Abstract
Spironolactone has been noted to attenuate cardiac fibrosis. We have observed that the cardiotonic steroid marinobufagenin plays an important role in the diastolic dysfunction and cardiac fibrosis seen with experimental renal failure. We performed the following studies to determine whether and how spironolactone might ameliorate these changes. First, we studied rats subjected to partial nephrectomy or administration of exogenous marinobufagenin. We found that spironolactone (20 mg/kg per day) attenuated the diastolic dysfunction as assessed by ventricular pressure-volume loops and essentially eliminated cardiac fibrosis as assessed by trichrome staining and Western blot. Next, we examined the effects of spironolactone and its major metabolite, canrenone (both 100 nM), on marinobufagenin stimulation of rat cardiac fibroblasts. Both spironolactone and canrenone prevented the stimulation of collagen production by 1 nM marinobufagenin but not 100 nM marinobufagenin, as assessed by proline incorporation and procollagen 1 expression, as well as signaling through the sodium-potassium-ATPase, as evidenced by protein kinase C isoform δ translocation and extracellular signal regulated kinase 1/2 activation. Both spironolactone and canrenone also altered ouabain binding to cultured porcine cells in a manner consistent with competitive inhibition. Our data suggest that some of the antifibrotic effects of spironolactone may be attributed to antagonism of marinobufagenin signaling through the sodium-potassium-ATPase.
Keywords: cardiomyopathy, renal failure, cardiotonic steroids, collagen, fibrosis
Cardiac fibrosis oftentimes complicates congestive cardiomyopathies, and it has been suggested that aldosterone may directly cause cardiac fibrosis.1 Spironolactone is a synthetic steroid molecule that has been characterized as a mineralocorticoid receptor antagonist.2,3 In recent years, it has been found that spironolactone can reduce the artery stiffness and left ventricular mass index in cardiomyopathic conditions.4–6 Mechanistically, spironolactone treatment inhibits angiotensin II and aldosterone-induced activation of epidermal growth factor receptor/extracellular signal regulated kinase (ERK), NAD(P)H oxidase/lectin-like oxidized low-density lipoprotein receptor 1, and ρ-kinase pathways.7
In addition to a well-defined ability to prevent the binding of aldosterone to the mineralocorticoid receptor, spironolactone and its major metabolite, canrenone,8,9 also interact with the plasmalemmal sodium-potassium-ATPase (Na/K-ATPase).10 Both have been shown to competitively inhibit ouabain and digoxin binding, and the latter has even been used clinically with the aim of combating digitalis toxicity.11 Depending on the experimental conditions, spironolactone or canrenone has been characterized as either a pure competitive antagonist of ouabain or a “partial inhibitor” of Na/K-ATPase enzymatic (ion pumping) function. We have observed previously that spironolactone functions as an inotrope in vitro.12 Moreover, this occurs at pharmacologically relevant concentrations of spironolactone and, in isolated cardiac myocytes, spironolactone stimulation causes very similar changes in calcium cycling to that seen with digoxin or ouabain.13
On this background, our laboratory has observed that the cardiotonic steroid marinobufagenin (MBG) is responsible for many of the clinical features of experimental uremic cardiomyopathy, including cardiac fibrosis.14–16 Interestingly, although the model of uremic cardiomyopathy that we use, specifically the fifth/sixth nephrectomy, is associated with marked increases in circulating aldosterone concentrations, antagonism by active immunization with an MBG-albumin conjugate, a process that produces a rather specific antibody response against MBG with negligible cross-reaction to aldosterone, ameliorates virtually all of the cardiac fibrosis.15 We have also observed that cardiotonic steroids directly stimulate cardiac fibroblasts to produce increased amounts of collagen through the Na/K-ATPase signal cascade. On the basis of these observations, we speculated that spironolactone might antagonize the signaling of cardiotonic steroids and have an ameliorative effect in the partial nephrectomy model of uremic cardiomyopathy. To test this hypothesis, the following studies were performed.
Methods
Animals
Male Sprague-Dawley rats were used for all of the studies. All of the animal experimentation described in the article was conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals using protocols approved by the University of Toledo, Medical Health Campus, Institutional Animal Use and Care Committee.
Experimental Groups
Rats weighing between 250 and 300 g were divided into 6 groups with 6 to 12 rats surviving surgery in each group. The first group was subjected to sham surgery, whereas the second group was subjected to fifth/sixth partial nephrectomy (PNx), as described previously.15 In the third group, sham surgery was performed, and a minipump (model 2004, Alzet) infusing spironolactone (Sigma-Aldrich) at 20 mg/kg per day was inserted SC through a flank incision.17 The fourth group was subjected to PNx with a minipump infusing spironolactone at 20 mg/kg per day. The fifth group was subjected to sham surgery with a minipump infusing MBG at 10 μg/kg per day, whereas the sixth group was subjected to sham surgery with minipumps infusing MBG at 10 μg/kg per day and spironolactone at 20 mg/kg per day.
Blood Pressure, Cardiac Physiological, and Other In Vivo Measurements
Conscious blood pressure (BP) was measured by the tail-cuff method. Some anesthetized rats were instrumented with a Millar 2.0F catheter placed into the carotid artery for measurement of left ventricular pressure and volume before euthanasia. MBG, aldosterone, and creatinine were also measured on blood drawn before euthanasia. All of these methods have been described previously.16,18
Isolation of Cardiac Fibroblasts
Isolation of cardiac fibroblasts was carried out as described previously by Brilla et al19 with modifications as reported previously.16
Western Blot Analysis
Western Blot analysis was performed on proteins isolated from cell lysates or from tissue homogenates as reported previously.14–16,20
Histology
Trichrome staining was performed on left ventricular tissues and fibrosis quantified as we have reported previously.15
Collagen Synthesis
(3H)Proline incorporation in cardiac fibroblasts was performed as described previously.16,19
(3H)Ouabain Binding
Ouabain binding studies were performed as described previously.21
Statistical Analysis and Expanded Methods
Statistical analysis and details regarding methods are provided in an online Data Supplement (available at http://hyper.ahajournals.org).
Results
Spironolactone Attenuates the Development of Cardiomyopathy After Fifth/Sixth Nephrectomy
Spironolactone at doses of 20 mg/kg per day reduced systolic BP modestly but did not significantly alter cardiac physiological function or heart weight compared with control animals subjected to sham surgery (Table 1). Both MBG infusion (10 μg/kg per day) and PNx surgery resulted in substantial increases in BP, as well as the heart weight. Coadministration of spironolactone with either MBG or PNx lowered conscious BP, as well as completely attenuated the increases in heart weight. PNx resulted in similar plasma concentrations of MBG as MBG infusion alone, whereas substantial increases in plasma aldosterone concentrations were restricted to those animals subjected to PNx. The addition of spironolactone did not significantly affect either aldosterone or MBG concentrations. These data are summarized in Table 1.
Table 1.
Effects of Spironolactone on Physiological Measurements After PNx or Infusion of MBG
| Group | Sham (n=8) | S (n=8) | PNx (n=10) | PNx+S (n=6) | MBG (n=8) | MBG+S (n=6) |
|---|---|---|---|---|---|---|
| Systolic BP | ||||||
| SBP 0 wk, mm Hg | 119±3 | 108±4* | 112±5 | 115±3 | 118±3 | 114±2 |
| SBP 1 wk, mm Hg | 123±4 | 101±4* | 160±4† | 144±5*‡ | 151±5† | 138±1*∥ |
| SBP 2 wk, mm Hg | 125±3 | 112±3* | 185±6† | 157±4†§ | 165±8† | 129±4¶ |
| SBP 3 wk, mm Hg | 122±3 | 101±3* | 183±3† | 148±3†§ | 154±6† | 125±7¶ |
| SBP 4 wk, mm Hg | 125±1 | 99±3 | 169±2† | 130±3§ | 162±1† | 123±1¶ |
| Heart weight | ||||||
| BW, g | 469±12 | 460±9 | 460±18 | 462±11 | 503±16 | 502±14 |
| HW, g | 1.32±0.03 | 1.30±0.2 | 1.54±0.06† | 1.30±0.03§ | 1.68±0.06† | 1.36±0.05¶ |
| HW/BW, ×103 | 2.95±0.05 | 2.82±0.09 | 3.46±0.08* | 2.83±0.07§ | 3.40±0.09* | 2.70±0.09¶ |
| Plasma measurements | ||||||
| Creatinine, mg/dL | 0.39±0.02 | 0.38±0.03 | 0.60±0.07* | 0.63±0.05* | 0.41±0.03 | 0.38±0.02 |
| Potassium, mEq/L | 5.8±0.3 | 5.6±0.4 | 6.3±0.3 | 6.6±0.4 | 6.2±0.3 | 6.1±0.3 |
| MBG, pmol/L | 371±43 | 321±86 | 903±135† | 994±154† | 887±140† | 762±98† |
| Aldosterone, pg/mL | 280±33 | 249±55 | 1890±277† | 1750±292† | 245±20 | 261±45 |
S indicates spironolactone; SBP, systolic BP measured in conscious animals with a tail cuff on weekly intervals after surgery; BW, body weight; HW, heart weight determined at the time of euthanasia.
P<0.05 vs sham.
P<0.01 vs sham.
P<0.05 vs PNx.
P<0.01 vs PNx.
P<0.05 vs MBG.
P<0.01 vs MBG.
In addition, we examined cardiac function using a pressure- and volume-sensing catheter placed into the left ventricle under anesthesia, as reported previously.16,18 We observed that PNx and MBG infusion both induced diastolic dysfunction, as assessed by increase in the time constant for isovolumic ventricular relaxation (τ, significantly increased only in the PNx group), as well as the slope of the pressure-volume relationship at end diastole assessed during inferior vena cava constriction. Additional evidence for this diastolic dysfunction could be inferred from the decreases in end diastolic volume seen in the PNx and MBG groups. These changes were significantly attenuated by concomitant spironolactone therapy in both PNx- and MBG-treated animals (Table 2).
Table 2.
Effects of Spironolactone on Left Ventricular Functional Measurements After PNx or Infusion of MBG
| Group | Sham (n=12) | S (n=10) | PNx (n=12) | PNx+S (n=8) | MBG (n=10) | MBG+S (n=8) |
|---|---|---|---|---|---|---|
| EDV, μL | 193±15 | 197±22 | 129±9† | 200±15§ | 147±11* | 207±181¶ |
| ESV, μL | 95±9 | 97±5 | 39±7† | 85±10§ | 57±6† | 99±12∥ |
| SV, μL | 115±8 | 120±16 | 106±10 | 109±9 | 98±9 | 108±11 |
| EF, % | 57±2 | 55±3 | 76±3† | 54±8†§ | 64±3* | 60±4 |
| Tau, ms | 11.9±0.7 | 10.6±0.9 | 17.6±2.0* | 13.3±1.2‡ | 12.8±0.9 | 9.9±1.4 |
| EDPVR, mm Hg/μL | 0.031±0.004 | 0.031±0.001 | 0.046±0.006* | 0.027±0.005‡ | 0.042±0.004* | 0.031±0.005∥ |
S indicates spironolactone; EDV, end-diastolic volume; ESR, end-systolic volume; SV, stroke volume; EF, ejection fraction; Tau, time constant for isovolumic relaxation; EDPVR, slope of the regression line fit to the end-diastolic pressure and volume data generated by inferior vena caval constriction.
P<0.05 vs sham.
P<0.01 vs sham.
P<0.05 vs PNx.
P<0.01 vs PNx.
P<0.05 vs MBG.
P<0.01 vs MBG.
Spironolactone Attenuates Cardiac Fibrosis
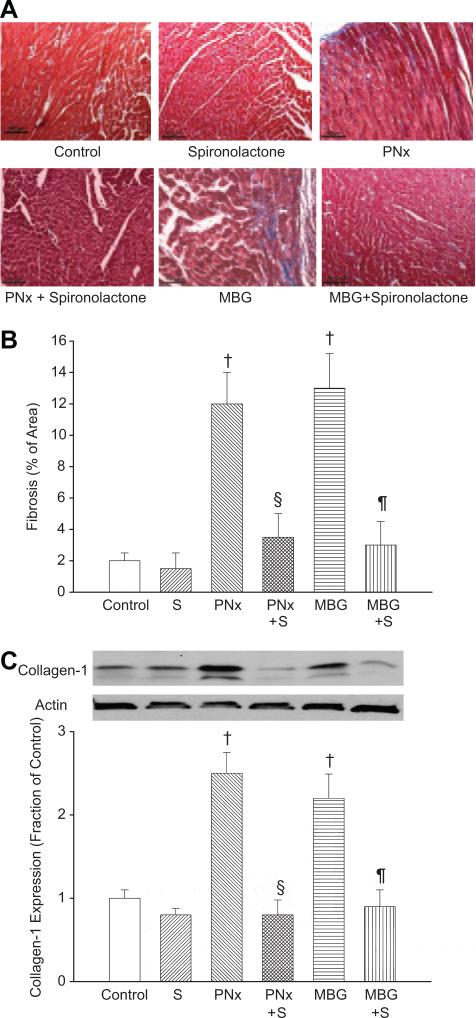
We assessed this fibrosis with both histological analysis (trichrome staining, Figure 1A and 1B), as well as collagen 1 content determined by Western blot (Figure 1C). Both PNx and MBG infusion caused dramatic increases in cardiac scarring and collagen content. Coadministration of spironolactone essentially prevented the cardiac fibrosis (Figure 1A through 1C).
Figure 1.
A, Representative trichrome-stained photomicrographs obtained from cardiac tissue derived from the different experimental groups. B, Amount of fibrosis expressed as the mean±SEM measured using computer-assisted morphometry, as we have described previously.15 C, Representative (top) and quantitative analysis of collagen 1 (mean±SEM) Western blots performed on cardiac tissues from the different groups. Actin was used to control loading. Control refers to animals subjected to sham surgery (n=8); S refers to spironolactone infusion using minipumps (n=8); PNx refers to partial nephrectomy (n=10); and MBG refers to MBG infusions using minipumps (n=8). PNx S (n=6) and MBG+S (n=6) refer to combining those 2 maneuvers. †P<0.01 vs control, §P<0.01 vs PNx, ¶P<0.01 vs MBG.
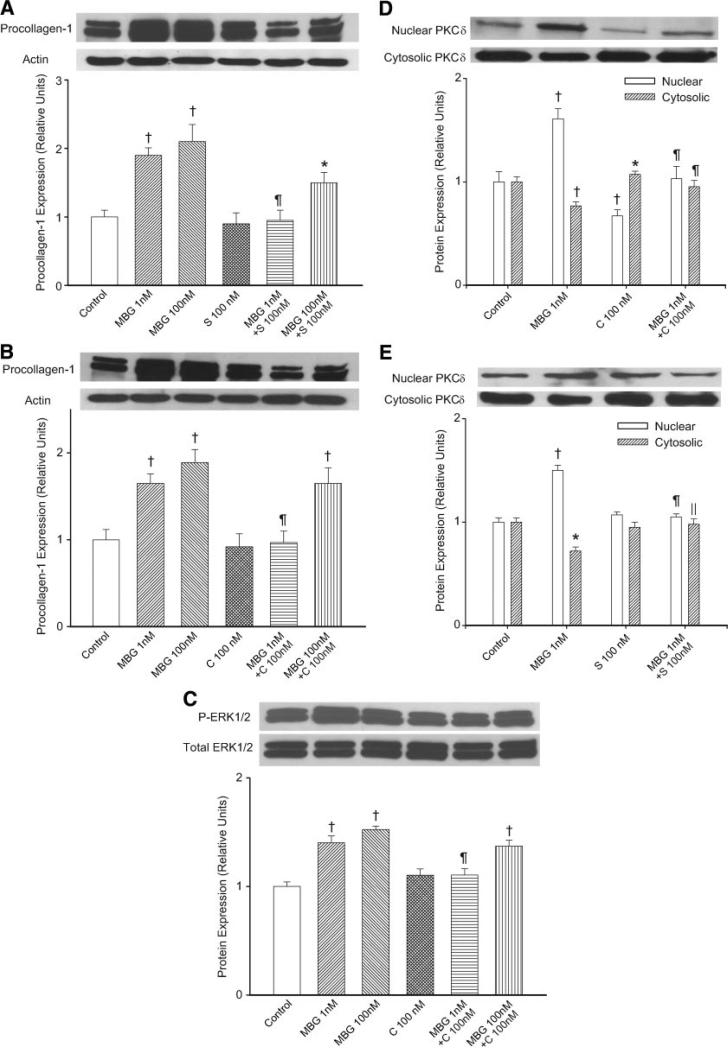
Spironolactone and Canrenone Attenuate Procollagen Expression and Radiolabeled Proline Incorporation Induced by MBG in Primary Culture of Cardiac Fibroblasts
Using cultured cardiac fibroblasts, MBG increased procollagen 1 expression determined with Western blot (Figure 2) and collagen production determined by radiolabeled proline incorporation into the cellular matrix (Table 3). MBG also increased proline incorporation into the supernatant to a similar degree as before (data not shown). Aldosterone applied at concentrations ranging from 1 to 100 nM did not significantly affect radiolabeled proline incorporation (Table 3) or procollagen expression determined by Western blot (data not shown). Both spironolactone (100 nM and 1 μmol/L) and canrenone (100 nM and 1 μmol/L) attenuated proline incorporation at baseline and blocked MBG stimulation of collagen production (Table 3). This was also confirmed with Western blot (Figure 2A and 2B). Interestingly, increasing the amount of MBG to 100 nM could overwhelm the inhibition of spironolactone (100 nM) or canrenone (100 nM), as demonstrated in Figure 2A and 2B.
Figure 2.
A, Representative Western blot against procollagen 1 derived from cardiac fibroblasts treated with MBG (1 or 100 nM), spironolactone (100 nM), or a combination with the corresponding quantitative data shown as the mean±SEM of 6 experiments shown below. B, Representative Western blot against procollagen 1 derived from cardiac fibroblasts treated with MBG (1 or 100 nM), canrenone (100 nM), or a combination with the corresponding quantitative data shown as the mean±SEM of 6 experiments shown below. C, Representative phosphorylated ERK1/2 (P-ERK1/2) and total ERK 1/2 (Total ERK1/2) blots as well as the quantitative ratio of P-ERK1/2/Total ERK1/2 (mean±SEM; n=9 each group) in cells treated similarly to B but harvested at 2 hours. D, The effects of MBG (1 nM), canrenone (100 nM), or a combination of the 2 treatments (15 minutes exposure) on PKC-δ expression in the cytosol and nucleus. E, The effects of MBG (1 nM), spironolactone (100 nM), or a combination of the 2 treatments (15-minute exposure) on PKC-δ expression in the cytosol and nucleus. For both D and F, representative Western blots are shown in the top portion of the panel with quantitative data (mean±SEM of 5 determinations) shown below. *P<0.05 and †P<0.01 vs control; ∥P<0.05; ¶P<0.01 vs MBG.
Table 3.
Effect of MBG, Spironolactone, and Canrenone on Radiolabeled Proline Incorporation
| Group (N) | Proline Incorporation (Fraction of Control Values) |
|---|---|
| Control (64) | 1.00±0.02 |
| MBG, 1 nmol/L (20) | 1.58±0.06* |
| MBG, 10 nmol/L (36) | 1.67±0.04* |
| MBG, 100 nmol/L (8) | 1.64±0.10* |
| Aldosterone, 1 nmol/L (24) | 1.04±0.03 |
| Aldosterone, 10 nmol/L (10) | 0.93±0.08 |
| Aldosterone, 100 nmol/L (24) | 0.97±0.04 |
| Spironolactone, 100 nmol/L (20) | 0.85±0.03* |
| Spironolactone, 1 μmol/L (32) | 0.80±0.05* |
| Canrenone, 100 nmol/L (12) | 0.82±0.04* |
| Canrenone, 1 μmol/L (12) | 0.66±0.05* |
| MBG, 1 nmol/L+spironolactone, 100 nmol/L (20) | 0.98±0.04† |
| MBG, 1 nmol/L+spironolactone, 1 μmol/L (20) | 0.80±0.03*† |
| MBG, 1 nmol/L+canrenone, 100 nmol/L (12) | 0.76±0.06*† |
| MBG, 1 nmol/L+canrenone, 1 μmol/L (12) | 0.65±0.07*† |
| MBG, 100 nmol/L+spironolactone, 100 nmol/L (8) | 1.65±0.05* |
| MBG, 100 nmol/L+canrenone, 100 nmol/L (8) | 1.72±0.06* |
P<0.01 vs control.
P<0.01 vs MBG, 1 nmol/L.
To examine the effect of canrenone on actual MBG signaling through the Na/K-ATPase, we first examined activation of ERK1/2 (also called p42-44 mitogen activated protein kinase). We found that MBG treatment stimulated increases in phosphorylated ERK1/2; the increases seen with 1 nM MBG could be prevented by coincubation with canrenone at 100 nM, but 100 nM MBG overwhelmed most of the inhibition by this dose (100 nM) of canrenone (Figure 2C). We further examined MBG signaling by examining the effects of MBG with and without canrenone on the translocation of protein kinase C (PKC)-δ, because we have demonstrated previously that MBG induces the translocation of PKC-δ to the nucleus and that this translocation appears necessary for increases in collagen synthesis.22 MBG (1 nM) led to a substantial decrease in cytosolic but an increase in nuclear PKC-δ.22 Canrenone (100 nM) alone appeared to actually increase the amount of PKC-δ in the cytosol and decrease PKC-δ in the nucleus, whereas the coincubation of cells with MBG (1 nM) and canrenone (100 nM) did not substantially change the distribution of PKC-δ compared with controls (Figure 2D).
Spironolactone and Canrenone Inhibit Ouabain Binding
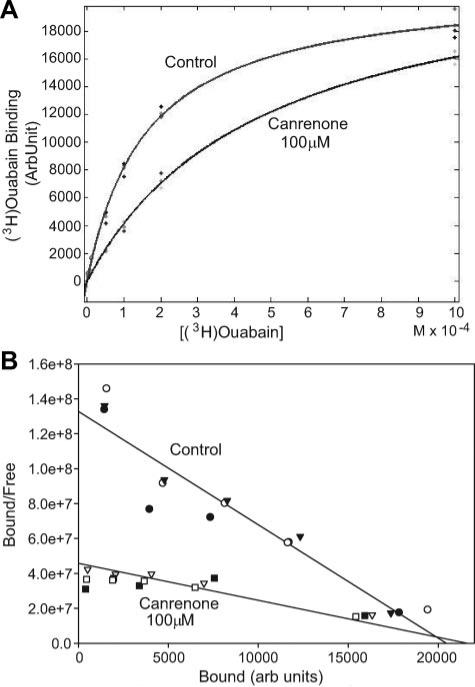
To further examine the interactions among spironolactone, canrenone, and cardiotonic steroids, we performed experiments in LLC-PK1 cells, as well as with the Na/K-ATPase isolated from a porcine kidney. The reason that we moved to porcine samples was because the α1 isoform from the pig binds ouabain quite tightly, allowing for easy measurement of ouabain binding. Because no radiolabeled form of MBG was available to us, we purchased (3H)ouabain and performed binding studies as described in the Methods section. We found that both canrenone and spironolactone (at high concentrations, eg, 50 and 100 μmol/L) significantly shifted binding of ouabain to LLC-PK1 cells (representative data in Figure 3A; quantitative curve fitting data shown in Table S1, please see the online Data Supplement at http://hyper.ahajournals.org). On the basis of analysis of these data, both canrenone and spironolactone increased the apparent dissociation constants for ouabain without significantly affecting the Bmax (Table S1). This was confirmed for canrenone using the purified Na/K-ATPase isolated from porcine kidney or heart tissues (Table S1). A Scatchard plot of these data also demonstrates that the slope of the lines fit to data were shifted dramatically by the addition of canrenone, whereas the intercept with the x axis was not significantly affected (Figure 3B), further illustrating the competitive nature of the interaction between canrenone and cardiotonic steroids.
Figure 3.
A, (3H)ouabain binding data expressed as a function of (3H)ouabain concentration either without (control) or with canrenone at 100 μmol/L. Curves shown are fit to the average of 3 separate sets of experiments in the control and canrenone groups. The R2 value for both curve fits exceeded 0.97. B, The same experimental data transformed into bound/free vs bound (Scatchard plot) with regression lines fit to both the control and canrenone data. Note that the slopes of the lines (reflecting the reciprocal of the dissociation constant values) are quite different, whereas the intersections of these lines with the x axis (reflecting the reciprocal of the Bmax values) occur at essentially the same point. Quantifications of the dissociation constant and Bmax data were performed using nonlinear regression methods (MATLAB) on data represented in A; these data are presented in the online Data Supplement (Table S1).
Discussion
Cardiac fibrosis complicates a number of clinical cardiomyopathies, including those seen with renal failure.23–26 Spironolactone improves outcomes in patients with dilated cardiomyopathy27,28 and attenuates fibrosis in several experimental cardiomyopathies.17,29–32 It also appears that spironolactone or canrenone may ameliorate renal injury33–35 and potentially attenuate cardiac hypertrophy in experimental renal failure.29
The beneficial effects of spironolactone have been attributed to antagonism of the profibrotic effects of aldosterone in early studies, because spironolactone and its major metabolite, canrenone, are known to compete with aldosterone at the level of the mineralocorticoid receptor.36 That said, it should be stressed that much of the data supporting a profibrotic effect of aldosterone are represented by the beneficial effects of spironolactone or other mineralocorticoid antagonists.29,31,32,37 In vivo, the profibrotic effects of aldosterone appear to be confined to conditions where extracellular volume is expanded.30,38 When studied in vitro using cardiac fibroblasts, a stimulation of collagen production has been noted by some authors,19 but many workers report having observed no stimulation,39–41 similar to that which we observed in this report. This complex area has been the topic of a number of recent reviews.42–44
In the current study, we observed that spironolactone therapy markedly attenuated the cardiomyopathy induced by either experimental renal failure induced by partial nephrectomy or by administration of MBG in a manner designed to mimic the increases in the circulating levels of this hormone observed after partial nephrectomy.15,16 Plasma MBG concentrations have been reported in normal subjects typically within the 300 to 400 pM range, depending on diet.45 The concentrations seen in vivo in experimental animals that we observed in this study (900 pM) were slightly higher than that which we have reported previously,15,16 but the ratio of experimental to sham values was similar. The cardiomyopathy is characterized by diastolic dysfunction and cardiac hypertrophy. The diastolic dysfunction, in particular, is evidenced by the decreases in end-diastolic and end-systolic volumes, as well as increases in τ values and the pressure-volume relationship at end diastole assessed during inferior vena cava constriction, representing impairment of both active and passive relaxation. We note that the magnitude of the diastolic dysfunction did appear to be somewhat more severe in the PNx animals as compared with the MBG infusion group, suggesting that other neurohumoral changes resulting from impaired renal function (eg, increases in parathyroid hormone and abnormalities in vitamin D metabolism) may contribute to the cardiomyopathy induced by PNx.26,46 Spironolactone therapy dramatically attenuated these hemodynamic abnormalities, as well as the cardiac growth seen in both the PNx- and MBG-infused groups. We had reported previously that, whereas the creation of partial nephrectomy resulted in marked increases in aldosterone, administration of MBG did not cause significant increases in the circulating concentrations of this hormone.15,16 These findings were confirmed in the current study, and it did not appear that administration of spironolactone substantially altered either the plasma concentrations of MBG or aldosterone. Both PNx and MBG resulted in substantial increases in cardiac collagen content and fibrosis, as we have reported previously15,16; spironolactone administration also dramatically attenuated these changes, which have been linked to the passive component of the diastolic dysfunction. Although the administration of spironolactone also attenuated the hypertension seen with both PNx and MBG, other work that we have presented suggests that at least some of the cardiac fibrosis caused by PNx appears to be independent of BP increases.14,15,18 Recently, Michea et al47 studied the effects of spironolactone on the cardiac alterations induced by experimental renal failure. These workers also noted that spironolactone attenuated cardiac growth, as well as oxidant stress, induced by PNx.
Moving to the in vitro setting, we observed that MBG at concentrations similar to those seen in our experimental models of cardiac fibrosis induced increases in collagen production by rat cardiac fibroblasts as assessed by proline incorporation and procollagen 1 expression determined by Western blot, as we have reported previously16; both spironolactone and its major metabolite, canrenone, prevented these changes. Canrenone also appeared to prevent the signaling of MBG through the Na/K-ATPase, as assessed by ERK1/2 activation and PKC-δ translocation. When we increased MBG to much higher concentrations, the effects of both spironolactone and canrenone on collagen synthesis and ERK1/2 activation could be overwhelmed. Finally, we performed binding studies using (3H)ouabain in LLC-PK1 cells, as well as the Na/K-ATPase isolated from porcine kidneys. (3H)ouabain was used rather than MBG because of its easy availability, and cells or enzymes obtained from pig were used because of their high affinity for ouabain. We observed that ouabain binding was inhibited by both spironolactone and canrenone in what appeared to be a competitive manner. In aggregate, our data suggest that spironolactone and its major metabolite, canrenone, appear to competitively inhibit MBG signaling and stimulation of collagen production in vitro, a phenomenon that may explain the beneficial effects of spironolactone in vivo.
The idea that canrenone (or spironolactone) may function as a competitive inhibitor for cardiotonic steroids is not a novel concept. Selye et al48 found that spironolactone therapy could dramatically attenuate experimental digitoxin toxicity in the rat. Erdmann et al49 demonstrated that canrenone could ameliorate digitalis-induced cardiac arrhythmias in some patients, as well as displace labeled strophanthin from red cell-binding sites. Finotti and Palatini11 suggested that that canrenone interacted with isolated Na/K-ATPase at the same site. Garay et al50 demonstrated that canrenone attenuated digitalis-induced inhibition of the NaK-ATPase in human red blood cells, whereas Balzan et al10 made similar observations in human red blood cells and placenta. Sorrentino et al51 observed that canrenone could antagonize the vasoconstrictor effects of ouabain. In addition to the aforementioned data suggesting that spironolactone and canrenone can act as classical competitive inhibitors, however, there are also data suggesting that both spironolactone and canrenone may influence signaling through Na/K-ATPase through alteration of its lipid environment.52–54
Perspectives
Our study demonstrates that administration of spironolactone attenuates cardiac fibrosis in both partial nephrectomy and MBG infusion models. Both spironolactone and canrenone appear to competitively antagonize cardiotonic steroid-induced fibroblast stimulation. On the basis of these findings in concert with existing literature, we would propose that MBG may be responsible for some of the cardiac injury that has been attributed to aldosterone and that spironolactone, canrenone, and other so-called mineralocorticoid antagonists may actually produce some of their beneficial effects by antagonism of cardiotonic steroid hormone signaling through the plasmalemmal Na/K-ATPase. If these data are confirmed in humans, this may open up new therapeutic indications for spironolactone, as well as allow for the design of other agents that specifically target cardiotonic steroid–induced processes.
Supplementary Material
Acknowledgments
We thank Carol Woods for her excellent secretarial assistance.
Sources of Funding
Portions of this work were supported by grants from the National Institutes of Health (HL-67963, HL-36573, HL-071556, and GM-78565), as well as intramural support from the National Institute on Aging Laboratory of Cardiovascular Science.
Footnotes
Disclosures
None.
References
- 1.Campbell SE, Janicki JS, Weber KT. Temporal differences in fibroblast proliferation and phenotype expression in response to chronic administration of angiotensin II or aldosterone. J Mol Cell Cardiol. 1995;27:1545–1560. doi: 10.1016/s0022-2828(95)90359-3. [DOI] [PubMed] [Google Scholar]
- 2.Fanestil DD. Mode of spirolactone action: competitive inhibition of aldosterone binding to kidney mineralocorticoid receptors. Biochem Pharmacol. 1968;17:2240–2242. doi: 10.1016/0006-2952(68)90203-7. [DOI] [PubMed] [Google Scholar]
- 3.Funder JW. Steroids, hypertension and cardiac fibrosis. Blood Press. 1995;2(suppl):39–42. [PubMed] [Google Scholar]
- 4.Edwards NC, Steeds RP, Stewart PM, Ferro CJ, Townend JN. Effect of spironolactone on left ventricular mass and aortic stiffness in early-stage chronic kidney disease: a randomized controlled trial. J Am Coll Cardiol. 2009;54:505–512. doi: 10.1016/j.jacc.2009.03.066. [DOI] [PubMed] [Google Scholar]
- 5.Nehme JA, Lacolley P, Labat C, Challande P, Robidel E, Perret C, Leenhardt A, Safar ME, Delcayre C, Milliez P. Spironolactone improves carotid artery fibrosis and distensibility in rat post-ischaemic heart failure. J Mol Cell Cardiol. 2005;39:511–519. doi: 10.1016/j.yjmcc.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 6.Taniguchi I, Kawai M, Date T, Yoshida S, Seki S, Taniguchi M, Shimizu M, Mochizuki S. Effects of spironolactone during an angiotensin II receptor blocker treatment on the left ventricular mass reduction in hypertensive patients with concentric left ventricular hypertrophy. Circ J. 2006;70:995–1000. doi: 10.1253/circj.70.995. [DOI] [PubMed] [Google Scholar]
- 7.Nakano S, Kobayashi N, Yoshida K, Ohno T, Matsuoka H. Cardioprotective mechanisms of spironolactone associated with the angiotensin-converting enzyme/epidermal growth factor receptor/extracellular signal-regulated kinases, NAD(P)H oxidase/lectin-like oxidized low-density lipoprotein receptor-1, and Rho-kinase pathways in aldosterone/salt-induced hypertensive rats. Hypertens Res. 2005;28:925–936. doi: 10.1291/hypres.28.925. [DOI] [PubMed] [Google Scholar]
- 8.Sadee W, Abshagen U, Finn C, Rietbrock N. Conversion of spironolactone to canrenone and disposition kinetics of spironolactone and canrenoate-potassium in rats. Naunyn Schmiedebergs Arch Pharmacol. 1974;283:303–318. doi: 10.1007/BF00499190. [DOI] [PubMed] [Google Scholar]
- 9.Karim A, Kook C, Zitzewitz DJ, Zagarella J, Doherty M, Campion J. Species differences in the metabolism and disposition of spironolactone. Drug Metab Dispos. 1976;4:547–555. [PubMed] [Google Scholar]
- 10.Balzan S, Nicolini G, Bellitto L, Ghione S, Biver P, Montali U. Effect of canrenone on the digitalis site of Na+/K(+)-ATPase in human placental membranes and in erythrocytes. J Cardiovasc Pharmacol. 2003;42:32–36. doi: 10.1097/00005344-200307000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Finotti P, Palatini P. Canrenone as a partial agonist at the digitalis receptor site of sodium-potassium-activated adenosine triphosphatase. J Pharmacol Exp Ther. 1981;217:784–790. [PubMed] [Google Scholar]
- 12.Barbato JC, Mulrow PJ, Shapiro JI, Franco-Saenz R. Rapid effects of aldosterone and spironolactone in the isolated working rat heart. Hypertension. 2002;40:130–135. doi: 10.1161/01.hyp.0000025879.29822.24. [DOI] [PubMed] [Google Scholar]
- 13.Barbato JC, Rashid S, Mulrow PJ, Shapiro JI, Franco-Saenz R. Mechanisms for aldosterone and spironolactone-induced positive inotropic actions in the rat heart. Hypertension. 2004;44:751–757. doi: 10.1161/01.HYP.0000144466.11568.7e. [DOI] [PubMed] [Google Scholar]
- 14.Kennedy D, Omran E, Periyasamy SM, Nadoor J, Priyadarshi A, Willey JC, Malhotra D, Xie Z, Shapiro JI. Effect of chronic renal failure on cardiac contractile function, calcium cycling, and gene expression of proteins important for calcium homeostasis in the rat. J Am Soc Nephrol. 2003;14:90–97. doi: 10.1097/01.asn.0000037403.95126.03. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy DJ, Vetteth S, Periyasamy SM, Kanj M, Fedorova L, Khouri S, Kahaleh MB, Xie Z, Malhotra D, Kolodkin NI, Lakatta EG, Fedorova OV, Bagrov AY, Shapiro JI. Central role for the cardiotonic steroid marinobufagenin in the pathogenesis of experimental uremic cardiomyopathy. Hypertension. 2006;47:488–495. doi: 10.1161/01.HYP.0000202594.82271.92. [DOI] [PubMed] [Google Scholar]
- 16.Elkareh J, Kennedy DJ, Yashaswi B, Vetteth S, Shidyak A, Kim EG, Smaili S, Periyasamy SM, Hariri IM, Fedorova L, Liu J, Wu L, Kahaleh MB, Xie Z, Malhotra D, Fedorova OV, Kashkin VA, Bagrov AY, Shapiro JI. Marinobufagenin stimulates fibroblast collagen production and causes fibrosis in experimental uremic cardiomyopathy. Hypertension. 2007;49:215–224. doi: 10.1161/01.HYP.0000252409.36927.05. [DOI] [PubMed] [Google Scholar]
- 17.Feria I, Pichardo I, Juarez P, Ramirez V, Gonzalez MA, Uribe N, Garcia-Torres R, Lopez-Casillas F, Gamba G, Bobadilla NA. Therapeutic benefit of spironolactone in experimental chronic cyclosporine A nephrotoxicity. Kidney Int. 2003;63:43–52. doi: 10.1046/j.1523-1755.2003.00707.x. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy DJ, Elkareh J, Shidyak A, Shapiro AP, Smaili S, Mutgi K, Gupta S, Tian J, Morgan E, Khouri S, Cooper CJ, Periyasamy SM, Xie Z, Malhotra D, Fedorova OV, Bagrov AY, Shapiro JI. Partial nephrectomy as a model for uremic cardiomyopathy in the mouse. Am J Physiol Renal Physiol. 2008;294:F450–F454. doi: 10.1152/ajprenal.00472.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brilla CG, Zhou G, Matsubara L, Weber KT. Collagen metabolism in cultured adult rat cardiac fibroblasts: response to angiotensin II and aldosterone. J Mol Cell Cardiol. 1994;26:809–820. doi: 10.1006/jmcc.1994.1098. [DOI] [PubMed] [Google Scholar]
- 20.Periyasamy SM, Liu J, Tanta F, Kabak B, Wakefield B, Malhotra D, Kennedy DJ, Nadoor A, Fedorova OV, Gunning W, Xie Z, Bagrov AY, Shapiro JI. Salt loading induces redistribution of the plasmalemmal Na/K-ATPase in proximal tubule cells. Kidney Int. 2005;67:1868–1877. doi: 10.1111/j.1523-1755.2005.00285.x. [DOI] [PubMed] [Google Scholar]
- 21.Liang M, Tian J, Liu L, Pierre S, Liu J, Shapiro J, Xie ZJ. Identification of a pool of non-pumping Na/K-ATPase. J Biol Chem. 2007;282:10585–10593. doi: 10.1074/jbc.M609181200. [DOI] [PubMed] [Google Scholar]
- 22.Elkareh J, Periyasamy SM, Shidyak A, Vetteth S, Schroeder J, Raju V, Hariri IM, El-Okdi N, Gupta S, Fedorova L, Liu J, Fedorova OV, Kahaleh MB, Xie Z, Malhotra D, Watson DK, Bagrov AY, Shapiro JI. Marinobufagenin induces increases in procollagen expression in a process involving protein kinase C and Fli-1: implications for uremic cardiomyopathy. Am J Physiol Renal Physiol. 2009;296:F1219–F1226. doi: 10.1152/ajprenal.90710.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.London GM, Parfrey PS. Cardiac disease in chronic uremia: pathogenesis. Adv Ren Replace Ther. 1997;4:194–211. doi: 10.1016/s1073-4449(97)70029-3. [DOI] [PubMed] [Google Scholar]
- 24.Burlew BS, Weber KT. Cardiac fibrosis as a cause of diastolic dysfunction. Herz. 2002;27:92–98. doi: 10.1007/s00059-002-2354-y. [DOI] [PubMed] [Google Scholar]
- 25.Vohringer M, Mahrholdt H, Yilmaz A, Sechtem U. Significance of late gadolinium enhancement in cardiovascular magnetic resonance imaging (CMR). Herz. 2007;32:129–137. doi: 10.1007/s00059-007-2972-5. [DOI] [PubMed] [Google Scholar]
- 26.Gross ML, Ritz E. Hypertrophy and fibrosis in the cardiomyopathy of uremia–beyond coronary heart disease. Semin Dial. 2008;21:308–318. doi: 10.1111/j.1525-139X.2008.00454.x. [DOI] [PubMed] [Google Scholar]
- 27.Effectiveness of spironolactone added to an angiotensin-converting enzyme inhibitor and a loop diuretic for severe chronic congestive heart failure (the Randomized Aldactone Evaluation Study [RALES]). Am J Cardiol. 1996;78:902–907. doi: 10.1016/s0002-9149(96)00465-1. [DOI] [PubMed] [Google Scholar]
- 28.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure: Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 29.Greene EL, Kren S, Hostetter TH. Role of aldosterone in the remnant kidney model in the rat. J Clin Invest. 1996;98:1063–1068. doi: 10.1172/JCI118867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Funder JW. Aldosterone, salt and cardiac fibrosis. Clin Exp Hypertens. 1997;19:885–899. doi: 10.3109/10641969709083193. [DOI] [PubMed] [Google Scholar]
- 31.Okada T, Nagai M, Taniguchi I, Kuno M, Imamoto S, Seki S, Taniguchi M, Mochizuki S. Combined treatment with valsartan and spironolactone prevents cardiovascular remodeling in renovascular hypertensive rats. Int Heart J. 2006;47:783–793. doi: 10.1536/ihj.47.783. [DOI] [PubMed] [Google Scholar]
- 32.Susic D, Varagic J, Ahn J, Matavelli L, Frohlich ED. Long-term mineralocorticoid receptor blockade reduces fibrosis and improves cardiac performance and coronary hemodynamics in elderly SHR. Am J Physiol Heart Circ Physiol. 2007;292:H175–H179. doi: 10.1152/ajpheart.00660.2006. [DOI] [PubMed] [Google Scholar]
- 33.Sato A, Hayashi K, Saruta T. Antiproteinuric effects of mineralocorticoid receptor blockade in patients with chronic renal disease. Am J Hypertens. 2005;18:44–49. doi: 10.1016/j.amjhyper.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 34.Epstein M. Aldosterone blockade: an emerging strategy for abrogating progressive renal disease. Am J Med. 2006;119:912–919. doi: 10.1016/j.amjmed.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 35.Nagase M, Yoshida S, Shibata S, Nagase T, Gotoda T, Ando K, Fujita T. Enhanced aldosterone signaling in the early nephropathy of rats with metabolic syndrome: possible contribution of fat-derived factors. J Am Soc Nephrol. 2006;17:3438–3446. doi: 10.1681/ASN.2006080944. [DOI] [PubMed] [Google Scholar]
- 36.Brilla CG. Aldosterone and myocardial fibrosis in heart failure. Herz. 2000;25:299–306. doi: 10.1007/s000590050024. [DOI] [PubMed] [Google Scholar]
- 37.Zannad F, Radauceanu A. Effect of MR blockade on collagen formation and cardiovascular disease with a specific emphasis on heart failure. Heart Fail Rev. 2005;10:71–78. doi: 10.1007/s10741-005-2351-3. [DOI] [PubMed] [Google Scholar]
- 38.Sato A, Saruta T. Aldosterone-induced organ damage: plasma aldosterone level and inappropriate salt status. Hypertens Res. 2004;27:303–310. doi: 10.1291/hypres.27.303. [DOI] [PubMed] [Google Scholar]
- 39.Fullerton MJ, Funder JW. Aldosterone and cardiac fibrosis: in vitro studies. Cardiovasc Res. 1994;28:1863–1867. doi: 10.1093/cvr/28.12.1863. [DOI] [PubMed] [Google Scholar]
- 40.Kohler E, Bertschin S, Woodtli T, Resink T, Erne P. Does aldosterone-induced cardiac fibrosis involve direct effects on cardiac fibroblasts? J Vasc Res. 1996;33:315–326. doi: 10.1159/000159159. [DOI] [PubMed] [Google Scholar]
- 41.Lijnen P, Petrov V. Antagonism of the renin-angiotensin-aldosterone system and collagen metabolism in cardiac fibroblasts. Methods Find Exp Clin Pharmacol. 1999;21:215–227. doi: 10.1358/mf.1999.21.3.534832. [DOI] [PubMed] [Google Scholar]
- 42.Lijnen P, Petrov V. Induction of cardiac fibrosis by aldosterone. J Mol Cell Cardiol. 2000;32:865–879. doi: 10.1006/jmcc.2000.1129. [DOI] [PubMed] [Google Scholar]
- 43.Young MJ, Funder JW. Mineralocorticoid receptors and pathophysiological roles for aldosterone in the cardiovascular system. J Hypertens. 2002;20:1465–1468. doi: 10.1097/00004872-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Funder JW. Minireview: aldosterone and the cardiovascular system: genomic and nongenomic effects. Endocrinology. 2006;147:5564–5567. doi: 10.1210/en.2006-0826. [DOI] [PubMed] [Google Scholar]
- 45.Anderson DE, Fedorova OV, Morrell CH, Longo DL, Kashkin VA, Metzler JD, Bagrov AY, Lakatta EG. Endogenous sodium pump inhibitors and age-associated increases in salt sensitivity of blood pressure in normotensives. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1248–R1254. doi: 10.1152/ajpregu.00782.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rostand SG, Drueke TB. Parathyroid hormone, vitamin D, and cardiovascular disease in chronic renal failure. Kidney Int. 1999;56:383–392. doi: 10.1046/j.1523-1755.1999.00575.x. [DOI] [PubMed] [Google Scholar]
- 47.Michea L, Villagran A, Urzua A, Kuntsmann S, Venegas P, Carrasco L, Gonzalez M, Marusic ET. Mineralocorticoid receptor antagonism attenuates cardiac hypertrophy and prevents oxidative stress in uremic rats. Hypertension. 2008;52:295–300. doi: 10.1161/HYPERTENSIONAHA.107.109645. [DOI] [PubMed] [Google Scholar]
- 48.Selye H, Krajny M, Savoie L. Digitoxin poisoning: prevention by spironolactone. Science. 1969;164:842–843. doi: 10.1126/science.164.3881.842. [DOI] [PubMed] [Google Scholar]
- 49.Erdmann E, Krawietz W, Poppert D, Kruger R, von Arnim T, Vogt W, Bolte HD. Cardiac effects of antikaliuretic diuretics-clinical and biochemical investigation [in German]. Klin Wochenschr. 1977;55:985–994. doi: 10.1007/BF01488185. [DOI] [PubMed] [Google Scholar]
- 50.Garay RP, Diez J, Nazaret C, Dagher G, Abitbol JP. The interaction of canrenone with the Na+,K+ pump in human red blood cells. Naunyn Schmiedebergs Arch Pharmacol. 1985;329:311–315. doi: 10.1007/BF00501886. [DOI] [PubMed] [Google Scholar]
- 51.Sorrentino R, Cirino G, Calignano A, Mancuso F, Sorrentino L, Andriuoli G, Pinto A. Increase in the basal tone of guinea pig thoracic aorta induced by ouabain is inhibited by spironolactone canrenone and potassium canrenoate. J Cardiovasc Pharmacol. 1996;28:519–525. doi: 10.1097/00005344-199610000-00007. [DOI] [PubMed] [Google Scholar]
- 52.Miner PB, Jr, Sneller M, Crawford SS. Spironolactone- and canrenone-induced changes in hepatic (Na+,K+)ATPase activity, surface membrane cholesterol and phospholipid, and fluorescence polarization in the rat. Hepatology. 1983;3:481–488. doi: 10.1002/hep.1840030403. [DOI] [PubMed] [Google Scholar]
- 53.Wehling M, Kasmayr J, Theisen K. Rapid effects of mineralocorticoids on sodium-proton exchanger: genomic or nongenomic pathway? Am J Physiol. 1991;260:E719–E726. doi: 10.1152/ajpendo.1991.260.5.E719. [DOI] [PubMed] [Google Scholar]
- 54.Wehling M. Looking beyond the dogma of genomic steroid action: insights and facts of the 1990s. J Mol Med. 1995;73:439–447. doi: 10.1007/BF00202262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.