Abstract
It remains a challenge to synthesize functional materials that can develop advanced scaffolding architectures for tissue engineering. In this study, a series of biodegradable amphiphilic poly(hydroxyalkyl (meth)acrylate)-graft-poly(L-lactic acid) (PHAA-g-PLLA) copolymers have been synthesized and fabricated into nano-fibrous scaffolds. These copolymers can be further functionalized, are more hydrophilic, and have faster degradation rates than the PLLA homopolymer, which are advantageous for certain tissue engineering applications. First, PLLA-based macromonomers were prepared by using functional hydroxyalkyl (meth)acrylates (HAA) as initiators. The PHAA-g-PLLA copolymers were then synthesized using free radical copolymerization of PLLA-based macromonomers and HAA. Nano-fibrous architecture was created using a thermally induced phase separation technique from these functional PHAA-g-PLLA copolymers. The nano-fibrous structure mimics the architecture of natural collagen matrix at the nanometer scale. The effects of the macromonomer composition, copolymer composition, blending ratio, and solvent selection on nano-scale structures were studied. In general, the nano-fibrous structure was created when the amount of HAA in the macromonomer was low. By increasing the amount of HAA in the macromonomer, microspheres with nano-fibrous surfaces were obtained. Further increasing the amount of HAA led to the creation of microspheres with leaf-like surfaces. These PLLA-based materials had much faster degradation rates than the PLLA, and could be completely degraded from several weeks to a few months depending on their composition and molecular weight. Furthermore, the PHAA-g-PLLA copolymers possess functional hydroxyl groups, which can be used to couple with bioactive molecules to control cell-material interactions. Therefore, these biodegradable functional copolymers have the design flexibility to fabricate various biomimetic materials for tissue engineering applications.
Introduction
It remains a challenge to synthesize functional materials that can develop advanced scaffolding architectures for tissue engineering [1-4]. A good scaffolding material should mimic the critical features (including physical architecture and chemical composition) of the natural extracellular matrix (ECM) [5, 6]. In the body, the ECM is a natural web of nanoscale structures and serves an important role in the maintenance of cell and tissue structure and function [7-9]. Therefore, considerable efforts have been made to mimic the physical architecture of natural ECM at the nanoscale using synthetic biomaterials [10-13].
Poly(L-lactic acid) (PLLA) has been widely used as a scaffolding biomaterial because it is biodegradable and among the few synthetic polymers approved by the Food and Drug Administration (FDA) for certain human clinical applications [14-16]. PLLA has been fabricated into nano-fibrous scaffolds to mimic the physical architecture of natural collagen (a main component of ECM) [10]. The nano-fibrous PLLA scaffolds have been demonstrated to enhance cell adhesion and differentiation [17, 18]. However, there are limitations to use PLLA as a scaffolding material for certain tissue engineering applications. First, PLLA is a relatively hydrophobic material and its degradation rate is too slow for some applications [19-21]. To accelerate the degradation of PLLA, a variety of strategies have been developed, such as imparting hydrophilic segments to PLLA by the use of suitable functionality and copolymerization techniques [22, 23]. However, the copolymerization of L-lactide (LLA) with other hydrophilic monomers generates random copolymers and changes the physical properties, such as crystallinity and mechanical strength. The change in crystallinity would affect the formation of nano-fibrous structure [10]. Second, there are no functional groups available on a PLLA homopolymer, which limit its capacity to incorporate biologically active moieties onto the surface. The incorporation of reactive sites on PLLA chains that are amenable to modification with a variety of biologically active molecules would provide a host of opportunities to control cell-material interactions [5, 24]. While there have been a few reports of synthesizing functional PLLA-based biomaterials [25-27], it is unknown whether those materials can be fabricated into three-dimensional nano-fibrous scaffolds with the appropriate degradation rate.
In this study, we aim at designing and synthesizing a series of poly(L-lactic acid)-based (PLLA-based) polymeric biomaterials which can form nano-fibrous architecture and have faster degradation rates. These PLLA-based biomaterials also possess functional groups on the polymer chains, which can be further utilized to couple with bioactive molecules. First, functional hydroxyalkyl (meth)acrylates (HAA) were selected as initiators to synthesize PLLA-based macromonomers. Hydroxyl groups were then incorporated onto copolymer side chains by free radical polymerization of the macromonomers and HAA. These copolymers were fabricated into three-dimensional (3D) matrices, and the nano-fibrous architecture as well as their in vitro degradation was investigated.
Materials and method
(3s)-cis-3,6-dimethyl-1,4-dioxane-2,5-dione (L-lactide) was purchased from Sigma-Aldrich Inc. (St. Louis, MO) and was purified by recrystalization from toluene. 2-hydroxyethyl acrylate (HEA), 2-hydroxyethyl methacrylate (HEMA), hydroxypropyl methacrylate (HPMA), 4-hydroxybutyl acrylate (HBA), and stannous 2-ethylhexanoate (Sn(Oct)2) were purchased from Sigma-Aldrich Inc. (St. Louis, MO), and were distilled under reduced pressure prior to use. 1,4-Dioxane (99+%) was purchased from Aldrich Chemical (Milwaukee, WI) and was dehydrated with calcium chloride (St. Louis, MO). 2,2′-Azoisobutyronitrile (AIBN) was obtained from Sigma-Aldrich Inc. (St. Louis, MO) and was recrystallized from ethanol. PLLA homopolymer with an inherent viscosity of 1.6 dL/g was purchased from Boehringer Ingelheim (Ingelheim, Germany).
Synthesis of PLLA macromonomers
PLLA-based macromonomers with various lactide chain lengths were synthesized by the ring opening polymerization of L-lactide (LLA) using the corresponding hydroxyalkyl (meth)acrylate (HEA, HEMA, HPMA, or HBA) as the initiator. The synthesized PLLA-based macromonomers are abbreviated as HAA-PLLAX, where HAA is HEA, HEMA, HPMA, or HBA, and X is the molar percentage of HAA-to-LLA (X=5 means HAA/LLA=5%). A typical procedure to synthesize HEA-PLLA5 is as follows: LLA (40 mmol, 5.760 g), HEA (2 mmol, 0.232 g) and Sn(Oct)2 (0.4 mmol, 0.162 g) were mixed in a 50 mL round-bottom flask with stirring and nitrogen purging. The mixture was heated to 120°C under nitrogen protection for complete melting. The polymerization was carried out at 140°C for 2 h. The crude product was dissolved in 20 mL chloroform, precipitated in 100 mL cold methanol, and then vacuum dried.
Copolymerization of PLLA-based macromonomers with HAA
The PLLA-based macromonomers were used to prepare graft copolymers with various HAA monomers via free radical copolymerization. The synthesized copolymers are abbreviated as PHAAY-g-PLLAX, where HAA is HEA, HEMA, HPMA, or HBA, Y is the molar percentage of macromonomer-to-HAA used to synthesize copolymers, and X is the molar percentage of HAA-to-LLA used to synthesize the macromonomers (The feed ratio of HAA-PLLA/HAA=Y% for the free radical copolymerization, and the feed ratio of HAA/LLA=X% for the macromonomer synthesis). A typical procedure to synthesize PHEA10-g-PLLA10 is as follows: HEA-PLLA10 macromonomer (0.3 mmol, 0.771 g), with an average number molecular weight of 2570 (around 35 lactic acid repeating units), HEA (3 mmol, 0.348 g), and AIBN (0.06 mmol, 9.8 mg) were added into dioxane (5 mL) and were stirred until dissolved. The polymerization was carried out at 70°C for 24 h. After polymerization, the crude product was purified by repeated re-precipitations from chloroform to methanol for 3 times, and finally vacuum dried at 40°C for 48 h.
Fabrication of nano-fibrous matrices
Nano-fibrous PLLA-based matrices were fabricated by using the thermally induced phase separation technique [10]. Briefly, 1.0 mL polymer solution (10% (wt/v)) in THF was cast into a Teflon vial. The polymer solution was phase separated at −20°C for 4 h and then immersed into cyclohexane to exchange THF for 2 days. The resulting gel was freeze-dried for 4 days. The dried porous matrices were then stored in a desiccator until characterization.
NMR measurements
1H spectra of the macromonomers and copolymers were recorded with an Inova 400 NMR instrument operating at 400 MHz at room temperature using CDCl3 as the solvent. Molecular weight measurement: the molecular weight was measured using a Waters gel permeation chromatograph (GPC) model 440. Tetrahydrofuran (THF) was used as the mobile phase at a flow rate of 1.0 mL/min. Molecular weight and polydispersity of macromonomers and copolymers were calibrated with polystyrene standards.
Scanning electron microscopy (SEM) observation
The surface morphology of the copolymer matrices and nano spheres was examined using SEM (Philips XL30 FEG) with an accelerating voltage of 10 kV. The samples were coated with gold for 200 s using a sputter coater (DeskII, Denton vacuum Inc). During the coating, the gas pressure was kept at 50 mtorr and the current was 40 mA.
Fiber diameter determination
The average fiber diameter was calculated from the SEM micrographs. At least 100 fibers were measured for each sample, and their averages and standard deviations were reported.
Porosity
Porosity ε was calculated as: ε = 1-Dp/D0 [10], where Dp was the skeletal density of copolymer foam, and D0 was the density of the copolymer. Dp was determined by: Dp = 4m/(πd2h). Where m was the mass, d was the diameter, and h was the thickness of the disk-shaped foam.
Mechanical test
Compressive modulus of the matrices was measured using an MTS Synergie 200 mechanical tester (MTS Systems Corporation, Eden Prairie, MN) [28]. All samples were circular disks (16 mm in diameter and 2 mm in thickness). Six specimens were tested for each sample. The averages and standard deviations were reported.
In vitro degradation
The copolymer matrices (diameter=7.2 mm, height=2.0 mm) were immersed in phosphate buffer solution (10 mL, 0.1 M, pH 7.4) at 37°C with a shaking speed of 50 rpm. The buffer solution was renewed every other day. At preset time intervals, the samples were removed from the buffer solution and dried under vacuum at room temperature to the constant weights. Morphology change was examined using SEM and weight loss was determined.
Results
Synthesis of macromonomers and copolymers
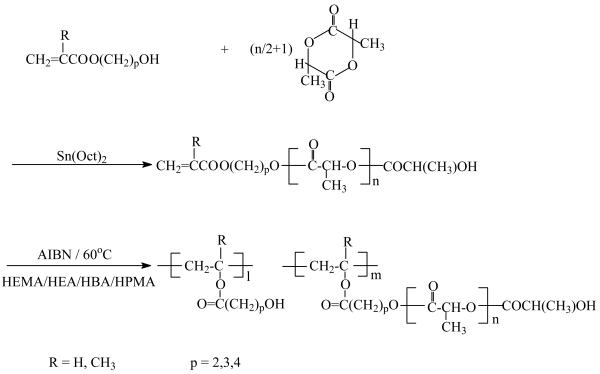
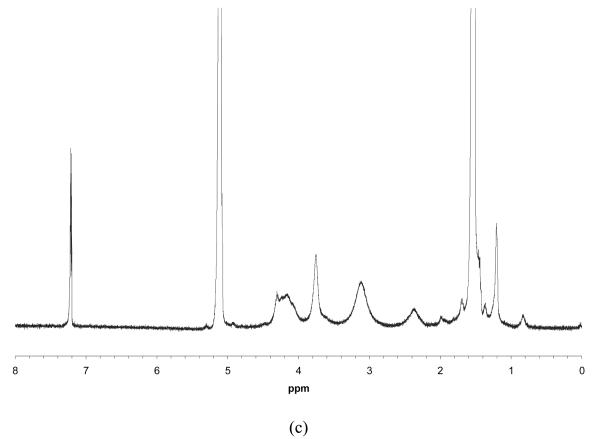
PLLA-based macromonomers were synthesized by ring-opening polymerization of LLA in the presence of a HAA as an initiator. Stannous octoate (Sn(Oct)2), which is highly efficient to initiate the polymerization of various lactones and lactides, was used as a catalyst. A schematic synthetic procedure of a series of PLLA-based macromonomers and their copolymers is illustrated in Figure 1. Figure 2 shows the 1H NMR spectra of HEA-PLLA5 macromonomer (a & b) and its graft copolymer PHEA10-g-PLLA5 (c). Peak assignments are indicated in Figure 2a & b. The three peaks between 5.81 and 6.42 ppm are attributed to CH2=CH of HEA. The assignments are better appreciated at a higher magnification (Figure 2b). It is clearly shown that the CH is split into quartets by the two neighboring CH2 protons, which have different chemical shifts (δ = 5.81, 6.42). Both of the two protons of CH2 are split into doublets by the neighboring CH proton. The yield of macromonomer synthesis was approximately 90%. The HEA-PLLA5 macromonomer was further copolymerized with HEA to yield the graft copolymer via free radical polymerization. After polymerization, the peaks between 5.81 ppm and 6.42 ppm disappeared and new broad peaks between 2.34 ppm and 3.75 ppm appeared, indicating that the double bonds of the HEA-PLLA5 macromonomers have been converted into carbon-carbon single bonds to form the main chains after the copolymerization (Figure 2c). The molecular weights of various macromonomers and copolymers are summarized in Table 1 and Table 2. The molecular weight of the macromonomer was controlled by varying the initiator-to-monomer molar ratio, that is to say, the molecular weight decreased with an increasing initiator-to-monomer molar ratio. When the initiator-to-monomer molar ratio was more than 20/100, the molecular weight of the resulting macromonomer was too low to be precipitated in cold methanol. The molecular weights of graft copolymers ranged from 10k (g/mol) to 40k (g/mol), and did not change significantly with the ratio of macromonomer/HAA.
Figure 1.
Synthesis of PLLA-based macromonomers and graft copolymers
Figure 2.
Typical 1H NMR spectra of HEA-PLLA5 (5 mol% HEA) macromonomer (a) & (b), and PHEA10-g-PLLA5 (macromonomer/HEA = 10/100 (mol/mol)) graft copolymer (c).
Table 1.
Molecular weight and molecular weight distribution of macromonomers*
| Macromonomer | Feed molar ratio | Mn | Mw | Mw/Mn |
|---|---|---|---|---|
| HEMA-PLLA2 | HEMA/LLA = 2/100 | 6200 | 7990 | 1.29 |
| HEMA-PLLA5 | HEMA/LLA = 5/100 | 4300 | 5900 | 1.37 |
| HEMA-PLLA10 | HEMA/LLA = 10/100 | 3700 | 4000 | 1.08 |
| HEMA-PLLA20 | HEMA/LLA = 20/100 | 930 | 1770 | 1.90 |
| HEA-PLLA10 | HEA/LLA = 10/100 | 2570 | 3900 | 1.52 |
| HEA-PLLA20 | HEA/LLA = 20/100 | 490 | 530 | 1.08 |
| HPMA-PLLA10 | HPMA/LLA = 10/100 | 3200 | 4900 | 1.53 |
| HBA-PLLA10 | HBA/LLA = 10/100 | 720 | 950 | 1.32 |
Reaction conditions: [LLA]/[Sn(Oct)2] = 100; Temperature = 140°C, Time = 2 h.
Table 2.
Molecular weight and molecular weight distribution of copolymers*
| copolymers | Macromono mers feed molar ratio |
Copolymers feed molar ratio |
Mn | Mw | Mw/Mn |
|---|---|---|---|---|---|
| PHEMA10-g-PLLA2 | HEMA/LLA = 2/100 |
Macromonomer/HEMA = 10/100 |
17000 | 33000 | 1.94 |
| PHEMA10-g-PLLA5 | HEMA/LLA = 5/100 |
Macromonomer/HEMA = 10/100 |
16400 | 20100 | 1.23 |
| PHEMA5-g-PLLA10 | HEMA/LLA = 10/100 |
Macromonomer/HEMA = 5/100 |
15200 | 20400 | 1.34 |
| PHEMA10-g-PLLA10 | HEMA/LLA = 10/100 |
Macromonomer/HEMA = 10/100 |
13500 | 22300 | 1.65 |
| PHEMA20-g-PLLA10 | HEMA/LLA = 10/100 |
Macromonomer/HEMA = 20/100 |
14200 | 29000 | 2.04 |
| PHEMA10-g-PLLA20 | HEMA/LLA = 20/100 |
Macromonomer/HEMA = 10/100 |
20300 | 32700 | 1.61 |
| PHEA10-g-PLLA10 | HEA/LLA = 10/100 |
Macromonomer/HEA = 10/100 |
14200 | 20000 | 1.41 |
| PHPMA10-g-PLLA10 | HPMA/LLA = 10/100 |
Macromonomer/HPMA = 10/100 |
15600 | 22300 | 1.43 |
Reaction conditions: [Macromonomer]/[AIBN] = 5; Temperature = 70°C, Time = 24 h, Solvent: 1,4-dioxane.
Nanofibrous Architecture
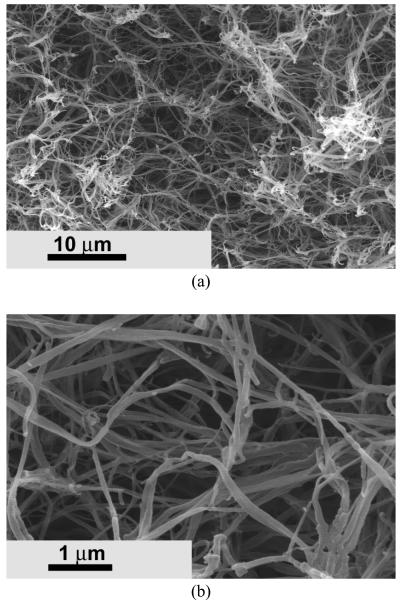
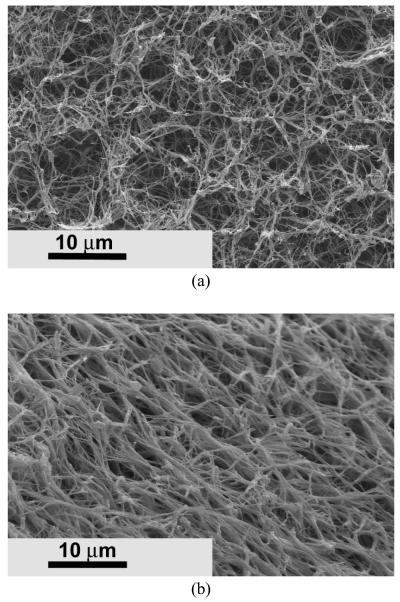
Porous nanofibrous matrix was created from the biodegradable PHEMA10-g-PLLA10 copolymer (Figure 3) by using a thermally induced phase separation technique [28]. These synthetic nano-fibrous matrices mimicked the architecture of natural collagen. The 3D continuous fibrous network was similar to the natural collagen matrix. The diameter of the fibers ranged from 50 to 500 nm, which was in the same diameter range as natural collagen fibers. The synthetic matrices also possessed high porosity. For example, the porosity of the matrix was 95.2% when the copolymer concentration was 5.0%.
Figure 3.
SEM micrographs of the PHEMA10-g-PLLA10 fibrous matrix prepared from 10% (wt/v) THF solution at a gelation temperature of −18 °C. (a) × 2500; (b) × 20000.
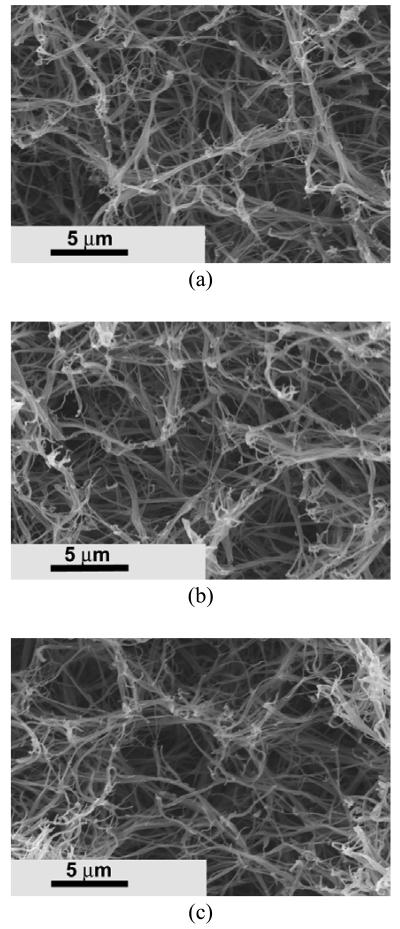
When PHEMA10-g-PLLA10 was blended with PLLA, the same nano-fibrous architecture was formed after phase separation (Figure 4a). Interestingly, these nano-fibers had similar lengths and diameters to those from either PHEMA10-g-PLLA10 or PLLA (Figure 4b, 4c).
Figure 4.
SEM micrographs of fibrous matrices prepared from (a) PHEMA10-g-PLLA10/PLLA=50/50, (b) PHEMA10-g-PLLA10, (c) PLLA. The polymer matrices were fabrication using 10% (wt/v) solutions in THF at a gelation temperature of −18 °C.
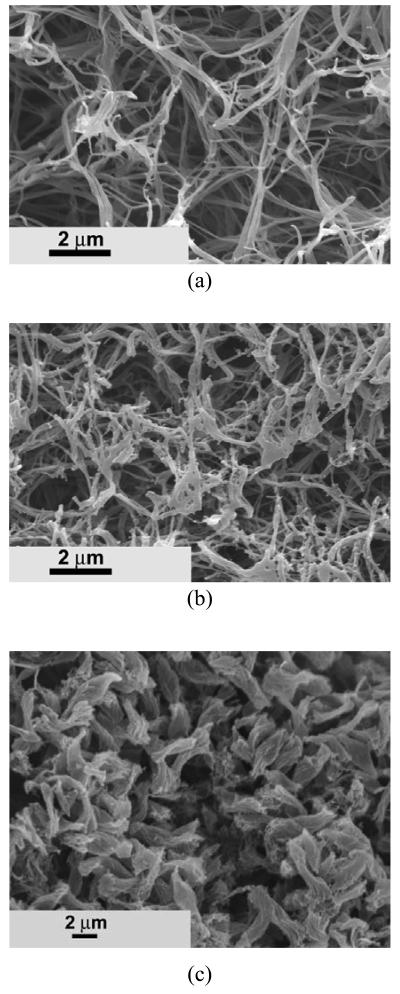
Other copolymers, such as PHEA10-g-PLLA10 and PHPMA10-g-PLLA10, could also be fabricated into nano-fibrous matrices similar to that of PHEMA10-g-PLLA10 (Figure 5). Their fiber diameters also ranged from tens of nanometers to hundreds of nanometers. However, the average diameter of PHPMA10-g-PLLA10 matrix (280±87 nm) was larger than that of PHEA10-g-PLLA10 (160±58 nm).
Figure 5.
SEM micrographs of fibrous matrices prepared from 10% (wt/v) THF solution of PLLA-based graft copolymer at a gelation temperature of −18 °C. (a) PHEA10-g-PLLA10; (b) PHPMA10-g-PLLA10.
As a representative of all PLLA-based graft copolymers, PHEMA-g-PLLA was used to study the effects of copolymer composition, macromonomer composition, and solvent selection on nano-scale structures.
The copolymer composition had significant effect on the matrix microstructure (Figure 6). A typical nano-fibrous structure was observed as the HEMA-PLLA10/HEMA molar ratio was 10/100 (Figure 6a). Nano-fibers could still be created at a HEMA-PLLA10/HEMA molar ratio of 8/100 (Figure 6b). However, some of the fibers were stuck together, which led to the increase of the average fiber diameter. At this copolymer composition, nano-beads were formed along with the nano-fibers. Short rod structures, instead of nano fibers, were created at a HEMA-PLLA10/HEMA molar ratio of 5/100 (Figure 6c). The short rods had an average length of around 4.0 μm.
Figure 6.
SEM micrographs of PHEMA-g-PLLA10 matrices prepared from 10% (wt/v) THF solution with different copolymer compositions. Copolymers feed molar ratio: (a) [HEMA-PLLA10]/[HEMA]=10/100; (b) [HEMA-PLLA10]/[HEMA]=8/100; (c) [HEMA-PLLA10]/[HEMA]=5/100.
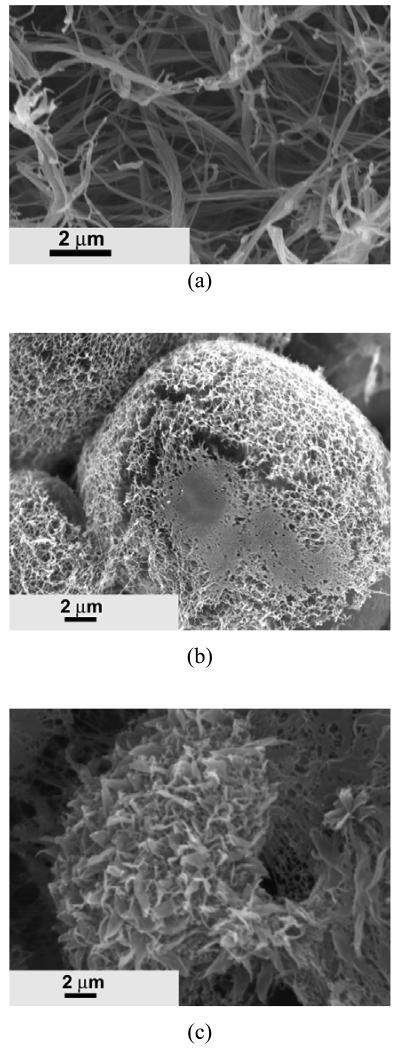
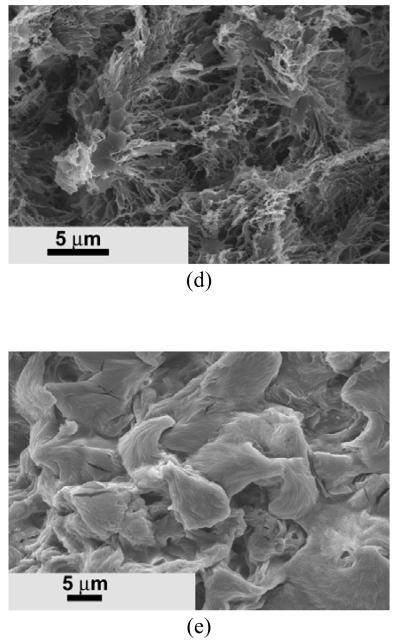
The nano architecture was also controlled by the macromonomer composition. As shown in Figure 7a, the low ratio of [HEMA]/[LLA] (5/100) usually led to the creation of a nano-fibrous structure. Microspheres with an average diameter of about 10 μm were observed at a molar ratio of [HEMA]/[LLA] of 12/100 (Figure 7b). Interestingly, the surface of the microspheres were composed of nano fibers, and the length of nano fibers was shorter than that made of [HEMA]/[LLA] (5/100). As the molar ratio of [HEMA]/[LLA] was further increased to 20/100, the resulting copolymer could also form microspheres (Figure 7c). However, instead of nano-fibrous structure, a leaf-like structure was formed on the surfaces of the microspheres.
Figure 7.
SEM micrographs of PHEMA10-g-PLLA) matrices prepared from 10% (wt/v) THF solution with different macromonomer compositions. Macromonomer feed molar ratio and molecular weight: (a) [HEMA]/[LLA]=5/100, Mn=4300; (b) [HEMA]/[LLA]=12/100, Mn=3200; (c) [HEMA]/[LLA]=20/100, Mn = 930.
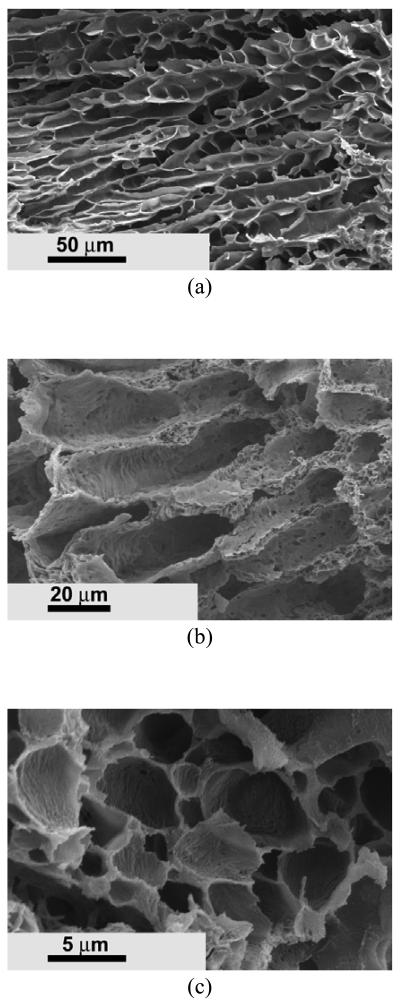
The nano architecture formation from these graft copolymers was also investigated by using other solvent systems. Ladder-like microtubular architecture was achieved when using 1,4-dioxane as solvent (Figure 8a). Very different morphologies appeared in the matrices when a solvent mixture was used (Figure 8b-8e). A tubular structure with an average diameter of about 20 μm was formed when water/dioxane ratio was 1/10 (v/v) (Figure 8b). The tubular wall was much rougher compared to that fabricated in dioxane. When the water/dioxane ratio increased to 1/8 (v/v), the tubular structure disappeared, and micropores with an average size of about 5 μm were obtained (Figure 8c). A nano-fibrous structure could also be created when using the dioxane/methanol (5/1, v/v) solvent mixture system (Figure 8d). However, some nano-fibers were bound and formed platelet structures. When the dioxane/methanol ratio was 3/1 (v/v), the fibers were stuck together and formed a relative solid and smooth surface (Figure 8e).
Figure 8.
SEM micrographs of PHEMA10-g-PLLA10 matrices prepared from different solvents. (a) 10% (wt/v) dioxane solution; (b) 10% (wt/v) dioxane/water (10/1,v/v) solution; (c) 10% (wt/v) dioxane/water (8/1,v/v) solution; (d) 10% (wt/v) dioxane/methanol (5/1,v/v) solution; (e) 10% (wt/v) dioxane/methanol (3/1,v/v) solution.
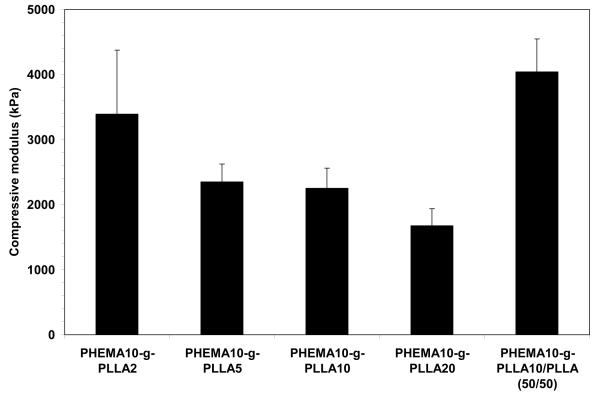
The compressive modulus of the nano-fibrous matrices fabricated from these PLLA-based copolymers was in the range of 1800 to 3500 kPa, and increased with the monomer/initiator ratio of the macromonomers (Figure 9). By adding the PLLA homopolymer to the PLLA-based copolymer, the compressive modulus of the nano-fibrous matrix significantly increased (Figure 9).
Figure 9.
Compressive moduli of the nano-fibrous matrices fabricated from 10% (wt/v) THF solution of the PLLA-based copolymers and a blend with PLLA.
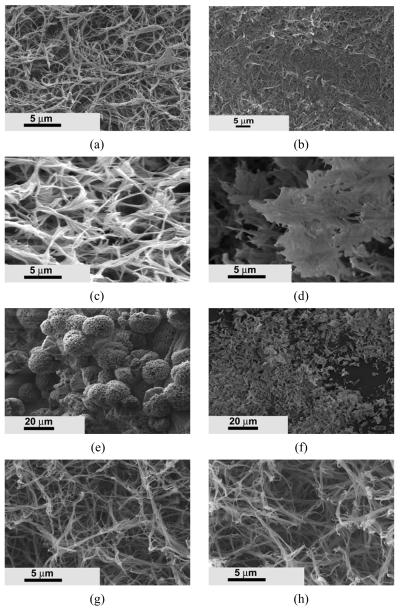
The in vitro degradation of these synthesized PLLA-based copolymers was examined using SEM. Prior to incubation in phosphate buffered saline (PBS), the nano-fibers were well separated and evenly distributed throughout the matrix for PHEMA10-g-PLLA2 (Figure 10a). After 10 weeks in PBS, the nano-fibrous morphology was still observable (Figure 10b). However, most fibers became stuck together and the pores between fibers decreased. For PHEMA10-g-PLLA10, the fibers were bundled together and some pieces had broken from the bulk after 10 weeks of incubation (compare Figure 10 c and d). The PHEMA10-g-PLLA20 spheres were totally broken into pieces after 10 weeks of incubation (compare Figure 10 e and f). For the PHEMA10-g-PLLA10/PLLA (50/50) matrix, the nano-fibrous architecture was unchanged after being incubated in PBS for 10 weeks (compare Figure 10 g and h).
Figure 10.
Comparison of morphology change of various copolymer matrices before and after immersion in PBS for 10 weeks. (a) PHEMA10-g-PLLA2, 0 week; (b) PHEMA10-g-PLLA2, 10 weeks; (c) PHEMA10-g-PLLA10, 0 week; (d) PHEMA10-g-PLLA10, 10 weeks; (e) PHEMA10-g-PLLA20, 0 week; (f) PHEMA10-g-PLLA10, 10 weeks; (g) PHEMA10-g-PLLA10/PLLA (50/50), 0 week; (h) PHEMA10-g-PLLA10/PLLA (50/50), 10 weeks.
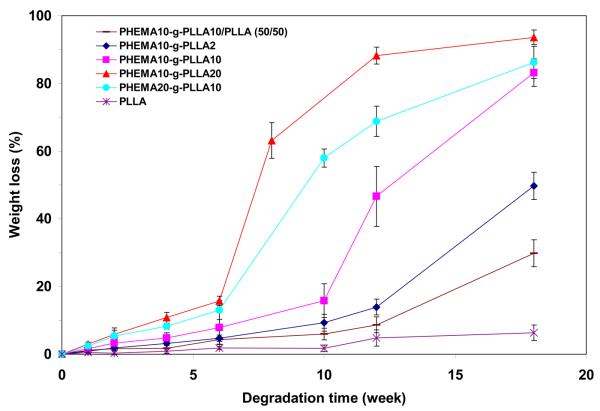
The weight loss measurement also showed that PHEMA10-g-PLLA20 had the fastest degradation rate than the others (Figure 11). More than 88.2% of the weight was lost for PHEMA10-g-PLLA20 after incubation in PBS for 12 weeks. For PHEMA10-g-PLLA10 and PHEMA10-g-PLLA2, the weight loss after 12 weeks of incubation was 46.6% and 13.9%, respectively. By mixing 50% PLLA homopolymer, the weight loss of the PHEMA10-g-PLLA2/PLLA was only 8.6% after 12 weeks of incubation.
Figure 11.
Weight loss of PLLA-based copolymer matrices with time incubated in PBS under 37°C.
Discussion
Due to its excellent set of properties (for example, biocompatibility, biodegradability, mechanical strength and ease of fabrication), PLLA is one of the most widely used scaffolding materials in tissue engineering [29]. Nano-fibrous PLLA (NF-PLLA) scaffolds have been fabricated to mimic the physical architecture of natural collagen fibers by using a thermally induced phase separation method developed in our lab [10]. To further mimic the ECM features and provide biological signals to cells seeded in the scaffolds, the NF-PLLA scaffolds need to be modified with biomacromolecules or peptides. The chemical coupling reaction is considered an effective method to accurately introduce bioactive molecules onto the scaffold surfaces. But there are no functional groups on PLLA chains, which make the coupling reaction unfeasible. The slow degradation rate of PLLA is also a disadvantage when used as a scaffolding material for certain tissue engineering applications, although the nano-fibrous architecture of PLLA significantly accelerates the hydrolysis process [20]. Furthermore, PLLA is a relative hydrophobic biomaterial with a contact angle of about 78° [30]. The PLLA scaffolds often need to be pre-soaked in ethanol solution and subsequently a solvent exchange before the cells are seeded [31]. The ethanol-soaking process may reduce the mechanical strength of the scaffold.
To improve on PLLA scaffold, we designed and synthesized a series of PLLA-based copolymers in this work. They possess functional hydroxyl groups on the polymer chains for further chemical modification and have fast degradation rates as well as the capability to form the biomimetic nano-fibrous architecture. We selected HAA as initiators to prepare PLLA-based macromonomers. The hydroxyl groups of HAA were used to initiate the polymerization of LLA and as functional groups to couple with bioactive molecules. The HAA monomers, such as HEA, and HEMA, are hydrophilic and allow easy access to water molecules. Therefore, the incorporation of the functional HAA in the copolymers can accelerate the hydrolytic degradation rate.
The PLLA-based macromonomers were fabricated by the ring-opening polymerization of LLA. The polymerization temperature was important for macromonomers syntheses. It is known that the melting point of LLA monomer is 92-94°C. The ring-opening polymerization temperature of LLA, therefore, should be above the melting point of LLA. On the other hand, the double bond of HAA in the macromonomers may be activated to react at high temperatures. In fact, a crosslinking side reaction occurs when the temperature rises above 160°C. In our study, the ring-opening polymerization was carried out at 140°C for 2 h. The 1H NMR spectra clearly showed the existence of double bonds in the macromonomer chains (Figure 2b). The molecular weights of the macromonomers were several thousands (g/mol), varying with the feed ratio of monomer/initiator.
Although linear PLLA homopolymer has been fabricated into nano-fibrous matrix using a thermally induced phase separation technique [10], it is unknown whether PLLA-based copolymers can also form nano architecture by using the phase separation technique. In this work, we demonstrated the capability of expanding this technique to create nano-fibrous matrices from the PLLA-based graft copolymers (Figure 3). The nano-fibrous matrices mimic the architecture of the natural collagen fibers at the nanometer scale. These synthetic nanofibers combine the advantages of synthetic biodegradable polymers and the architecture of natural collagen fibers, which are considered to play an important role in cell adhesion, migration, growth, and function [32, 33]. In addition to avoiding the immunogenicity, these synthetic polymers are advantageous due to their controllable degradation rate and mechanical properties to be tailored for specific applications.
Interestingly, when PHEMA10-g-PLLA10 was blended with PLLA, the nano-fibrous architecture maintained essentially the same fiber length and diameter regardless of the ratio of PHEMA10-g-PLLA10/PLLA mixture (Figure 4). Therefore, it is very convenient to tailor the blend matrix degradation rate and mechanical properties by simply changing the blend ratio, without altering the nano-fibrous architecture.
While there could be other ways to incorporate functional HAA units onto the copolymer chains, the polymerization of macromonomers allowed us to prepare well-defined PLLA-based graft copolymers in this study. The length of the graft copolymer chains, which is determined by the molecular weight of the macromonomer, can be tailored in advance by controlling the molar feed ratio of monomer/initiator. Different molecular weights of the macromonomer (the length of the graft chains) lead to different architectures of the copolymer matrices (Figure 7). Typical nano-fibrous structure was observed when the average molecular weight (Mn) of the HEMA-PLLA macromonomer was 4300 (g/mol). Microspheres with nano-fibrous structure were formed when the Mn of HEMA-PLLA macromonomer was 3200 (g/mol). Microspheres with leaf-like structure were observed when the Mn of HEMA-PLLA macromonomer was 930 (g/mol). Therefore, the macromonomer strategy provides a convenient way to control nano architecture formation.
Besides the molecular weight of the macromonomer, the nano-fibrous architecture could also be tailored by controlling the copolymer feed ratio and solvent composition. As shown in Figure 6, the copolymer architecture was changed from nano-fibers to micro rods as the ratio of HEMA-PLLA10/HEMA decreased from 10/100 to 5/100. As the ratio of dioxane/methanol mixture decreased from 5/1 to 3/1, the copolymer architecture was changed from nano-fibers to smooth solid aggregates (Figure 8).
Because of the incorporation of hydrophilic HAA into the copolymer chains, the degradation rate of these PLLA-based copolymers could be substantially accelerated. Due to capability of tailoring the molecular weight of macromonomer, the degradation rate could also be tuned up or down in certain range. As shown in Figure 11, the PHEMA10-g-PLLA20 could lose more than 88.2% of its weight after incubation in PBS for 12 weeks, while the PLLA only lost 4.8% of its weight with the same incubation time. The SEM images also confirmed that the PHEMA10-g-PLLA20 matrix was completely broken down to pieces, while the nano-fibrous architecture of PLLA was unchanged after 10 weeks of incubation in PBS (Figure 10). By increasing the molecular weight of macromonomers, the degradation time of the copolymer matrices were increased from several weeks to several months. In addition, the degradation rate could also be tailored by varying the copolymer composition and/or blending with PLLA. Therefore, this copolymer system provides us the design flexibility to tailor architectures and properties for various biomedical applications.
Conclusions
In this work, we designed and synthesized a series of PLLA-based functional graft copolymers. We demonstrated that these copolymers can be fabricated into nano-fibrous matrices. The nanofiber formation is dependent on the macromonomer composition, copolymer composition, and solvent composition. Faster degradation rates than that of PLLA matrix can be easily achieved with these PLLA-based copolymer matrices. The time for complete degradation can be varied from several weeks to several months by adjusting the molecular weight of macromonomer, the copolymer composition, and the blending ratio with PLLA homopolymer. Similarly, the mechanical properties of the PLLA-based copolymer matrices are also adjustable. Thus, these PLLA-based copolymers have the potential to be tailored into ideal scaffolds for various tissue engineering applications.
Acknowledgements
The authors would like to acknowledge the financial support from the National Institutes of Health (Research Grants DE015384, GM075840 and DE017689: PXM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Langer R, Vacanti JP. Tissue Engineering. Science. 1993;260(5110):920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 2.Ma PX. Biomimetic materials for tissue engineering. Adv Drug Deliv Rev. 2008;60(2):184–198. doi: 10.1016/j.addr.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hench LL, Polak JM. Third-generation biomedical materials. Science. 2002;295(5557):1014–1017. doi: 10.1126/science.1067404. [DOI] [PubMed] [Google Scholar]
- 4.Liu XH, Ma PX. Polymeric scaffolds for bone tissue engineering. Annals of Biomedical Engineering. 2004;32(3):477–486. doi: 10.1023/b:abme.0000017544.36001.8e. [DOI] [PubMed] [Google Scholar]
- 5.Shin H, Jo S, Mikos AG. Biomimetic materials for tissue engineering. Biomaterials. 2003;24(24):4353–4364. doi: 10.1016/s0142-9612(03)00339-9. [DOI] [PubMed] [Google Scholar]
- 6.Wei G, Ma PX. Nanostructured Biomaterials for Regeneration. Advanced Functional Materials. 2008;18:3568–3582. doi: 10.1002/adfm.200800662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevens MM, George JH. Exploring and engineering the cell surface interface. Science. 2005;310(5751):1135–1138. doi: 10.1126/science.1106587. [DOI] [PubMed] [Google Scholar]
- 8.Gullberg D, Ekblom P. Extracellular matrix and its receptors during development. International Journal of Developmental Biology. 1995;39(5):845–854. [PubMed] [Google Scholar]
- 9.Rosso F, Giordano A, Barbarisi M, Barbarisi A. From cell-ECM interactions to tissue engineering. Journal of Cellular Physiology. 2004;199(2):174–180. doi: 10.1002/jcp.10471. [DOI] [PubMed] [Google Scholar]
- 10.Ma PX, Zhang RY. Synthetic nano-scale fibrous extracellular matrix. Journal of Biomedical Materials Research. 1999;46(1):60–72. doi: 10.1002/(sici)1097-4636(199907)46:1<60::aid-jbm7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 11.Pham QP, Sharma U, Mikos AG. Electrospun poly(epsilon-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: Characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules. 2006;7(10):2796–2805. doi: 10.1021/bm060680j. [DOI] [PubMed] [Google Scholar]
- 12.Bognitzki M, Czado W, Frese T, Schaper A, Hellwig M, Steinhart M, et al. Nanostructured fibers via electrospinning. Advanced Materials. 2001;13(1):70–72. [Google Scholar]
- 13.Kenawy ER, Layman JM, Watkins JR, Bowlin GL, Matthews JA, Simpson DG, et al. Electrospinning of poly(ethylene-co-vinyl alcohol) fibers. Biomaterials. 2003;24(6):907–913. doi: 10.1016/s0142-9612(02)00422-2. [DOI] [PubMed] [Google Scholar]
- 14.Bergsma JE, de Bruijn WC, Rozema FR, Bos RR, Boering G. Late degradation tissue response to poly(L-lactide) bone plates and screws. Biomaterials. 1995;16(1):25–31. doi: 10.1016/0142-9612(95)91092-d. [DOI] [PubMed] [Google Scholar]
- 15.Ma PX, Choi JW. Biodegradable polymer scaffolds with well-defined interconnected spherical pore network. Tissue Engineering. 2001;7(1):23–33. doi: 10.1089/107632701300003269. [DOI] [PubMed] [Google Scholar]
- 16.Holy CE, Shoichet MS, Davies JE. Engineering three-dimensional bone tissue in vitro using biodegradable scaffolds: Investigating initial cell-seeding density and culture period. Journal of Biomedical Materials Research. 2000;51(3):376–382. doi: 10.1002/1097-4636(20000905)51:3<376::aid-jbm11>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 17.Woo KM, Chen VJ, Ma PX. Nano-fibrous scaffolding architecture selectively enhances protein adsorption contributing to cell attachment. Journal of Biomedical Materials Research Part A. 2003;67A(2):531–537. doi: 10.1002/jbm.a.10098. [DOI] [PubMed] [Google Scholar]
- 18.Woo KM, Jun JH, Chen VJ, Seo JY, Baek JH, Ryoo HM, et al. Nano-fibrous scaffolding promotes osteoblast differentiation and biomineralization. Biomaterials. 2007;28(2):335–343. doi: 10.1016/j.biomaterials.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 19.Walton M, Cotton NJ. Long-term in vivo degradation of poly-L-lactide (PLLA) in bone. Journal of Biomaterials Applications. 2007;21(4):395–411. doi: 10.1177/0885328206065125. [DOI] [PubMed] [Google Scholar]
- 20.Chen VJ, Ma PX. The effect of surface area on the degradation rate of nano-fibrous poly(L-lactic acid) foams. Biomaterials. 2006;27(20):3708–3715. doi: 10.1016/j.biomaterials.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 21.Eppley BL, Reilly M. Degradation characteristics of PLLA-PGA bone fixation devices. Journal of Craniofacial Surgery. 1997;8(2):116–120. doi: 10.1097/00001665-199703000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Barrera DA, Zylstra E, Lansbury PT, Langer R. Copolymerization and Degradation of Poly(Lactic Acid Co-Lysine) Macromolecules. 1995;28(2):425–432. [Google Scholar]
- 23.Kimura Y, Shirotani K, Yamane H, Kitao T. Copolymerization of 3-(S)-[(Benzyloxycarbonyl)Methyl]-1,4-Dioxane-2,5-Dione and L-Lactide - a Facile Synthetic Method for Functionalized Bioabsorbable Polymer. Polymer. 1993;34(8):1741–1748. [Google Scholar]
- 24.Quirk RA, Chan WC, Davies MC, Tendler SJB, Shakesheff KM. Poly(L-lysine)-GRGDS as a biomimetic surface modifier for poly(lactic acid) Biomaterials. 2001;22(8):865–872. doi: 10.1016/s0142-9612(00)00250-7. [DOI] [PubMed] [Google Scholar]
- 25.Noga DE, Petrie TA, Kumar A, Weck M, Garcia AJ, Collard DM. Synthesis and modification of functional poly(lactide) copolymers: Toward biofunctional materials. Biomacromolecules. 2008;9(7):2056–2062. doi: 10.1021/bm800292z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerhardt WW, Noga DE, Hardcastle KI, Garcia AJ, Collard DM, Weck M. Functional lactide monomers: Methodology and polymerization. Biomacromolecules. 2006;7(6):1735–1742. doi: 10.1021/bm060024j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim DW, Choi SH, Park TG. A new class of biodegradable hydrogels stereocomplexed by enantiomeric oligo(lactide) side chains of poly(HEMA-g-OLA)s. Macromolecular Rapid Communications. 2000;21(8):464–471. [Google Scholar]
- 28.Liu XH, Won YJ, Ma PX. Porogen-induced surface modification of nano-fibrous poly(L-lactic acid) scaffolds for tissue engineering. Biomaterials. 2006;27(21):3980–3987. doi: 10.1016/j.biomaterials.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 29.Peter SJ, Miller MJ, Yasko AW, Yaszemski MJ, Mikos AG. Polymer concepts in tissue engineering. Journal of Biomedical Materials Research. 1998;43(4):422–427. doi: 10.1002/(sici)1097-4636(199824)43:4<422::aid-jbm9>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 30.Liu XH, Smith L, Wei G, Won YJ, Ma PX. Surface Engineering of Nano-Fibrous Poly(L-lactic Acid) Scaffolds via Self-Assembly Technique for Bone Tissue Engineering. Journal of Biomedical Nanotechnology. 2005;1(1):54–60. [Google Scholar]
- 31.Ma PX, Zhang RY. Microtubular architecture of biodegradable polymer scaffolds. Journal of Biomedical Materials Research. 2001;56(4):469–477. doi: 10.1002/1097-4636(20010915)56:4<469::aid-jbm1118>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 32.Meredith JE, Fazeli B, Schwartz MA. The Extracellular-Matrix as a Cell-Survival Factor. Molecular Biology of the Cell. 1993;4(9):953–961. doi: 10.1091/mbc.4.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grinnell F, Bennett MH. Ultrastructural Studies of Cell-Collagen Interactions. Methods in Enzymology. 1982;82:535–544. doi: 10.1016/0076-6879(82)82085-5. [DOI] [PubMed] [Google Scholar]