Abstract
Altekar, W. W. (National Chemical Laboratory, Poona, India) and M. R. Raghavendra Rao. Microbiological dissimilation of tricarballylate and trans-aconitate. J. Bacteriol. 85:604–613. 1963.—Two fluorescent pseudomonads capable of metabolizing tricarballylate and trans-aconitate were isolated by the soil-enrichment culture technique. These and some other species of bacteria were tested for their ability to utilize for growth the salts of many di- and tricarboxylic acids. Alloisocitrate and mesaconate were not utilized by any of the ten strains tested; only two strains grew on tricarballylate and itaconate. trans-Aconitate was utilized by many strains which had not been previously exposed to this compound. The resting cells of two strains could adapt to oxidize two acids (tricarballylate and trans-aconitate), and this induction was chloramphenicol-sensitive. The tricarballylate-grown cells were simultaneously induced to oxidize trans-aconitate and other tricarboxylates, whereas the trans-aconitate-grown cells were not induced to oxidize tricarballylate, and their subsequent induction was inhibited by chloramphenicol. This trans-aconitate or tricarballylate. But tricarballylate dehydrogenase was present only in tricarballylate-grown cells. The cell-free extracts of the two organisms contained the enzymes of the Krebs cycle and isocitritase. These enzymes are most probably operative during growth on and oxidation of these two acids as sole carbon sources.
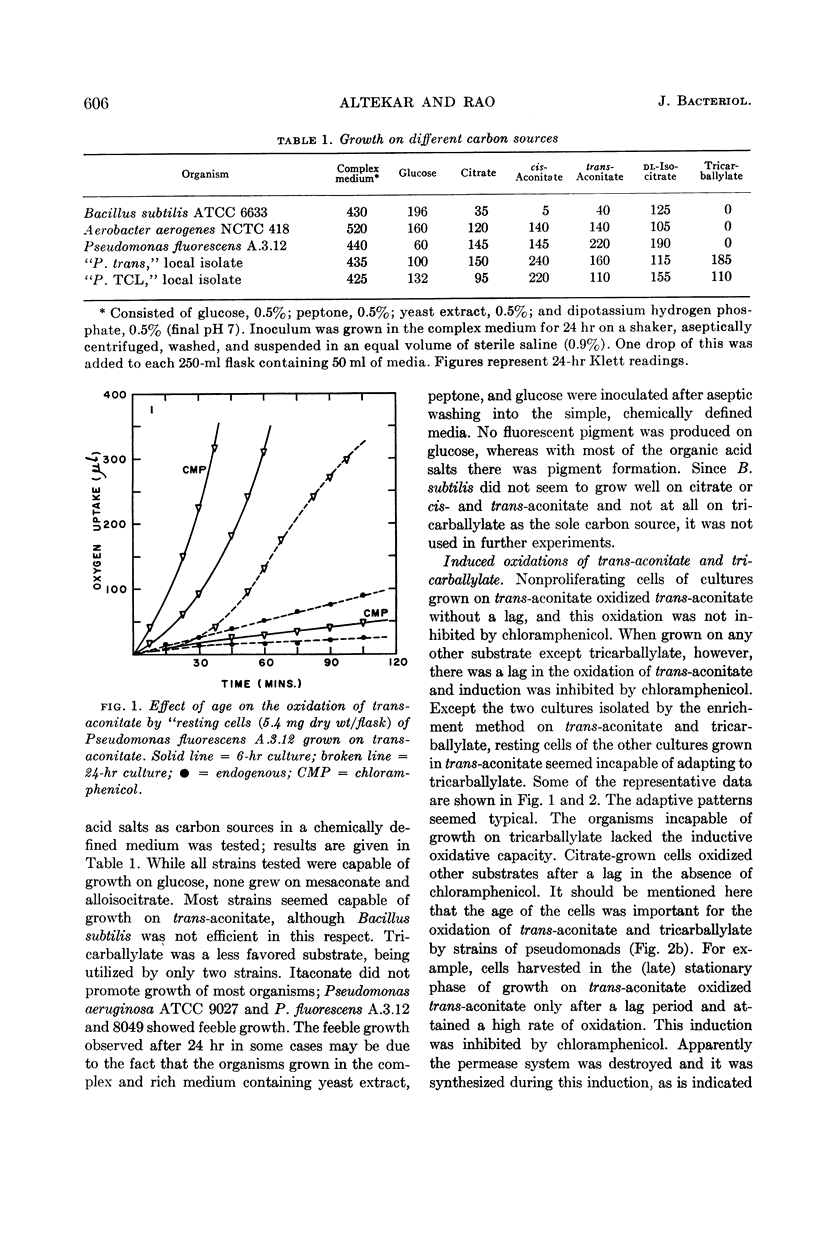
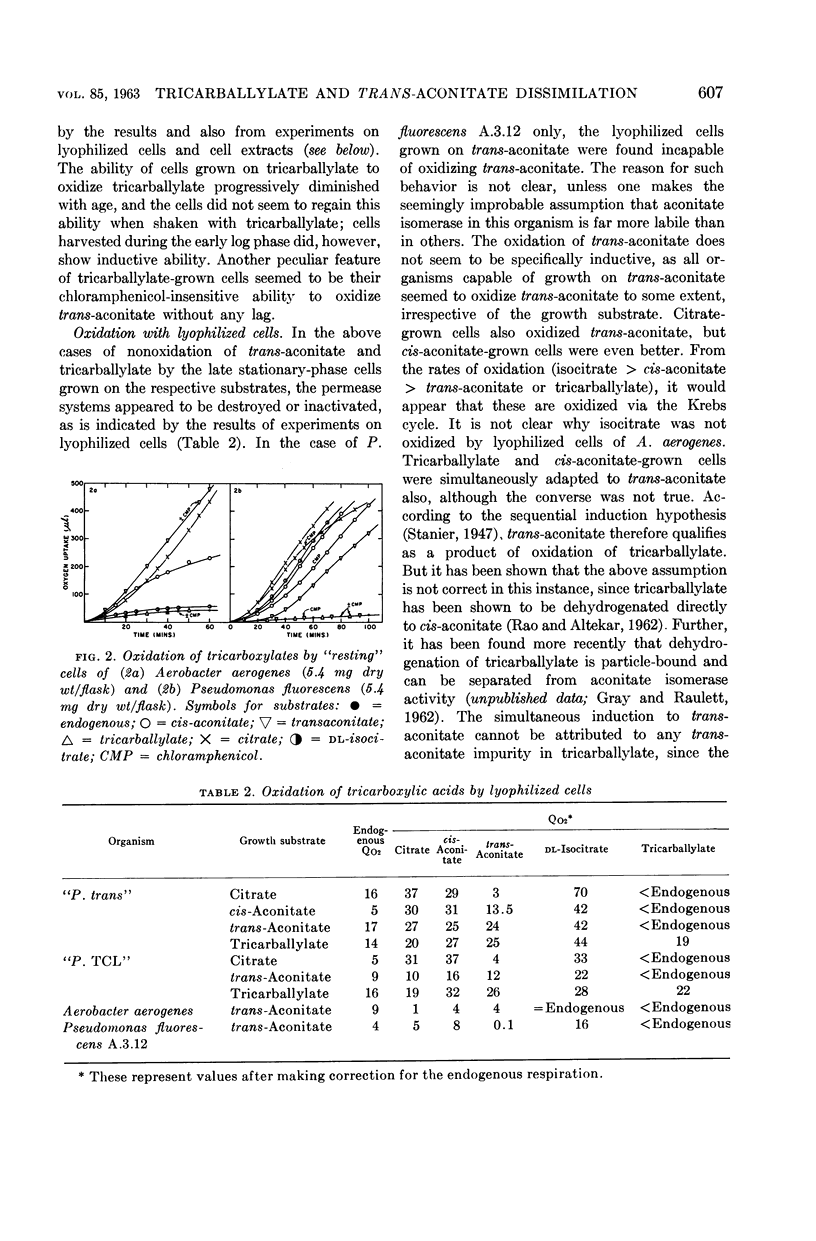
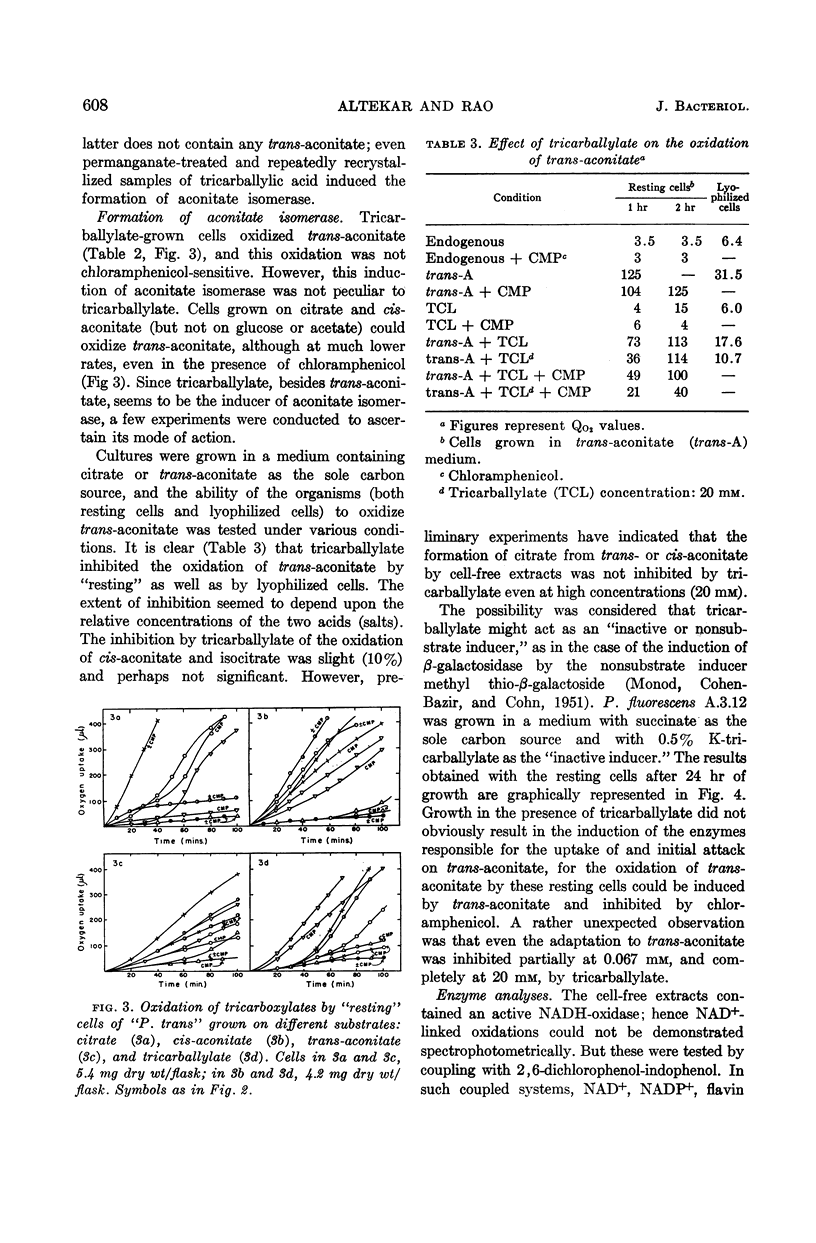
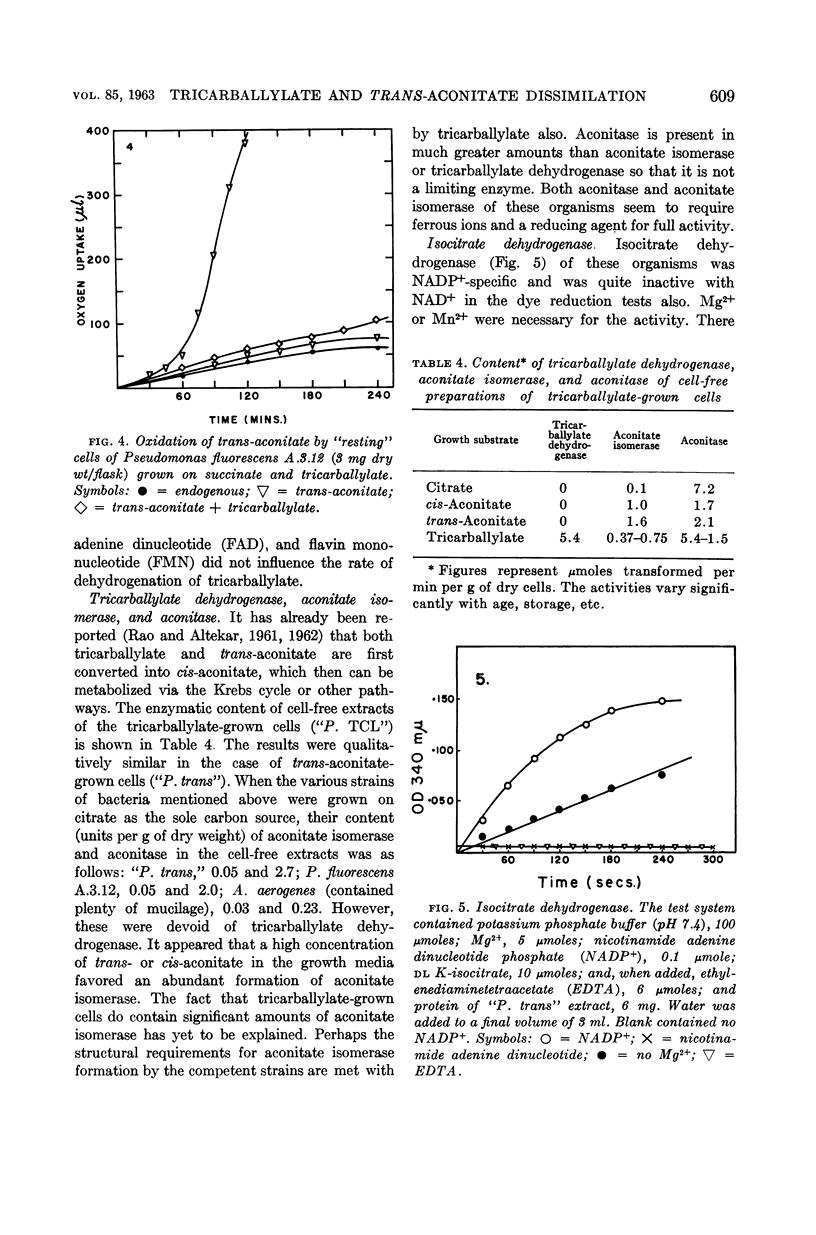
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DICKMAN S. R., CLOUTIER A. A. Factors affecting the activity of aconitase. J Biol Chem. 1951 Jan;188(1):379–388. [PubMed] [Google Scholar]
- FUKUMOTO J., YAMAMOTO T., TSURU D. Effects of carbon sources and base analogues of nucleic acid on the formation of bacterial amylase. Nature. 1957 Aug 31;180(4583):438–439. doi: 10.1038/180438b0. [DOI] [PubMed] [Google Scholar]
- MONOD J., COHEN-BAZIRE G., COHN M. Sur la biosynthèse de la beta-galactosidase (lactase) chez Escherichia coli; la spécificité de l'induction. Biochim Biophys Acta. 1951 Nov;7(4):585–599. doi: 10.1016/0006-3002(51)90072-8. [DOI] [PubMed] [Google Scholar]
- OCHOA S., STERN J. R., SCHNEIDER M. C. Enzymatic synthesis of citric acid. II. Crystalline condensing enzyme. J Biol Chem. 1951 Dec;193(2):691–702. [PubMed] [Google Scholar]
- RACKER E. Spectrophotometric measurements of the enzymatic formation of fumaric and cis-aconitic acids. Biochim Biophys Acta. 1950 Jan;4(1-3):211–214. doi: 10.1016/0006-3002(50)90026-6. [DOI] [PubMed] [Google Scholar]
- RAO M. R., ALTEKAR W. W. Aconitate isomerase. Biochem Biophys Res Commun. 1961 Feb 24;4:101–105. doi: 10.1016/0006-291x(61)90355-2. [DOI] [PubMed] [Google Scholar]
- RAO M. R., ALTEKAR W. W. Tricarballylate dehydrogenase. Biochem Biophys Res Commun. 1962 Feb 20;7:62–66. doi: 10.1016/0006-291x(62)90146-8. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y. Simultaneous Adaptation: A New Technique for the Study of Metabolic Pathways. J Bacteriol. 1947 Sep;54(3):339–348. doi: 10.1128/jb.54.3.339-348.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]


