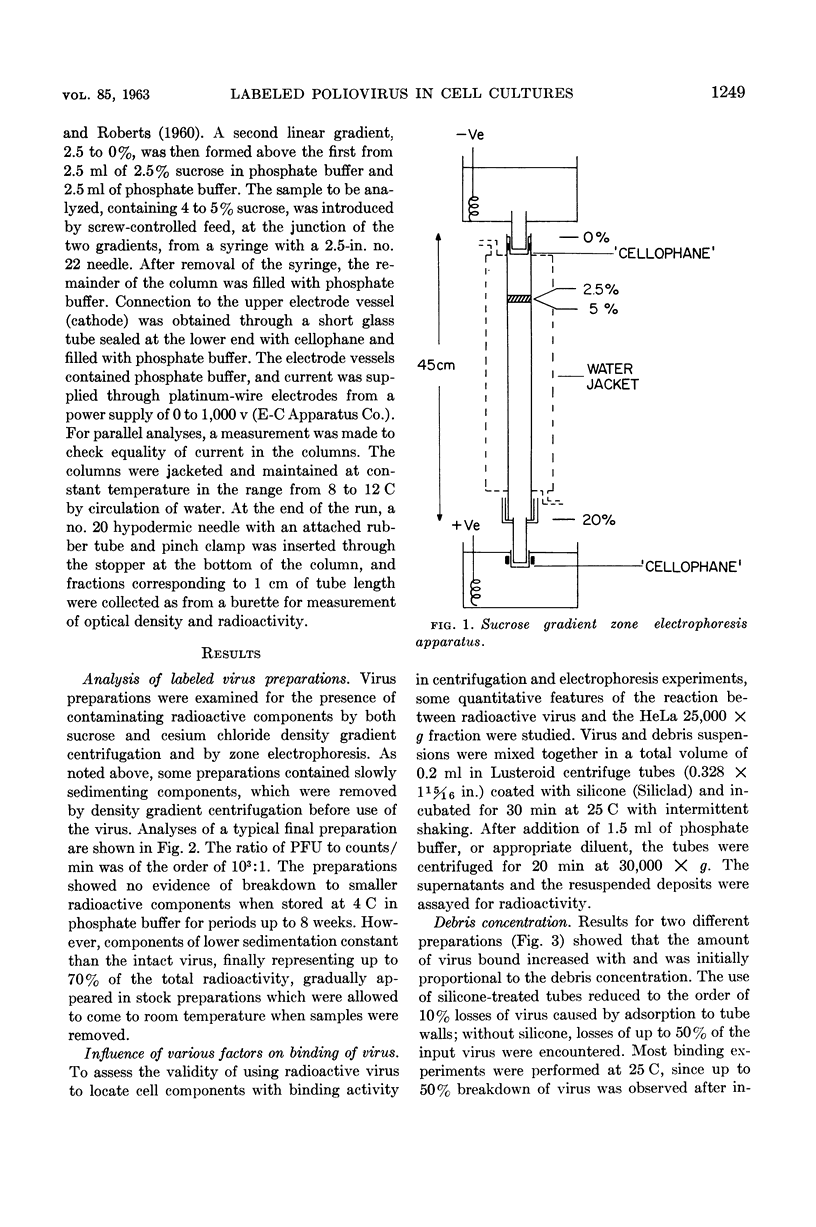
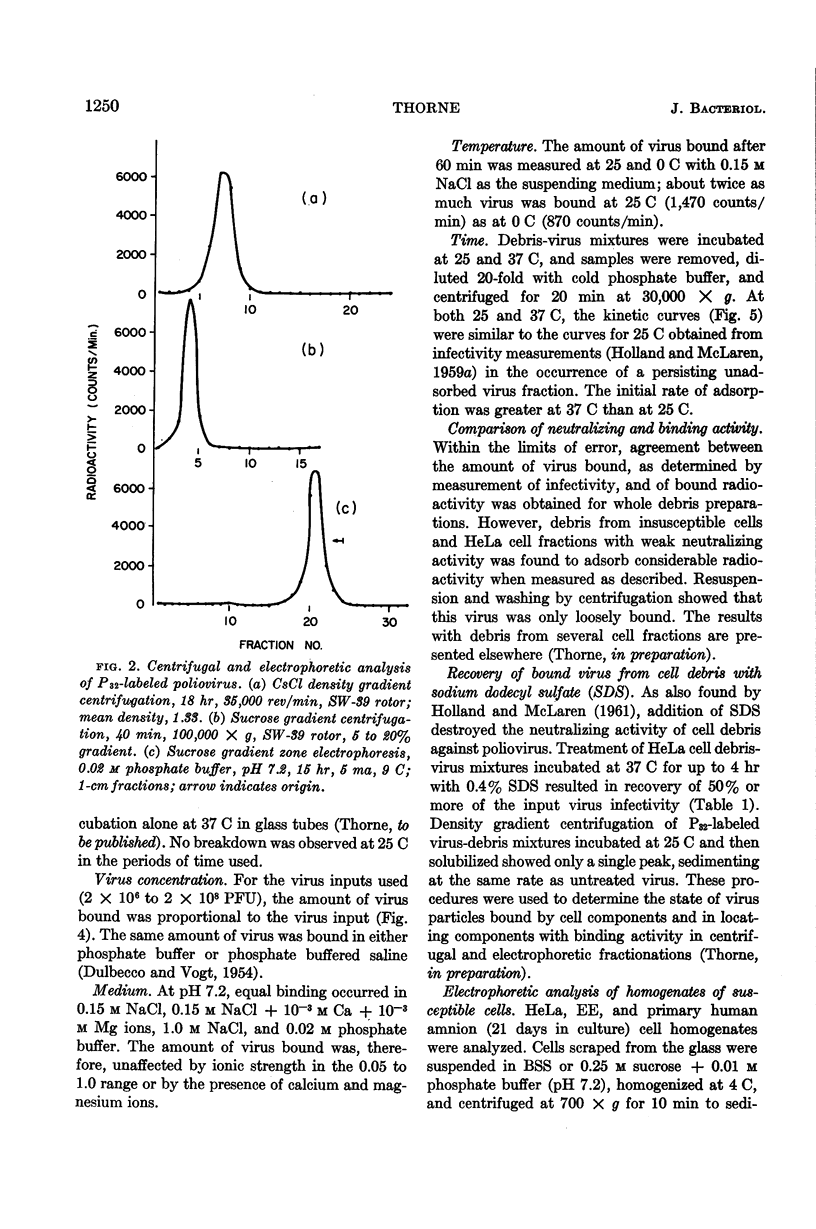
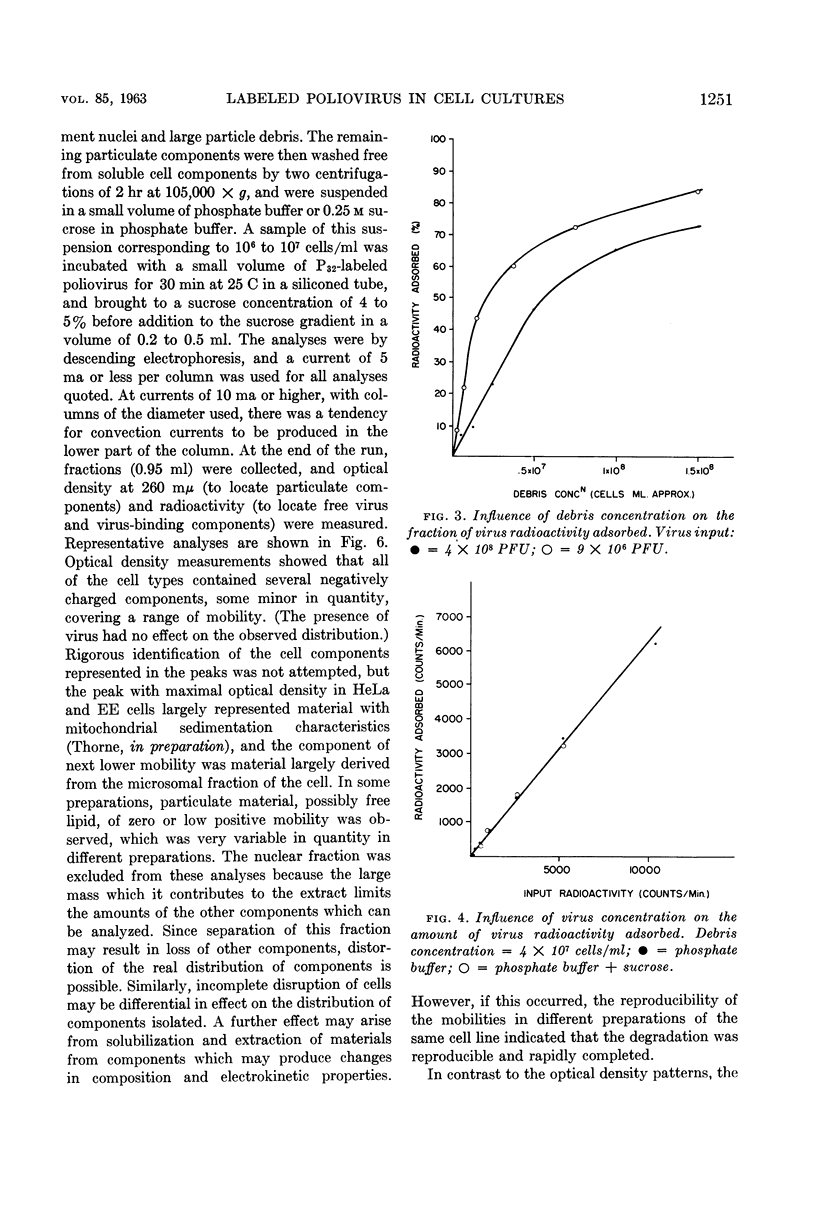
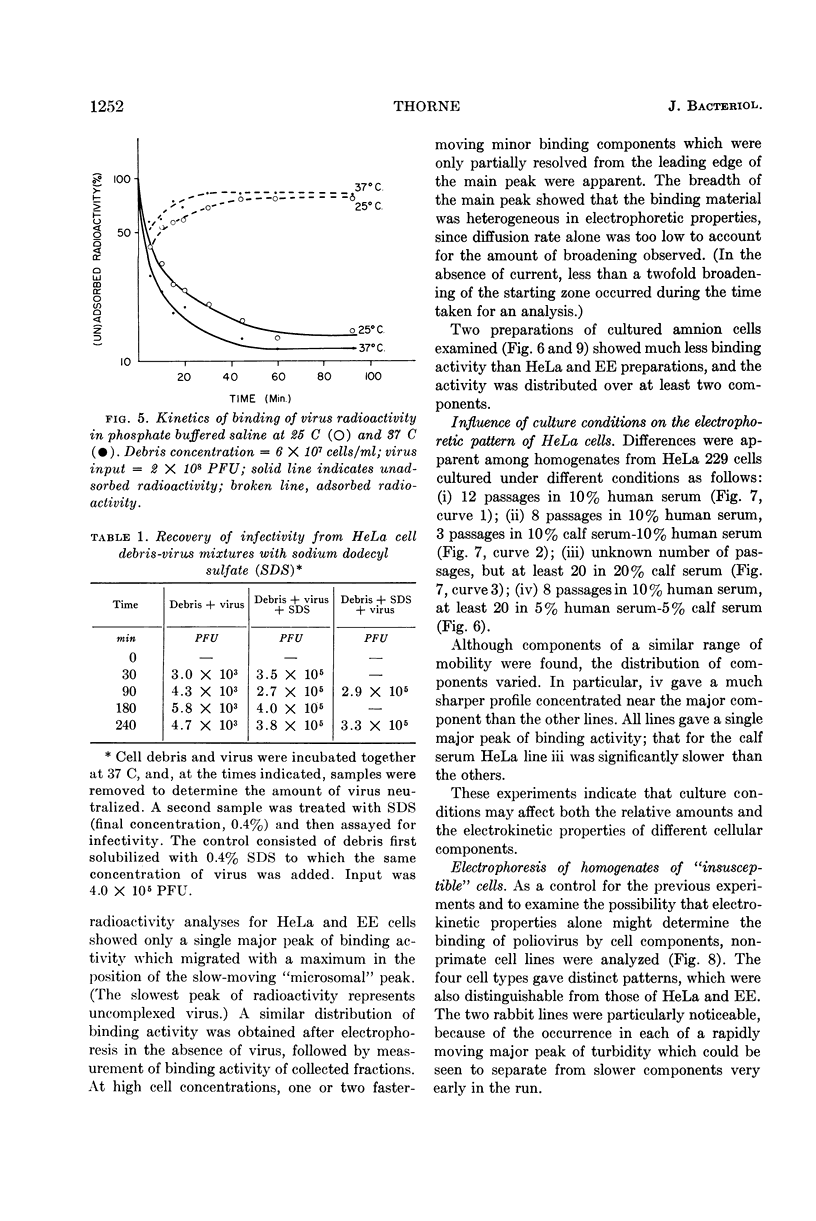
Abstract
Thorne, H. V. (University of Minnesota, Minneapolis). Electrophoretic study of the interaction of radioactive poliovirus with components of cultured cells. J. Bacteriol. 85:1247–1255. 1963.—The interaction of P32-labeled poliovirus with the postnuclear fraction of HeLa cell homogenates was first examined using radioactivity to quantitate the reaction. The effects of virus and debris concentration, suspending medium, temperature, and time on the reaction were determined. Binding was independent of salt concentration up to 1.0 m and unaffected by calcium and magnesium ions at 10−3m. The postnuclear particulate components of homogenates of mammalian cells in culture were examined by sucrose gradient zone electrophoresis at pH 7.2 with a simple apparatus which permitted several simultaneous analyses. The distributions of components for different cell types were distinct and appeared to be influenced by conditions of culture. Addition of radioactive poliovirus to the homogenate before analysis was used to identify components with virus-binding activity. Activity of HeLa and human esophageal epithelium cell homogenates was found mainly in membranous fractions of relatively low electrophoretic mobility. Components with a broader spread of mobility were moderately active in cultured human amnion, but uncultured amnion had almost undetectable virus-binding activity and a distinct distribution of components. Rat heart cells and L cells did not bind poliovirus, but binding components were present in both ERK-1 and CRE rabbit lines.
Full text
PDF

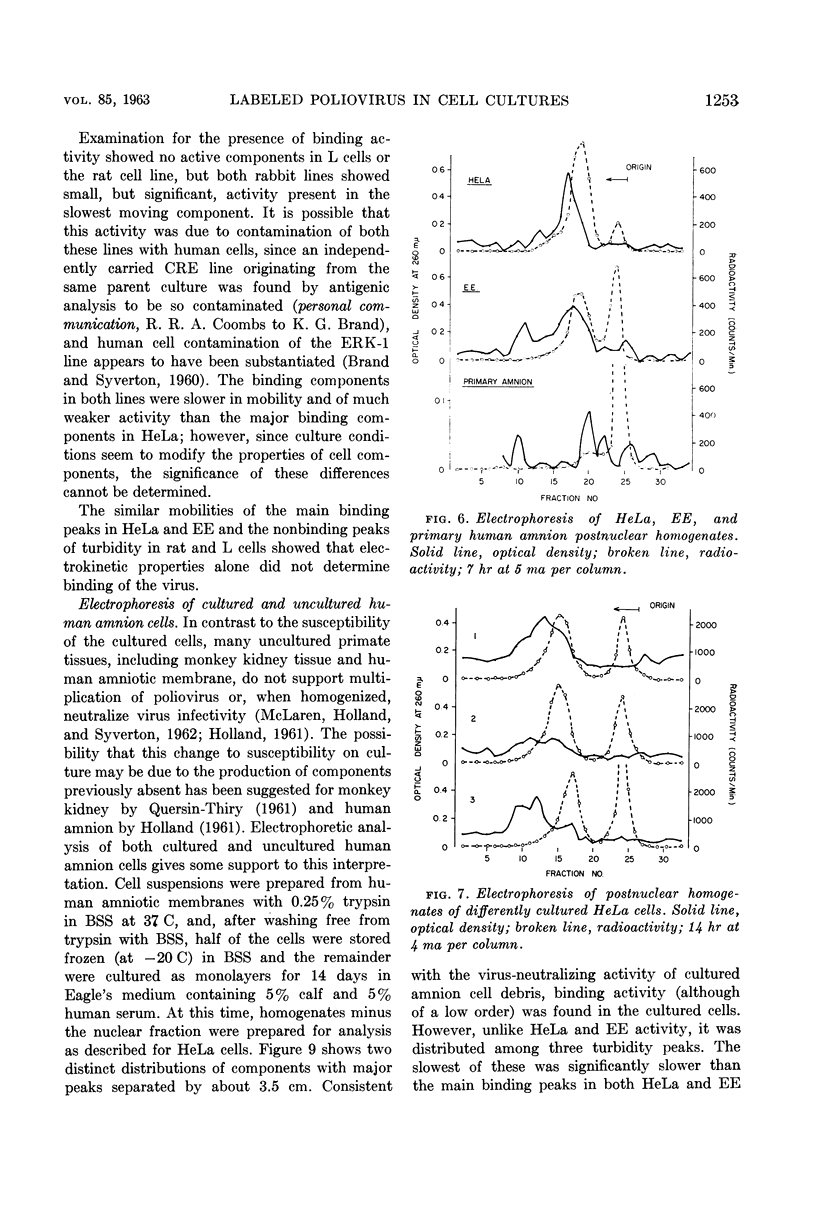
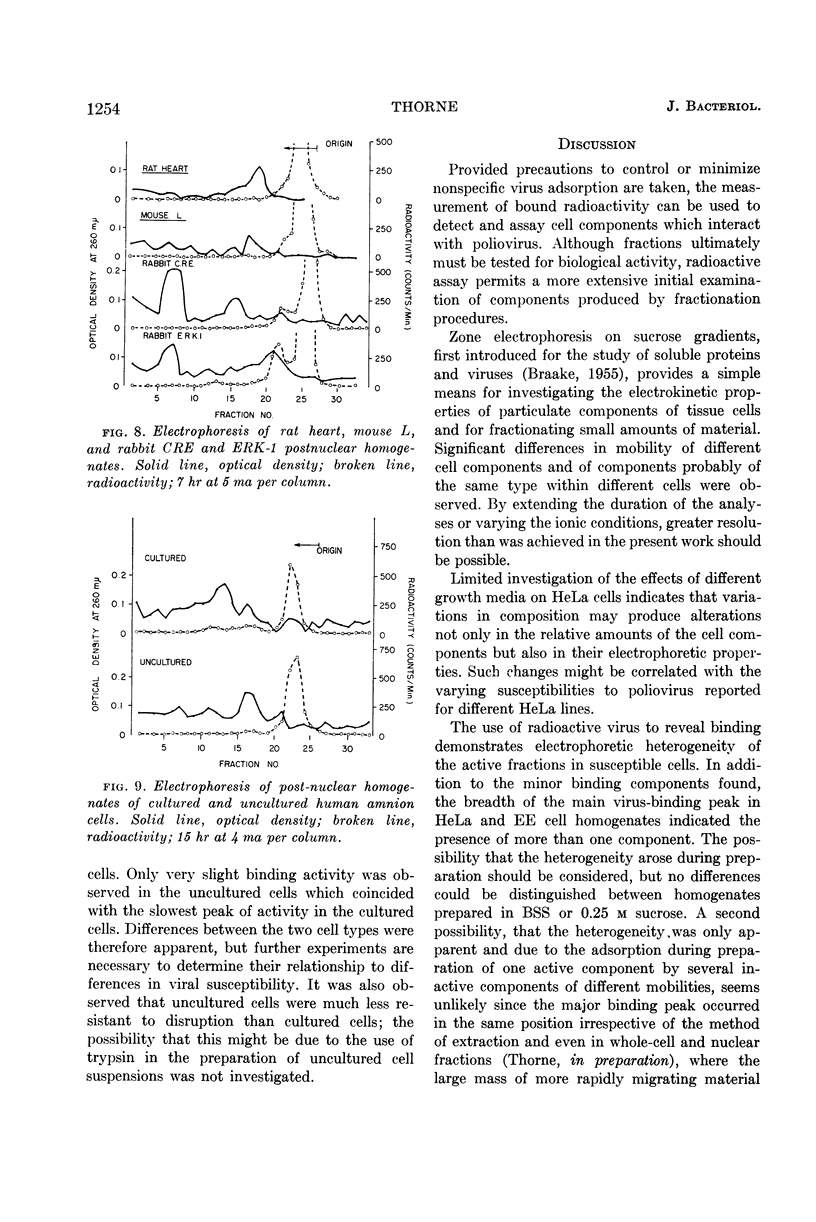

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRAND K. G., SYVERTON J. T. Immunology of cultivated mammalian cells. I. Species specificity determined by hemagglutination. J Natl Cancer Inst. 1960 May;24:1007–1019. [PubMed] [Google Scholar]
- Britten R. J., Roberts R. B. High-Resolution Density Gradient Sedimentation Analysis. Science. 1960 Jan 1;131(3392):32–33. doi: 10.1126/science.131.3392.32. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLAND J. J., McLAREN L. C. Improved method for staining cell monolayers for virus plaque counts. J Bacteriol. 1959 Oct;78:596–597. doi: 10.1128/jb.78.4.596-597.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLAND J. J., McLAREN L. C., SYVERTON J. T. The mammalian cell-virus relationship. IV. Infection of naturally insusceptible cells with enterovirus ribonucleic acid. J Exp Med. 1959 Jul 1;110(1):65–80. doi: 10.1084/jem.110.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLAND J. J., McLAREN L. C. The location and nature of enterovirus receptors in susceptible cells. J Exp Med. 1961 Aug 1;114:161–171. doi: 10.1084/jem.114.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLAND J. J., McLAREN L. C. The mammalian cell-virus relationship. II. Adsorption, reception, and eclipse of poliovirus by HeLa cells. J Exp Med. 1959 May 1;109(5):487–504. doi: 10.1084/jem.109.5.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLAND J. J. Receptor affinities as major determinants of enterovirus tissue tropisms in humans. Virology. 1961 Nov;15:312–326. doi: 10.1016/0042-6822(61)90363-4. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K., DARNELL J. E., Jr The adsorption and early fate of purified poliovirus in HeLa cells. Virology. 1961 Apr;13:439–447. doi: 10.1016/0042-6822(61)90275-6. [DOI] [PubMed] [Google Scholar]
- SYVERTON J. T., McLAREN L. C. Human cells in continuous culture. I. Derivation of cell strains from esophagus, palate, liver, and lung. Cancer Res. 1957 Oct;17(9):923–926. [PubMed] [Google Scholar]
- THORNE H. V., CARTWRIGHT S. F. Reactions of the virus of foot-and-mouth disease with cells and cell debris. Virology. 1961 Nov;15:245–257. doi: 10.1016/0042-6822(61)90355-5. [DOI] [PubMed] [Google Scholar]


