzed by mass spectrometry. Two peptides in the mass spectrometry spectra showed m/z ratios at 2173.19 and 1987.08, corresponding to acetylated Lys34 (supplemental Fig. S1A) and Lys140 (supplemental Fig. S1B), respectively. This acetylation could indicate that hNaa50p is a substrate for an unknown KAT, or that it acetylates itself. To test the possibility of autoacetylation, hNaa50p from an E. coli extract was purified and incubated with [1-14C]acetyl-CoA at 37 or 4 °C. Incubation at 37 °C showed a time-dependent increase in radioactively labeled protein, whereas the hNaa50p protein being incubated at 4 °C had no detectable level of acetylation (Fig. 5A). To quantify the autoacetylation reaction, purified GST-hNaa50p was incubated with [1-14C]acetyl-CoA at 37 °C, and aliquots were collected at 6 different time points. GST-hNaa50p was isolated by reverse phase HPLC and the extent of modification determined by scintillation counting (Fig. 5B). Given that 150 pmol of GST-hNaa50p incorporated 2.7 pmol of acetyl groups over a time period of 5 min, this assay suggests an enzyme turnover that is almost 2000 times slower than the highest recorded value for the acetylation of NAT substrate (Table 2).
FIGURE 5.
The in vitro autoacetyltransferase activity of purified wild type hNaa50p. A, purified hNaa50p was incubated with [1-14C]acetyl-CoA for the indicated times and analyzed by autoradiography and Western blotting with anti-hNaa50p antibody. Upper panel indicates the increased level of [1-14C]acetyl incorporation as a response to increased incubation time at 37 °C. No acetylation is detectable in the sample incubated for 120 min at 4 °C. Lower panel represents Western blot analyses with anti-hNaa50p showing an equal amount of hNaa50p present in the assay. B, purified GST-hNaa50p (3 μm) was incubated with acetyl-CoA (500 μm with 33% radioactive acetyl-CoA) at 37 °C, and samples were collected at 6 different time points. After isolating the oligopeptides with reverse phase HPLC, the acetylation was quantified by scintillation counting of incorporated radioactivity.
Because the autoacetylation assay does not discriminate between Nα-terminal acetylation and Nϵ-acetylation, the site(s) of modification were identified. GST-hNaa50p was autoacetylated for 60 min and analyzed by mass spectrometry. Moreover, the protein was digested with the Lys-C endoprotease, which is unable to cleave C-terminal acetylated lysines (19). The mass spectrometry analyses of the resulting peptides revealed three MALDI molecular ions of m/z 1552.769, 1986.980, and 2173.072, corresponding to predicted peptides with a 42-Da increase compared with their theoretical molecular masses (supplemental Fig. S2). An acetylated internal lysine was verified in all peptides by MS/MS analyses and established y- and b-ion series covering the acetylated lysine residues (supplemental Fig. S2, B–D). In addition, a specific acetyllysine marker ion at m/z 126 was detected in all MALDI MS/MS experiments further confirming that an acetylated lysine is present in the peptides (19). Sequence mapping of hNaa50p using the MS results identified lysines 34 and 140 as acetylated, the same residues as detected in vivo. In addition, lysine 37 was also observed as acetylated in vitro.
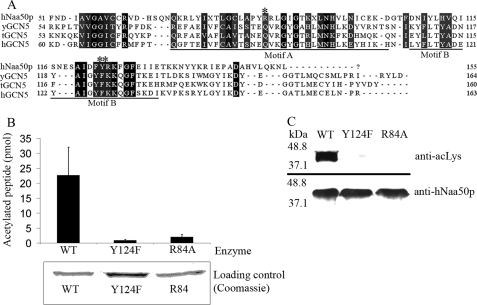
In KAT assays, a significant amount of acetyl-CoA spontaneously reacts with substrate lysines (20), a reaction that can lead to a false-positive interpretation of the acetylation assays. To verify that the observed autoacetylation of hNaa50p was due to enzymatic activity and not a chemical acetylation, we attempted to generate mutants of hNaa50p that were expected to have reduced enzyme activity. Alignment of the amino acid sequence of hNaa50p with proteins in the GCN5 family from yeast, Tetrahymena, and human, show that Arg84 of hNaa50p is central in the acetyl-CoA binding motif 84RRLGIG (Fig. 6A). The same alignment indicated that Tyr124 is positioned in Motif B, which is a region of the GCN5 superfamily that was predicted to be essential for catalytic activity (11, 21). Therefore, a mutant hNaa50p was generated in which Arg84 was substituted by Ala, and Tyr124 was replaced by Phe. In a NAT assay, using 1MLGP-RRR24 as oligopeptide substrate, more than 80% of the activity was lost for the R84A mutant, whereas the Y124F mutant had ∼90% less activity than the wild type enzyme (Fig. 6B). Although both mutants still contained the three lysine residues identified as autoacetylation targets, a clear decrease in the acetylation signal was observed (Fig. 6C). Therefore, the contribution of non-enzymatic acetylation in the KAT assays presented is negligible. Collectively these data indicate that hNaa50p is acetylated in vivo and that it can perform autoacetylation in vitro in the same residues, consistent with a possible in vivo autoacetylation.
FIGURE 6.
Enzymatic activity of hNaa50p Arg84 to Ala or Tyr124 to Phe mutants. A, alignment of amino acid sequences of hNaa50p with GCN5 from Saccharomyces cerevisiae (yGCN5), Tetrahymena (tGCN5), and human (hGCN5). The alignment indicates that Arg84 (*) and Tyr124 (**) are located in Motif A and Motif B, respectively, of the GCN5 protein fold. B, in vitro acetyltransferase assay comparing the enzyme activity of purified GST-hNat5 WT with GST-hNat5 Arg84 to Ala mutant (R84A) and GST-hNat5 Tyr124 to Phe mutant (Y124F). The 1MLGP-RRR24 oligopeptide (30 μm) was used as substrate. Error bars (S.D.) are based on three independent experiments. Coomassie-stained SDS-PAGE verifying an equal amount of each enzyme. C, purified GST-hNaa50p WT and mutants were incubated for 1 h 37 °C with acetyl-CoA. Western blot analyses using anti-acetylated lysine (anti-acLys) antibody showed a significant difference in the autoacetylation pattern between the WT and mutants. An equal protein amount is shown by Western blotting using anti-hNaa50p.
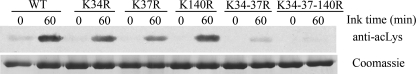
The observed autoacetylation pattern was further supported using mutants of GST-hNaa50p in which Lys residues 34, 37, or 140 were replaced by Arg. In addition, a double mutant, hNaa50p K34R/K37R, and a triple mutant hNaa50p K34R/K37R/K140R were made. KAT assays with GST-hNaa50p WT and these mutants showed significantly reduced acetylation for the mutants, confirming the identification of these three Lys residues as the major targets for hNaa50p autoacetylation (Fig. 7).
FIGURE 7.
Comparison of the autoacetylation pattern for GST-hNaa50p WT and different lysine mutants. Upper panel, Western blotting analyses of GST-hNaa50p WT and different lysine to arginine mutants using anti-acetylated lysine (anti-acLys) antibody. 2.4 μm enzymes were incubated for 1 h at 37 °C with 500 μm acetyl-CoA. Lower panel, Coomassie-stained SDS-PAGE presenting protein loading.
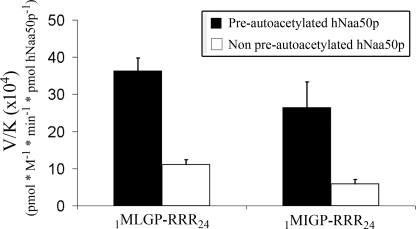
The potential effect of autoacetylation on NAT activity was explored by determining the kinetic constants for the best in vitro substrate 1MLGP-RRR24 and 1MIGP-RRR24 using pre-autoacetylated and non-preautoacetylated GST-hNaa50p. These assays show that autoacetylation leads to an increase in the enzyme specificity for both oligopeptide substrates (Fig. 8). The autoacetylation led to a 2-fold decrease of the Km values and a doubling of the maximal reaction rate (Table 3).
FIGURE 8.
The specificity constants (V/K) for two selected in vitro oligopeptide substrates using preautoacetylated and non-preautoacetylated GST-hNaa50p. 10 μm purified GST-hNaa50p was incubated for 5 days at 37 °C with either 300 μm acetyl-CoA (Pre-autoacetylated) or H2O (Non pre-autoacetylated). The Km values for 1MLGP-RRR24 or 1MIGP-RRR24 were then determined with these enzyme variants. 30 nm GST-hNaa50p were incubated with either 1MLGP-RRR24 (20–400 μm) or 1MIGP-RRR24 (50–500 μm) oligopeptide substrate at 37 °C for 30 min with saturated levels of acetyl-CoA (300 μm). The acetylation kinetics were determined with reverse phase HPLC. V/K is the Vmax/Km (oligopeptides). Error bars indicate the S.D. Experiments were performed in triplicates.
TABLE 3.
Effect of autoacetylation on kinetic constants
Experiments were performed in duplicate or triplicate. The recorded values are average ± S.D. For more experimental details, see “Experimental Procedures” and supplemental data.
| Non pre-autoacetylated hNaa50p |
Pre-autoacetylated hNaa50p |
|||
|---|---|---|---|---|
| Km | Vmaxa | Km | Vmaxa | |
| μm | μm | |||
| 1MLGP-RRR24 | 62 ± 10 | 7 ± 1.5 | 33 ± 5.5 | 12 ± 3 |
| 1MIGP-RRR24 | 186 ± 37 | 10.9 ± 3 | 80 ± 8.3 | 20.7 ± 3.6 |
a Vmax is given in picomole of acetylated peptide min−1 pmol of hNaa50p−1.
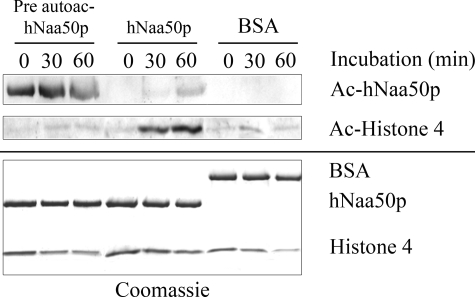
It was also investigated whether the KAT activity of hNaa50p might be directed toward other proteins. Several proteins known to be KAT modified in vivo were tested. No modification was observed when using GST-p53, GST-ODD (Oxygen dependent degradation domain within hypoxia-inducible factor-1α), or human rhSP1 (transcription factor specificity protein 1 (22)) as substrates (data not shown). However, hNaa50p KAT activity was detectable when using histone 4 as substrate. As shown in Fig. 9, a time-dependent acetylation of histone 4 was observed, as detected by anti-acLys antibodies, but only for the non-preautoacetylated GST-hNaa50p variant (Fig. 9). As negative control, GST-hNaa50p was replaced with an equal concentration of bovine serum albumin.
FIGURE 9.
GST-hNaa50p KAT activity on histone 4. 10 μm purified GST-hNaa50p was incubated for 5 days at 37 °C with either 300 μm acetyl-CoA (Pre-autoacetylated) or H2O (Non pre-autoacetylated). 2 μm of these enzyme variants were then tested for KAT activity toward Histone 4 protein (2 μm). Equal amounts of bovine serum albumin (BSA) replaced hNaa50p in the negative control. The samples were incubated at 37 °C, and aliquots were collected as indicated. Upper panel, Western blotting analyses of GST-hNaa50ps KAT activity using anti-acetylated lysine (anti-acLys) antibody. Lower panel, Coomassie-stained SDS-PAGE presenting the protein loading.
Potential Structural Basis for the Dual Activity of hNaa50p
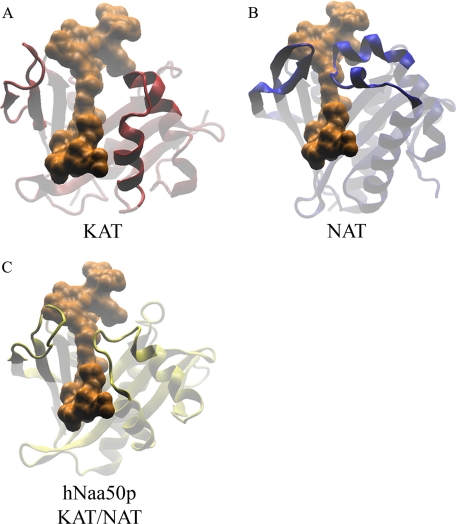
To find some indications of how hNaa50p can perform both NAT and KAT functions, a structural superimposition of hNaa50p (Protein data bank code 2OB0) on a known NAT enzyme from Salmonella typhimurium RimL (PDB code 1S7N), and a known KAT enzyme, GNAT from Bacillus cereus (PDB code 3BLN) was performed (Fig. 10). All three structures were also superimposed on a KAT from Tetrahymena (PDB code 1PU9) that had been co-crystallized with a 19-residue oligopeptide of the H3 substrate (residue 5–23). The purpose of this structural comparison was to get an indication of how the Nϵ-substrate could interact with hNaa50p (Fig. 10).
FIGURE 10.
Structural superimposing of hNaa50p, RimL from S. thyphimurium (NAT), and GNAT enzyme from B. cereus (histone acetyltransferases). A, protein structure of GNAT (B. cereus; PDB code 3BLN) was structurally compared with tGCN5 (Tetrahymena; PDB code 1PU9) that had been co-crystallized with a stretch of the H3 substrate (residue 5–23) shown in orange. B, protein structure of RimL (S. thyphimurium; PDB code 1S7N) with the same H3 substrate stretch present in orange. C, structural comparison of hNaa50p (PDB code 2OB0) with the H3 substrate stretch in orange. All structures are with similar orientations, and parts the protein structures are indicated as partly transparent to clarify the point.
The analyses showed that hNaa50p shares some of the flexible structural elements with GCN5. They both have loops in the entry of the substrate binding cleft (Fig. 10, A and C). In contrast, the corresponding area of RimL is composed of an antiparallel β-sheet on one side of the cleft and two short α-helixes on the other side (Fig. 10B).
DISCUSSION
Proteomic analyses of HeLa cell lysates, where the NatA complex was knocked down with small interfering RNAs, demonstrated reduced acetylation levels of a protein with an N-terminal sequence that did not fit with the expected hNatA substrate specificity (8). Based on the N-terminal sequence (1MLGPEGG7), the protein was identified to be hnRNP F, a protein that is known to interact with members of the nuclear cap-binding complex (23). Because knockdown of the human NatA complex causes reduced levels of the physical interactor hNaa50p (11) (supplemental Fig. S3), it is reasonable to speculate that the reduction of hnRNP F acetylation could be explained by a reduced amount of hNaa50p. Immunoprecipitated hNaa50p did indeed acetylate 1MLGP-RRR24, whereas only very low activity was observed using the NatA substrate 1SESS-RRR24 (Fig. 1). The latter also excluded the potential presence of the catalytical subunit of NatA (hNaa10p) in the precipitates.
Kinetic experiments show that NAT substrates with the third residue small and hydrophobic, 1MLGP-RRR24, or negatively charged, 1MLDP-RRR24, function equally well as substrates. If the third position is positively charged as in 1MLRP-RRR24, acetylation is more than 5-fold reduced, based on the V/K constant (Fig. 3). Also, Leu in the third position, 1MLLP-RRR24, is suboptimal for enzyme activity. Interestingly, Pro in the fourth position, 1MLGP-RRR24, is preferred in the hNaa50p substrates, because the V/K constant of the 1MLGTE-RRR24 peptide decreased by a factor of 2 (Fig. 3). The change from Pro to Thr has a minor effect on the kcat, but the Km is more than 4-fold increased (Table 2), indicating a weakened enzyme-substrate interaction. In contrast, the presence of Pro in position 2 is associated with reduced N-terminal acetylation by the NatA complex (5, 8).
The specificity of hNaa50p appears relatively similar to the specificity of the yeast NatC complex. In yeast, NatC acetylate peptides with N termini ML, MF, and MW (5). In contrast to yNatC, Phe in the second position leads to a dramatic increase in the Km value (Table 2). Recent findings on the human NatC complex suggest that hNaa30p (hMak3, the catalytic subunit of hNatC) has a conserved substrate specificity from yeast (18). In vitro acetylation assays showed that hNaa30p-acetylated oligopeptides with N-terminal sequences of 1MLALI-RRR24, 1MLGTG-RRR24, and 1MLGTE-RRR24 are more than 2-fold better than 1MLGPE-RRR24. Therefore, the substrate preference regarding the ML group of peptides differs between hNaa50p and hNatC. This implies that hNaa50p and hNaa30p acetylate similar, but distinct N-terminal substrates in vivo, and that the hNaa50p activity is restricted to a specific subset of substrates previously assumed to be NatC substrates.
hNaa50p was earlier reported to perform autoacetylation, which most likely represents Nϵ-acetylation. The results of the present study verify this supposition. Moreover, they strongly suggest that autoacetylation of hNaa50p is of physiological relevance. Lysine residues 34 and 140 were identified as acetylation target sites both when the protein was immunoprecipitated from HeLa cells or autoacetylated in vitro. Moreover, autoacetylation led to an increase in its NAT, but not KAT activity.
Autoacetylation of hNaa50p leads to an increased enzyme specificity as indicated by a decreased Km value and increased enzyme activity. This appears to be a general effect as it was observed for both a highly preferred (1MLGP-RRR24) and suboptimal substrate (1MIGP-RRR24). The decrease in Km indicates an improved enzyme-substrate interaction and autoacetylation may thus reflect a potential specificity switch in vivo.
Besides its autoacetylation, hNaa50p might also exert KAT activity toward other proteins. Although no such activity could be detected using GST-p53, GST-ODD, or rhSP1 as substrates, histone 4 was modified. Interestingly, whereas the NAT activity of hNaa50p was enhanced by autoacetylation, the KAT activity toward histone 4 was clearly reduced. This could indicate that autoacetylation mediates increased enzyme specificity. Further studies are required to establish whether or not histone 4 is a hNaa50p KAT substrate in vivo.
The observed phenotypes following silencing of Naa50p/San in Drosophila or human cells (9, 11) indicate that important in vivo substrates are involved in sister chromatid cohesion. Blastp searches in the Swiss-Prot data base (version 56.0) for human proteins that match the preferred N-terminal sequence for the NAT activity of hNaa50p resulted in several hits, including TIMELESS-interacting protein, a protein that is reportedly involved in chromatid cohesion (24, 25) (supplemental Table S3).
hNaa50p is the first acetyltransferase to be identified as having both NAT and KAT activity. Several KAT enzymes have been structurally determined, but only a few NAT structures from prokaryotic organisms were solved. Therefore it is difficult to explain the dual acetylation capacity of hNaa50p in structural terms. However, comparison of the structures provided some hints. hNaa50p is constructed with flexible loops in the entry site of the substrate binding cleft (Fig. 10C). This is a structural property that hNaa50p shares with the KAT enzymes (Fig. 10A). These loops could allow for flexibility to accommodate larger substrates, such as protein surfaces that could make a productive interaction with hNaa50p. RimL, representing the NAT enzymes, has in the corresponding area an antiparallel β-sheet on one side and two short α-helixes on the other (Fig. 10B). These secondary structures are expected to be more rigid and hence reduce the area of interaction possibly required for ϵ-acetylation.
In conclusion, hNaa50p displays Nα-acetyltransferase activity and prefers substrates that have ML at their N termini. In addition, hNaa50p has the capacity for Nϵ-autoacetylation, targeting Lys34, Lys37, and Lys140. It is thus the first identified bifunctional protein acetyltransferase exhibiting both KAT and NAT activities.
Supplementary Material
Acknowledgments
We thank E. Skjelvik, L. Vikebø, M. Algrøy, H. Halseth, and N. Glomnes for technical assistance.
This work was supported by The Norwegian Cancer Society, The Meltzer Foundation, and the Norwegian Health Region West.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables S1–S3 and Figs. S1–S3.
- KAT
- lysine Nϵ-acetyltransferases
- NAT
- N-terminal acetyltransferase
- hnRNP
- heterogeneous nuclear ribonucleoprotein
- MS
- mass spectrometry
- V/K
- Vmax/Km
- acLys
- acetylated lysine
- CBP
- CREB-binding protein
- CREB
- cAMP-response element-binding protein
- HPLC
- high pressure liquid chromatography
- MALDI-TOF
- matrix-assisted laser desorption ionization time-of-flight
- GST
- glutathione S-transferase
- WT
- wild type.
REFERENCES
- 1.Marmorstein R. (2004) Methods Enzymol. 376, 106–119 [DOI] [PubMed] [Google Scholar]
- 2.Kalkhoven E. (2004) Biochem. Pharmacol. 68, 1145–1155 [DOI] [PubMed] [Google Scholar]
- 3.Polevoda B., Brown S., Cardillo T. S., Rigby S., Sherman F. (2008) J. Cell. Biochem. 103, 492–508 [DOI] [PubMed] [Google Scholar]
- 4.Gautschi M., Just S., Mun A., Ross S., Rücknagel P., Dubaquié Y., Ehrenhofer-Murray A., Rospert S. (2003) Mol. Cell. Biol. 23, 7403–7414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polevoda B., Sherman F. (2003) J. Mol. Biol. 325, 595–622 [DOI] [PubMed] [Google Scholar]
- 6.Polevoda B., Arnesen T., Sherman F. (2009) BMC Proc. 3, Suppl. 6, S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park E. C., Szostak J. W. (1992) EMBO J. 11, 2087–2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnesen T., Van Damme P., Polevoda B., Helsens K., Evjenth R., Colaert N., Varhaug J. E., Vandekerckhove J., Lillehaug J. R., Sherman F., Gevaert K. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 8157–8162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams B. C., Garrett-Engele C. M., Li Z., Williams E. V., Rosenman E. D., Goldberg M. L. (2003) Curr. Biol. 13, 2025–2036 [DOI] [PubMed] [Google Scholar]
- 10.Arnesen T., Anderson D., Torsvik J., Halseth H. B., Varhaug J. E., Lillehaug J. R. (2006) Gene 371, 291–295 [DOI] [PubMed] [Google Scholar]
- 11.Hou F., Chu C. W., Kong X., Yokomori K., Zou H. (2007) J. Cell Biol. 177, 587–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pimenta-Marques A., Tostões R., Marty T., Barbosa V., Lehmann R., Martinho R. G. (2008) Dev. Biol. 323, 197–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnesen T., Anderson D., Baldersheim C., Lanotte M., Varhaug J. E., Lillehaug J. R. (2005) Biochem. J. 386, 433–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnesen T., Kong X., Evjenth R., Gromyko D., Varhaug J. E., Lin Z., Sang N., Caro J., Lillehaug J. R. (2005) FEBS Lett. 579, 6428–6432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evjenth R., Hole K., Ziegler M., Lillehaug J. R. (2009) BMC Proc. 3, Suppl. 6, S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shevchenko A., Wilm M., Vorm O., Mann M. (1996) Anal. Chem. 68, 850–858 [DOI] [PubMed] [Google Scholar]
- 17.Gobom J., Nordhoff E., Mirgorodskaya E., Ekman R., Roepstorff P. (1999) J. Mass Spectrom. 34, 105–116 [DOI] [PubMed] [Google Scholar]
- 18.Starheim K. K., Gromyko D., Evjenth R., Ryningen A., Varhaug J. E., Lillehaug J. R., Arnesen T. (2009) Mol. Cell. Biol. 29, 3569–3581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dormeyer W., Ott M., Schnölzer M. (2005) Mol. Cell. Proteomics 4, 1226–1239 [DOI] [PubMed] [Google Scholar]
- 20.Tanner K. G., Trievel R. C., Kuo M. H., Howard R. M., Berger S. L., Allis C. D., Marmorstein R., Denu J. M. (1999) J. Biol. Chem. 274, 18157–18160 [DOI] [PubMed] [Google Scholar]
- 21.Trievel R. C., Rojas J. R., Sterner D. E., Venkataramani R. N., Wang L., Zhou J., Allis C. D., Berger S. L., Marmorstein R. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 8931–8936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pugh B. F., Tjian R. (1990) Cell 61, 1187–1197 [DOI] [PubMed] [Google Scholar]
- 23.Gamberi C., Izaurralde E., Beisel C., Mattaj I. W. (1997) Mol. Cell. Biol. 17, 2587–2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshizawa-Sugata N., Masai H. (2007) J. Biol. Chem. 282, 2729–2740 [DOI] [PubMed] [Google Scholar]
- 25.Gotter A. L., Suppa C., Emanuel B. S. (2007) J. Mol. Biol. 366, 36–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.