Abstract
The human mitochondrial electron transfer flavoprotein (ETF) accepts electrons from at least 10 different flavoprotein dehydrogenases and transfers electrons to a single electron acceptor in the inner membrane. Paracoccus denitrificans ETF has the identical function, shares the same three dimensional structure and functional domains, and exhibits the same conformational mobility. It has been proposed that the mobility of the αII domain permits the promiscuous behavior of ETF with respect to a variety of redox partners. Double electron-electron resonance (DEER) measurements between a spin label and an enzymatically reduced flavin adenine dinucleotide (FAD) cofactor in P. denitrificans ETF gave two distributions of distances: a major component centered at 4.2 ± 0.1 nm and a minor component centered at 5.1 ± 0.2 nm. Both components had widths of approximately 0.3 nm. A distance of 4.1 nm was calculated using the crystal structure of P. denitrificans ETF, which agrees with the major component obtained from the DEER measurement. The observation of a second distribution suggests that ETF, in the absence of substrate, adopts some conformations that are intermediate between the predominant free and substrate-bound states.
Protein structures are commonly determined by X-ray crystallography and NMR spectroscopy but there are drawbacks to these methods. For example, some proteins can be crystallized, but there are examples in which crystallization conditions generate non-biologically relevant conformations.1,2 A complementary method of obtaining structural information for proteins without growing crystals is site-directed spin labeling (SDSL) and pulsed electron paramagnetic resonance (EPR) spectroscopy.3 Double electron-electron resonance (DEER) experiments can provide information on the distribution of distances between two paramagnetic sites in macromolecules (including nucleic acids) and has proven to be an effective way to study the conformational changes induced by protein-protein interactions.4 In most cases DEER measurements are made between two nitroxyl spin labels, such as MTSL (1-oxyl-2,2,5,5-tetramethylpyrroline-3-methyl-methanethiosulfonate, Toronto Research Chemicals), bound to cysteine residues introduced at desired locations by site-directed mutagenesis.5
Interpretation of DEER data between two nitroxyls is complicated because both labels can adopt multiple conformations. Utilizing a tightly bound, natural paramagnetic cofactor in place of a spin label can lower the uncertainty that arises from the rotational freedom of a spin label. Previously DEER has been used to determine a distance of 26 Å between flavin radicals in augmenter liver regeneration (ALR) dimers.6 It has also been used to study complex formation in E. coli ribonucleotide reductase by measuring the distance between a tyrosyl radical on the R2 subunit and a radical formed by the inhibitor 2′-azido-2′-deoxyuridine-5′-diphosphate in the active site of the R1 subunit.7 The work of Borovykh et al. on the photosynthetic reaction center of Rhodobacter sphaeroides is the only example of DEER measurements between a spin label and a tightly bound, natural organic cofactor; the photochemically generated anionic semiquinone of QA.8 In the present study DEER was used to measure the distance between a spin label and an enzymatically reduced flavin adenine dinucleotide (FAD) cofactor in electron transfer flavoprotein (ETF) from Paracoccus denitrificans. Enzymatic formation of the anionic semiquinone makes the method applicable to a larger number of proteins.
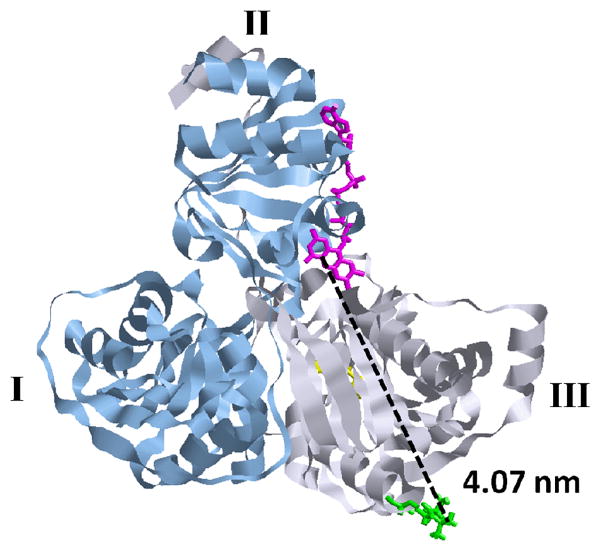
ETF (Figure 1) is a soluble heterodimeric flavoprotein located in the mitochondrial matrix. X-ray crystal structures of human and P. denitrificans ETF are similar and both show 3 distinct structural domains.9,10 Mammalian ETF contains a single FAD redox center, located in the αII domain, which shuttles electrons from at least 10 different flavoprotein dehydrogenases to the membrane-bound electron transfer flavoprotein ubiquinone oxidoreductase (ETF-QO).11,12 Because of this promiscuous behavior it has been postulated that ETF must be able to adopt a range of conformations. Evidence from low angle x-ray scattering suggests that the αII domain of human and P. denitrificans ETFs can rotate by 30 to 50° relative to an axis defined by domains I and III.13 Domain III is the β subunit and provides an anchoring recognition site for the interaction between ETF and the electron donors and presumably also the electron acceptor. The ability of the αII domain to rotate is proposed to permit the flavin-containing αII domain to assume the most favorable position for electron transfer with a variety of electron donors and its electron acceptor.
Figure 1.
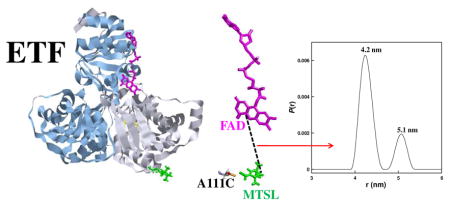
Crystal structure of Paracoccus denitrificans ETF (PDB id: 1efp) with the α (blue ribbon) and β (grey ribbon) subunits, FAD (pink), AMP (yellow) and MTSL spin label (green) highlighted. Structural domains are labeled using roman numerals. The PDB file was modified, using the Insight II software (Accelyrs), by substituting a cysteine for an alanine at position 111 of the β chain and then attaching MTSL. A distance of 4.07 nm between C4 of the FAD and the N-O bond of the MTSL label (dashed line) was calculated using the program RasTop.
Site-directed mutagenesis was used to substitute Ala111 of the β-subunit of P. denitrificans ETF with a cysteine. Unlike the mammalian enzyme, Paracoccus ETF has no exposed cysteine residues, making it ideal for site-directed spin-labeling experiments. A111C ETF was spin labeled using the cysteine-specific, nitroxyl spin label MTSL.5 Stoichiometric incorporation of MTSL into A111C ETF was confirmed by continuous wave (CW) EPR spectroscopy (Figure 2a).
Figure 2.
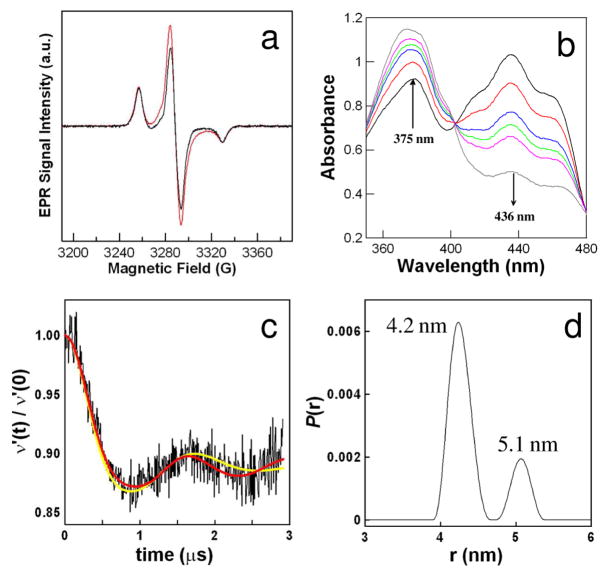
(a) CW EPR spectra of spin-labeled A111C ETF with (red) and without (black) enzymatic reduction to FAD SQ−•. (b) Time dependence of visible spectrum of ETF during enzymatic reduction to FAD SQ−•. (c) Time domain DEER data from enzymatically-reduced, spin-labeled A111C ETF and DEER analysis fit using a single Gaussian distribution (yellow) or Tikhonov regularization (red). (d) Distance distribution calculated from DEER data by Tikhonov regularization.
Spin-labeled A111C ETF was enzymatically reduced under anaerobic conditions at pH 8.0 to FAD SQ−• using a coupled reaction with glutaryl-CoA and glutaryl-CoA dehydrogenase. FAD SQ−• formation was followed by monitoring the increase in absorbance at 375 nm (Figure 2b). About 60 % of the ETF flavin was reduced to FAD SQ−• before decrease in the A375 indicated disproportionation by the dehydrogenase/enoyl-CoA complex.14 The difference between CW EPR spectra before and after reduction matches the spectrum of FAD SQ−• from unlabeled, reduced wild-type ETF.
Four-pulse DEER measurements were performed at 60 K on a Bruker E580 spectrometer equipped with a split-ring resonator and Oxford CF 935 cryostat. DEER data were analyzed using the DeerAnalysis2008 program.15 Figure 2c shows the DEER trace at 60 K after background correction. DEER data were fit using a single Gaussian model or Tikhonov regularization.16 A better fit was obtained using Tikonov regularization (Figure 2c) than with a single Gaussian (distribution centered at 4.3 nm). Two distributions of distances were obtained from Tikonov regularization: a major component centered at 4.2 ± 0.1 nm and a minor component centered at 5.1 ± 0.2 nm (Figure 2d). Both components of the distribution had widths of approximately 0.3 ± 0.25 nm at half height. Uncertainties are estimates based on variation with fitting parameters (see supporting information).
DEER results were compared with the crystal structure in the closed conformation (pdb id: 1EFP) using Insight II software (Accelrys). The distance between the C4a of the FAD (the approximate centroid of spin density) and the N-O group of the MTSL at position 111 is approximately 4.1 nm. This distance is in agreement with the center of the major distance distribution found in the DEER experiment. P. denitrificans ETF bound to MCAD (medium chain acyl-CoA dehydrogenase) was modeled using the structure of the human ETF:MCAD complex (pdb id: 2A1T) as a template.17 This model predicts that there would be a change of about 1.6 nm in the interspin distance between the free and bound conformations of Paracoccus ETF (see supporting information), which is larger than the difference of 0.9 nm between the two distributions found in the DEER experiment. Modeling indicates that the two distributions seen in the DEER analysis are not the result of multiple conformations of the spin label. The maximum increase in interspin distance caused by varying the dihedral angles of the spin label is approximately 0.5 nm. The room temperature CW EPR spectrum (supporting information) shows no evidence of multiple spin label conformations.18 We propose that the longer distance distribution at 5.1 nm is due to a protein conformation that is intermediate between the substrate-free and the substrate-bound forms.
In conclusion we have demonstrated enzymatic reduction of a FAD cofactor in a spin-labeled protein, without destruction of the spin label. The reduced ETF was used to determine the distribution of distances between the spin label and the FAD SQ−•. This method has the potential to characterize conformational changes in ETF that occur when it interacts with various redox partners.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health NIBIB EB002807 (G.RE. and S.S.E.), the Children’s Hospital Research Foundation, Denver, CO and a Center Grant to the Colorado Intellectual and Developmental Research Center supported by the National Institutes of Health NICHD, HD 004024 (F.E.F.). Mark Tseitlin (University of Denver) assisted with analysis of the DEER data.
Footnotes
Supporting Information Available: Details of the expression and purification of A111C ETF, enzymatic reduction, EPR spectroscopy and homology modeling. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Hanson SM, Van Eps N, Francis DJ, Altenbach C, Vishnivetskiy SA, Arshavsky VY, Klug CS, Hubbell WL, Gurevich W. EMBO Journal. 2007;26:1726–1736. doi: 10.1038/sj.emboj.7601614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim M, Xu Q, Murray D, Cafiso DS. Biochemistry. 2008;47:670–679. doi: 10.1021/bi7016415. [DOI] [PubMed] [Google Scholar]
- 3.Berliner LJ, Eaton GR, Eaton SS, editors. Biol Mag Res. Vol. 19. Kluwer; New York: 2000. [Google Scholar]
- 4.Banham JE, Timmel CR, Abbott RJM, Lea SM, Jeschke G. Angew Chemie Int Ed. 2006;45:1058–1061. doi: 10.1002/anie.200503720. [DOI] [PubMed] [Google Scholar]
- 5.Hubbell WL, Altenbach C, Hubbell CM, Khorana HG. Adv Prot Chem. 2003;63:243–290. doi: 10.1016/s0065-3233(03)63010-x. [DOI] [PubMed] [Google Scholar]
- 6.Kay CWM, Elsasser C, Bittl R, Farrell SR, Thorpe C. J Am Chem Soc. 2006;128:76–77. doi: 10.1021/ja057308g. [DOI] [PubMed] [Google Scholar]
- 7.Bennati M, Robblee JH, Mugnaini V, Stubbe J, Freed JH, Borbat P. J Am Chem Soc. 2005;127:15014–15015. doi: 10.1021/ja054991y. [DOI] [PubMed] [Google Scholar]
- 8.Borovykh IV, Ceola S, Gajula P, Gast P, Steinhoff HJ, Huber M. J Mag Res. 2006;180:178–185. doi: 10.1016/j.jmr.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Roberts DL, Frerman FE, Kim JJP. Proc Nat Acad Sci U S. 1996;93:143555–14360. doi: 10.1073/pnas.93.25.14355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts DL, Salazar D, Fulmer JP, Frerman FE, Kim J-JP. Biochemistry. 1999;38:1977–1989. doi: 10.1021/bi9820917. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J, Frerman FE, Kim JJ. Proc Nat Acad Sci U S. 2006;103:16212–16217. doi: 10.1073/pnas.0604567103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toogood HS, Leys D, Scrutton NS. FEBS Journal. 2007;274:5481–5504. doi: 10.1111/j.1742-4658.2007.06107.x. [DOI] [PubMed] [Google Scholar]
- 13.Chohan KK, Jones M, Grossmann JG, Frerman FE, Scrutton NS, Sutcliffe MJ. J Biol Chem. 2001;276:34142–34147. doi: 10.1074/jbc.M101341200. [DOI] [PubMed] [Google Scholar]
- 14.Gorelick RJ, Schopfer LM, Ballou DP, Massey V, Thorpe C. Biochemistry. 1985;24:6830–6839. doi: 10.1021/bi00345a015. [DOI] [PubMed] [Google Scholar]
- 15.Jeschke G, Chechik V, Ionita P, Godt A, Zimmermann H, Banham JC, Timmel R, Hilger D, Jung H. Appl Magn Reson. 2006;30:473–498. [Google Scholar]
- 16.Chiang YW, Borbat PP, Freed JH. J Mag Res. 2005;172:279–295. doi: 10.1016/j.jmr.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 17.Lambert C, Leonard N, De Bolle X, Depiereux E. Bioinformatics. 2002;18:1250–1256. doi: 10.1093/bioinformatics/18.9.1250. [DOI] [PubMed] [Google Scholar]
- 18.Columbus L, Kalai T, Jeko J, Hideg K, Hubbell WL. Biochemistry. 2001;40:3828–3846. doi: 10.1021/bi002645h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.