Abstract
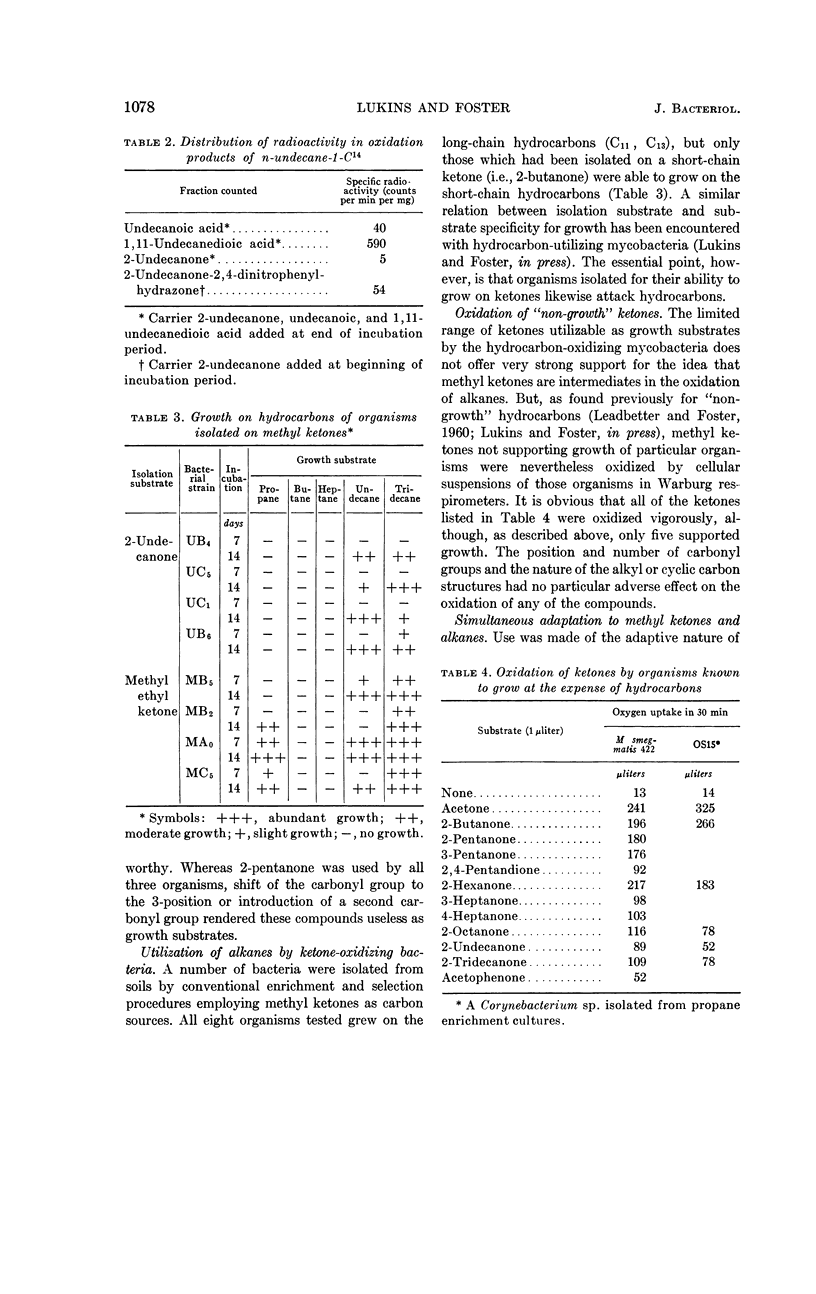
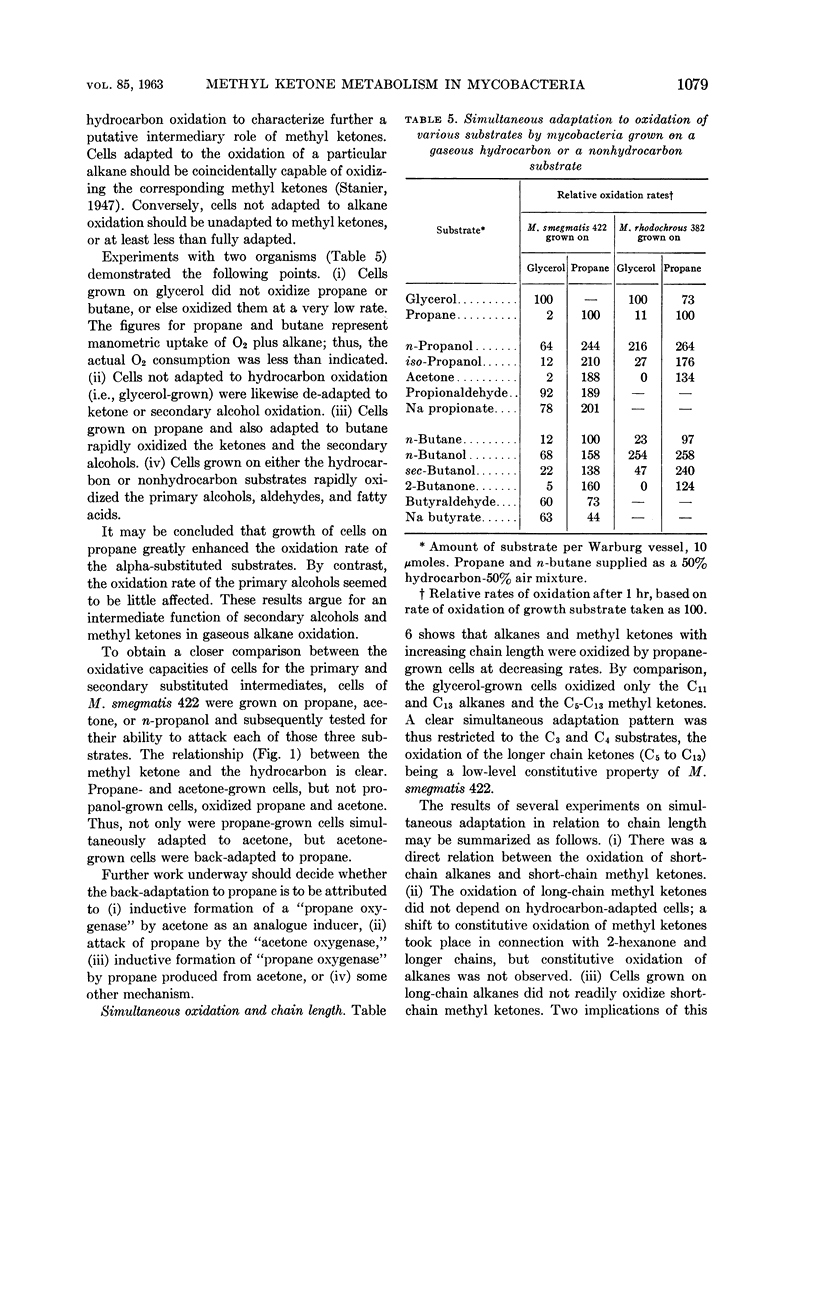
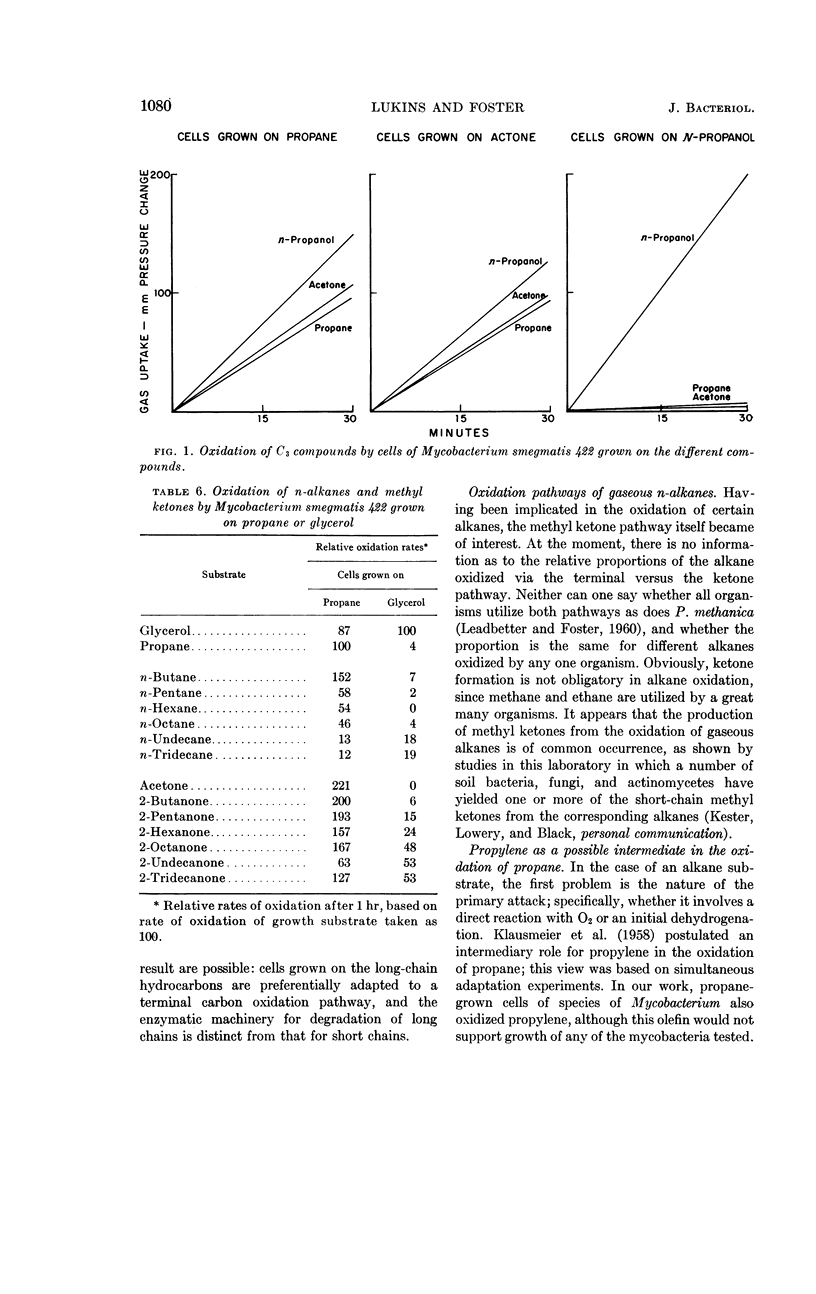
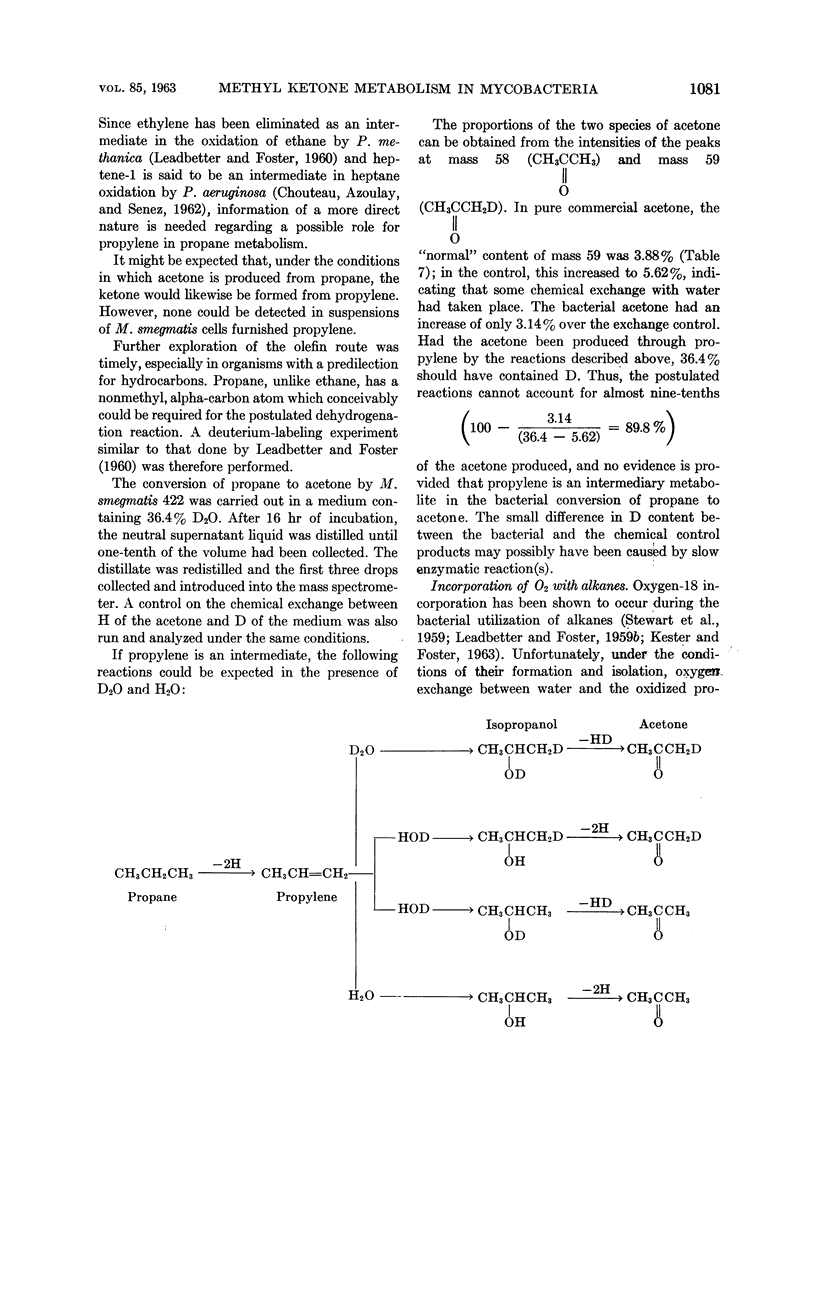
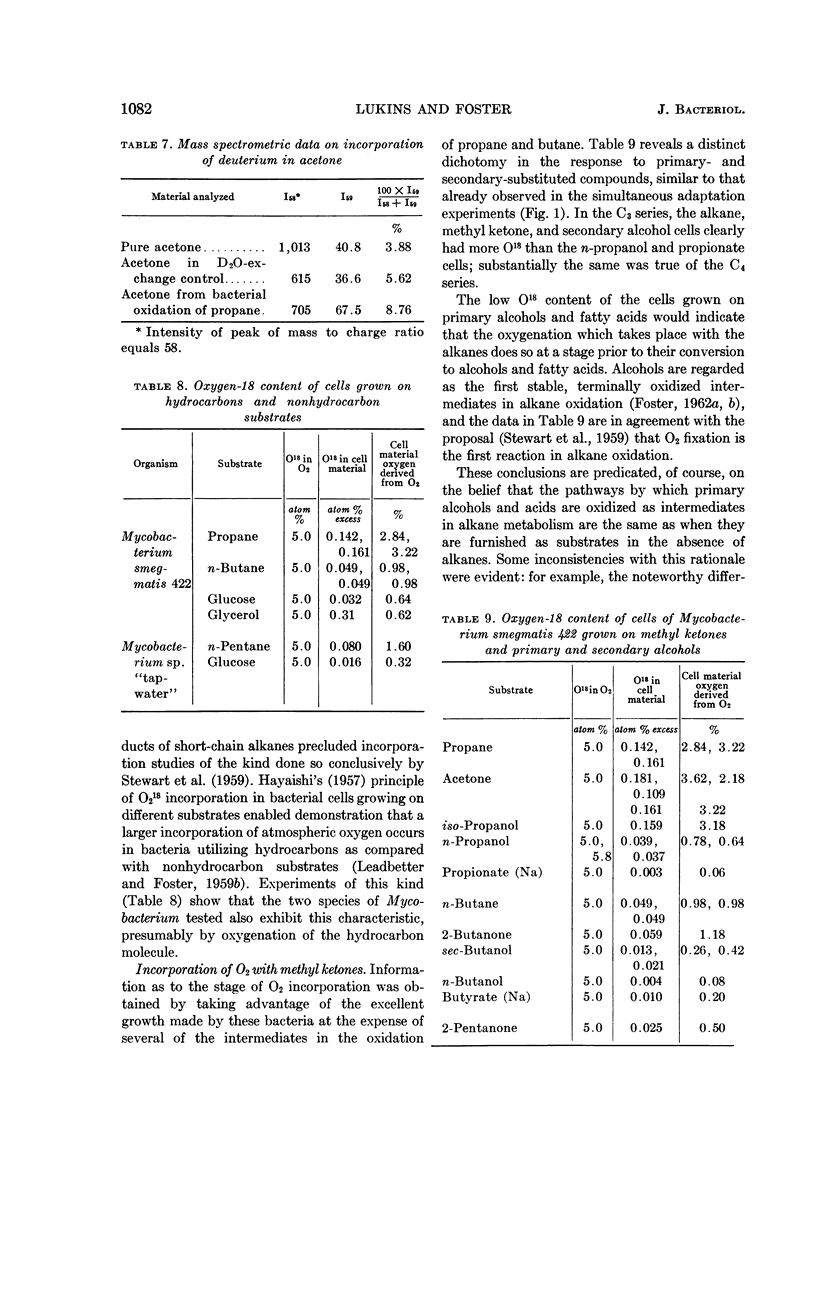
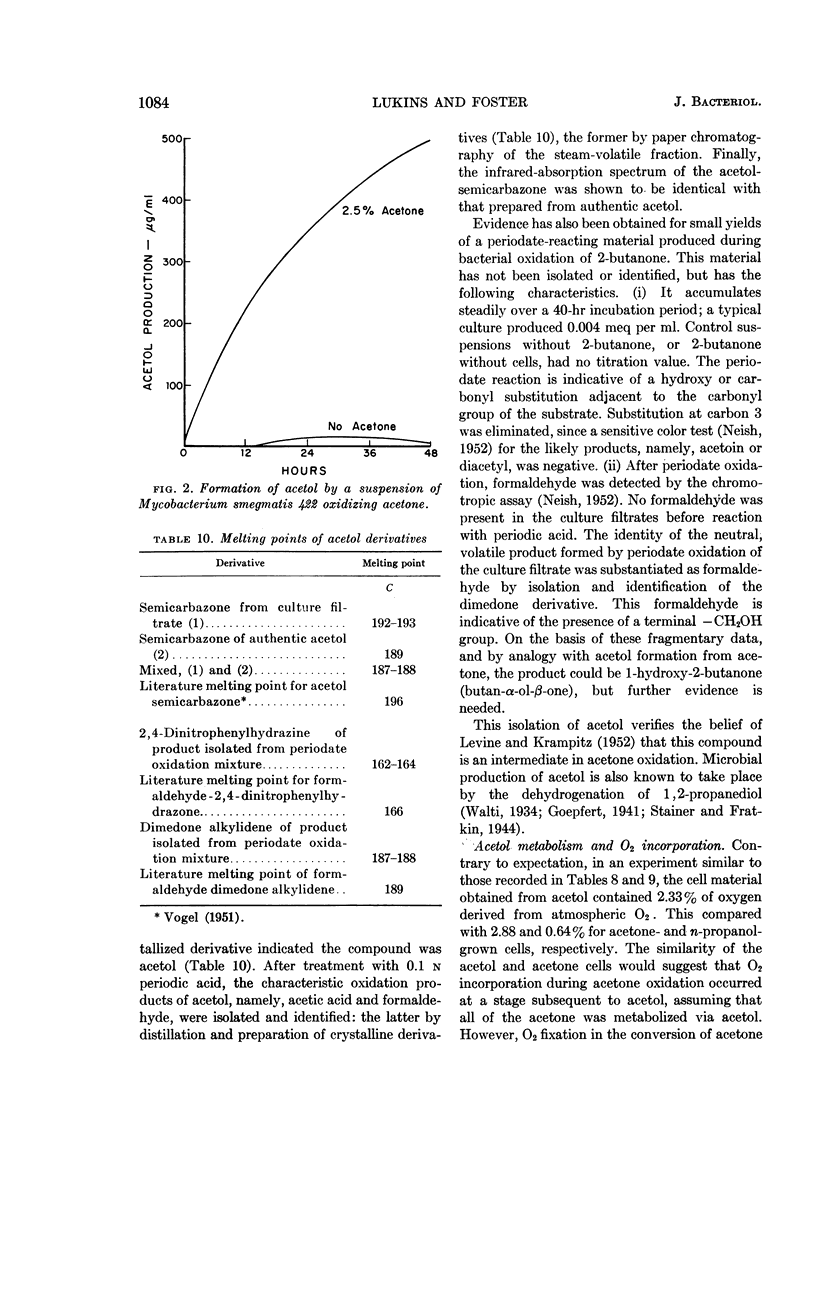
Lukins, H. B. (University of Texas, Austin) and J. W. Foster. Methyl ketone metabolism in hydrocarbon-utilizing mycobacteria. J. Bacteriol. 85: 1074–1087. 1963.—Species of Mycobacterium especially M. smegmatis 422, produced the homologous methyl ketones during the oxidation of propane, n-butane, n-pentane, or n-hexane. A carrier-trapping experiment demonstrated the formation of 2-undecanone, as well as 1,11-undecanedioic acid, during the oxidation of undecane-1-C14. Aliphatic alkane-utilizing mycobacteria were able to grow at the expense of several aliphatic methyl ketones as sole sources of carbon. Other ketones which did not support growth were oxidized by resting bacterial suspensions. M. smegmatis 422 cells grown on propane or acetone were simultaneously adapted to oxidize both substrates, as well as n-propanol. n-Propanol cells were unadapted to propane or acetone. Acetone produced from propane in a medium enriched in D2O contained a negligible quantity of D, presumably eliminating propylene as an intermediate in the oxidation. Cells grown at the expense of alkanes or methyl ketones in the presence of O218 had a higher content of O18 than did cells grown on terminally oxidized compounds, e.g., primary alcohols or fatty acids. An oxygenase reaction is postulated for the attack on methyl ketones. Acetol was isolated and characterized as an oxidation product of acetone by M. smegmatis 422. Acetol-grown cells had a higher O18 content than did n-propanol cells, and its utilization appears to involve at least one oxygenase reaction. Acetol produced from acetone in the presence of O218 was not enriched in the isotope, indicating the occurrence of exchange reactions or of oxygenation reactions at a later stage in the assimilation of acetone and acetol.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FOSTER J. W. Hydrocarbons as substrates for microorganisms. Antonie Van Leeuwenhoek. 1962;28:241–274. doi: 10.1007/BF02538739. [DOI] [PubMed] [Google Scholar]
- FUHS G. W. [Microbial catabolism of hydrocarbons]. Arch Mikrobiol. 1961;39:374–422. [PubMed] [Google Scholar]
- Hopkins S. J. Growth of Aspergillus versicolor on higher paraffins. Biochem J. 1932;26(1):133–142. doi: 10.1042/bj0260133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ITADA N., ICHIHARA A., MAKITA T., HAYAISHI O., SUDA M., SASAKI N. L-Lysine oxidase, a new oxygenase. J Biochem. 1961 Aug;50:118–121. doi: 10.1093/oxfordjournals.jbchem.a127418. [DOI] [PubMed] [Google Scholar]
- KESTER A. S., FOSTER J. W. DITERMINAL OXIDATION OF LONG-CHAIN ALKANES BY BACTERIA. J Bacteriol. 1963 Apr;85:859–869. doi: 10.1128/jb.85.4.859-869.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEADBETTER E. R., FOSTER J. W. Bacterial oxidation of gaseous alkanes. Arch Mikrobiol. 1960;35:92–104. doi: 10.1007/BF00425597. [DOI] [PubMed] [Google Scholar]
- LEADBETTER E. R., FOSTER J. W. Incorporation of molecular oxygen in bacterial cells utilizing hydrocarbons for growth. Nature. 1959 Oct 31;184(Suppl 18):1428–1429. doi: 10.1038/1841428a0. [DOI] [PubMed] [Google Scholar]
- LEADBETTER E. R., FOSTER J. W. Oxidation products formed from gaseous alkanes by the bacterium Pseudomonas methanica. Arch Biochem Biophys. 1959 Jun;82(2):491–492. doi: 10.1016/0003-9861(59)90154-7. [DOI] [PubMed] [Google Scholar]
- LEVINE S., KRAMPITZ L. O. The oxidation of acetone by a soil diphtheroid. J Bacteriol. 1952 Nov;64(5):645–650. doi: 10.1128/jb.64.5.645-650.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUKHERJEE S. Studies on degradation of fats by microorganisms. I. Preliminary investigations on enzyme systems involved in the spoilage of fats. Arch Biochem Biophys. 1951 Oct;33(3):364–376. doi: 10.1016/0003-9861(51)90124-5. [DOI] [PubMed] [Google Scholar]
- PONTICORVO L., RITTENBERG D. A method for the determination of the O18 concentration of the oxygen of organic compounds. Int J Appl Radiat Isot. 1956 Nov;1(3):208–214. doi: 10.1016/0020-708x(56)90008-4. [DOI] [PubMed] [Google Scholar]
- RUDNEY H. Propanediol phosphate as a possible intermediate in the metabolism of acetone. J Biol Chem. 1954 Sep;210(1):361–371. [PubMed] [Google Scholar]
- SELLINGER O. Z., MILLER O. N. The metabolism of acetol phosphate. II. 1,2-Propanediol-1-phosphate dehydrogenase. J Biol Chem. 1959 Jul;234(7):1641–1646. [PubMed] [Google Scholar]
- SMITH J. N., SMITHIES R. H., WILLIAMS R. T. Studies in detoxication. 56. The metabolism of alkylbenzenes: stereochemical aspects of the biological hydroxylation of ethylbenzene to methylphenylcarbinol. Biochem J. 1954 Feb;56(2):320–324. doi: 10.1042/bj0560320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEWART J. E., KALLIO R. E., STEVENSON D. P., JONES A. C., SCHISSLER D. O. Bacterial hydrocarbon oxidation. I. Oxidation of n-hexadecane by a gram-negative coccus. J Bacteriol. 1959 Sep;78:441–448. doi: 10.1128/jb.78.3.441-448.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUTTON W. B. Mechanism of action and crystallization of lactic oxidative decarboxylase from Mycobacterium phlei. J Biol Chem. 1957 May;226(1):395–405. [PubMed] [Google Scholar]
- Stanier R. Y. Simultaneous Adaptation: A New Technique for the Study of Metabolic Pathways. J Bacteriol. 1947 Sep;54(3):339–348. doi: 10.1128/jb.54.3.339-348.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]


