Abstract
Mast cells (MCs) play a critical role in innate and adaptive immunity through the release of cytokines, chemokines, lipid mediators, biogenic amines, and proteases. We recently showed that the activities of MC proteases are transcriptionally regulated by intracellularly retained interleukin-15 (IL-15), and we provided evidence that this cytokine acts as a specific regulator of mouse mast cell protease-2 (mMCP-2). Here, we show that in wild-type bone marrow-derived mast cells (BMMCs) IL-15 inhibits mMCP-2 transcription indirectly by inducing differential expression and mMCP-2 promoter binding of the bifunctional transcription factors C/EBPβ and YY1. In wild-type BMMCs, C/EBPβ expression predominates over YY1 expression, and thus C/EBPβ preferentially binds to the mMCP-2 promoter. In IL-15-deficient BMMCs, the opposite is found: YY1 expression predominates and binds to the mMCP-2 promoter at the expense of C/EBPβ. Hypertranscription of the mMCP-2 gene in IL-15-deficient BMMCs is associated with histone acetylation and, intriguingly, with methylation of non-CpG dinucleotides within the MCP-2 promoter. This suggests a novel model of cytokine-controlled protease transcription: non-CpG methylation maintains a chromosomal domain in an “open” configuration that is permissive for gene expression.
Introduction
Mast cells (MCs)3 are key cellular mediators of host innate defense (1). Upon activation, MC secretory granules release a number of preformed inflammatory molecules that include cytokines, histamine, serglycin proteoglycans, and several MC-specific proteases (chymases, tryptases, and carboxypeptidase A) (2, 3). Upon release into the extracellular space, these mediators regulate a broad variety of responses ranging from acute phase immediate reactions over recruitment of specific cell populations to sites of infection to long term tissue-remodeling reactions (1).
In the mouse, several mast cell proteases (mMCPs) have been identified and partially characterized, including the chymases mMCP-1, -2, -4, -5, and -9 and the tryptases mMCP-6 and -7 (4). Proteases play an important role in extracellular matrix remodeling, extravascular coagulation, and fibrinolysis as well as angiogenesis. Our recent investigation of the mechanisms that regulate mMCP gene expression has shown that intracellular cytokines can regulate mMCP activities (5). Murine MCs express constitutive and lipopolysaccharide-inducible IL-15 and store IL-15 intracellularly, where it colocalizes with tumor necrosis factor-α and proteases in MC granula. IL-15−/− MCs exhibit markedly elevated chymase activity, leading to increased bactericidal activity and processing of neutrophil-recruiting chemokines. In particular, intracellular IL-15 operates as a specific negative transcriptional regulator of mMCP-2 (5).
In this report, we show that transcriptional repression of mMCP-2 by intracellular IL-15 is indirect. IL-15-mediated repression is effected by changing the expression levels of the transcription factors (TFs) C/EBPβ and YY1 and thus the amount of each TF bound to the mMCP-2 promoter. C/EBPβ binding is correlated with repression of mMCP-2 in wild-type (WT) BMMCs whereas YY1 binding is correlated with activation of mMCP-2 in IL-15−/− BMMCs. In IL-15−/− BMMCs, activation of mMCP-2 is associated with histone acetylation throughout the promoter and the body of the gene. Finally, we show that the genomic DNA of the active mMCP-2 locus is hypermethylated at non-CpG dinucleotides, and we suggest a novel role for non-CpG-methylation in gene activation.
EXPERIMENTAL PROCEDURES
Cell Culture
Mouse bone marrow cells were cultivated in complete medium consisting of 10% heat-inactivated fetal calf serum (Biochrom), 50 μm β-mercaptoethanol, nonessential amino acids, 2 mm l-glutamine, penicillin, streptomycin, and 1 mm sodium pyruvate (all from Invitrogen) in Iscove's modified Dulbecco's medium (PAA) supplemented with 5 ng/ml IL-3 and 10 ng/ml stem cell factor (both from R&D). After 4 weeks of culture, BMMCs represented >98% of the total cells according to fluorescence-activated cell sorter analysis of cell surface expression of CD117 (c-Kit) and FcϵRI and were negative for CD11c, CD45R/B220, F4/80 and Gr-1 expression. COS-7 cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% inactivated fetal bovine serum and penicillin (1 unit/ml) streptomycin (1 unit/ml).
Plasmid Construction
The full-length mMCP-2 promoter reporter construct (−1311 to +28, p1311) as well as the 5′ truncated constructs (′591 to +28 (Δp591), −397 to +28 (Δp397), −189 to +28(Δp189) were amplified by PCR from mouse genomic DNA using primers described in supplemental “Experimental Procedures”. Inserts were cloned upstream from the β-lactamase reporter gene into the pGeneBlazer TOPO TA reporter vector (Invitrogen) according to the instructions of the manufacturer. Mouse IL-15 SSP (with short signal peptide)-expressing vector was generated by recombination of pEntry-SSP-IL-15 with expression destination vector pDest 53 (Invitrogen). The human IL-15-expressing vector was generated as described previously (6).
Real Time Quantitative PCR and Western Blotting
Total RNA from WT or IL-15−/− BMMCs was extracted using TRIzol reagent (Invitrogen) according to the manufacturer's recommendations, and RNA quality controls were determined using routine agarose gel control of the 28 S/18 S ratio. Five micrograms of total RNA were treated with RNase free DNase I (Invitrogen) and were reverse-transcribed to cDNA using oligo(dT)12–18 primer and SuperScriptII reverse transcription (Invitrogen) in the presence of 10 units of RNase inhibitor (Invitrogen) according to the manufacturer's recommendations. cDNAs of BMMCs were routinely diluted 5-fold, and 2-μl aliquots were used in 10-μl light cycler PCRs containing 5 μl of QuantiTect SYBR Green PCR kit (Qiagen) and 5 μm gene-specific primers described in supplemental “Experimental Procedures”. The PCR was performed in a Light Cycler 3 (Roche Diagnostics) for 45–50 cycles of a four-step program: initial denaturation of 95 °C for 12 min, 95 °C for 10 s, 63 °C-53 °C touchdown (0.5 °C per step) for 10 s, 72 °C for 15 s, and fluorescence read step for 1 s. Quantitative PCRs were performed twice in triplicate; threshold cycles were obtained using cycler software version 3.0 (Roche Diagnostics). Mouse 18 S RNA was used for normalization of expression values. Relative quantification was calculated using the Relquant software (Roche Diagnostics), according to calibrator normalized quantification procedure with correction of differences in PCR efficiencies between target and reference. The difference in PCR amplification efficiencies was determined by amplification of target and reference genes (18 S RNA) in five dilution steps of P815 mastocytoma cell line cDNA in triplicate to generate a fit coefficient file according to the recommendation of Light Cycler 3.0 (Roche Diagnostics) instructions. For Western blots, nuclear extracts obtained from 107 WT or IL-15−/− BMMCs were electrophoresed in Novex 4–12% gradient SDS-bis-Tris gels (Invitrogen). The separated proteins were electroblotted onto nitrocellulose membranes (Schleicher and Schuell) in a transfer buffer consisting of 20 mm Tris-HCl, 150 mm glycine, and 20% methanol. The membranes were incubated sequentially in synthetic 2× blocking solution (Roti-Immunoblock; Carl Roth) in 1× phosphate-buffered saline for 1 h, in 1:250 dilution (0.75 μg/ml) anti-C/EBPβ IgG, clone sc-150 or anti-YY1 IgG, clone sc-281 (Santa Cruz Biotechnology), or 1:1000 of anti-β-actin IgG clone 13E5 (Cell Signaling Technology), in 2× blocking solution with 0.1% Tween 20 for 1 h and 3 × 5 min in wash buffer (1× phosphate-buffered saline with 0.1% Tween 20). For visualization of bound primary antibodies, membranes were incubated in 1:10,000 goat polyclonal anti-rabbit-IRDye IgG 800 nm (Biomol) for 1 h following three 5- min incubations of membranes in wash buffer. Proteins were detected using the Odyssey® imaging system (Li-Cor Biosciences). Mouse polyclonal anti-β-actin IgG purchased from Santa Cruz was routinely used to control equal sample loadings.
GeneBlazer Promoter Activity Assay
0.5 × 106 COS-7 cells were incubated for 48 h with 2 μg of mMCP-2 reporter plasmid and 3 μl of FuGENE transfection reagent (Roche Diagnostics) at 37 °C. β-Lactamase reporter expression in cell lysates was determined using the fluorescence resonance energy transfer (FRET)-based GeneBlazer in vitro detection system (Invitrogen) according to the manufacturer's instructions. Briefly, replicate samples of cells were washed once in cold 1× Hanks' balanced salt solution (HBSS), lysed by three freeze-thaw cycles in 100 μl of Hanks' balanced salt solution, and centrifuged at 13,000 rpm for 5 min at 4 °C to separate cell debris. Fifty microliters of cell lysate was mixed with 50 μl of 1:200 diluted Lytic Blazer-h-FRET B/G substrate in homogeneous lysis buffer (Invitrogen) and incubated in the dark for up to 3 h at room temperature. Fluorescence was then quantified on a TECAN infinite M200 fluorescence plate reader using 409/25 nm excitation, and emission was detected via 460/40 nm (blue) and 530/30 nm (green) bandpass filters. Emission intensities from blank wells were subtracted from wells containing cell lysates. Ratiometric fluorescence intensities of blue to green have been calculated as a value for mMCP-2 promoter activity. Lysates of untransfected COS-7 cells and Hanks' balanced salt solution alone served as negative controls, and transfection of COS-7 cells with the human ubiquitin promoter construct was routinely performed as positive control.
Chromatin Immunoprecipitation (ChIP) Assays
ChIP has been performed as described previously (7). Briefly, 6 × 106 mast cells (WT or IL-15 −/−) were washed twice with phosphate-buffered saline and cross-linked for 10 min with 1% formaldehyde in serum-free medium at 65 °C. Cross-linking was stopped with 1.25 m glycine, and genomic DNAs were sonificated (7). For precipitation of acetylated chromatin, we used 2 μl of polyclonal rabbit antibody specific for acetylated histone 3 (Upstate). The transcription factor assays were carried out with 5 μg of anti-C/EBPβ antibody or anti-YY1 antibody (both from Santa Cruz Biotechnology). As negative controls, we used rabbit normal serum (Dianova) or anti-muscle-specific kinase antibody (Abcam).
Bisulfite Sequencing
Genomic DNA was purified from WT and IL-15−/− BMMCs with the Easy-DNA kit (Invitrogen). One microgram of genomic DNA was bisulfite-converted using the EpiTect bisulfite kit (Qiagen) according to the manufacturer's instructions. The DNA was ethanol-precipitated and resuspended in 20 μl of TE buffer. For bisulfite sequencing, PCR was performed with 1 μl of the converted genomic DNA solution from WT and IL-15−/− BMMCs as template with primer pairs described in supplemental “Experimental Procedures”. PCR products were cloned into the pGEM-T Easy vector (Promega), and a minimum of 15 clones from each sample was cycle sequenced with the BigDye Terminator kit (version 3.1; PE Biosystems) and an ABI automated DNA sequencer (PE Biosystems).
Statistical Analysis
The statistical significance threshold values for differential expression with the individual methods were calculated for genes showing changes >2-fold. Furthermore, to be regarded as differentially expressed between IL-15−/− and WT BMMCs, a gene should differ in the same direction between a minimum of two different samples (taken at two time points) when analyzed by at least two different methods. Real time quantitative reverse transcription (RT)-PCR and Western blotting were performed in triplicate, and the values were imported into Excel worksheets for determination of means and standard errors. Student's t test for unpaired samples was calculated with p values of <0.05 accepted as significant.
RESULTS
IL-15 Regulates Repression of mMCP-2 Promoter Activity
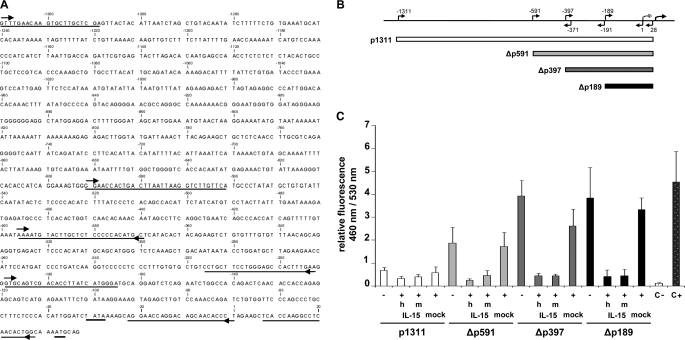
To investigate the role of IL-15 in transcriptional repression of the mMCP-2 promoter, we generated a series of constructs where either the full-length mMCP-2 promoter (p1311) or 5′ truncations of the promoter (Δp591, Δp397, and Δp189) were linked to the β-lactamase reporter gene (Fig. 1, A and B). Using a FRET-based GeneBlazer in vitro assay, we then measured the β-lactamase activity driven by these constructs in COS-7 cells in the presence or absence of (mouse/human) IL-15. As shown (Fig. 1, B and C) each of the truncations showed significantly higher β-lactamase activity compared with p1311, indicating that the p1311 promoter contains an additional element(s) that operates independently of IL-15, resulting in a lower basal mMCP-2 activity. Importantly, all constructs showed a significant decrease in β-lactamase activity when exogenous mouse or human IL-15 was coexpressed in the cells, showing that the minimal mMCP-2 fragment downstream of nucleotide −591 is sufficient for IL-15-regulated transcriptional repression of the mMCP-2 promoter.
FIGURE 1.
IL-15 is a transcriptional repressor of mMCP-2 promoter activity. A, nucleotide sequence (from −1311 to +38) of the mMCP-2 promoter region. Numbering corresponds to the positioning of the 1311-bp mMCP-2 region relative to the transcription start point. The TATA box as well as the translation start are underlined. Forward and reverse primers used in this study are marked with arrows. B, schematic representation of full-length and 5′ truncation reporter plasmids of the mMCP-2 promoter tested in COS-7 cells. C, relative activity of the full-length mouse mMCP-2 promoter (p1311) and the 5′ deletion mutants Δp591, Δp397, and Δp189 was assessed in the presence or absence of a plasmid coding for human or mouse IL-15 (hIL-15 and mIL-15, respectively) under the control of the cytomegalovirus promoter in COS-7 cells. Relative mMCP-2 promoter activity was calculated as the ratio between blue (460 nm) and green fluorescence (530 nm). Expression of pcDNA3.1 (mock) under similar conditions was used as control. C−, untransfected COS-7 cells; C+, COS-7 cells transfected with a GeneBlazer vector carrying the human ubiquitin promoter used here as a positive control. Values for all plasmid combinations are the means ± S.E.M. of at least three independent transfections with at least two independent preparations of each plasmid.
IL-15 Regulates C/EBPβ and YY1 Binding to the Minimal mMCP-2 Promoter
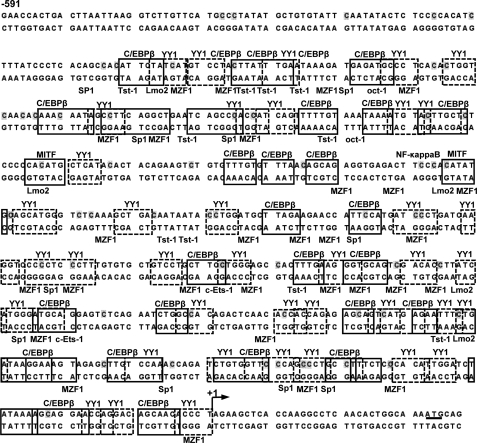
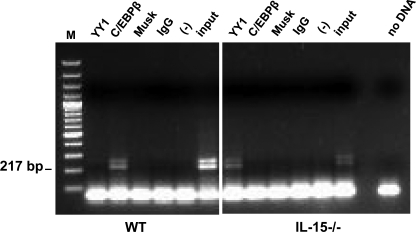
A bioinformatic search for potential TF binding sites within the mMCP-2 promoter (−591 to +28) revealed a high number of putative C/EBPβ and YY1 binding sites along with binding sites for Tst-1, Sp1, Oct-1, NF-κb, MITF, MZF-1, Lmo2, and c-Ets-1 TFs (Fig. 2 and supplemental Figs. 1 and 2). We decided to focus on the bifunctional C/EBPβ and YY1 TFs that are known to act either as suppressors or as activators of gene activity depending on the context (cell type, relative concentration, and interacting partners) (8–12). To test whether C/EBPβ and YY1 do indeed bind within the mMCP-2 promoter, we performed a ChIP assay with BMMCs derived from WT or IL-15−/− mice with TF-specific antibodies. The primers used for amplification of the ChIPped material were taken from the limits that define the Δp189 promoter, which we had previously shown to be the minimal promoter exhibiting IL-15-dependent regulation (Fig. 1C). As shown in Fig. 3, we observed reciprocity in the binding of the C/EBPβ and YY1 to the Δp189 truncated promoter that was dependent on the presence or absence of IL-15. In WT BMMCs, C/EBPβ antibodies immune precipitated Δp189-containing chromatin, whereas hardly any amplification was observed when the ChIP was performed with anti-YY1 antibodies (Fig. 3). In contrast, when IL-15−/− BMMCs were used, anti-YY1 immunoprecipitated Δp189-containing chromatin, whereas anti-C/EBPβ antibodies did not. These data indicate that the IL-15-dependent transcriptional repression of Δp189 (Fig. 1, A–C) correlates with the IL-15-dependent binding of C/EBPβ. Conversely, activity of Δp189 correlates with IL-15-dependent binding of YY1. Using antibodies specific for intracellular IL-15, we could prove that IL-15 does not bind the mMCP-2 promoter directly (data not shown).
FIGURE 2.
mMCP-2 promoter sequence (−591 to translation start site) is shown with sites predicted to bind C/EBPβ and YY1 based on bioinformatic analysis (supplemental Fig. 1). Transcription factor binding sites are shown from the transcription start indicated by an arrow to −502 bp. Potential and predominant in number are the C/EBPβ and YY1 sites located throughout the region. Potential MITF, MZF1, Tst-1, c-ETS-1(p54), Lmo-complex, Sp1, and NF-κb, which in many positions overlap with C/EBPβ or YY1, are also shown. Fifty-seven methylated mainly non-CpG cytosine nucleotide sites (only one CpG) in IL-15−/− BMMCs with a threshold frequency higher than 40 percentage points in sequenced clones, as indicated in Fig. 7, are shaded. The transcription start site is denoted by a black arrow, and the translation start is underlined.
FIGURE 3.
IL-15 regulates C/EBPβ and YY1 binding to the mMCP-2 promoter in mouse mast cells. WT and IL-15−/− BMMCs were analyzed by ChIP using monoclonal Abs to C/EBPβ, YY1, or with an equal amount of unspecific (anti-muscle-specific kinase receptor, Musk) Abs, or isotype-matched control Abs (rabbit IgG), or without Abs, all used as negative control. Immunoprecipitated DNA was analyzed by PCR using mouse mMCP-2-specific primers, which amplify the −189 to +28 bp (Δp189) region. Each panel displays one experiment representative of three that were performed. Input, DNA purified from chromatin that has not been subjected to ChIP. M, 100-bp ladder markers.
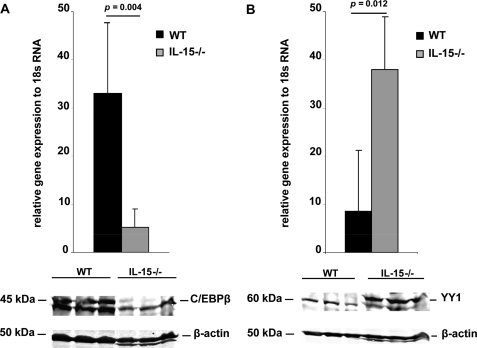
IL-15 Modulates C/EBPβ and YY1 Expression in Mouse MCs
We next investigated whether the IL-15-dependent binding of C/EBPβ and YY1 to Δp189 (Fig. 3) results from changes in the expression levels of C/EBPβ and YY1 that are dependent upon IL-15 activity. Accordingly, we determined the expression of C/EBPβ and YY1 at the transcript and the protein levels in WT and IL-15−/− BMMCs. As shown in Fig. 4A (upper panel), quantitative real-time RT-PCR using PCR primers specific for C/EBPβ showed significant overexpression in WT compared with IL-15−/− BMMCs. We also observed the typical “doublet” of the C/EBPβ protein in WT nuclear BMMCs extracts, whereas the C/EBPβ doublet was barely detectable in IL-15−/− BMMCs (Fig. 4A, lower panels). The converse was true for YY1. In IL-15−/− BMMCs, quantitative real-time RT-PCR demonstrated significant overexpression of YY1 in IL-15 compared with WT BMMCS (Fig. 4B, upper panel). Consistent with the RT-PCR data, we found that YY1 protein expression in IL-15−/− BMMC nuclear extracts was higher than that observed in WT BMMC (Fig. 4B, lower panels). These data suggest that IL-15 represses mMCP-2 expression indirectly through regulation of the C/EBPβ and YY1 transcription factors which bind to the mMCP-2 promoter resulting in either its repression (C/EBPβ) or activation (YY1).
FIGURE 4.
IL-15 modulates C/EBPβ and YY1 expression in mouse mast cells. C/EBPβ expression (A) and YY1 expression (B) in WT and IL-15−/− BMMCs was analyzed by quantitative real-time RT-PCR (upper panels) and by Western blotting (lower panels) using specific primers and monoclonal Abs to mouse C/EBPβ and YY1. In real time quantitative RT-PCR experiments, 18 S RNA expression was used for normalization. β-Actin detection is used as a loading control in Western blotting. BMMC lysates from three mice from each genotype are shown.
Exogenous C/EBPβ Expression Represses mMCP-2 Promoter Activity in COS-7 Cells
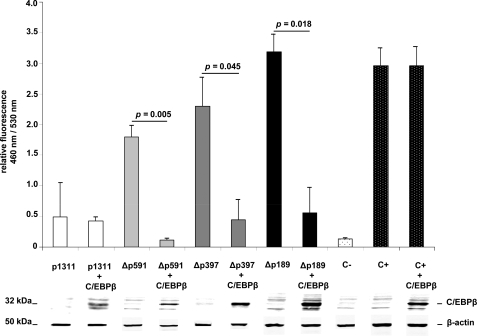
Guided by our observation that IL-15 repression of mMCP-2 in WT cells is indirect (Figs. 2–4) and likely to operate via binding of C/EBPβ to the mMCP-2 promoter, we decided to test the effect of exogenously expressing C/EBPβ in COS-7 cells that were cotransfected with the p1311 Δp591, Δp397, and Δp189 which we had previously shown to exhibit IL-15-dependent regulation (see Fig. 1). As a control, the C/EBPβ-expressing plasmid was cotransfected into COS-7 cells with an irrelevant ubiquitin promoter construct. As shown in Fig. 5, exogenous expression of mouse C/EBPβ results in specific suppression of the activity of Δp591, Δp397, and Δp189 truncated promoter constructs, indicating that C/EBPβ represses the mMCP-2 promoter directly.
FIGURE 5.
C/EBPβ specifically suppresses mMCP-2 downstream promoter activity (−591 to +28). COS-7 cells transfected with reporter plasmids p1311 (white bars), Δp591 (gray bars), Δp397 (dark gray bars), and Δp189 (black bars) or ubiquitin promoter (C+) as control (dotted black bars) show promoter activities in the absence or presence of exogenous C/EBPβ expression. Activations of Δp591, Δp397, and Δp189 promoters rather than irrelevant ubiquitin promoter are exclusively suppressed by cotransfection of the same cells with a mouse C/EBPβ-expressing construct. C−, untransfected COS-7 cells. Results are representative of three independent experiments.
mMCP-2 Locus Is Hyperacetylated in IL-15-deficient MCs
To investigate whether the repression and activation of mMCP-2 seen in WT and IL-15-deficient BMMCs, respectively, resulted in any changes in chromatin structure, we undertook a ChIP analysis of the mMCP-2 promoter using an antibody specific for acetylated histone H3 (anti-AcK9H3), which is a marker for gene activity (13). ChIP analysis encompassed the promoter region from nucleotide −591 to +28 and also the body of the gene, going from nucleotide +28 through exons 1 and 2 to nucleotide +670. As shown in Fig. 6A, the three fragments that encompass the promoter region, from position −591 to +28, were precipitated exclusively in IL-15−/− BMMCs and not in WT cells using the anti-AcK9H3 antibody. This was also true for the body of the gene where the anti-AcK9H3 antibody precipitated the region from nucleotide +28 to +670 of mMCP-2 in IL-15−/− BMMCs but not in WT nuclear extracts (Fig. 6B). An additional histone modification that is associated with gene activity, namely trimethylation of lysine 4 on histone H3 Me(3)K4H3 (14), was also tested for the body of the gene. Comparable with the anti-AcK9H3 antibody, the anti-Me(3)K4H3 precipitates the region from +28 to +670 from nuclear extracts from IL-15−/− BMMCs but not from WT cell extracts (data not shown). This shows that in WT cells the promoter and the body of the gene are hypoacetylated and further that the body of the gene is deficient in the Me(3)K4H3 modification. In contrast, in IL-15−/− BMMCs the mMCP-2 promoter and gene display epigenetic histone modifications characteristic of active genes.
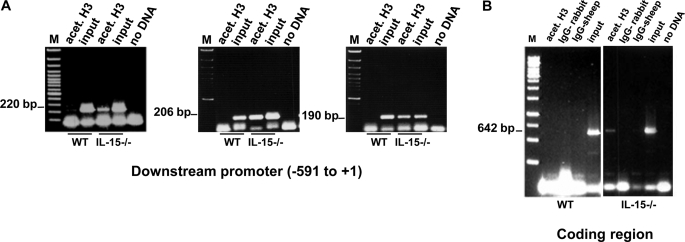
FIGURE 6.
IL-15 contributes to the epigenetic regulation of the mMCP-2 promoter. A, ChIP precipitated genomic DNA of WT and IL-15−/− BMMCs with anti-acetylated H3-Abs were amplified with primers: mcp2-pm-591 and mcp2-pm-371 (left panel), mcp2-pm-397 and mcp2-pm-191 (center panel), and mcp2-pm-189 and mcp2 + 1 (right panel). Specific precipitation of the coding region of mMCP-2 in IL-15−/− BMMCs with anti-acetylated H3-Abs and amplification using primers mcp2-expF and mcp2-expR (B) shows that the acetylation procedure proceeds from the promoter into the coding region of mMCP-2. Each panel displays one experiment representative of three performed. Input, DNA purified from chromatin that has not been subjected to ChIP. M, 100-bp ladder markers.
(Non-)CpGs Are Methylated in the Active mMCP-2 Promoter in IL-15-deficient MCs
Cytosine methylation is the principal base modification found in mammals (15). It typically occurs at CpG dinucleotides and is almost exclusively associated with epigenetic silencing of gene expression (16), although there are examples where CpG methylation is associated with gene activity (17). Methylation of cytosine residues can also occur in the context of CpA, CpT, and CpC dinucleotides as has been observed in cultured embryonic stem cells; however, the role of non-CpG methylation in epigenetic regulation of gene activity is less well explored (18, 19). To investigate the methylation status of individual cytosines in the proximal region of the mMCP-2 promoter between nucleotides −591 and +28 in WT and IL-15−/− BMMCs, bisulfite genomic conversion and DNA sequencing were employed. Sequencing of at least 15 clones each from WT and IL-15−/− BMMCs identified 57 hypermethylated non-CpG cytosines in IL-15−/− BMMCs with an average degree of methylation being at least 90 percentage points higher than in WT cells (Fig. 7). Because non-CpG methylation correlates with the high mMCP-2 expression in IL-15−/− BMMCs, this could represent an epigenetic mechanism that keeps the mMCP-2 chromosomal domain in an “open,” active, configuration and/or may prevent binding of suppressor transcription factors.
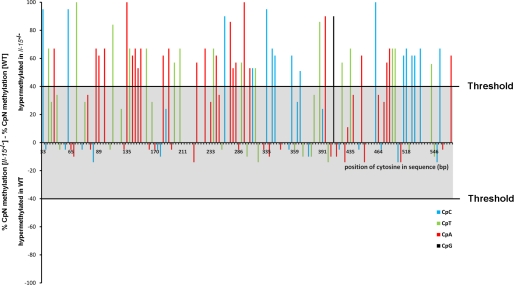
FIGURE 7.
IL-15 prevents non-CpG methylation of cytosines in downstream mMCP-2 promoter from −591 to the transcription start. Genomic DNA of WT and IL-15−/− BMMCs were bisulfite-converted, amplified with primers covering the region −591 to +28, cloned and sequenced (n > 15). The degree of methylation was calculated for every single cytosine of the sequence (x axis). The difference between the methylation rate of IL-15−/− and WT BMMCs has been computed and plotted. Fifty-seven cytosines are hypermethylated in the IL-15−/− BMMCs (difference >40 percentage points threshold). Different colors of the columns represent alternative cytosine-containing dinucleotides. As shown in Fig. 2B, many of the methylated cytosines in IL-15−/− BMMCs occur at C/EBPβ and YY1 binding sites.
DISCUSSION
The choice between IL-15-dependent repression or activation of the mMCP-2 gene in MCs is indirect and appears to be mediated by the binding of either the C/EBPβ (for repression) or the YY1 (for activation) TFs to the mMCP-2 promoter (Figs. 2 and 3). Dependence on IL-15 is founded upon the observation that IL-15 regulates the expression of C/EBPβ and YY1, where the levels of C/EBPβ are elevated in WT cells and reduced in IL-15-deficient cells (Fig. 3), whereas the opposite is found for YY1 (Fig. 3). The binding sites for these two TFs lie in close proximity to each other in the mMCP-2 promoter. Within the Δp591 promoter fragment there are potentially 27 C/EBPβ and 39 YY1 binding sites, several of which are overlapping (Fig. 2). We suggest that in WT cells the elevated levels of C/EBPβ lead to its binding to the mMCP-2 promoter (Fig. 5) with little or no binding of YY1, resulting in repression of mMCP-2. In IL-15-deficient MCs, the reverse occurs where YY1, whose levels are elevated, binds to the mMCP-2 promoter, with little or no binding of C/EBPβ, resulting in activation of mMCP-2 (Figs. 3 and 4). In addition to the reciprocal effects of IL-15 on the expression levels of C/EBPβ and YY1, and their resultant binding to the mMCP-2 promoter, the close proximity of the C/EBPβ and YY1 binding sites (some sites overlap) indicates that there may be a competition of the TFs for their recognition sequences within the mMCP-2 promoter, where binding of one TF to the mMCP-2 promoter occurs at the expense of the other.
To explore whether changes in chromatin structure are involved in the IL-15-dependent regulation of the mMCP-2 gene, we undertook ChIP analysis with antibodies to histone modifications, AcK9H3 and Me(3)K4H3 (Fig. 6). We could show that the promoter and the body of the gene are hypoacetylated in WT BMMCs compared with IL-15-deficient BMMCs, where the promoter and gene are hyperacetylated. These data are consistent with many previous studies which have shown that acetylation of histones is an epigenetic marker of gene activity that is involved in the cell-to-cell inheritance of patterns of gene activity (20).
Our DNA methylation analysis revealed that in IL-15-deficient BMMCs, where the mMCP-2 gene is hyperactive, we find that its promoter (−581 to +2) is hypermethylated at both CpG and non-CpG dinucleotides (Fig. 7). This is, to our knowledge, the first time that non-CpG methylation in mammals has been correlated with gene activity; CpG methylation has previously been correlated with the allele-specific expression of the maternal Igf2r gene (17) although, in the vast majority of cases, CpG methylation is associated with gene inactivity. What could be the purpose of this cytosine methylation at the promoter of the active mMCP-2 gene in IL-15−/− BMMCs? We suggest that the methylation at the active mMCP-2 gene acts to inhibit the binding of suppressor transcription factors, such as C/EBPβ, or a protein complex. It is known that the binding of both C/EBPβ and YY1 is affected by DNA methylation (21, 22).
Based on our data, we suggest that the mechanism responsible for the dramatic 103-fold increase in mMCP-2 expression in IL-15-deficient BMMCs occurs in two steps. First, there is the preferential (competitive) binding of YY1 to the mMCP-2 promoter at the expense of C/EBPβ (Figs. 1–4). Second, YY1 recruits the chromatin-remodeling machinery to “open” the chromatin so that it is transcribed by the basal transcriptional machinery, and this open chromatin state is epigenetically inherited through cell division, via histone acetylation. In addition, stable epigenetic (cell-to-cell) inheritance of this open chromatin state might be reinforced by the cytosine methylation at CpG and non-CpG sites within the promoter (Figs. 6 and 7). Our future work will be focused on testing this model for regulation of mMCP-2 by IL-15. Conclusively, our study delineates a novel concept in cytokine-mediated control and fine tuning of intracellular protease activities in primary mast cells.
Supplementary Material
Acknowledgment
We thank Manuel Hein and Anja Lüdemann for excellent technical assistance.
This study was supported in part by the Deutsche Forschungsgemeinschaft Grant SFB415/A10 (to S. B.-P.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1 and 2, “Experimental Procedures,” and additional references.
- MC
- mast cell
- BMMC
- bone marrow-derived MC
- Ab
- antibody
- bis-Tris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- ChIP
- chromatin immunoprecipitation
- FRET
- fluorescence resonance energy transfer
- IL-15
- interleukin-15
- mMCP-2
- mouse mast cell protease-2
- RT
- reverse transcription
- TF
- transcription factor
- WT
- wild type.
REFERENCES
- 1.Galli S. J., Grimbaldeston M., Tsai M. (2008) Nat. Rev. Immunol. 8, 478–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalesnikoff J., Galli S. J. (2008) Nat. Immunol. 9, 1215–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C., Sali A., Stevens R. L. (1998) J. Clin. Immunol. 18, 169–183 [DOI] [PubMed] [Google Scholar]
- 4.Pejler G., Abrink M., Ringvall M., Wernersson S. (2007) Adv. Immunol. 95, 167–255 [DOI] [PubMed] [Google Scholar]
- 5.Orinska Z., Maurer M., Mirghomizadeh F., Bulanova E., Metz M., Nashkevich N., Schiemann F., Schulmistrat J., Budagian V., Giron-Michel J., Brandt E., Paus R., Bulfone-Paus S. (2007) Nat. Med. 13, 927–934 [DOI] [PubMed] [Google Scholar]
- 6.Duitman E. H., Orinska Z., Bulanova E., Paus R., Bulfone-Paus S. (2008) Mol. Cell. Biol. 28, 4851–4861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bullwinkel J., Baron-Lühr B., Lüdemann A., Wohlenberg C., Gerdes J., Scholzen T. (2006) J. Cell. Physiol. 206, 624–635 [DOI] [PubMed] [Google Scholar]
- 8.Ko C. Y., Hsu H. C., Shen M. R., Chang W. C., Wang J. M. (2008) J. Biol. Chem. 283, 30919–30932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bauknecht T., Shi Y. (1998) J. Virol. 72, 2113–2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bauknecht T., See R. H., Shi Y. (1996) J. Virol. 70, 7695–7705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaston K., Fried M. (1995) Nucleic Acids Res. 23, 901–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi Y., Lee J. S., Galvin K. M. (1997) Biochim. Biophys. Acta 1332, F49–F66 [DOI] [PubMed] [Google Scholar]
- 13.Jenuwein T., Allis C. D. (2001) Science 293, 1074–1080 [DOI] [PubMed] [Google Scholar]
- 14.Santos-Rosa H., Schneider R., Bannister A. J., Sherriff J., Bernstein B. E., Emre N. C., Schreiber S. L., Mellor J., Kouzarides T. (2002) Nature 419, 407–411 [DOI] [PubMed] [Google Scholar]
- 15.Delcuve G. P., Rastegar M., Davie J. R. (2009) J. Cell. Physiol. 219, 243–250 [DOI] [PubMed] [Google Scholar]
- 16.Walsh C. P., Bestor T. H. (1999) Genes Dev. 13, 26–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stöger R., Kubicka P., Liu C. G., Kafri T., Razin A., Cedar H., Barlow D. P. (1993) Cell 73, 61–71 [DOI] [PubMed] [Google Scholar]
- 18.Ramsahoye B. H., Biniszkiewicz D., Lyko F., Clark V., Bird A. P., Jaenisch R. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 5237–5242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dodge J. E., Ramsahoye B. H., Wo Z. G., Okano M., Li E. (2002) Gene 289, 41–48 [DOI] [PubMed] [Google Scholar]
- 20.Yang X. J., Seto E. (2008) Mol. Cell 31, 449–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J., Kollhoff A., Bergmann A., Stubbs L. (2003) Hum. Mol. Genet. 12, 233–245 [DOI] [PubMed] [Google Scholar]
- 22.Ikeda R., Nishida T., Watanabe F., Shimizu-Saito K., Asahina K., Horikawa S., Teraoka H. (2008) Int. J. Biochem. Cell Biol. 40, 1956–1969 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.