Abstract
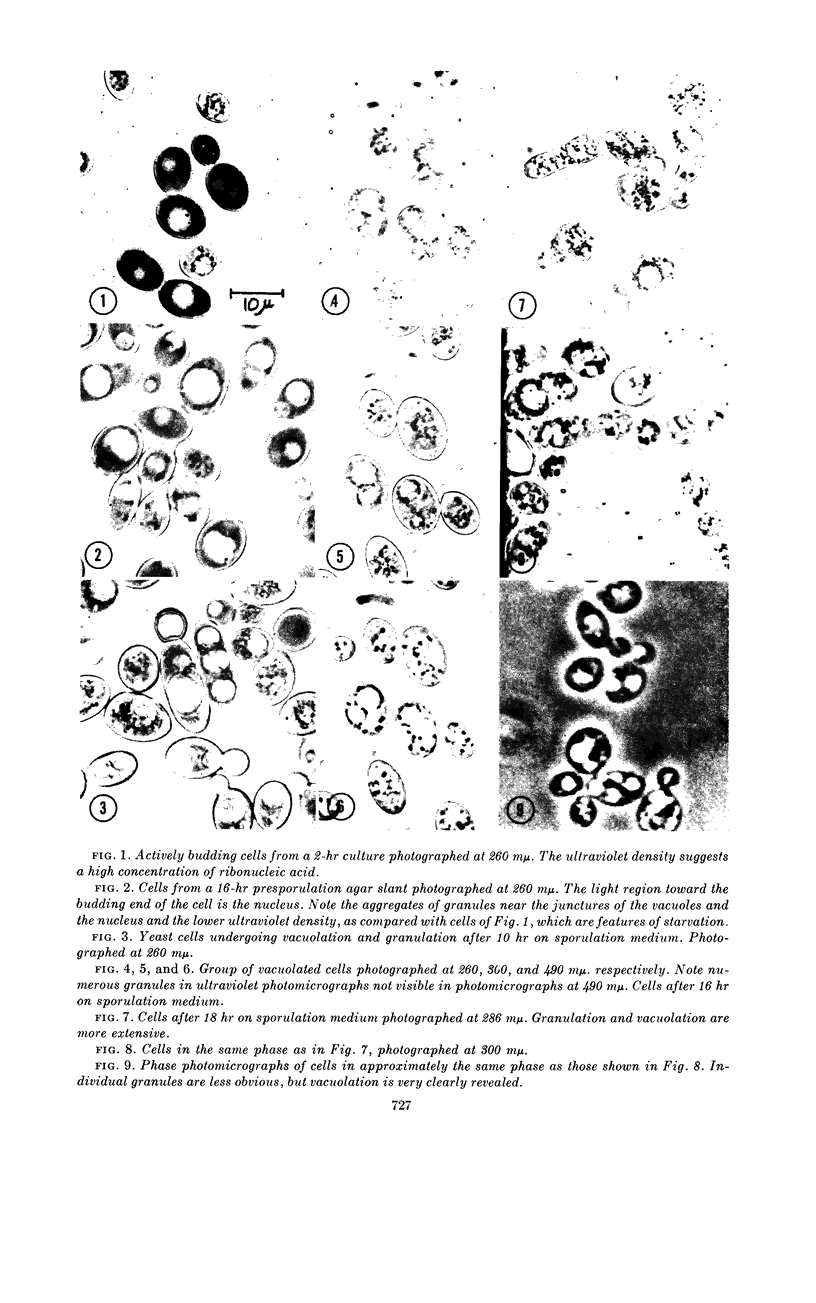
Miller, Glendon R. (Southern Illinois University, Carbondale), Dan O. McClary, and Wilbert D. Bowers, Jr. Ultraviolet and phase microscopy of sporulating Saccharomyces. J. Bacteriol. 85:725–731. 1963.—During active growth, the cytoplasm of yeast cells is densely absorbent to ultraviolet light at 260 mμ, whereas the nucleus is only faintly so, and the vacuole is nonabsorbent. After 24 hr on presporulation medium (about the age of transfer to acetate sporulation medium), the cells manifest many characteristics of starvation. The cytoplasm is weakly absorbent to ultraviolet light except for a dense zone immediately surrounding the vacuole and one or two groups of highly refractile granules clustered on one or both sides of the juncture of the nucleus and the vacuole. After several hours on the acetate sporulation medium, the cells undergo a progressive vacuolation until four or more large vacuoles appear, separated by granular cytoplasm. The nucleus is obscured to ultraviolet light during the vacuolation stage, at which time previous studies have shown its division to occur, and remains so during the rest of the cycle. Photographs of densely granulated cells, at various wavelengths of ultraviolet and visible light, indicated several different types of granules with respect to their absorption spectra. With continued development of the ascus, the granules increase in number until they fill the entire cell and obscure the vacuoles, after which they condense into a compact mass, leaving much of the cell empty. Spores emerge from the granular mass as separate, ultraviolet-absorbent regions without distinguishable cell walls, which seem to be the last structures formed. The rudimentary spores contain a central cluster of dense granules and are separated within the ascus by optically dense, granular partitions which diminish as spore walls are laid down. With the maturing of the ascus, the granules and epiplasm disappear, and the ascus wall is drawn tightly around the more or less homogeneously absorbent spores.
Full text
PDF


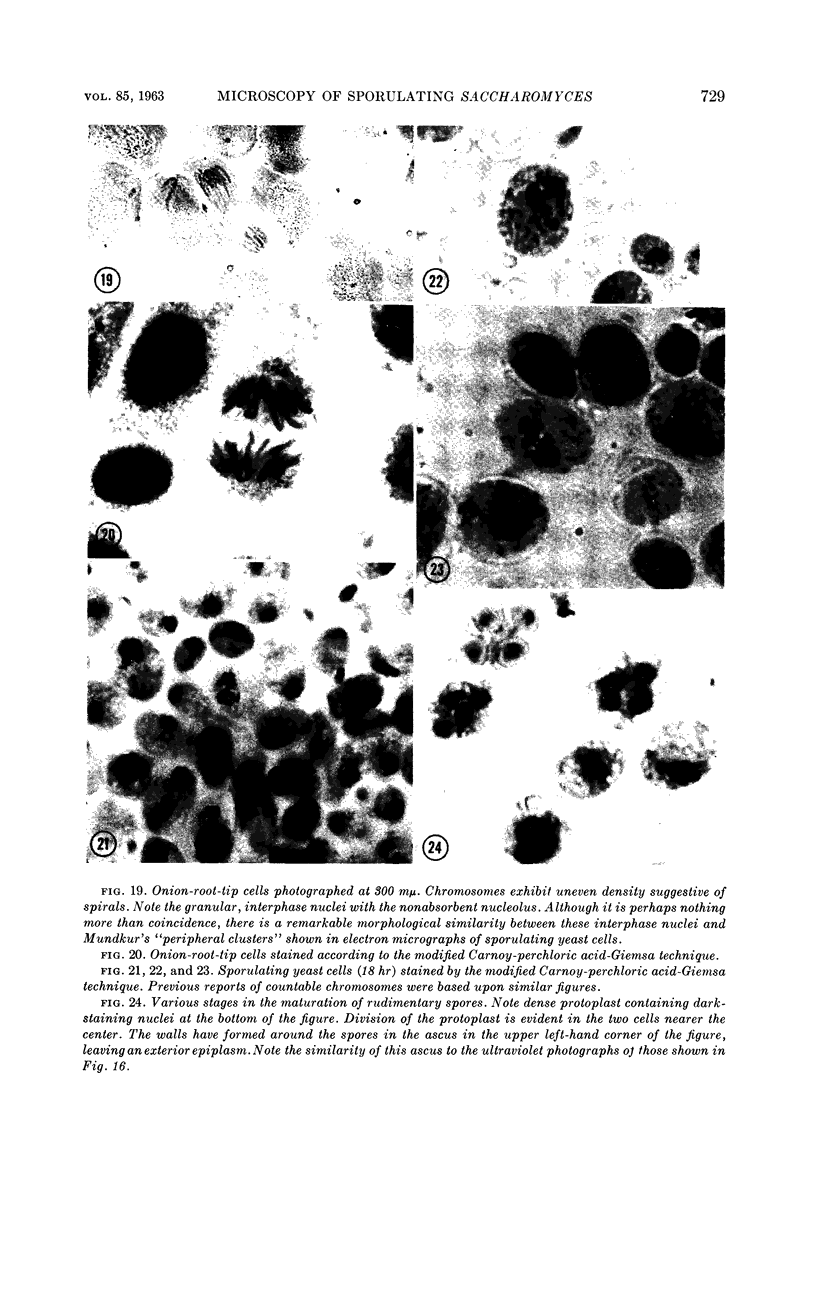


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- HASHIMOTO T., CONTI S. F., NAYLOR H. B. Fine structure of microorganisms. III. Electron microscopy of resting and germinating ascospores of Saccharomyces cerevisiae. J Bacteriol. 1958 Oct;76(4):406–416. doi: 10.1128/jb.76.4.406-416.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASHIMOTO T., GERHARDT P., CONTI S. F., NAYLOR H. B. Studies on the fine structure of microorganisms. V. Morphogenesis of nuclear and membrane structures during ascospore formation in yeast. J Biophys Biochem Cytol. 1960 Apr;7:305–310. doi: 10.1083/jcb.7.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCLARY D. O., WILLIAMS M. A., LINDEGREN C. C. Nuclear changes in the life cycle of Saccharomyces. J Bacteriol. 1957 Jun;73(6):754–757. doi: 10.1128/jb.73.6.754-757.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUNDKUR B. Electron microscopical studies of frozendried yeast. III. Formation of the tetrad in Saccharomyces. Exp Cell Res. 1961 Oct;25:24–40. doi: 10.1016/0014-4827(61)90304-4. [DOI] [PubMed] [Google Scholar]
- McClary D. O., Bowers W. D., Miller G. R. ULTRAVIOLET MICROSCOPY OF BUDDING SACCHAROMYCES. J Bacteriol. 1962 Feb;83(2):276–283. doi: 10.1128/jb.83.2.276-283.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVIHLA G., SCHLENK F. Localization of S-adenosylmethionine in Candida utilis by ultraviolet microscopy. J Bacteriol. 1959 Oct;78:500–505. doi: 10.1128/jb.78.4.500-505.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVIHLA G., SCHLENK F. S-adenosylmethionine in the vacuole of Candida utilis. J Bacteriol. 1960 Jun;79:841–848. doi: 10.1128/jb.79.6.841-848.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slepecky R. A., Law J. H. SYNTHESIS AND DEGRADATION OF POLY-beta-HYDROXYBUTYRIC ACID IN CONNECTION WITH SPORULATION OF BACILLUS MEGATERIUM. J Bacteriol. 1961 Jul;82(1):37–42. doi: 10.1128/jb.82.1.37-42.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOTSUYANAGI Y. [Electron-microscopic demonstration of chromosomes in yeasts by means of specific staining]. C R Hebd Seances Acad Sci. 1960 Feb 22;250:1522–1524. [PubMed] [Google Scholar]