Abstract
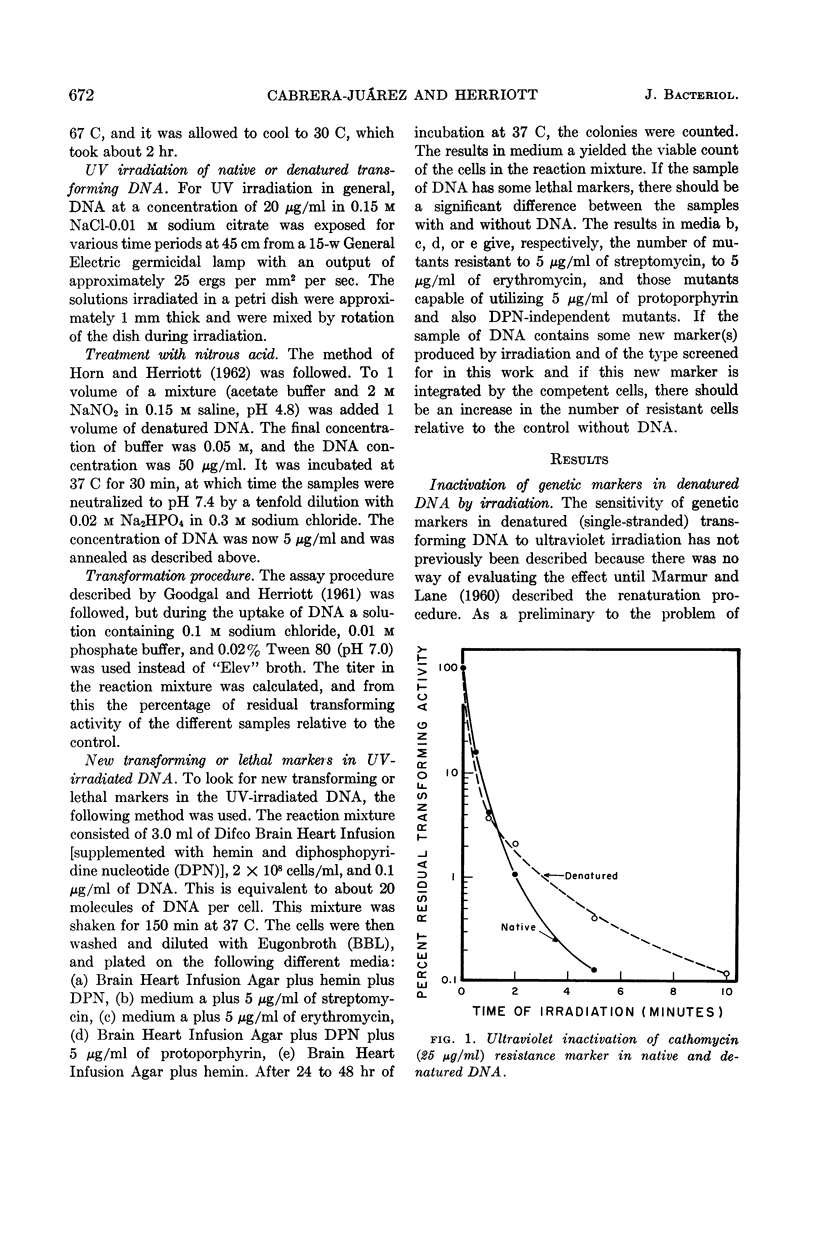
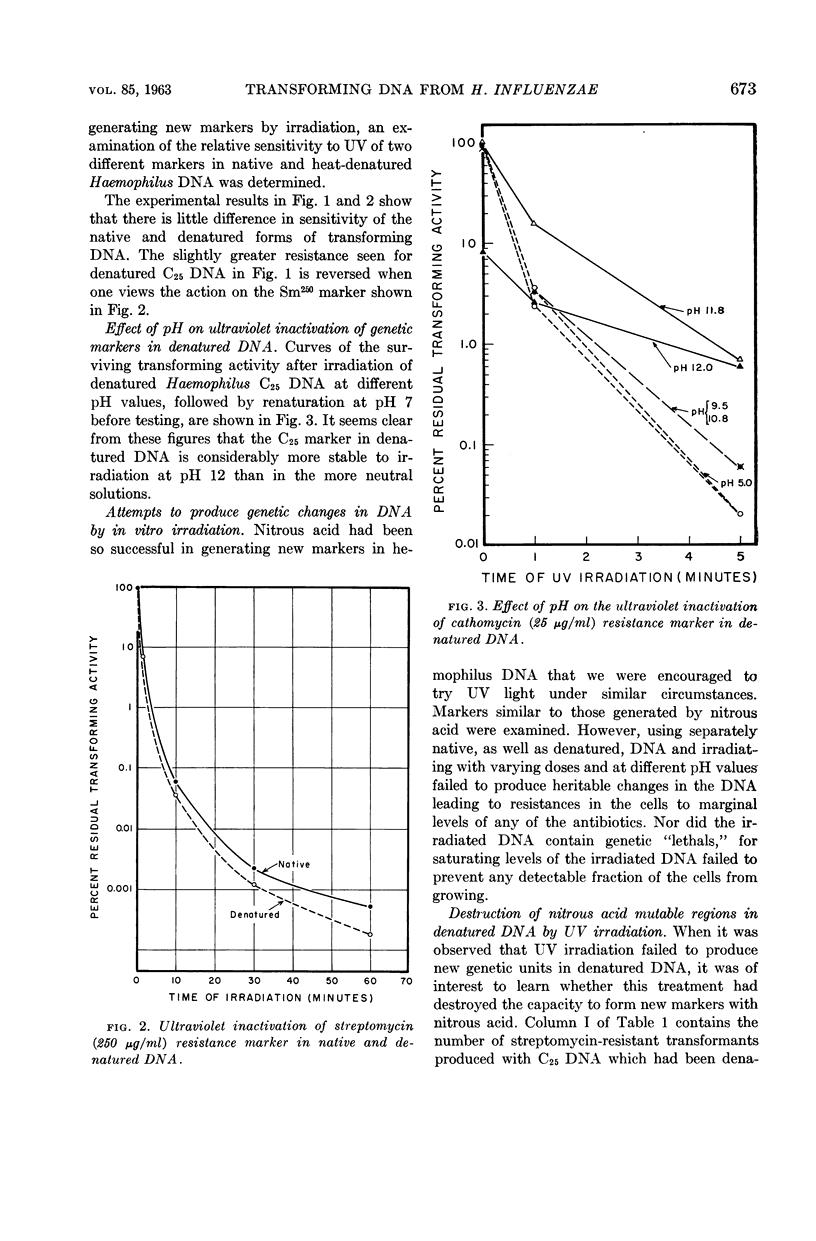
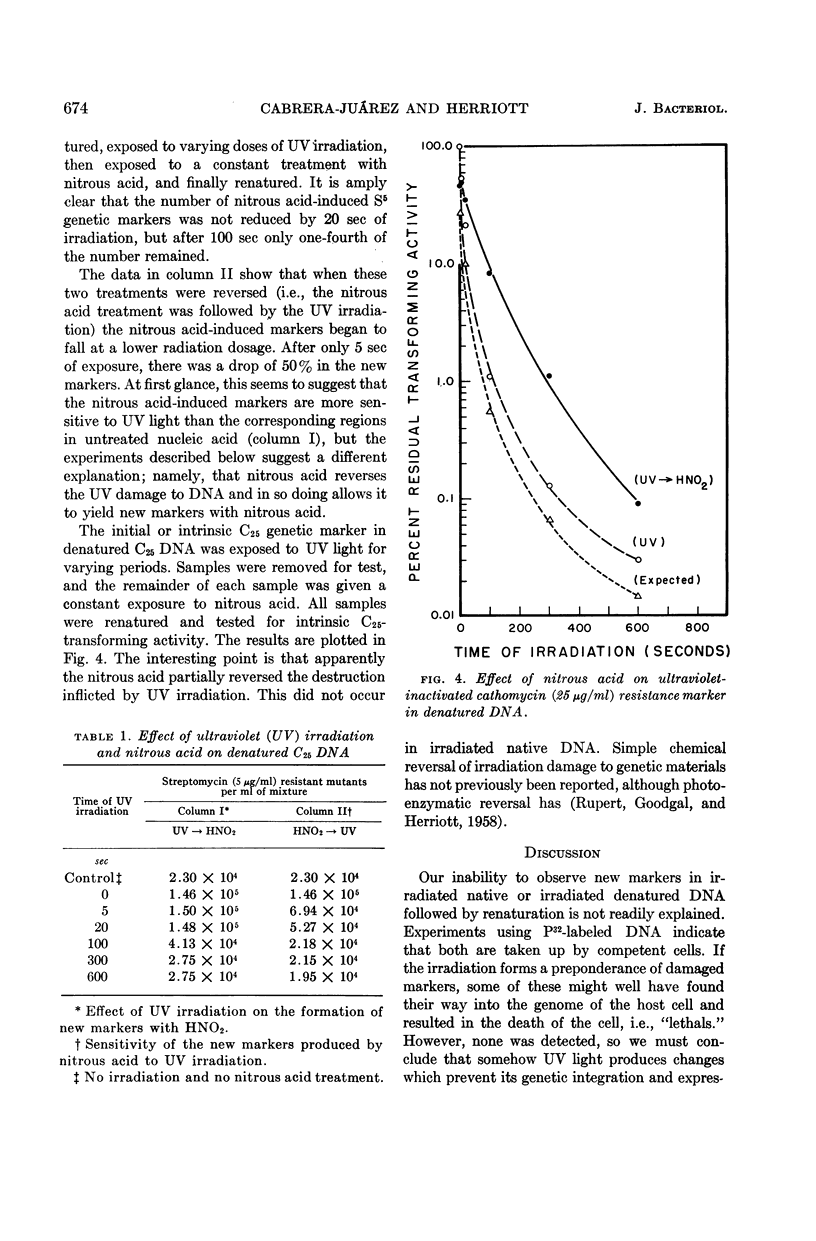
Cabrera-Juarez, Emiliano (Johns Hopkins University School of Hygiene and Public Health, Baltimore, Md.) and Roger M. Herriott. Ultraviolet irradiation of native and denatured transforming deoxyribonucleic acid from Haemophilus influenzae. J. Bacteriol. 85:671–675. 1963.—Two antibiotic-resistance markers in denatured deoxyribonucleic acid (DNA) from Haemophilus influenzae showed about the same sensitivity to ultraviolet irradiation as in native DNA. The resistance of markers in denatured DNA to ultraviolet (UV) light increases with alkalinity up to pH 12. New transforming markers or genetic “lethals” were not produced by in vitro irradiation of either native or denatured DNA. The UV irradiation of the denatured DNA reduces its capacity to form new markers with nitrous acid. Nitrous acid reactivated some of the intrinsic marker destroyed by UV irradiation of denatured transforming DNA.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEXANDER H. E., LEIDY G. Induction of streptomycin resistance in sensitive Hemophilus influenzae by extracts containing desoxyribonucleic acid from resistant Hemophilus influenzae. J Exp Med. 1953 Jan;97(1):17–31. doi: 10.1084/jem.97.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEIDUSCHEK E. P. "Reversible" DNA. Proc Natl Acad Sci U S A. 1961 Jul 15;47:950–955. doi: 10.1073/pnas.47.7.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODGAL S. H., HERRIOTT R. M. Studies on transformations of Hemophilus influenzae. I. Competence. J Gen Physiol. 1961 Jul;44:1201–1227. doi: 10.1085/jgp.44.6.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORN E. E., HERRIOTT R. M. The mutagenic action of nitrous acid on "single-stranded" (denatured) Hemophilus transforming DNA. Proc Natl Acad Sci U S A. 1962 Aug;48:1409–1416. doi: 10.1073/pnas.48.8.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HSU Y. C., HERRIOTT R. M. Studies on transformations of Hemophilus influenzae. III. The genotypes and phenotypic patterns of three streptomycin-resistant mutants. J Gen Physiol. 1961 Nov;45:197–204. doi: 10.1085/jgp.45.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB F. Mutation d'un bactériophage induite par l'irradiation des seules bactéries-hotes avant l'infection. C R Hebd Seances Acad Sci. 1954 Feb 8;238(6):732–734. [PubMed] [Google Scholar]
- KRIEG D. R. Induced reversion of T4rII mutants by ultraviolet irradiation of extracellular phage. Virology. 1959 Oct;9:215–227. doi: 10.1016/0042-6822(59)90116-3. [DOI] [PubMed] [Google Scholar]
- Marmur J., Lane D. STRAND SEPARATION AND SPECIFIC RECOMBINATION IN DEOXYRIBONUCLEIC ACIDS: BIOLOGICAL STUDIES. Proc Natl Acad Sci U S A. 1960 Apr;46(4):453–461. doi: 10.1073/pnas.46.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NESTER E. W., LEDERBERG J. Linkage of genetic units of Bacillus subtilis in DNA transformation. Proc Natl Acad Sci U S A. 1961 Jan 15;47:52–55. doi: 10.1073/pnas.47.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUPERT C. S., GOODGAL S. H., HERRIOTT R. M. Photoreactivation in vitro of ultraviolet-inactivated Hemophilus influenzae transforming factor. J Gen Physiol. 1958 Jan 20;41(3):451–471. doi: 10.1085/jgp.41.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIGLE J. J., DULBECCO R. Induction of mutations in bacteriophage T3 by ultraviolet light. Experientia. 1953 Oct 15;9(10):372–373. doi: 10.1007/BF02167637. [DOI] [PubMed] [Google Scholar]
- WITKIN E. M. Time, temperature, and protein synthesis: a study of ultraviolet-induced mutation in bacteria. Cold Spring Harb Symp Quant Biol. 1956;21:123–140. doi: 10.1101/sqb.1956.021.01.011. [DOI] [PubMed] [Google Scholar]
- Weigle J. J. Induction of Mutations in a Bacterial Virus. Proc Natl Acad Sci U S A. 1953 Jul;39(7):628–636. doi: 10.1073/pnas.39.7.628. [DOI] [PMC free article] [PubMed] [Google Scholar]


