Abstract
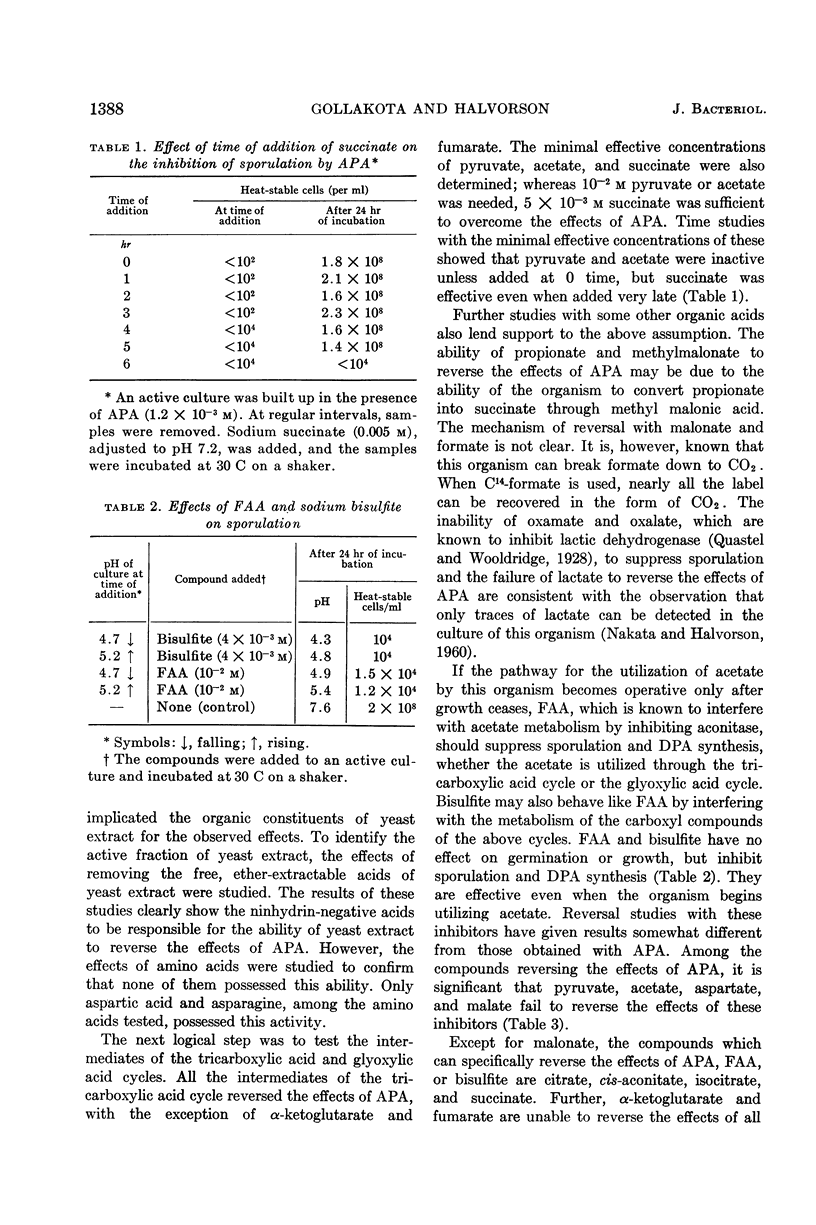
Gollakota, K. G. (University of Illinois, Urbana) and H. Orin Halvorson. Biochemical changes occurring during sporulation of Bacillus cereus T. II. Effect of esters of organic acids on sporulation. J. Bacteriol. 85:1386–1393. 1963.—Sporulation of Bacillus cereus T in yeast extract-glucose-minerals medium was specifically inhibited by α-picolinic acid (APA), if the acid was added before the pH of the culture began to rise. The effects of APA could be reversed by aspartic acid or asparagine, among the amino acids, and by intermediates of the tricarboxylic acid cycle, with the exception of α-ketoglutarate and fumarate. Formate, malonate, and certain other organic acids also possessed this ability. Succinate was the best reversing agent. Fluoroacetic acid (FAA) also inhibited sporulation, but had no effect on vegetative growth or germination of spores of B. cereus T. Unlike APA, FAA inhibited sporulation even when added after the pH of the culture had started to rise. Bisulfite was similar to FAA in its effects on sporulation. With the exception of pyruvate, acetate, aspartate, and malate, most of the compounds reversing the effects of APA also overcame the effects of FAA or bisulfite on sporulation. Esters of some of the acids reversing the effects of the above inhibitors were studied for their action on germination, growth, and sporulation. Ethyl pyruvate prevented germination of the spores, slowed down growth, and inhibited sporulation. Ethyl malonate and ethyl succinate inhibited only sporulation. All the above inhibitors prevented the synthesis of dipicolinic acid (DPA) also. When B. cereus T was grown in the absence of glucose (in extracted yeast extract-minerals medium), the above inhibitors had no effect on sporulation. Ethyl oxamate permitted sporulation, but the spores produced were heat-sensitive. Ethyl pimelate caused lysis when added before the pH of the culture began to rise. When added after the pH of the culture began to rise, it also permitted sporulation, and the spores were sensitive to heat. (These heat-sensitive spores were refractile and dormant, and did not stain with crystal violet. However, they germinated normally, losing refractibility and became stainable.) The effect of ethyl oxamate and ethyl pimelate could be overcome by DPA. APA, FAA, ethyl malonate, and ethyl succinate also inhibit the sporulation of a number of other bacilli.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHURCH B. D., HALVORSON H. Dependence of the heat resistance of bacterial endospores on their dipicolinic acid content. Nature. 1959 Jan 10;183(4654):124–125. doi: 10.1038/183124a0. [DOI] [PubMed] [Google Scholar]
- GOLLAKOTA K. G., HALVORSON H. O. Biochemical changes occurring during sporulation of Bacillus cereus. Inhibition of sporulation by alpha-picolinic acid. J Bacteriol. 1960 Jan;79:1–8. doi: 10.1128/jb.79.1.1-8.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANSSEN F. W., LUND A. J., ANDERSON L. E. Colorimetric assay for dipicolinic acid in bacterial spores. Science. 1958 Jan 3;127(3288):26–27. doi: 10.1126/science.127.3288.26. [DOI] [PubMed] [Google Scholar]
- KORNBERG H. L., GOTTO A. M., LUND P. Effect of growth substrates on isocitratase formation by Pseudomonas ovalis Chester. Nature. 1958 Nov 22;182(4647):1430–1431. doi: 10.1038/1821430a0. [DOI] [PubMed] [Google Scholar]
- KRISHNA MURTY G. G., HALVORON H. O. Effect of duration of heating, L-alanine and spore concentration on the oxidation of glucose by spores of Bacillus cereus var. terminalis. J Bacteriol. 1957 Feb;73(2):235–240. doi: 10.1128/jb.73.2.235-240.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A. Metabolism of amino-acids: Deamination of amino-acids. Biochem J. 1935 Jul;29(7):1620–1644. doi: 10.1042/bj0291620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN H. H., FOSTER J. W. Biosynthesis of dipicolinic acid in Bacillus megaterium. J Bacteriol. 1958 Aug;76(2):167–178. doi: 10.1128/jb.76.2.167-178.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKATA H. M., HALVORSON H. O. Biochemical changes occurring during growth and sporulation of Bacillus cereus. J Bacteriol. 1960 Dec;80:801–810. doi: 10.1128/jb.80.6.801-810.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVOA W. B., WINER A. D., GLAID A. J., SCHWERT G. W. Lactic dehydrogenase. V. Inhibition by oxamate and by oxalate. J Biol Chem. 1959 May;234(5):1143–1148. [PubMed] [Google Scholar]
- PERRY J. J., FOSTER J. W. Studies on the biosynthesis of dipicolinic acid in spores of Bacillus cereus var. mycoides. J Bacteriol. 1955 Mar;69(3):337–346. doi: 10.1128/jb.69.3.337-346.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POWELL J. F., STRANGE R. E. Synthesis of dipicolinic acid from 2,6-diketopimelic acid. Nature. 1959 Sep 19;184:878–880. doi: 10.1038/184878a0. [DOI] [PubMed] [Google Scholar]
- Quastel J. H., Wooldridge W. R. Some properties of the dehydrogenating enzymes of bacteria. Biochem J. 1928;22(3):689–702. doi: 10.1042/bj0220689. [DOI] [PMC free article] [PubMed] [Google Scholar]


