Abstract
Animal eyes can vary in complexity ranging from a single photoreceptor cell shaded by a pigment cell to elaborate arrays of these basic units, which allow image formation in compound eyes of insects or camera-type eyes of vertebrates. The evolution of the eye requires involvement of several distinct components—photoreceptors, screening pigment and genes orchestrating their proper temporal and spatial organization. Analysis of particular genetic and biochemical components shows that many evolutionary processes have participated in eye evolution. Multiple examples of co-option of crystallins, Gα protein subunits and screening pigments contrast with the conserved role of opsins and a set of transcription factors governing eye development in distantly related animal phyla. The direct regulation of essential photoreceptor genes by these factors suggests that this regulatory relationship might have been already established in the ancestral photoreceptor cell.
Keywords: eye, evolution, crystallin, opsin, pigment, gene
1. Introduction
Eyes of some sort occur in many animal phyla, but their anatomy, ontogenetic origin and degree of sophistication vary enormously (Land & Nilsson 2002). For the purpose of this text, we use the minimal definition of an eye as a photoreceptor cell in the close vicinity of a screening pigment (Arendt & Wittbrodt 2001; Land & Nilsson 2002)—a situation found, for example, in Hesse eye cups of amphioxus (Lacalli 2004). As these two components are formed by different genes and genetic pathways, which have different evolutionary histories, the evolution of an eye then becomes a question of evolutionary history of these separate components and their continuous or repeated integration to an organ called ‘eye’. The aim of this review is to gather available genetic and biochemical data regarding evolution of particular eye components, i.e. phototransduction (which is extensively reviewed elsewhere in this issue), pigmentation and regulatory genes involved, and unite these processes.
2. Genetic components of photoreceptors
Despite a remarkable variation in size and complexity, the common indispensable basis of all animal eyes is the photoreceptor cell containing photosensitive molecules, which are connected to a downstream phototransduction cascade. The photoreceptor cells are classified according to the morphology of membrane protrusions bearing visual pigments as ‘rhabdomeric’, which form microvilli, and ‘ciliary’, where the membrane surface is increased by folding the membrane of the cilium (Eakin 1979; Arendt 2003). Primary observations revealed the rhabdomeric photoreceptors as being predominantly present in the eyes of invertebrates, whereas the vertebrate eyes employ the ciliary type; however, several exceptions from this rule do exist (Arendt & Wittbrodt 2001; Land & Nilsson 2002). Both photoreceptor cell types have always co-existed in bilaterians, as suggested by both types found in amphioxus (Lacalli 2004) and confirmed by recent morphological and molecular studies (Arendt et al. 2004; Velarde et al. 2005). Still, it is not clear why the two types of photoreceptors were employed in the eyes of invertebrates versus vertebrates in a mutually exclusive way.
(a). Opsins: variation on ancestral theme
The first step of photoreception is mediated by light-sensitive transmembrane proteins containing retinal chromophore, generally termed rhodopsins. They have been found in most groups of organisms including archeal prokaryotes (Blanck & Oesterhelt 1987), unicellular eukaryotes (Nagel et al. 2002), fungi (Bieszke et al. 1999) and metazoa. The functions of rhodopsins in these organisms vary from photon-driven ionic pumps in prokaryotes (Blanck & Oesterhelt 1987), sensory molecules in fungi, to a light-gated ion channel in the eyespot of green algae (Spudich et al. 2000; Nagel et al. 2002). Metazoan opsins are seven transmembrane proteins belonging to the superfamily of G-protein coupled receptors (GPCRs) and often are coupled to a Gα protein to mediate a phototransduction cascade. Despite these distinct functions of rhodopsins, several common features, such as a transmembrane structure, conserved retinal group covalently bound to a lysine residue and similarities in exon–intron structure, suggested the possibility of common evolutionary origin of all opsins. However, a recent bioinformatic study (Larusso et al. 2008) provided strong evidence that at least prokaryotic and metazoan opsins are not homologous, thus revealing their common features as a remarkable example of convergent evolution.
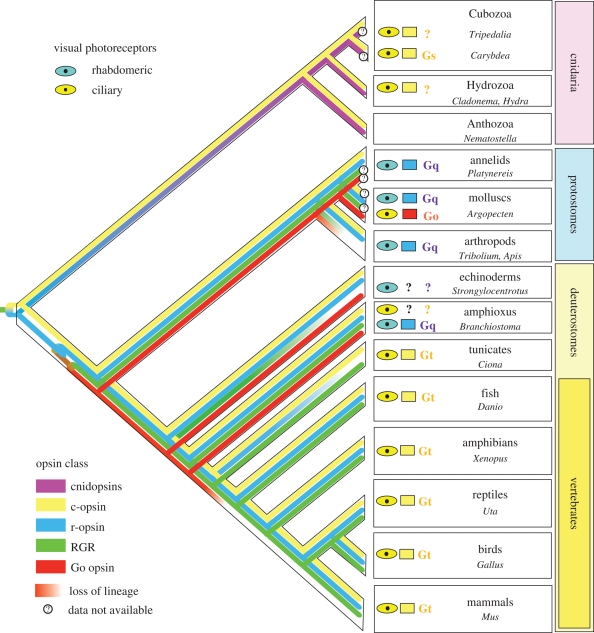
With increasing number of sequenced genomes available, the opsin gene repertoire has been described in several animal species (Raible et al. 2006; Plachetzki et al. 2007; Holland et al. 2008). This information then enables the understanding of metazoan opsin evolution and origin (figure 1), which is inseparably connected with the origin of eyes itself. The metazoan opsins seem to originate early in metazoan evolution from a single GPCR gene by acquisition of light sensitivity. As no opsin genes have been found in available genomic data for the choanoflagellate Monosiga and the poriferan Amphimedon (Plachetzki et al. 2007; Suga et al. 2008), it is probable that this event pre-dated the cnidarian–bilaterian ancestor. Before the split of cnidarians and bilaterians, the newly formed ur-opsin gene underwent duplication producing two ancestral classes of opsins: ciliary opsin class (c-opsin) conserved in cnidarians and bilaterians and a second opsin gene eventually giving rise to the rest of bilaterian opsins (see below) and persisting in cnidarians as ‘cnidopsin’ class (figure 1) (Plachetzki et al. 2007).
Figure 1.
A schematic diagram of opsin distribution among eyes in different animal phyla. Particular opsins subfamilies are distinguished by different colouring. The lines leading to different phyla depict the hypothetical evolutionary fate of given opsin lineage based on available genomic and other data. The question mark denotes such animals where the presence of a given opsin lineage has not been confirmed yet. The colour of the eye-like pictogram corresponds to the type of photoreceptor cell employed in the eye. The class of opsin employed in the eye is represented by the colour of the rectangle next to the eye-like pictogram. If known, the Gα subunit interacting with the opsin is shown. Note that a small subset of vertebrate retinal ganglion cells expresses melanopsin coupled to a Gq signalling cascade (Panda et al. 2005). Although these cells fulfil the definition of a minimal eye, they are not the major photoreceptors of the eye and are not considered in this figure.
In cnidarians, the c-opsins are expressed in the ciliary photoreceptors of adult eyes of hydrozoan Cladonema, cubozoans Tripedalia and Carybdea (Koyanagi et al. 2008; Kozmik et al. 2008a; Suga et al. 2008).
The bilaterian opsin repertoire comprises the c-opsin class and the second ancestral class which has diversified into several subclasses termed as rhabdomeric opsins (r-opsins), Go-coupled opsins, neuropsins and RGR (Terakita 2005). From these, only three groups (namely c-, r- and Go-coupled opsins) seem to have been recruited for visual purposes, whereas the function of other subclasses is probably supportive, as, for example, photoisomerases (RGR-opsin) involved in the retinal visual cycle (Radu et al. 2008). With one exception of Go-coupled opsin mediating the phototransduction in the ciliary part of the retina in scallop Patinopecten yessoensis (Kojima et al. 1997), the rhabdomeric and ciliary photoreceptors of bilaterians consistently employ r- and c-opsins, respectively.
(b). Phototransduction in ciliary photoreceptors: promiscuity in Gα coupling
The different morphology of rhabdomeric and ciliary photoreceptors is further reflected in the level of phototransduction cascades operating in these cells, although some common elements, such as opsins or arrestins, participate in both. The rhabdomeric phototransduction cascade, which is mediated by Gαq and phospholipase C (Suzuki et al. 1995), seems to be evolutionarily conserved from protostomes to vertebrate retinal ganglion cells (Koyanagi et al. 2005; Panda et al. 2005; Contin et al. 2006; Graham et al. 2008). In contrast, the ciliary photoreceptors may employ both Go-opsin (Kojima et al. 1997) and c-opsins, whose Gα protein partners may be rather variable. In vertebrate rods and cones, the c-opsin couples via transducin Gαt to downstream hyperpolarizing phototransduction cascade. Because the Gαt subfamily originated together with other rod and cone specific phototransduction genes during vertebrate-specific whole-genome duplications (Nordstrom et al. 2004; Milligan & Kostenis 2006), the Gαt subunits are not present in invertebrates. A question arises—what then are the Gα proteins involved in ciliary phototransduction cascades in invertebrates and cnidarians? Apparently, the members of the Gαi/o protein subfamily, from which Gαt proteins evolved, could be plausible candidates for this function. Consistent with this assumption, a Gαi1 protein subunit is expressed in the ciliary photoreceptor cells of Ciona intestinalis (Yoshida et al. 2002). In a reptile Uta stansburiana, a vertebrate c-opsin (parietopsin), expressed in the parietal eye retina, signals via a Gαo protein (Su et al. 2006), and in the same cell, another vertebrate visual/non-visual opsin, pinopsin, couples with gustducin—a third vertebrate paralogue of transducin not used in rods and cones. In contrast to its reptile counterpart, chicken pinopsin has been shown to interact with Gα11 subunit (Kasahara et al. 2002), which is closely related to Gαq protein. An exciting surprise came from a recent study (Koyanagi et al. 2008), which revealed that phototransduction in cubomedusan Carybdea rastonii is mediated by Gαs cascade.
Taken together, these findings indicate that the coupling specificity of ciliary opsins could be rather promiscuous in comparison to the rhabdomeric opsins retaining more strictly the Gαq specificity. Since the origin of G proteins pre-dated the origin of opsins, the opsin–Gα protein interaction evolved by co-option and subsequent coevolution (Plachetzki & Oakley 2007). Multiple co-option events during c-opsin evolution might explain their ‘promiscuity’ and reconcile an apparent discrepancy between the data pointing to Gi/o phototransduction cascade in Tripedalia (Kozmik et al. 2008a) and Gs-mediated transduction in Carybdea (Koyanagi et al. 2008). Although Carybdea and Tripedalia are closely related, the opsin sequences identified in the studies are rather diversified and suggest that two different phototransduction cascades in structurally the same eyes might be possible. Moreover, all the opsins found to be expressed in the eye of hydrozoan Cladonema radiatum and assigned to the ciliary class (Suga et al. 2008) show even more sequence diversification (figure S1, electronic supplementary material). Together with low bootstrap support in phylogenetic trees and discrepancies in total number of Hydra and Nematostella opsins identified in two independent studies (Plachetzki et al. 2007; Suga et al. 2008), further analyses are required to fully resolve the relationships among cnidarian opsins and address their Gα coupling specificity and role in phototransduction.
3. Dark pigments: redeployment without logic
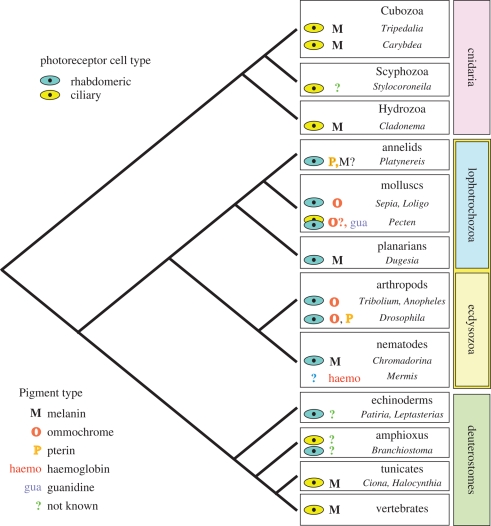
The second essential component of a postulated minimal eye is a dark shielding pigment, which brings the organism additional information of light direction. Generally, three types of compounds—melanins, ommochromes and pterins—serve as shielding pigments in most animal eyes (see electronic supplementary material for detailed information). The distribution of screening pigments in the animal kingdom (figure 2) does not follow any rule and is not correlated with a certain eye or photoreceptor cell. This leads to an assumption that the union of a dark pigment and photoreceptor cannot be traced to any ancestral condition, but is rather an outcome of independent assembly of these two components in different animal phyla. Another possibility is that the pigments coexisted in ancient pigmented photoreceptor cells and were lost in several extant pigment cells.
Figure 2.
A schematic diagram of distribution of screening pigments in different animal phyla. The photoreceptor cell type screened by particular pigments is depicted on the left side of the box of each phylum. There is no apparent correlation between the photoreceptor cell type and shielding pigment used. Note the unknown type of photoreceptor and unique deployment of haemoglobin as a shielding pigment of nematode Mermis, caused probably by independent origin of the eye in this organism. See electronic supplementary material for further details and references.
4. Lens-containing eyes: a story of endless crystallin diversity
Lens-containing eyes are a feature of a surprisingly broad spectrum of organisms across the animal kingdom, which represent a significant improvement of the simple eye composed of just photoreceptor cells and pigment cells. Highly abundant, water-soluble proteins that contribute to the main optical properties of lens, transparency and refractive power, are collectively called crystallins (reviewed in Piatigorsky 2007). The molecular mechanism of their optical function is based on their exceptional solubility and stability at high concentration. In some cases, crystallins may account for up to 90 per cent of the dry weight of the lens. Remarkably and in striking contrast to the universal conservation of opsins as the visual pigments in the photoreceptors, the lens crystallins are enormously diverse proteins that are often taxon-specific, i.e. different proteins function as crystallins in different species (Wistow & Piatigorsky 1988; de Jong et al. 1989; Piatigorsky & Wistow 1989). Crystallin diversity might be a consequence, at least in some cases, of multiple independent origins of lenses during evolution. A surprising number of proteins has biophysical properties that enable them to fulfil an optical role as lens crystallins in different taxonomic groups. Nevertheless, the selection of a particular polypeptide as a crystallin appears not to be entirely random since certain proteins are preferentially recruited as lens crystallins. Proteins used as lens crystallins are often related or identical to ubiquitously expressed metabolic enzymes or physiological stress proteins. For example, all vertebrate lenses contain the α-crystallins that belong to the family of small heat shock proteins (Ingolia & Craig 1982; de Jong et al. 1993) and the β/γ-crystallins that are related to microbial stress proteins (Wistow 1990; D'Alessio 2002). Vertebrate α-crystallins are indeed effective chaperones that protect partially denatured proteins from aggregating in the lens (Horwitz 1992). The co-option of a protein with chaperone-like activity as a lens crystallin makes sense, in light of the fact that proteins in vertebrate lenses must remain functional and soluble for the entire lifespan of an organism. The selective advantage of recruiting enzyme-crystallins is much less clear. First, they do not fall within a common metabolic category. The catalytic activities include, but are not limited to, lactate dehydrogenase (ε-crystallin in crocodiles and birds), argininosuccinate lyase (δ-crystallin in reptiles and birds), α-enolase (τ-crystallin in turtles), glutathione S-transferase (S-crystallin in cephalopods), ADP-ribosylglycohydrolase (J1-crystallin in cubozoan jellyfish) or glyceraldehyde 3-phosphate dehydrogenase (π-crystallin in diurnal geckos) (Piatigorsky 2007). Furthermore, some enzyme-crystallins have no or diminished catalytic activity, e.g. aldehyde dehydrogenase/Ω-crystallin in scallops (Piatigorsky et al. 2000) or argininosuccinate lyase/δ1-crystallin in chickens (Piatigorsky et al. 1988), suggesting that their function in the lens is limited to a refractive role.
It turned out that most crystallins are not lens-restricted proteins being expressed at a low level in other tissues as well. This means that apart from their optical role in the lens, crystallins serve an apparently different function elsewhere. A concept of ‘gene sharing’ has emerged from crystallin studies (Piatigorsky et al. 1988; Piatigorsky & Wistow 1989; Piatigorsky 2007). Gene sharing proposes that the protein encoded by a single gene may perform two entirely different functions: a structural (refractive) function in the lens as a lens crystallin and a catalytic or stress function elsewhere in the organism. An important implication of gene sharing is that a protein can evolve a new role, without losing its original function, simply by a change in gene expression (Piatigorsky & Wistow 1991). It has become apparent over time that gene sharing and repeated use of proteins for new tasks are not limited to crystallins and lenses, but they rather represent a common evolutionary strategy (Piatigorsky 2007).
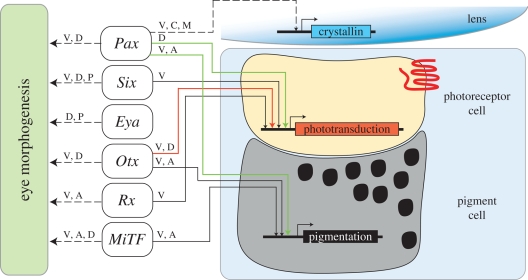
The overall diversity of crystallins throughout the animal kingdom is indicative of convergent evolutionary solution: lens cells simply choose to synthesize a suitable protein to high levels to make their lenses optically useful. Such opportunistic molecular strategy emphasizes the key role of transcriptional regulation in crystallin gene recruitment. The high level lens-preferred expression of crystallin genes is often regulated by transcription factors implicated in eye development as well (Duncan et al. 2004). Perhaps the best example of such a dual role of transcription factors as regulators of both eye development and crystallin gene expression is the case of Pax proteins (reviewed in Cvekl & Piatigorsky 1996; Gehring & Ikeo 1999; Cvekl et al. 2004; Kondoh et al. 2004; Kozmik 2005). The presence of Pax regulatory elements in non-homologous crystallin genes in phylogenetically distant animal species is due to convergent evolution (Carosa et al. 2002; Cvekl et al. 2004; Kozmik et al. 2008b). It reflects the situation that the diverse crystallin genes have been recruited independently during evolution and hence their regulatory elements to achieve lens-preferred expression must have been acquired by independent events as well (figure 3).
Figure 3.
Dual role of transcription factors in regulation of both eye development and differentiation genes. The box on the left-hand side represents the sum of largely unknown developmental genes regulated by corresponding transcription factors based on functional data. The letters represent different animals (V, vertebrates; A, ascidians; D, Drosophila; C, cnidarians; M, molluscs; P, planarians). The arrows on the right-hand side represent a direct influence of a given factor on differentiation set of genes proved by biochemical methods (DNA-binding assay, ChIP, transgenesis, luciferase assays, etc.) The green arrows indicate the ancestral interaction proposed by the ‘bipartite’ model. The red arrow highlights the proposed role of Otx in the regulation of ancestral phototransduction genes. Co-option of a certain transcription factor to a new role is indicated by dashed line. We propose that the transcription factors were independently co-opted for regulation of genes governing eye development in different species and these downstream genes may vary among species. Please note that cross-regulatory interactions of transcription factors are not considered in this scheme for simplicity.
5. Redeployment of a selected set of transcription factors for animal eye development
Structural components of simple or complex (lens-containing) eyes described above are encoded by genes expressed during terminal cell differentiation at the end of developmental processes. Any developmental process to be completed properly requires tight regulation by a dedicated set of transcription factors. Given the enormous diversity of animal eyes, it came as a surprise that certain transcription factors are redeployed for visual system development far more often than others. In addition to governing eye morphogenesis, some of these transcription factors are directly involved in the regulation of differentiation genes encoding structural eye components.
(a). Pax family of transcription factors
Pax transcription factors are defined by the presence of a highly conserved DNA binding domain, the paired domain (Burri et al. 1989; Treisman et al. 1991). In addition to a paired domain, some Pax proteins (such as Pax6) contain a second DNA binding domain, a homeodomain. Because a PaxB-like gene was identified in porifera (Hoshiyama et al. 1998) and placozoa (Hadrys et al. 2005), the origin of Pax genes predates the origin of eyes and the nervous system. The widespread use of Pax genes in the genetic programme underlying eye formation throughout the animal kingdom is remarkable. Mutations in the Pax6 gene disrupt eye development in both mammals (Hill et al. 1991) and insects (Quiring et al. 1994). The ability to induce ectopic eyes through Pax6 misexpression has furthermore been demonstrated in Drosophila and vertebrates (Halder et al. 1995; Chow et al. 1999). The key role of the Pax6 gene for eye morphogenesis in such diverse species led to the proposal of Pax6 being a ‘master control gene’ in animal visual system development (Gehring & Ikeo 1999). Such a simplified scenario, however, has to be dismissed. The term ‘master control gene’ implies that the Pax6 transcription factor is located at the top of a gene cascade and initiates eye development in almost any tissue where it is ectopically expressed. However, neither seems to be the case. For instance, in the absence of Pax6, presumptive retina can develop up to the optic cup stage, albeit abnormally (Grindley et al. 1995). Moreover, within the lens placode, Pax6 expression is under the control of other transcription factors, Meis1 and Meis2 (Zhang et al. 2002). In Drosophila, the ability of the two Pax6 paralogues, ey (Halder et al. 1995) and toy (Czerny et al. 1999), to induce ectopic eyes is restricted both spatially and temporally. These limitations suggest that ey/toy modify an existing programme of sensory organ development rather than initiate the entire eye morphogenesis. Furthermore, toy controls more than just eye morphogenesis because a loss-of-function mutation produces flies missing an entire head (Kronhamn et al. 2002). Likewise, in mice, Pax6 is a pleiotropic regulator of development (Simpson & Price 2002).
Despite these limitations, there is no doubt that Pax6 and other Pax genes have been frequently redeployed for visual system development. Apart from Pax6/ey/toy, three other Pax genes (Pax2, Eyg, toe) might have a role in the genetic programme underlying Drosophila eye morphogenesis (Fu & Noll 1997; Jang et al. 2003; Dominguez et al. 2004). Likewise, in mice, Pax2 cooperates with Pax6 in the development of the retinal pigment epithelium (Baumer et al. 2003) and the mutual repression of Pax6 and Pax2 is responsible for morphogenesis of the entire mouse optic primordium (Schwarz et al. 2000). Even in species where genetic studies have not been done, there has been a generally good correlation between the presence of eyes and Pax gene expression (Loosli et al. 1996; Glardon et al. 1997, 1998; Tomarev et al. 1997; Callaerts et al. 1999; Arendt et al. 2002; Hartmann et al. 2003; Kozmik et al. 2003; Quigley et al. 2007), although the expression is never eye-restricted. There are few notable examples, however, known so far among bilaterians of eyes developing in the absence of Pax6. Pax6 is apparently not expressed in developing Limulus eyes (Blackburn et al. 2008), developing Platynereis adult eyes (Arendt et al. 2002), Hesse eye cups of amphioxus (Glardon et al. 1998) and its function is not required for the eye regeneration in planarians (Pineda et al. 2002) as well as the Bolwig organ in Drosophila (Suzuki & Satoh 2000). Nonetheless, Pax genes arguably have an ancient and fundamental role in visual system development. The bipartite model (Kozmik 2005) proposes that the two independent DNA binding domains within a single Pax transcription factor have been co-opted for two essential features of the prototypical eye, production of a dark shielding pigment and production of a photopigment. Frequent deployment of Pax genes in eye development in phylogenetically diverse species may reflect their ancestral role in the regulation of key differentiation genes (figure 3).
(b). Sine oculis (Six) and eyes absent (Eya)
Eye specification in Drosophila is governed by the members of the retinal determination gene network that includes, apart from Pax6 paralogues (ey, toy), eyes absent (eya), sine oculis (so/six) and dachshund (dac) (Pappu & Mardon 2004; Silver & Rebay 2005; Friedrich 2006). This highly interactive network of genes is sometimes referred to as Pax–Six–Eya–Dach network (PSEDN; Kawakami et al. 2000) to reflect the situation that it has been co-opted for non-retinal roles in other species and developmental contexts (Heanue et al. 1999; Xu et al. 1999; Ozaki et al. 2004; Kozmik et al. 2007). Six and Eya are evolutionarily old gene families, whose origin like in the case of Pax predates the origin of eyes and that are characterized by the conserved biochemical roles of the encoded proteins (Bebenek et al. 2004; Silver & Rebay 2005). This is perhaps best documented by Eya gene that encodes a protein phosphatase (Li et al. 2003; Rayapureddi et al. 2003; Tootle et al. 2003). Eya phosphatase activity is required for eye development in Drosophila, yet the same phosphatase activity is already found in the plant orthologue (Silver & Rebay 2005). Eya functions in the transcription factor complex with members of Six gene family (Ohto et al. 1999), which might explain consistent co-expression in many different developmental settings (Silver & Rebay 2005).
Nonetheless, the current evidence strongly suggests that Eya and Six have an ancient role in eye development. Orthologues of both genes are involved in visual system development in both invertebrates and vertebrates. In Drosophila, deficiency mutations of so and eya are characterized by loss of all visual sense organs (Bonini et al. 1993; Cheyette et al. 1994; Zimmerman et al. 2000; Friedrich 2006). Likewise, eya and so are expressed in the embryonic visual system of directly developing insects (Dong & Friedrich 2005) and their knockdown induces a long-term arrest of eye development (Dong & Friedrich in press). In addition, a divergent Six3/6-like gene, optix, does not synergize with eya and contributes to compound eye morphogenesis in Drosophila by a mechanism that is apparently Pax6/ey independent (Seimiya & Gehring 2000). In the annelid Platynereis, the orthologue of Six1/2 is expressed in all components of the larval and adult visual system (Arendt et al. 2002). Orthologues of eya and so are functionally required for planarian eye regeneration (Pineda et al. 2002; Mannini et al. 2004). In the hydrozoan jellyfish Cladonema, a species with well-developed eyes, orthologues of Six1/2 and Six3/6 are expressed, both during normal development and during the process of regeneration (Stierwald et al. 2004). Eya and Six4/5 genes in the chordate amphioxus are transiently expressed in the two rhabdomeric photoreceptive neurons that flank a biconcave pigment cell to comprise the first Hesse eyecup (Kozmik et al. 2007). Among the Six genes that are expressed in vertebrate eyes only Six3/6-like genes appear to have a critical role during development (Zuber et al. 1999; Kobayashi et al. 2001; Carl et al. 2002; Li et al. 2002; Zhu et al. 2002; Lopez-Rios et al. 2003; Liu et al. 2006). In addition, Six3 has been implicated in the regulation of mouse rhodopsin gene (Manavathi et al. 2007). The analysis of a functional role of Eya in vertebrate eye development is complicated by the fact that three mouse Eya genes (Eya1–3) are expressed in the developing eye (Xu & Saunders 1997) and so their combined loss-of-function phenotype has to be generated in order to unmask possible redundancy. Unlike the other trascriptional regulators discussed in this review, Eya has so far not been associated with the expression of key differentiation genes such as opsins or genes regulating dark pigment formation (figure 3).
(c). Orthodenticle-related homeobox (Otx)
The first member of the Otx gene family, orthodenticle (Otd), has been isolated from Drosophila and shown to be necessary for development of photoreceptors in the compound eye, Bolwig organ and the ocelli (Finkelstein et al. 1990; Royet & Finkelstein 1995; Vandendries et al. 1996). Otd also participates in terminal photoreceptor differentiation. It has been shown to directly regulate opsins (Tahayato et al. 2003) and influence the expression of genes involved in rhabdomeric phototransduction cascade (Ranade et al. 2008). In other invertebrates, the expression of Otx genes in photoreceptors has been reported in planarians (Umesono et al. 1999), putative eye-field precursor of the annelid Hydroides elegans (Arenas-Mena & Wong 2007), ciliary and rhabdomeric photoreceptor cells of Platynereis dumerilii (D. Arendt 2008, personal communication), sensory pigment cells of ascidians (Wada et al. 1996) and the frontal eye region of amphioxus (Williams & Holland 1996). Reciprocal rescue experiments with Drosophila and mammalian Otx orthologues demonstrated that at least part of ancestral genetic and biochemical interactions is still conserved between vertebrates and invertebrates (Acampora et al. 1998; Nagao et al. 1998).
Multiple vertebrate orthologoues of otd termed Otx1, Otx2 and Crx/Otx5 (Germot et al. 2001; Plouhinec et al. 2003) probably arose during whole genome duplication, since a single Otx gene is present in the genome of C. intestinalis (Wada et al. 2003) and amphioxus (Williams & Holland 1998). Besides the role of Otx genes in early vertebrate development of anterior neural structures and the brain (Simeone et al. 2002; Acampora et al. 2005), these genes are necessary for the proper development of the pineal gland and the eye (Martinez-Morales et al. 2001; Nishida et al. 2003; Plouhinec et al. 2005). Later in development, Otx genes play a crucial role in the terminal differentiation of photoreceptors and their maintenance during postnatal development (Nishida et al. 2003; Koike et al. 2007). The expression of Otx genes has been also detected in immature retinal ganglion cells (Bovolenta et al. 1997; Martinez-Morales et al. 2001; Rath et al. 2007)—putative descendants of the rhabdomeric photoreceptor line in vertebrates (Arendt 2003).
The role of vertebrate Otx genes in the regulation of eye-specific genes has been extensively studied and led to the discovery of many direct target genes. Crx, strongly expressed in differentiated photoreceptor cells, directly regulates the phototransduction genes, rhodopsin, β-PDE, arrestin and guanylate cyclase, via binding the PCE element in the promoters (Chen et al. 1997; Furukawa et al. 1997; Qian et al. 2005) (for a review, see Hennig et al. 2008). The expression of ciliary-phototransduction cascade genes in the vertebrate pineal gland is mediated by the action of Otx genes as well (Appelbaum & Gothilf 2006; Takechi et al. 2008). Besides the direct regulation of photoreceptor-specific genes, Otx genes are involved in the regulation of pigmentation. In ascidians, the Tyrp gene is a direct target of Otx (Wada et al. 2002). The vertebrate homologue Otx2 has been shown to bind to the promoters of Mitf, tyrosinase and Tyrp1 (Martinez-Morales et al. 2003) as well as Tyrp2 in the retinal pigmented epithelium (Takeda et al. 2003).
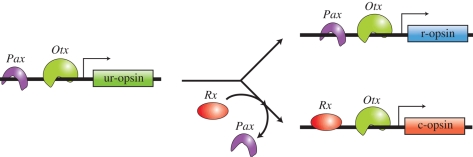
The fact that Otx genes are expressed in both rhabdomeric and ciliary photoreceptors across animal phyla points to their ancient role in photoreceptor cell differentiation (Ranade et al. 2008). The differentiation processes and regulatory subcircuits composed of differentiation genes regulated by certain transcription factors seem to be well conserved during evolution (Arendt 2008). The direct regulation of opsins by Otx could be an example of such a differentiation subcircuit dating back before the split of r- and c-opsins. Based on the scenario of opsin evolution proposed in Plachetzki et al. (2007), the regulatory relationship must have been already established between an ancestral Otx and ur-opsin before the split of cnidarians and bilaterians. Then, after the diversification of c-opsins and the second opsin class, this regulatory unit has been preserved in both rhabdomeric and ciliary photoreceptors (figure 4).
Figure 4.
A hypothetical scenario suggesting the ancient regulatory relationship between Otx and ur-opsin predating the cnidarian–bilaterian split. The ur-opsin has been already regulated by the Otx gene and probably by Pax (not excluding other transcription factors involved). After the duplication and diversification of r- and c-opsin, the regulation by Otx has been preserved in both lineages, whereas Pax-dependent regulation has been lost in c-opsin lineage. Retinal homeobox Rx might have been recruited for regulation of c-opsin. Alternatively, Rx has regulated the ur-opsin and this role has been lost in r-opsin lineage. With increasing complexity of animal body plans, all the transcription factors have consequently acquired additional roles in eye development.
(d). Retinal homeobox (Rx)
During vertebrate development, Rx genes are expressed in the anterior forebrain, retinal primordia and pineal gland (reviewed in Bailey et al. 2004). The over-expression of Rx in Xenopus leads to ectopic formation of retinal tissue (Mathers et al. 1997). In zebrafish chokh mutants, a non-sense mutation in Rx3 paralogue leads to the loss of eyes (Loosli et al. 2003) and Rx knock-out mice lack the eye and the anterior brain structures (Mathers et al. 1997). Similar to vertebrates, the ascidian homologue of Rx is expressed in the anterior brain and the knock-down resulted in the loss of photoreceptor cells (D'Aniello et al. 2006). In contrast, Rx is not expressed in planarian eyes (Salo et al. 2002) and genetic studies in Drosophila have shown a clear dispensability of Rx for compound eye development (Davis et al. 2003). The explanation of this result came from the emerging concept of sister cell types (Arendt 2003) and the fundamental discovery of Rx expression in the ciliary photoreceptors of Platynereis brain (Arendt et al. 2004). These findings led to the identification of Rx as a ciliary photoreceptor-specific marker. One may speculate, what is the reason for keeping the expression of Rx in differentiated ciliary photoreceptors in distantly related species. Analogous to the Otx scenario, the direct regulation of vertebrate c-opsins by Rx (Kimura et al. 2000; Wang et al. 2004; Pan et al. 2006) may point to an ancestral condition of ciliary photoreceptor cell type. In these cells, the regulation of c-opsins by Rx might have been already established and, being a differentiation subcircuit, it was conserved throughout evolution. Rx might be later co-opted for new roles in developmental regulatory networks operating in the anterior body part. Since this hypothesis is based on an Rx/c-opsin regulatory relationship so far confirmed solely in vertebrates, one has to keep in mind a possible co-option of Rx for this function.
(e). Microphthalmia-associated transcription factor (Mitf)
Mitf is a member of the Mitf/TFE family of bHLH-lecine-zipper transcription factors (Hodgkinson et al. 1993). Homozygous-mutant Mitf mice show severe defects of body pigmentation, have small unpigmented eyes, lack melanocytes in the inner ear and are deaf (reviewed in Steingrimsson et al. 2004). Genetic experiments have shown that multiple isoforms of Mitf are responsible for driving the expression of Tyr and Tyrp in melanocytes and retinal pigmented epithelium in cooperation with Otx and Pax genes (Martinez-Morales et al. 2004; Murisier & Beermann 2006; Bharti et al. 2008). Besides its role in melanogenesis, Mitf has been suggested to be involved in the regulation of the pteridine synthesis pathway in zebrafish (Ziegler 2003). In ascidians, Mitf is expressed in the precursors of pigmented cells in the brain vesicle (Yajima et al. 2003). Although the regulatory mechanism of ascidian Tyr and Tyrp genes is not yet fully understood (Toyoda et al. 2000), ascidian Tyrp gene has been shown to be directly regulated by Otx (Wada et al. 2002) and over-expression experiments suggest that Mitf and Pax are involved as well (Yajima et al. 2003; Toyoda et al. 2004).
The role of Mitf in eye development does not seem to be restricted to deuterostomes. Drosophila homologue of Mitf has been shown to be expressed in the eye-antennal imaginal disc and expression of dominant negative form of Drosophila Mitf resulted in enlarged photoreceptor field (Hallsson et al. 2004). Strikingly, a cnidarian homologue of Mitf has been isolated (Kozmik et al. 2008a) and shown to be expressed in the melanin-pigmented photoreceptor cells of the camera-type eye of Tripedalia cystophora. The expression of Mitf in cnidarians as well as bilaterian eyes and its interaction with Otx and Pax genes in deutorostomes raises speculations about Mitf–Pax–Otx cooperation within an ancestral pigmented photoreceptor cell.
6. Conclusions
The commonalities in the use of structurally similar seven-transmembrane receptors (opsins) as animal eye photopigments stem from their shared evolutionary history. The handfull of dark (shielding) pigments that animals can make through various biosynthetic pathways are all apparently used for screening purposes without any evolutionary pattern or developmental logic. The recruitment of lens crystallins provides an extreme example of an opportunistic use of almost any soluble cytoplasmic protein for a refractive role. In contrast, there is a homology at the level of transcriptional regulators operating in developmental programmes in structurally diverse eyes. Although the expression of genes in all metazoa is generally regulated by a large number of diverse transcription factors often belonging to distinct families, certain transcription factors (i.e. Pax, Six, Eya, Otx, Mitf) have been deployed for the regulation of eye development far more often than others. The reasons for this phenomenon are not entirely clear at the moment, however, might be due to several underlying molecular mechanisms that are not necessarily mutually exclusive. Anteriorly expressed transcription factors are more likely to be co-opted for the regulation of an eye programme due to the fact that eyes are located more often at the front rather than at the back of the animal. Perhaps the most important aspect contributing to the observed homology of transcription factors is due to stochastically chosen ancestral regulatory connections. We can reasonably well argue that certain transcription factors were more or less randomly chosen for the regulation of essential genes in the ancestral (pigmented?) photoreceptor cell such as the gene encoding an ur-opsin. Gene duplication events generated opsin gene duplicates that began the process of divergence both in the coding sequence and in the regulatory regions. One might expect that the diverging genes encoding essential eye components have retained in their promoters binding sites of any transcription factor provided that such regulatory link was useful and the spatio-temporal expression pattern of the regulator was maintained. Such transcriptional regulators were later co-opted into new roles as ‘higher structure’ organizers of increasingly complex but diverse eyes. Frequent deployment in eye development in phylogenetically diverse species, thus, most likely reflects the ancestral role of a particular transcription factor in the regulation of a key differentiation gene.
Acknowledgements
We would like to thank three referees for valuable comments on our manuscript and Mr Jasper Manning for the correction of English. Work in Kozmik's laboratory is supported by grant nos. AV0Z50520514, IAA500520908 and IAA500520604 from the Academy of Sciences of the Czech Republic, by Center for Applied Genomics grant no. 1M6837805002 awarded by Ministry of Education, Youths and Sports of the Czech Republic and grant no. 91808 from the Grant Agency of the Charles University in Prague.
Footnotes
One contribution of 13 to a Theme Issue ‘The evolution of phototransduction and eyes’.
References
- Acampora D., Avantaggiato V., Tuorto F., Barone P., Reichert H., Finkelstein R., Simeone A.1998Murine Otx1 and Drosophila otd genes share conserved genetic functions required in invertebrate and vertebrate brain development. Development 125, 1691–1702 [DOI] [PubMed] [Google Scholar]
- Acampora D., Annino A., Tuorto F., Puelles E., Lucchesi W., Papalia A., Simeone A.2005Otx genes in the evolution of the vertebrate brain. Brain Res. Bull. 66, 410–420 (doi:10.1016/j.brainresbull.2005.02.005) [DOI] [PubMed] [Google Scholar]
- Appelbaum L., Gothilf Y.2006Mechanism of pineal-specific gene expression: the role of E-box and photoreceptor conserved elements. Mol. Cell. Endocrinol. 252, 27–33 (doi:10.1016/j.mce.2006.03.021) [DOI] [PubMed] [Google Scholar]
- Arenas-Mena C., Wong K. S.2007HeOtx expression in an indirectly developing polychaete correlates with gastrulation by invagination. Dev. Genes Evol. 217, 373–384 (doi:10.1007/s00427-007-0150-7) [DOI] [PubMed] [Google Scholar]
- Arendt D.2003Evolution of eyes and photoreceptor cell types. Int. J. Dev. Biol. 47, 563–571 [PubMed] [Google Scholar]
- Arendt D.2008The evolution of cell types in animals: emerging principles from molecular studies. Nat. Rev. Genet. 9, 868–882 (doi:10.1038/nrg2416) [DOI] [PubMed] [Google Scholar]
- Arendt D., Wittbrodt J.2001Reconstructing the eyes of Urbilateria. Phil. Trans. R. Soc. B 356, 1545–1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt D., Tessmar K., De Campos-Baptista M. I., Dorresteijn A., Wittbrodt J.2002Development of pigment-cup eyes in the polychaete Platynereis dumerilii and evolutionary conservation of larval eyes in Bilateria. Development 129, 1143–1154 [DOI] [PubMed] [Google Scholar]
- Arendt D., Tessmar-Raible K., Snyman H., Dorresteijn A. W., Wittbrodt J.2004Ciliary photoreceptors with a vertebrate-type opsin in an invertebrate brain. Science 306, 869–871 (doi:10.1126/science.1099955) [DOI] [PubMed] [Google Scholar]
- Bailey T. J., El-Hodiri H., Zhang L., Shah R., Mathers P. H., Jamrich M.2004Regulation of vertebrate eye development by Rx genes. Int. J. Dev. Biol. 48, 761–770 (doi:10.1387/ijdb.041878tb) [DOI] [PubMed] [Google Scholar]
- Baumer N., Marquardt T., Stoykova A., Spieler D., Treichel D., Ashery-Padan R., Gruss P.2003Retinal pigmented epithelium determination requires the redundant activities of Pax2 and Pax6. Development 130, 2903–2915 (doi:10.1242/dev.00450) [DOI] [PubMed] [Google Scholar]
- Bebenek I. G., Gates R. D., Morris J., Hartenstein V., Jacobs D. K.2004sine oculis in basal Metazoa. Dev. Genes Evol. 214, 342–351 (doi:10.1007/s00427-004-0407-3) [DOI] [PubMed] [Google Scholar]
- Bharti K., Liu W., Csermely T., Bertuzzi S., Arnheiter H.2008Alternative promoter use in eye development: the complex role and regulation of the transcription factor MITF. Development 135, 1169–1178 (doi:10.1242/dev.014142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieszke J. A., Braun E. L., Bean L. E., Kang S., Natvig D. O., Borkovich K. A.1999The nop-1 gene of Neurospora crassa encodes a seven transmembrane helix retinal-binding protein homologous to archaeal rhodopsins. Proc. Natl Acad. Sci. USA 96, 8034–8039 (doi:10.1073/pnas.96.14.8034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn D. C., Conley K. W., Plachetzki D. C., Kempler K., Battelle B. A., Brown N. L.2008Isolation and expression of Pax6 and atonal homologues in the American horseshoe crab, Limulus polyphemus. Dev. Dyn. 237, 2209–2219 (doi:10.1002/dvdy.21634) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanck A., Oesterhelt D.1987The halo-opsin gene. II. Sequence, primary structure of halorhodopsin and comparison with bacteriorhodopsin. EMBO J. 6, 265–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonini N. M., Leiserson W. M., Benzer S.1993The eyes absent gene: genetic control of cell survival and differentiation in the developing Drosophila eye. Cell 72, 379–395 (doi:10.1016/0092-8674(93)90115-7) [DOI] [PubMed] [Google Scholar]
- Bovolenta P., Mallamaci A., Briata P., Corte G., Boncinelli E.1997Implication of OTX2 in pigment epithelium determination and neural retina differentiation. J. Neurosci. 17, 4243–4252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burri M., Tromvoukis Y., Bopp D., Frigerio G., Noll M.1989Conservation of the paired domain in metazoans and its structure in three isolated human genes. EMBO J. 8, 1183–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaerts P., Munoz-Marmol A. M., Glardon S., Castillo E., Sun H., Li W. H., Gehring W. J., Salo E.1999Isolation and expression of a Pax-6 gene in the regenerating and intact Planarian Dugesia(G)tigrina. Proc. Natl Acad. Sci. USA 96, 558–563 (doi:10.1073/pnas.96.2.558) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carl M., Loosli F., Wittbrodt J.2002Six3 inactivation reveals its essential role for the formation and patterning of the vertebrate eye. Development 129, 4057–4063 [DOI] [PubMed] [Google Scholar]
- Carosa E., Kozmik Z., Rall J. E., Piatigorsky J.2002Structure and expression of the scallop Omega-crystallin gene. Evidence for convergent evolution of promoter sequences. J. Biol. Chem. 277, 656–664 (doi:10.1074/jbc.M107004200) [DOI] [PubMed] [Google Scholar]
- Chen S., Wang Q. L., Nie Z., Sun H., Lennon G., Copeland N. G., Gilbert D. J., Jenkins N. A., Zack D. J.1997Crx, a novel Otx-like paired-homeodomain protein, binds to and transactivates photoreceptor cell-specific genes. Neuron 19, 1017–1030 (doi:10.1016/S0896-6273(00)80394-3) [DOI] [PubMed] [Google Scholar]
- Cheyette B. N., Green P. J., Martin K., Garren H., Hartenstein V., Zipursky S. L.1994The Drosophila sine oculis locus encodes a homeodomain-containing protein required for the development of the entire visual system. Neuron 12, 977–996 (doi:10.1016/0896-6273(94)90308-5) [DOI] [PubMed] [Google Scholar]
- Chow R. L., Altmann C. R., Lang R. A., Hemmati-Brivanlou A.1999Pax6 induces ectopic eyes in a vertebrate. Development 126, 4213–4222 [DOI] [PubMed] [Google Scholar]
- Contin M. A., Verra D. M., Guido M. E.2006An invertebrate-like phototransduction cascade mediates light detection in the chicken retinal ganglion cells. FASEB J. 20, 2648–2650 (doi:10.1096/fj.06-6133fje) [DOI] [PubMed] [Google Scholar]
- Cvekl A., Piatigorsky J.1996Lens development and crystallin gene expression: many roles for Pax-6. Bioessays 18, 621–630 (doi:10.1002/bies.950180805) [DOI] [PubMed] [Google Scholar]
- Cvekl A., Yang Y., Chauhan B. K., Cveklova K.2004Regulation of gene expression by Pax6 in ocular cells: a case of tissue-preferred expression of crystallins in lens. Int. J. Dev. Biol. 48, 829–844 (doi:10.1387/ijdb.041866ac) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerny T., Halder G., Kloter U., Souabni A., Gehring W. J., Busslinger M.1999Twin of eyeless, a second Pax-6 gene of Drosophila, acts upstream of eyeless in the control of eye development. Mol. Cell 3, 297–307 (doi:10.1016/S1097-2765(00)80457-8) [DOI] [PubMed] [Google Scholar]
- D'Alessio G.2002The evolution of monomeric and oligomeric betagamma-type crystallins. Facts and hypotheses. Eur. J. Biochem. 269, 3122–3130 (doi:10.1046/j.1432-1033.2002.03004.x) [DOI] [PubMed] [Google Scholar]
- D'Aniello S., et al. 2006The ascidian homolog of the vertebrate homeobox gene Rx is essential for ocellus development and function. Differentiation 74, 222–234 (doi:10.1111/j.1432-0436.2006.00071.x) [DOI] [PubMed] [Google Scholar]
- Davis R. J., Tavsanli B. C., Dittrich C., Walldorf U., Mardon G.2003Drosophila retinal homeobox (drx) is not required for establishment of the visual system, but is required for brain and clypeus development. Dev. Biol. 259, 272–287 (doi:10.1016/S0012-1606(03)00201-X) [DOI] [PubMed] [Google Scholar]
- de Jong W. W., Hendriks W., Mulders J. W., Bloemendal H.1989Evolution of eye lens crystallins: the stress connection. Trends Biochem. Sci. 14, 365–368 [DOI] [PubMed] [Google Scholar]
- de Jong W. W., Leunissen J. A., Voorter C. E.1993Evolution of the alpha-crystallin/small heat-shock protein family. Mol. Biol. Evol. 10, 103–126 [DOI] [PubMed] [Google Scholar]
- Dominguez M., Ferres-Marco D., Gutierrez-Avino F. J., Speicher S. A., Beneyto M.2004Growth and specification of the eye are controlled independently by Eyegone and Eyeless in Drosophila melanogaster. Nat. Genet. 36, 31–39 (doi:10.1038/ng1281) [DOI] [PubMed] [Google Scholar]
- Dong Y., Friedrich M.2005Comparative analysis of Wingless patterning in the embryonic grasshopper eye. Dev. Genes Evol. 215, 177–197 (doi:10.1007/s00427-004-0465-6) [DOI] [PubMed] [Google Scholar]
- Dong Y., Friedrich M.In press Enforcing biphasic visual system development in a directly developing insect by transient knockdown of single eye selector genes. J. Exp. Zool. (Mol. Dev. Evol.) [DOI] [PubMed] [Google Scholar]
- Duncan M. K., Cvekl A., Kantorow M., Piatigorsky J.2004Lens crystallins New York, NY: Cambridge University Press [Google Scholar]
- Eakin R. M.1979Evolutionary significance of photoreceptors—retrospect. Am. Zool. 19, 647–653 [Google Scholar]
- Finkelstein R., Smouse D., Capaci T. M., Spradling A. C., Perrimon N.1990The orthodenticle gene encodes a novel homeo domain protein involved in the development of the Drosophila nervous system and ocellar visual structures. Genes Dev. 4, 1516–1527 (doi:10.1101/gad.4.9.1516) [DOI] [PubMed] [Google Scholar]
- Friedrich M.2006Ancient mechanisms of visual sense organ development based on comparison of the gene networks controlling larval eye, ocellus, and compound eye specification in Drosophila. Arthropod Struct. Dev. 35, 357–378 (doi:10.1016/j.asd.2006.08.010) [DOI] [PubMed] [Google Scholar]
- Fu W., Noll M.1997The Pax2 homolog sparkling is required for development of cone and pigment cells in the Drosophila eye. Genes Dev. 11, 2066–2078 (doi:10.1101/gad.11.16.2066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T., Morrow E. M., Cepko C. L.1997Crx, a novel otx-like homeobox gene, shows photoreceptor-specific expression and regulates photoreceptor differentiation. Cell 91, 531–541 (doi:10.1016/S0092-8674(00)80439-0) [DOI] [PubMed] [Google Scholar]
- Gehring W. J., Ikeo K.1999Pax 6: mastering eye morphogenesis and eye evolution. Trends Genet. 15, 371–377 (doi:10.1016/S0168-9525(99)01776-X) [DOI] [PubMed] [Google Scholar]
- Germot A., Lecointre G., Plouhinec J. L., Le Mentec C., Girardot F., Mazan S.2001Structural evolution of Otx genes in craniates. Mol. Biol. Evol. 18, 1668–1678 [DOI] [PubMed] [Google Scholar]
- Glardon S., Callaerts P., Halder G., Gehring W. J.1997Conservation of Pax-6 in a lower chordate, the ascidian Phallusia mammillata. Development 124, 817–825 [DOI] [PubMed] [Google Scholar]
- Glardon S., Holland L. Z., Gehring W. J., Holland N. D.1998Isolation and developmental expression of the amphioxus Pax-6 gene (AmphiPax-6): insights into eye and photoreceptor evolution. Development 125, 2701–2710 [DOI] [PubMed] [Google Scholar]
- Graham D. M., Wong K. Y., Shapiro P., Frederick C., Pattabiraman K., Berson D. M.2008Melanopsin ganglion cells use a membrane-associated rhabdomeric phototransduction cascade. J. Neurophysiol. 99, 2522–2532 (doi:10.1152/jn.01066.2007) [DOI] [PubMed] [Google Scholar]
- Grindley J. C., Davidson D. R., Hill R. E.1995The role of Pax-6 in eye and nasal development. Development 121, 1433–1442 [DOI] [PubMed] [Google Scholar]
- Hadrys T., Desalle R., Sagasser S., Fischer N., Schierwater B.2005The Trichoplax PaxB gene: a putative Proto-PaxA/B/C gene predating the origin of nerve and sensory cells. Mol. Biol. Evol. 22, 1569–1578 (doi:10.1093/molbev/msi150) [DOI] [PubMed] [Google Scholar]
- Halder G., Callaerts P., Gehring W. J.1995Induction of ectopic eyes by targeted expression of the eyeless gene in Drosophila. Science 267, 1788–1792 (doi:10.1126/science.7892602) [DOI] [PubMed] [Google Scholar]
- Hallsson J. H., Haflidadottir B. S., Stivers C., Odenwald W., Arnheiter H., Pignoni F., Steingrimsson E.2004The basic helix–loop–helix leucine zipper transcription factor Mitf is conserved in Drosophila and functions in eye development. Genetics 167, 233–241 (doi:10.1534/genetics.167.1.233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann B., Lee P. N., Kang Y. Y., Tomarev S., De Couet H. G., Callaerts P.2003Pax6 in the sepiolid squid Euprymna scolopes: evidence for a role in eye, sensory organ and brain development. Mech. Dev. 120, 177–183 (doi:10.1016/S0925-4773(02)00456-2) [DOI] [PubMed] [Google Scholar]
- Heanue T. A., Reshef R., Davis R. J., Mardon G., Oliver G., Tomarev S., Lassar A. B., Tabin C. J.1999Synergistic regulation of vertebrate muscle development by Dach2, Eya2, and Six1, homologs of genes required for Drosophila eye formation. Genes Dev. 13, 3231–3243 (doi:10.1101/gad.13.24.3231) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig A. K., Peng G. H., Chen S.2008Regulation of photoreceptor gene expression by Crx-associated transcription factor network. Brain Res. 1192, 114–133 (doi:10.1016/j.brainres.2007.06.036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R. E., et al. 1991Mouse small eye results from mutations in a paired-like homeobox-containing gene. Nature 354, 522–525 (doi:10.1038/354522a0) [DOI] [PubMed] [Google Scholar]
- Hodgkinson C. A., Moore K. J., Nakayama A., Steingrimsson E., Copeland N. G., Jenkins N. A., Arnheiter H.1993Mutations at the mouse microphthalmia locus are associated with defects in a gene encoding a novel basic-helix–loop–helix-zipper protein. Cell 74, 395–404 [DOI] [PubMed] [Google Scholar]
- Holland L. Z., et al. 2008The amphioxus genome illuminates vertebrate origins and cephalochordate biology. Genome Res. 18, 1100–1111 (doi:10.1101/gr.073676.107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz J.1992Alpha-crystallin can function as a molecular chaperone. Proc. Natl Acad. Sci. USA 89, 10 449–10 453 (doi:10.1073/pnas.89.21.10449) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshiyama D., Suga H., Iwabe N., Koyanagi M., Nikoh N., Kuma K., Matsuda F., Honjo T., Miyata T.1998Sponge Pax cDNA related to Pax-2/5/8 and ancient gene duplications in the Pax family. J Mol Evol 47, 640–648 (doi:10.1007/PL00006421) [DOI] [PubMed] [Google Scholar]
- Ingolia T. D., Craig E. A.1982Four small Drosophila heat shock proteins are related to each other and to mammalian alpha-crystallin. Proc. Natl Acad. Sci. USA 79, 2360–2364 (doi:10.1073/pnas.79.7.2360) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang C. C., et al. 2003Two Pax genes, eye gone and eyeless, act cooperatively in promoting Drosophila eye development. Development 130, 2939–2951 (doi:10.1242/dev.00522) [DOI] [PubMed] [Google Scholar]
- Kasahara T., Okano T., Haga T., Fukada Y.2002Opsin-G11-mediated signaling pathway for photic entrainment of the chicken pineal circadian clock. J. Neurosci. 22, 7321–7325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami K., Sato S., Ozaki H., Ikeda K.2000Six family genes—structure and function as transcription factors and their roles in development. Bioessays 22, 616–626 (doi:10.1002/1521-1878(200007)22:7<616::AID-BIES4>3.0.CO;2-R) [DOI] [PubMed] [Google Scholar]
- Kimura A., Singh D., Wawrousek E. F., KIkuchi M., Nakamura M., Shinohara T.2000Both PCE-1/RX and OTX/CRX interactions are necessary for photoreceptor-specific gene expression. J. Biol. Chem. 275, 1152–1160 (doi:10.1074/jbc.275.2.1152) [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Nishikawa K., Suzuki T., Yamamoto M.2001The homeobox protein Six3 interacts with the Groucho corepressor and acts as a transcriptional repressor in eye and forebrain formation. Dev. Biol. 232, 315–326 (doi:10.1006/dbio.2001.0185) [DOI] [PubMed] [Google Scholar]
- Koike C., et al. 2007Functional roles of Otx2 transcription factor in postnatal mouse retinal development. Mol. Cell. Biol. 27, 8318–8329 (doi:10.1128/MCB.01209-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima D., Terakita A., Ishikawa T., Tsukahara Y., Maeda A., Shichida Y.1997A novel Go-mediated phototransduction cascade in scallop visual cells. J. Biol. Chem. 272, 22 979–22 982 (doi:10.1074/jbc.272.37.22979) [DOI] [PubMed] [Google Scholar]
- Kondoh H., Uchikawa M., Kamachi Y.2004Interplay of Pax6 and SOX2 in lens development as a paradigm of genetic switch mechanisms for cell differentiation. Int. J. Dev. Biol. 48, 819–827 (doi:10.1387/ijdb.041868hk) [DOI] [PubMed] [Google Scholar]
- Koyanagi M., Kubokawa K., Tsukamoto H., Shichida Y., Terakita A.2005Cephalochordate melanopsin: evolutionary linkage between invertebrate visual cells and vertebrate photosensitive retinal ganglion cells. Curr. Biol. 15, 1065–1069 (doi:10.1016/j.cub.2005.04.063) [DOI] [PubMed] [Google Scholar]
- Koyanagi M., Takano K., Tsukamoto H., Ohtsu K., Tokunaga F., Terakita A.2008Jellyfish vision starts with cAMP signaling mediated by opsin-Gs cascade. Proc. Natl Acad. Sci. USA 105, 15 576–15 580 (doi:10.1073/pnas.0806215105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozmik Z.2005Pax genes in eye development and evolution. Curr. Opin. Genet. Dev. 15, 430–438 (doi:10.1016/j.gde.2005.05.001) [DOI] [PubMed] [Google Scholar]
- Kozmik Z., Daube M., Frei E., Norman B., Kos L., Dishaw L. J., Noll M., Piatigorsky J.2003Role of Pax genes in eye evolution: a cnidarian PaxB gene uniting Pax2 and Pax6 functions. Dev. Cell 5, 773–785 (doi:10.1016/S1534-5807(03)00325-3) [DOI] [PubMed] [Google Scholar]
- Kozmik Z., et al. 2007Pax–Six–Eya–Dach network during amphioxus development: conservation in vitro but context specificity in vivo. Dev. Biol. 306, 143–159 (doi:10.1016/j.ydbio.2007.03.009) [DOI] [PubMed] [Google Scholar]
- Kozmik Z., et al. 2008aAssembly of the cnidarian camera-type eye from vertebrate-like components. Proc. Natl Acad. Sci. USA 105, 8989–8993 (doi:10.1073/pnas.0800388105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozmik Z., Swamynathan S. K., Ruzickova J., Jonasova K., Paces V., Vlcek C., Piatigorsky J.2008bCubozoan crystallins: evidence for convergent evolution of pax regulatory sequences. Evol Dev 10, 52–61 [DOI] [PubMed] [Google Scholar]
- Kronhamn J., Frei E., Daube M., Jiao R., Shi Y., Noll M., Rasmuson-Lestander A.2002Headless flies produced by mutations in the paralogous Pax6 genes eyeless and twin of eyeless. Development 129, 1015–1026 [DOI] [PubMed] [Google Scholar]
- Lacalli T. C.2004Sensory systems in amphioxus: a window on the ancestral chordate condition. Brain Behav. Evol. 64, 148–162 (doi:10.1159/000079744) [DOI] [PubMed] [Google Scholar]
- Land M., Nilsson E.2002Animal eyes New York, NY: Oxford University Press [Google Scholar]
- Larusso N. D., Rutterberg B. E., Singh A. K., Oakley T. H.2008Type II opsins: evolutionary origin by internal domain duplication? J. Mol. Evol. 66, 417–423 (doi:10.1007/s00239-008-9076-6) [DOI] [PubMed] [Google Scholar]
- Li X., Perissi V., Liu F., Rose D. W., Rosenfeld M. G.2002Tissue-specific regulation of retinal and pituitary precursor cell proliferation. Science 297, 1180–1183 [DOI] [PubMed] [Google Scholar]
- Li X., et al. 2003Eya protein phosphatase activity regulates Six1–Dach–Eya transcriptional effects in mammalian organogenesis. Nature 426, 247–254 (doi:10.1038/nature02083) [DOI] [PubMed] [Google Scholar]
- Liu W., Lagutin O. V., Mende M., Streit A., Oliver G.2006Six3 activation of Pax6 expression is essential for mammalian lens induction and specification. EMBO J. 25, 5383–5395 (doi:10.1038/sj.emboj.7601398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loosli F., Kmita-Cunisse M., Gehring W. J.1996Isolation of a Pax-6 homolog from the ribbonworm Lineus sanguineus. Proc. Natl Acad. Sci. USA 93, 2658–2663 (doi:10.1073/pnas.93.7.2658) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loosli F., Staub W., Finger-Baier K. C., Ober E. A., Verkade H., Wittbrodt J., Baier H.2003Loss of eyes in zebrafish caused by mutation of chokh/rx3. EMBO Rep. 4, 894–899 (doi:10.1038/sj.embor.embor919) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Rios J., Tessmar K., Loosli F., Wittbrodt J., Bovolenta P.2003Six3 and Six6 activity is modulated by members of the groucho family. Development 130, 185–195 (doi:10.1242/dev.00185) [DOI] [PubMed] [Google Scholar]
- Manavathi B., et al. 2007Repression of Six3 by a corepressor regulates rhodopsin expression. Proc. Natl Acad. Sci. USA 104, 13 128–13 133 (doi:10.1073/pnas.0705878104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannini L., Rossi L., Deri P., Gremigni V., Salvetti A., Salo E., Batistoni R.2004Djeyes absent (Djeya) controls prototypic planarian eye regeneration by cooperating with the transcription factor Djsix-1. Dev. Biol. 269, 346–359 (doi:10.1016/j.ydbio.2004.01.042) [DOI] [PubMed] [Google Scholar]
- Martinez-Morales J. R., Signore M., Acampora D., Simeone A., Bovolenta P.2001Otx genes are required for tissue specification in the developing eye. Development 128, 2019–2030 [DOI] [PubMed] [Google Scholar]
- Martinez-Morales J. R., Dolez V., Rodrigo I., Zaccarini R., Leconte L., Bovolenta P., Saule S.2003OTX2 activates the molecular network underlying retina pigment epithelium differentiation. J. Biol. Chem. 278, 21 721–21 731 (doi:10.1074/jbc.M301708200) [DOI] [PubMed] [Google Scholar]
- Martinez-Morales J. R., Rodrigo I., Bovolenta P.2004Eye development: a view from the retina pigmented epithelium. Bioessays 26, 766–777 [DOI] [PubMed] [Google Scholar]
- Mathers P. H., Grinberg A., Mahon K. A., Jamrich M.1997The Rx homeobox gene is essential for vertebrate eye development. Nature 387, 603–607 (doi:10.1038/42475) [DOI] [PubMed] [Google Scholar]
- Milligan G., Kostenis E.2006Heterotrimeric G-proteins: a short history. Br. J. Pharmacol. 147(Suppl. 1), S46–S55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murisier F., Beermann F.2006Genetics of pigment cells: lessons from the tyrosinase gene family. Histol. Histopathol. 21, 567–578 [DOI] [PubMed] [Google Scholar]
- Nagao T., Leuzinger S., Acampora D., Simeone A., Finkelstein R., Reichert H., Furukubo-Tokunaga K.1998Developmental rescue of Drosophila cephalic defects by the human Otx genes. Proc. Natl Acad. Sci. USA 95, 3737–3742 (doi:10.1073/pnas.95.7.3737) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel G., Ollig D., Fuhrmann M., Kateriya S., Musti A. M., BAmberg E., Hegemann P.2002Channelrhodopsin-1: a light-gated proton channel in green algae. Science 296, 2395–2398 (doi:10.1126/science.1072068) [DOI] [PubMed] [Google Scholar]
- Nishida A., Furukawa A., Koike C., Tano Y., Aizawa S., Matsuo I., Furukawa T.2003Otx2 homeobox gene controls retinal photoreceptor cell fate and pineal gland development. Nat. Neurosci. 6, 1255–1263 (doi:10.1038/nn1155) [DOI] [PubMed] [Google Scholar]
- Nordstrom K., Larsson T. A., Larhammar D.2004Extensive duplications of phototransduction genes in early vertebrate evolution correlate with block (chromosome) duplications. Genomics 83, 852–872 (doi:10.1016/j.ygeno.2003.11.008) [DOI] [PubMed] [Google Scholar]
- Ohto H., Kamada S., Tago K., Tominaga S. I., Ozaki H., Sato S., Kawakami K.1999Cooperation of six and eya in activation of their target genes through nuclear translocation of Eya. Mol. Cell. Biol. 19, 6815–6824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki H., et al. 2004Six1 controls patterning of the mouse otic vesicle. Development 131, 551–562 (doi:10.1242/dev.00943) [DOI] [PubMed] [Google Scholar]
- Pan Y., Nekkalapudi S., Kelly L. E., El-Hodiri H. M.2006The Rx-like homeobox gene (Rx-L) is necessary for normal photoreceptor development. Invest. Ophthalmol. Vis. Sci. 47, 4245–4253 (doi:10.1167/iovs.06-0167) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S., Nayak S. K., Campo B., Walker J. R., Hogenesch J. B., Jegla T.2005Illumination of the melanopsin signaling pathway. Science 307, 600–604 (doi:10.1126/science.1105121) [DOI] [PubMed] [Google Scholar]
- Pappu K. S., Mardon G.2004Genetic control of retinal specification and determination in Drosophila. Int. J. Dev. Biol. 48, 913–924 (doi:10.1387/ijdb.041875kp) [DOI] [PubMed] [Google Scholar]
- Piatigorsky J.2007Gene sharing and evolution: the diversity of protein functions Cambridge, MA: Harvard University Press [Google Scholar]
- Piatigorsky J., Wistow G.1991The recruitment of crystallins: new functions precede gene duplication. Science 252, 1078–1079 (doi:10.1126/science.252.5009.1078) [DOI] [PubMed] [Google Scholar]
- Piatigorsky J., Wistow G. J.1989Enzyme/crystallins: gene sharing as an evolutionary strategy. Cell 57, 197–199 (doi:10.1016/0092-8674(89)90956-2) [DOI] [PubMed] [Google Scholar]
- Piatigorsky J., O'Brien W. E., Norman B. L., Kalumuck K., Wistow G. J., Borras T., Nickerson J. M., Wawrousek E. F.1988Gene sharing by delta-crystallin and argininosuccinate lyase. Proc. Natl Acad. Sci. USA 85, 3479–3483 (doi:10.1073/pnas.85.10.3479) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatigorsky J., Kozmik Z., Horwitz J., Ding L., Carosa E., Robison W. G., JR, Steinbach P. J., Tamm E. R.2000Omega-crystallin of the scallop lens. A dimeric aldehyde dehydrogenase class 1/2 enzyme-crystallin. J. Biol. Chem. 275, 41 064–41 073 (doi:10.1074/jbc.M005625200) [DOI] [PubMed] [Google Scholar]
- Pineda D., et al. 2002The genetic network of prototypic planarian eye regeneration is Pax6 independent. Development 129, 1423–1434 [DOI] [PubMed] [Google Scholar]
- Plachetzki D. C., Oakley T. H.2007Key transitions during the evolution of animal phototransduction: novelty, ‘tree-thinking,’ co-option, and co-duplication. Integr. Comp. Biol. 47, 759–769 (doi:10.1093/icb/icm050) [DOI] [PubMed] [Google Scholar]
- Plachetzki D. C., Degnan B. M., Oakley T. H.2007The origins of novel protein interactions during animal opsin evolution. PLoS ONE 2, e1054 (doi:10.1371/journal.pone.0001054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plouhinec J. L., et al. 2003The mammalian Crx genes are highly divergent representatives of the Otx5 gene family, a gnathostome orthology class of orthodenticle-related homeogenes involved in the differentiation of retinal photoreceptors and circadian entrainment. Mol. Biol. Evol. 20, 513–521 (doi:10.1093/molbev/msg085) [DOI] [PubMed] [Google Scholar]
- Plouhinec J. L., Leconte L., Sauka-Spengler T., Bovolenta P., Mazan S., Saule S.2005Comparative analysis of gnathostome Otx gene expression patterns in the developing eye: implications for the functional evolution of the multigene family. Dev. Biol. 278, 560–575 (doi:10.1016/j.ydbio.2004.11.019) [DOI] [PubMed] [Google Scholar]
- Qian J., Esumi N., Chen Y., Wang Q., Chowers I., Zack D. J.2005Identification of regulatory targets of tissue-specific transcription factors: application to retina-specific gene regulation. Nucleic Acids Res. 33, 3479–3491 (doi:10.1093/nar/gki658) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley I. K., Xie X., Shankland M.2007Hau-Pax6A expression in the central nervous system of the leech embryo. Dev. Genes Evol. 217, 459–468 (doi:10.1007/s00427-007-0156-1) [DOI] [PubMed] [Google Scholar]
- Quiring R., Walldorf U., Kloter U., Gehring W. J.1994Homology of the eyeless gene of Drosophila to the Small eye gene in mice and Aniridia in humans. Science 265, 785–789 (doi:10.1126/science.7914031) [DOI] [PubMed] [Google Scholar]
- Radu R. A., Hu J., Peng J., Bok D., Mata N. L., Travis G. H.2008Retinal pigment epithelium-retinal G protein receptor-opsin mediates light-dependent translocation of all-trans-retinyl esters for synthesis of visual chromophore in retinal pigment epithelial cells. J. Biol. Chem. 283, 19 730–19 738 (doi:10.1074/jbc.M801288200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raible F., Tessmar-Raible K., Arboleda E., Kaller T., Bork P., Arendt D., Arnone M. I.2006Opsins and clusters of sensory G-protein-coupled receptors in the sea urchin genome. Dev. Biol. 300, 461–475 (doi:10.1016/j.ydbio.2006.08.070) [DOI] [PubMed] [Google Scholar]
- Ranade S. S., Yang-Zhou D., Kong S. W., Mcdonald E. C., Cook T. A., Pignoni F.2008Analysis of the Otd-dependent transcriptome supports the evolutionary conservation of CRX/OTX/OTD functions in flies and vertebrates. Dev. Biol. 315, 521–534 (doi:10.1016/j.ydbio.2007.12.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath M. F., Morin F., Shi Q., Klein D. C., Moller M.2007Ontogenetic expression of the Otx2 and Crx homeobox genes in the retina of the rat. Exp. Eye Res. 85, 65–73 (doi:10.1016/j.exer.2007.02.016) [DOI] [PubMed] [Google Scholar]
- Rayapureddi J. P., Kattamuri C., Steinmetz B. D., Frankfort B. J., Ostrin E. J., Mardon G., Hegde R. S.2003Eyes absent represents a class of protein tyrosine phosphatases. Nature 426, 295–298 (doi:10.1038/nature02093) [DOI] [PubMed] [Google Scholar]
- Royet J., Finkelstein R.1995Pattern formation in Drosophila head development: the role of the orthodenticle homeobox gene. Development 121, 3561–3572 [DOI] [PubMed] [Google Scholar]
- Salo E., Pineda D., Marsal M., Gonzalez J., Gremigni V., Batistoni R.2002Genetic network of the eye in Platyhelminthes: expression and functional analysis of some players during planarian regeneration. Gene 287, 67–74 (doi:10.1016/S0378-1119(01)00863-0) [DOI] [PubMed] [Google Scholar]
- Schwarz M., Cecconi F., Bernier G., Andrejewski N., Kammandel B., Wagner M., Gruss P.2000Spatial specification of mammalian eye territories by reciprocal transcriptional repression of Pax2 and Pax6. Development 127, 4325–4334 [DOI] [PubMed] [Google Scholar]
- Seimiya M., Gehring W. J.2000The Drosophila homeobox gene optix is capable of inducing ectopic eyes by an eyeless-independent mechanism. Development 127, 1879–1886 [DOI] [PubMed] [Google Scholar]
- Silver S. J., Rebay I.2005Signaling circuitries in development: insights from the retinal determination gene network. Development 132, 3–13 (doi:10.1242/dev.01539) [DOI] [PubMed] [Google Scholar]
- Simeone A., Puelles E., Acampora D.2002The Otx family. Curr. Opin. Genet. Dev. 12, 409–415 (doi:10.1016/S0959-437X(02)00318-0) [DOI] [PubMed] [Google Scholar]
- Simpson T. I., Price D. J.2002Pax6; a pleiotropic player in development. Bioessays 24, 1041–1051 (doi:10.1002/bies.10174) [DOI] [PubMed] [Google Scholar]
- Spudich J. L., Yang C. S., Jung K. H., Spudich E. N.2000Retinylidene proteins: structures and functions from archaea to humans. Annu. Rev. Cell Dev. Biol. 16, 365–392 (doi:10.1146/annurev.cellbio.16.1.365) [DOI] [PubMed] [Google Scholar]
- Steingrimsson E., Copeland N. G., Jenkins N. A.2004Melanocytes and the microphthalmia transcription factor network. Annu. Rev. Genet. 38, 365–411 (doi:10.1146/annurev.genet.38.072902.092717) [DOI] [PubMed] [Google Scholar]
- Stierwald M., Yanze N., Bamert R. P., Kammermeier L., Schmid V.2004The Sine oculis/Six class family of homeobox genes in jellyfish with and without eyes: development and eye regeneration. Dev. Biol. 274, 70–81 (doi:10.1016/j.ydbio.2004.06.018) [DOI] [PubMed] [Google Scholar]
- Su C. Y., Luo D. G., Terakita A., Shichida Y., Liao H. W., Kazmi M. A., Sakmar T. P., Yau K. W.2006Parietal-eye phototransduction components and their potential evolutionary implications. Science 311, 1617–1621 (doi:10.1126/science.1123802) [DOI] [PubMed] [Google Scholar]
- Suga H., Schmid V., Gehring W. J.2008Evolution and functional diversity of jellyfish opsins. Curr. Biol. 18, 51–55 (doi:10.1016/j.cub.2007.11.059) [DOI] [PubMed] [Google Scholar]
- Suzuki M. M., Satoh N.2000Genes expressed in the amphioxus notochord revealed by EST analysis. Dev. Biol. 224, 168–177 (doi:10.1006/dbio.2000.9796) [DOI] [PubMed] [Google Scholar]
- Suzuki T., Terakita A., Narita K., Nagai K., Tsukahara Y., Kito Y.1995Squid photoreceptor phospholipase C is stimulated by membrane Gq alpha but not by soluble Gq alpha. FEBS Lett. 377, 333–337 (doi:10.1016/0014-5793(95)01364-4) [DOI] [PubMed] [Google Scholar]
- Tahayato A., Sonneville R., Pichaud F., Wernet M. F., Papatsenko D., Beaufils P., Cook T., Desplan C.2003Otd/Crx, a dual regulator for the specification of ommatidia subtypes in the Drosophila retina. Dev. Cell 5, 391–402 (doi:10.1016/S1534-5807(03)00239-9) [DOI] [PubMed] [Google Scholar]
- Takeda K., Yokoyama S., Yasumoto K., Saito H., Udono T., Takahashi K., Shibahara S.2003OTX2 regulates expression of DOPAchrome tautomerase in human retinal pigment epithelium. Biochem. Biophys. Res. Commun. 300, 908–914 (doi:10.1016/S0006-291X(02)02934-0) [DOI] [PubMed] [Google Scholar]
- Takechi M., Seno S., Kawamura S.2008Identification of cis-acting elements repressing blue opsin expression in zebrafish UV cones and pineal cells. J. Biol. Chem 283, 31 625–31 632 (doi:10.1074/jbc.M806226200) [DOI] [PubMed] [Google Scholar]
- Terakita A.2005The opsins. Genome Biol. 6, 213 (doi:10.1186/gb-2005-6-3-213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomarev S. I., Callaerts P., Kos L., Zinovieva R., Halder G., Gehring W., Piatigorsky J.1997Squid Pax-6 and eye development. Proc. Natl Acad. Sci. USA 94, 2421–2426 (doi:10.1073/pnas.94.6.2421) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootle T. L., Silver S. J., Davies E. L., Newman V., Latek R. R., Mills I. A., Selengut J. D., Parlikar B. E., Rebay I.2003The transcription factor Eyes absent is a protein tyrosine phosphatase. Nature 426, 299–302 (doi:10.1038/nature02097) [DOI] [PubMed] [Google Scholar]
- Toyoda R., Sato S., Ikeo K., Gojobori T., Numakunai T., Goding C. R., Yamamoto H.2000Pigment cell-specific expression of the tyrosinase gene in ascidians has a different regulatory mechanism from vertebrates. Gene 259, 159–170 (doi:10.1016/S0378-1119(00)00433-9) [DOI] [PubMed] [Google Scholar]
- Toyoda R., Kasai A., Sato S., Wada S., Saiga H., Ikeo K., Gojobori T., Numakunai T., Yamamoto H.2004Pigment cell lineage-specific expression activity of the ascidian tyrosinase-related gene. Gene 332, 61–69 (doi:10.1016/j.gene.2004.01.030) [DOI] [PubMed] [Google Scholar]
- Treisman J., Harris E., Desplan C.1991The paired box encodes a second DNA-binding domain in the paired homeo domain protein. Genes Dev. 5, 594–604 (doi:10.1101/gad.5.4.594) [DOI] [PubMed] [Google Scholar]
- Umesono Y., Watanabe K., Agata K.1999Distinct structural domains in the planarian brain defined by the expression of evolutionarily conserved homeobox genes. Dev. Genes Evol. 209, 31–39 (doi:10.1007/s004270050224) [DOI] [PubMed] [Google Scholar]
- Vandendries E. R., Johnson D., Reinke R.1996orthodenticle is required for photoreceptor cell development in the Drosophila eye. Dev. Biol. 173, 243–255 (doi:10.1006/dbio.1996.0020) [DOI] [PubMed] [Google Scholar]
- Velarde R. A., Sauer C. D., Walden K. K., Fahrbach S. E., Robertson H. M.2005Pteropsin: a vertebrate-like non-visual opsin expressed in the honey bee brain. Insect Biochem. Mol. Biol. 35, 1367–1377 (doi:10.1016/j.ibmb.2005.09.001) [DOI] [PubMed] [Google Scholar]
- Wada S., Katsuyama Y., Sato Y., Itoh C., Saiga H.1996Hroth an orthodenticle-related homeobox gene of the ascidian, Halocynthia roretzi: its expression and putative roles in the axis formation during embryogenesis. Mech. Dev. 60, 59–71 (doi:10.1016/S0925-4773(96)00600-4) [DOI] [PubMed] [Google Scholar]
- Wada S., Toyoda R., Yamamoto H., Saiga H.2002Ascidian otx gene Hroth activates transcription of the brain-specific gene HrTRP. Dev. Dyn. 225, 46–53 (doi:10.1002/dvdy.10135) [DOI] [PubMed] [Google Scholar]
- Wada S., et al. 2003A genomewide survey of developmentally relevant genes in Ciona intestinalis. II. Genes for homeobox transcription factors. Dev. Genes Evol. 213, 222–234 (doi:10.1007/s00427-003-0321-0) [DOI] [PubMed] [Google Scholar]
- Wang Q. L., et al. 2004QRX, a novel homeobox gene, modulates photoreceptor gene expression. Hum. Mol. Genet. 13, 1025–1040 (doi:10.1093/hmg/ddh117) [DOI] [PubMed] [Google Scholar]
- Williams N. A., Holland P. W. H.1996Old head on young shoulders. Nature 383, 490–490 (doi:10.1038/383490a0) [Google Scholar]
- Williams N. A., Holland P. W.1998Gene and domain duplication in the chordate Otx gene family: insights from amphioxus Otx. Mol. Biol. Evol. 15, 600–607 [DOI] [PubMed] [Google Scholar]
- Wistow G.1990Evolution of a protein superfamily: relationships between vertebrate lens crystallins and microorganism dormancy proteins. J. Mol. Evol. 30, 140–145 (doi:10.1007/BF02099940) [DOI] [PubMed] [Google Scholar]
- Wistow G. J., Piatigorsky J.1988Lens crystallins: the evolution and expression of proteins for a highly specialized tissue. Annu. Rev. Biochem. 57, 479–504 (doi:10.1146/annurev.bi.57.070188.002403) [DOI] [PubMed] [Google Scholar]
- Xu Z. P., Saunders G. F.1997Transcriptional regulation of the human PAX6 gene promoter. J. Biol. Chem. 272, 3430–3436 [DOI] [PubMed] [Google Scholar]
- Xu P. X., Adams J., Peters H., Brown M. C., Heaney S., Maas R.1999Eya1-deficient mice lack ears and kidneys and show abnormal apoptosis of organ primordia. Nat. Genet. 23, 113–117 [DOI] [PubMed] [Google Scholar]
- Yajima I., et al. 2003Cloning and functional analysis of ascidian Mitf in vivo: insights into the origin of vertebrate pigment cells. Mech. Dev. 120, 1489–1504 (doi:10.1016/j.mod.2003.08.009) [DOI] [PubMed] [Google Scholar]
- Yoshida R., Kusakabe T., Kamatani M., Daitoh M., Tsuda M.2002Central nervous system-specific expression of G protein alpha subunits in the ascidian Ciona intestinalis. Zool. Sci. 19, 1079–1088 (doi:10.2108/zsj.19.1079) [DOI] [PubMed] [Google Scholar]
- Zhang X., Friedman A., Heaney S., Purcell P., Maas R. L.2002Meis homeoproteins directly regulate Pax6 during vertebrate lens morphogenesis. Genes Dev. 16, 2097–2107 (doi:10.1101/gad.1007602) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C. C., Dyer M. A., Uchikawa M., Kondoh H., Lagutin O. V., Oliver G.2002Six3-mediated auto repression and eye development requires its interaction with members of the Groucho-related family of co-repressors. Development 129, 2835–2849 [DOI] [PubMed] [Google Scholar]
- Ziegler I.2003The pteridine pathway in zebrafish: regulation and specification during the determination of neural crest cell-fate. Pigment Cell Res. 16, 172–182 (doi:10.1034/j.1600-0749.2003.00044.x) [DOI] [PubMed] [Google Scholar]
- Zimmerman J. E., Bui Q. T., Liu H., Bonini N. M.2000Molecular genetic analysis of Drosophila eyes absent mutants reveals an eye enhancer element. Genetics 154, 237–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber M. E., Perron M., Philpott A., Bang A., Harris W. A.1999Giant eyes in Xenopus laevis by overexpression of XOptx2. Cell 98, 341–352 (doi:10.1016/S0092-8674(00)81963-7) [DOI] [PubMed] [Google Scholar]