Abstract
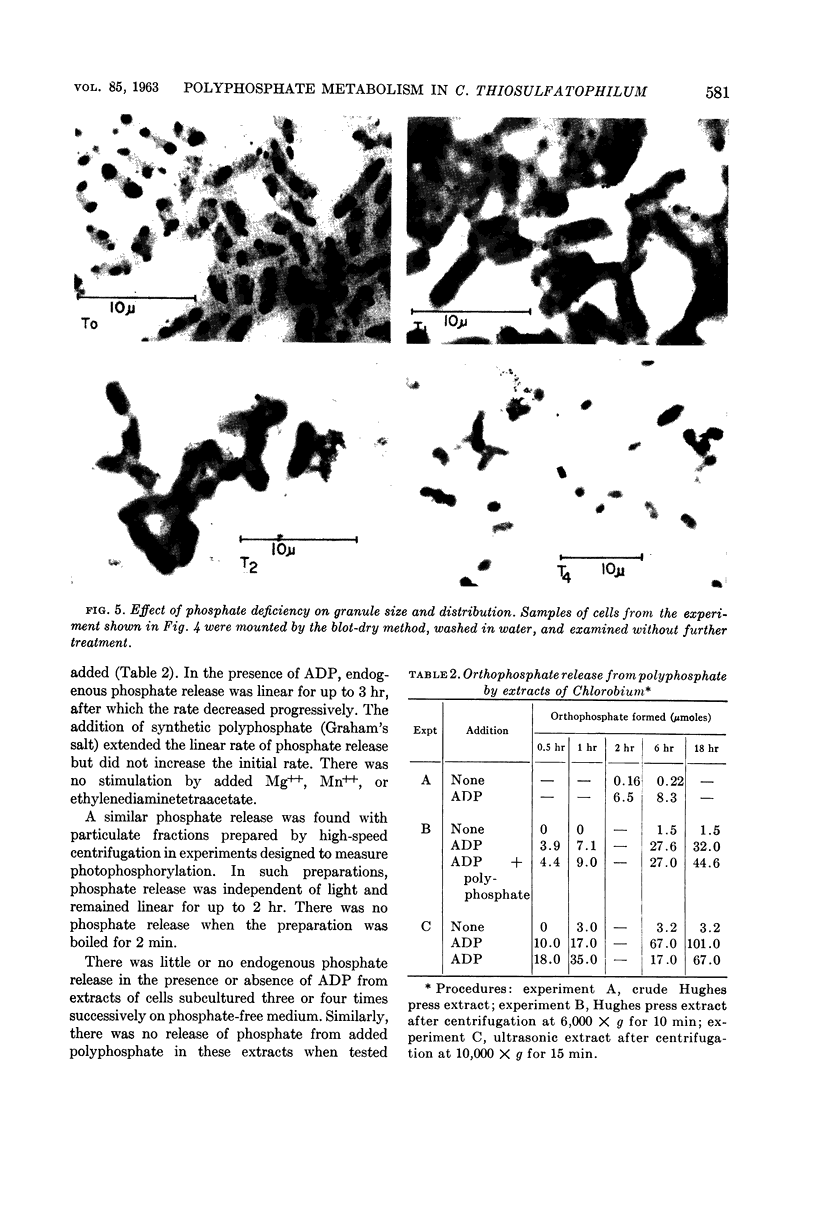
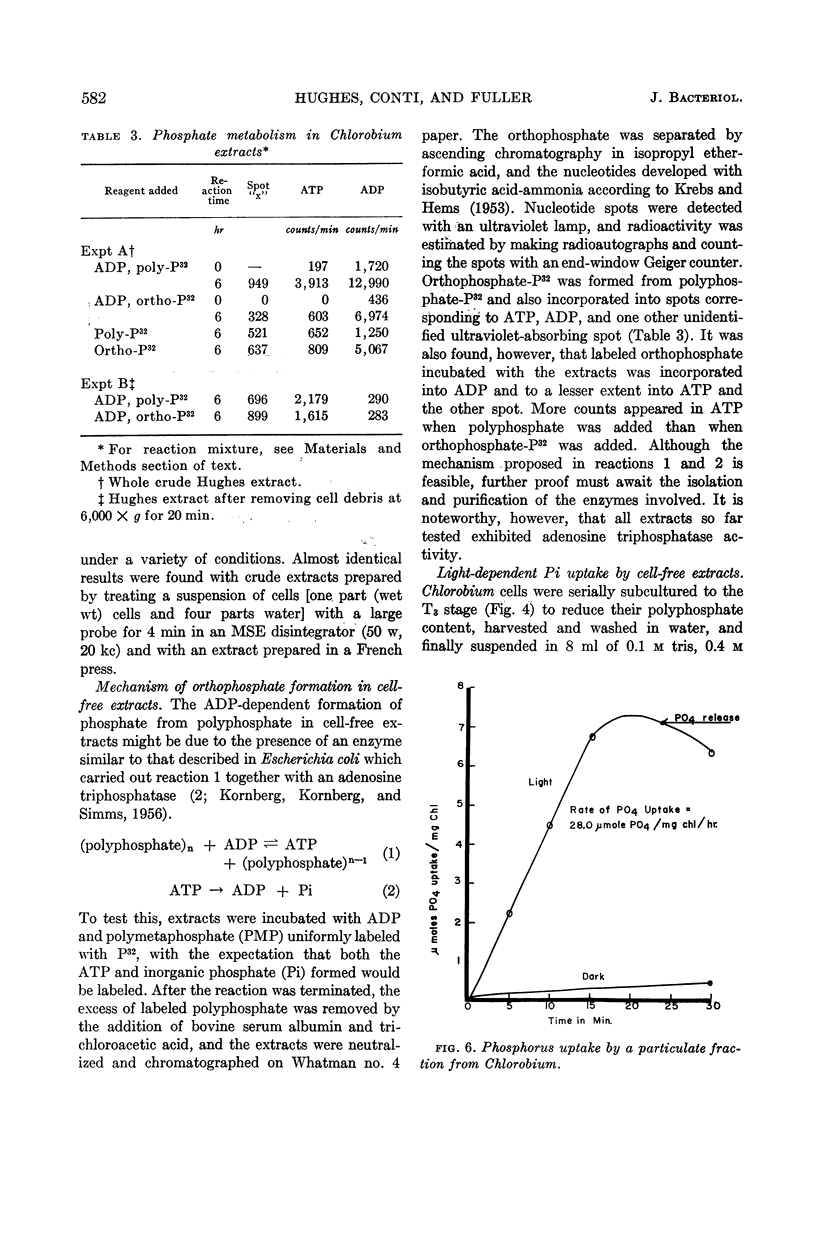
Hughes, D. E. (Dartmouth Medical School, Hanover, N.H.), S. F. Conti, and R. C. Fuller. Inorganic polyphosphate metabolism in Chlorobium thiosulfatophilum. J. Bacteriol. 85:577–584. 1963.—Cells of the obligate phototroph Chlorobium thiosulfatophilum, when grown on a normal concentration of inorganic phosphate, accumulated large intracellular metachromatic granules identified as polymetaphosphate. Inorganic phosphate was released from polymetaphosphate by cell-free extracts. This release was adenosine diphosphate-dependent and light-independent. When cells were subcultured through serial transfer in the absence of inorganic phosphate, the production of polymetaphosphate granules was almost completely stopped. After the serial transfer, these cells showed little or no endogenous release of inorganic phosphate or release from added polymetaphosphate. Using such polymetaphosphate-free cells, it has been possible to demonstrate photosynthetic phosphorylation by the naturally occurring photosynthetic macro-molecules isolated from this organism. These particles, of about 100 A in diameter with a molecular weight of approximately 1.5 million, are by far the simplest functional naturally occurring photosynthetic electron-transport units thus far described.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fuller R. C. Modified End-Window Counting Tube for Paper Chromatograms. Science. 1956 Dec 21;124(3234):1253–1253. doi: 10.1126/science.124.3234.1253. [DOI] [PubMed] [Google Scholar]
- HAROLD F. M. Depletion and replenishment of the inorganic polyphosphate pool in Neurospora crassa. J Bacteriol. 1962 May;83:1047–1057. doi: 10.1128/jb.83.5.1047-1057.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGHES D. E. A press for disrupting bacteria and other micro-organisms. Br J Exp Pathol. 1951 Apr;32(2):97–109. [PMC free article] [PubMed] [Google Scholar]
- HULCHER F. H., CONTI S. F. Cytochromes in chlorophyll-containing particles of Chromatium and Chlorobium thiosulfatophiulm. Biochem Biophys Res Commun. 1960 Nov;3:497–503. doi: 10.1016/0006-291x(60)90163-7. [DOI] [PubMed] [Google Scholar]
- KORNBERG A., KORNBERG S. R., SIMMS E. S. Metaphosphate synthesis by an enzyme from Escherichia coli. Biochim Biophys Acta. 1956 Apr;20(1):215–227. doi: 10.1016/0006-3002(56)90280-3. [DOI] [PubMed] [Google Scholar]
- KREBS H. A., HEMS R. Some reactions of adenosine and inosine phosphates in animal tissues. Biochim Biophys Acta. 1953 Sep-Oct;12(1-2):172–180. doi: 10.1016/0006-3002(53)90136-x. [DOI] [PubMed] [Google Scholar]
- MILNER H. W., LAWRENCE N. S., FRENCH C. S. Colloidal dispersion of chloroplast material. Science. 1950 Jun 9;111(2893):633–634. doi: 10.1126/science.111.2893.633. [DOI] [PubMed] [Google Scholar]
- MUHAMMED A., RODGERS A., HUGHES D. E. Purification and properties of a polymetaphosphatase from Corynebacterium xerosis. J Gen Microbiol. 1959 Jun;20(3):482–495. doi: 10.1099/00221287-20-3-482. [DOI] [PubMed] [Google Scholar]
- SALL T., MUDD S., TAKAGI A. Polyphosphate accumulation and utilization as related to synchronized cell division of Corynebacterium diphtheriae. J Bacteriol. 1958 Dec;76(6):640–645. doi: 10.1128/jb.76.6.640-645.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH I. W., WILKINSON J. F., DUGUID J. P. Volutin production in Aerobacter aerogenes due to nutrient imbalance. J Bacteriol. 1954 Oct;68(4):450–463. doi: 10.1128/jb.68.4.450-463.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIDRA A. Metachromatic granules of microorganisms. J Bacteriol. 1959 Nov;78:664–670. doi: 10.1128/jb.78.5.664-670.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINDER F. G., DENNENY J. M. The metabolism of inorganic polyphosphate in mycobacteria. J Gen Microbiol. 1957 Dec;17(3):573–585. doi: 10.1099/00221287-17-3-573. [DOI] [PubMed] [Google Scholar]