Abstract
Purpose
To define the prognosis in myelodysplastic syndrome and deletion 5q with or without other cytogenetic abnormalities.
Patients and Methods
Patients with MDS (<20% blasts) and evaluable cytogenetic studies (1966-present) were reviewed. Outcome of patients with deletion 5q or with loss of chromosome 5 (monosomy 5) was analyzed.
Results
Of 2743 patients analyzed, 287 (10%) had deletion 5q, and 216 (8%) had monosomy 5 abnormalities. The median survival was 9 months with deletion 5q, 6 months with monosomy 5, and 17 months without a chromosome 5 abnormality. Considering patients with deletion 5q, the median survival was 33 months with deletion 5q alone, 17 months with deletion 5q and one additional abnormality, but only 6–12 months with deletion 5q and ≥ 2 abnormalities or chromosome 7 abnormality. Only 93 patients (3.4% of total) had lower risk MDS (International Prognostic Scoring System low or intermediate 1) and deletion 5q; their median survival was 29 months (2-year survival 60%), and ranged from 13 to 41 months depending on the presence or absence of additional chromosomal abnormalities. Among 198 patients with MDS with deletion 5q and < 10% blasts (a subset now often offered lenalidomide) the median survival was 12 months. Monosomy 5 was significantly more frequently associated with advanced MDS and with other chromosomal abnormalities.
Conclusions
This study provides baseline expectations of outcome in different MDS subsets and deletion 5q.
INTRODUCTION
The prognosis of patients with myelodysplastic syndrome (MDS) is heterogeneous and is defined, in part, by several prognostic factors including the percentage of marrow blasts, cytogenetic abnormalities, and number of cytopenias.(1–3) Abnormalities involving chromosome 5 occur in 20% of patients with MDS; they are more frequent in secondary MDS. These may involve single chromosome 5 abnormalities such as a deletion in the long arm of chromosome 5 (deletion 5q) or total loss of chromosome 5 (monosomy 5), as well as additional abnormalities of one or more clones, including abnormalities in chromosome 7.(4,5) Abnormalities accompanying deletion 5q confer a very different prognosis than that of the “5q syndrome” or a single deletion 5q. Also, the MDS literature makes little distinction between deletion 5q and monosomy 5; these are often categorized as “chromosome 5 abnormalities”. Whether they are associated with different prognoses, after accounting for the MDS risk, is unknown.
The 5q syndrome typically describes a younger patient with MDS, often a female, who presents with low risk MDS, red blood cell (RBC) transfusion-dependent anemia, and normal or increased platelets counts. These patients have a favorable prognosis with a median survival longer than 8 to 10 years, and a low incidence of transformation to acute myeloid leukemia (AML) (about 10%). The end-stage course is associated with cytopenias, iron overload related to frequent RBC transfusions, and organ failure from iron overload. Patients with MDS and single deletion 5q may or may not have a “5q syndrome”.(6,7) Finally patients with deletion 5q and other abnormalities have a worse prognosis than either of these two MDS subgroups.7–10
Lenalidomide therapy in patients with “lower risk” MDS (International Prognostic Scoring System [IPSS] low or intermediate 1 risk; IPSS Score 0–1.0), RBC transfusion dependence, and with deletion 5q, has shown favorable results. In such patients, lenalidomide therapy was associated with a RBC transfusion independence rate of 66%, and a complete cytogenetic response rate of 44%. Responses were durable with a median remission duration of 116 weeks.(11) These observations led to the approval of lenalidomide by the Food and Drug Administration (FDA) in the United States for the treatment of lower risk MDS with RBC transfusion dependence and deletion 5q. Lenalidomide was not approved for this indication by the European Medicines Agency (EMEA), perhaps partly because of the occurrence of AML in some patients treated.(12, 13)
In the lenalidomide study, the hematologic and cytogenetic response rates were reported to be similar in patients with deletion 5q alone or with deletion 5q and other abnormalities, and were also similar within the IPSS risk groups, and across a range of percent marrow blasts. This resulted in a perception that lenalidomide may be equally active in all subsets, and its use across all MDS subsets with deletion 5q (i.e. with higher percentage marrow blasts and with deletion 5q with other abnormalities). The EMEA reservations regarding the “higher-than-expected” perceived incidence of AML would have been justified if these patients had only a “5q syndrome” or single deletion 5q, but not so in the setting of the other MDS subsets.
To define the potential benefit of lenalidomide in patients with MDS and deletion 5q, randomized trials would be required (e.g. lenalidomide versus hypomethylating agents). Alternatively, as for imatinib mesylate in chronic myelogenous leukemia (CML),( 14, 15) solid historical data bases would help establish baseline expectations against which the benefit of lenalidomide in single arm trials could be compared. The purpose of this analysis is to establish the baseline expectations and prognosis of patients with MDS and deletion 5q outside the context of lenalidomide therapy. In addition, the study evaluated the prognosis of patients with MDS and monosomy 5.
PATIENTS AND METHODS
All patients with MDS referred to the Leukemia Department at MD Anderson Cancer Center since 1966 were reviewed. Consistent with the new WHO MDS classification, only patients with <20% marrow blasts were included.(2) Among them, we focused on patients with chromosome 5 abnormalities. These were further categorized into the following cytogenetic subsets: 1) deletion 5q only; 2) monosomy 5 only; 3) deletion 5q or monosomy 5 with 1, 2, or ≥3 abnormalities and 4) deletion 5q or monosomy 5 and chromosome 7 abnormalities. Patients and disease characteristics including age, gender, hemoglobin levels, platelets counts, white blood cell counts, absolute granulocyte counts, percent of marrow blasts, duration of MDS, prior therapy, and other characteristics were recorded.
Statistical considerations
Survival was measured from the date of referral to M. D. Anderson Cancer Center. Survival curves were plotted by the Kaplan-Meier method and differences among survival curves were analyzed by the log rank test.
RESULTS
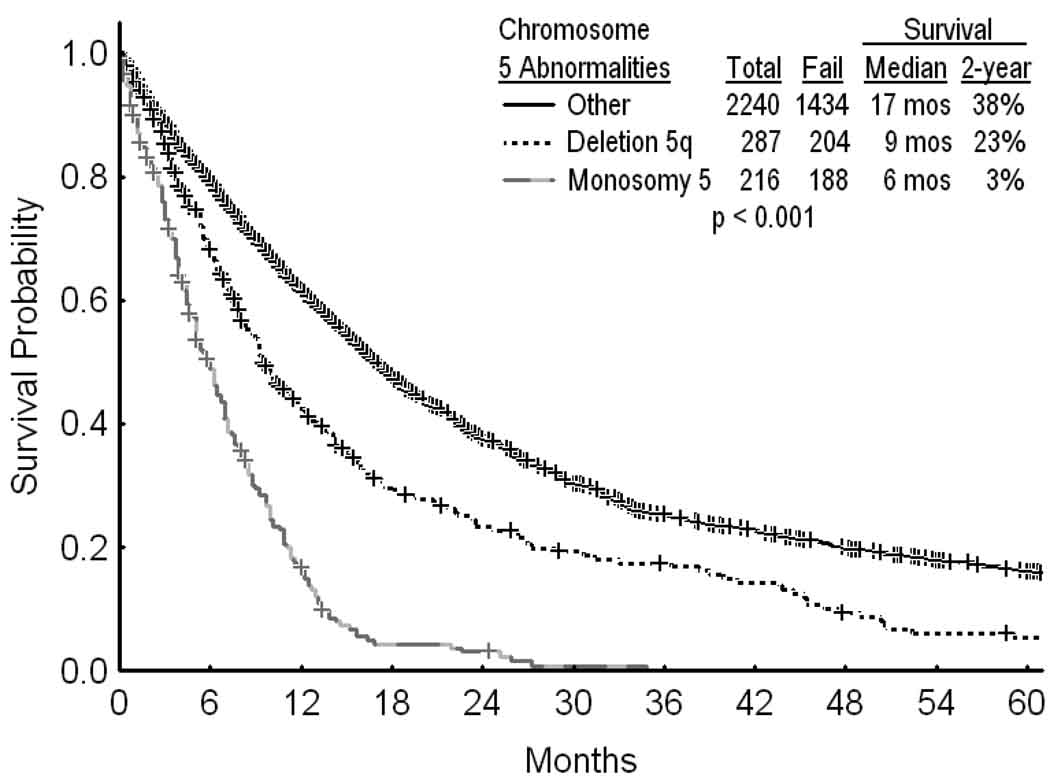
A total of 2974 patients with MDS and < 20% blasts had been referred; 2743 patients had adequate cytogenetic information. Among them, 503 (18%) had chromosome 5 abnormalities. These included 287 patients (10%) with deletion 5q, and 216 patients (8%) with monosomy 5. The characteristics of patients without and with deletion 5q or monosomy 5 are shown in Table 1. Compared with monosomy 5, deletion 5q was more frequently associated with lower IPSS risks (IPSS low-intermediate 1) (32% versus 7%; p < 0.001), and with a lower incidence of additional (3 or more) chromosomal abnormalities or chromosome 7 abnormality (60% versus 97 %; p<0.0001) (Table 1). The overall median survival in MDS was 14 months. The median survival was 9 months with deletion 5q, 6 months with monosomy 5, and 17 months without chromosome 5 abnormalities (Figure 1; p<0.001). Overall 1419/2240 patients (63%) with MDS and 326/503 patients (65%) with MDS and chromosome 5 abnormalities had no prior therapy. Among patients with deletion 5q, the median survival in subgroups without or with prior therapy were 9 months and 12 months, respectively (p=0.271). Because of the high percent of patients referred with prior therapy (998/2743=36%), we elected to categorize patients by IPSS risk group regardless of prior therapy.
Table 1.
Characteristics Of the Study Groups
| No. (%) Chromosome 5 Abnormalities |
||||
|---|---|---|---|---|
| Parameter | Category | Deletion 5q (n= 287) |
Monosomy 5 (n= 216) |
No (n=2240) |
| Age (years) | ≥ 60 | 213 (74) | 141 (65) | 1584 (71) |
| Gender | Female | 125 (44) | 73 (34) | 728 (33) |
| Hemoglobin (g/dl) | < 10 | 200 (70) | 149 (69) | 1178 (53) |
| Platelets (×109/L) | < 100 | 166 (58) | 187 (87) | 1357 (61) |
| WBC (×109/L) | >10 | 17 (6) | 18 (8) | 482 (22) |
| < 1 | 6 (2) | 5 (2) | 53 (2) | |
| Absolute granulocyte count (×109/L) |
< 1.0 | 91 (32) | 86 (40) | 729 (33) |
| < 5 | 115 (40) | 52 (24) | 1084 (48) | |
| Marrow blasts % | 5–9 | 83 (29) | 73 (34) | 561 (25) |
| 10–19 | 89 (31) | 91 (42) | 580 (26) | |
| RA | 74 (26) | 21 (10) | 601 (27) | |
| RA-RS | 25 (9) | 18 (8) | 190 (8) | |
| FAB morphology | RAEB | 141 (49) | 120 (56) | 710 (32) |
| RAEBT | 37 (13) | 48 (22) | 256 (11) | |
| CMML | 10 (4) | 9 (4) | 483 (22) | |
| Low | 27 (9) | 0 (0) | 460 (21) | |
| Intermediate 1 | 66 (23) | 15 (7) | 997 (45) | |
| IPSS risk | Intermediate 2 | 133 (46) | 122 (57) | 602 (27) |
| High | 61 (21) | 78 (36) | 137 (6) | |
| Not classifiable | 0 (0) | 1 (0.5) | 44 (2) | |
| Prior therapy | Yes | 116 (40) | 61 (28) | 821 (37) |
| < 3 | 118 (41) | 127 (59) | 776 (35) | |
| Duration of MDS | 3–6 | 65 (23) | 43 (20) | 446 (20) |
| (mos) | 7–12 | 36 (13) | 25 (12) | 303 (14) |
| > 12 | 68 (24) | 21 (10) | 715 (32) | |
| Diploid | 1394 (62) | |||
| Trisomy 8 ± other | 157 (7) | |||
| Cytogenetic | 20q ± other | 77 (3) | ||
| abnormalities | Chromosome 7 abnormalities | 223 (10) | ||
| Miscellaneous | 254 (11) | |||
| Insufficient metaphases | 135 (6) | |||
| Only | 65 (23) | 1 (0.5) | ||
| Chromosome 5 | 1 other | 32 (11) | 1 (0.5) | |
| abnormality with or | 2 others | 17 (6) | 5 (2) | |
| without other | ≥ 3 others | 74 (26) | 67 (31) | |
| abnormalities | Chromosome 7 | 99 (34) | 142 (66) | |
Figure 1.
Survival in MDS by the presence or absence of chromosome 5 abnormalities (A).
The distribution of patients with chromosome 5 abnormalities (n=503) was: deletion 5q only-65 (13%); deletion 5q and one other abnormality – 32 (6%); deletion 5q and two other abnormalities – 17 (3%); deletion 5q and three or more abnormalities – 74 (15%); deletion 5q and chromosome 7 abnormality 99 (20%); monosomy 5 only or with one or two other abnormalities – 7 (1.5%); monosomy 5 and three or more abnormalities – 67 (13%); monosomy 5 and chromosome 7 abnormality 142 (28%).
Survival of patients with monosomy 5 was worse than with deletion 5q. Much of this worse prognosis is related to its association with more advanced MDS and with more adverse cytogenetic abnormalities (Table 1; analyzed later). To provide a clear assessment of the prognosis of MDS and deletion 5q, the primary concern of this study, we first evaluated these patients separately.
Outcome of patients with deletion 5q
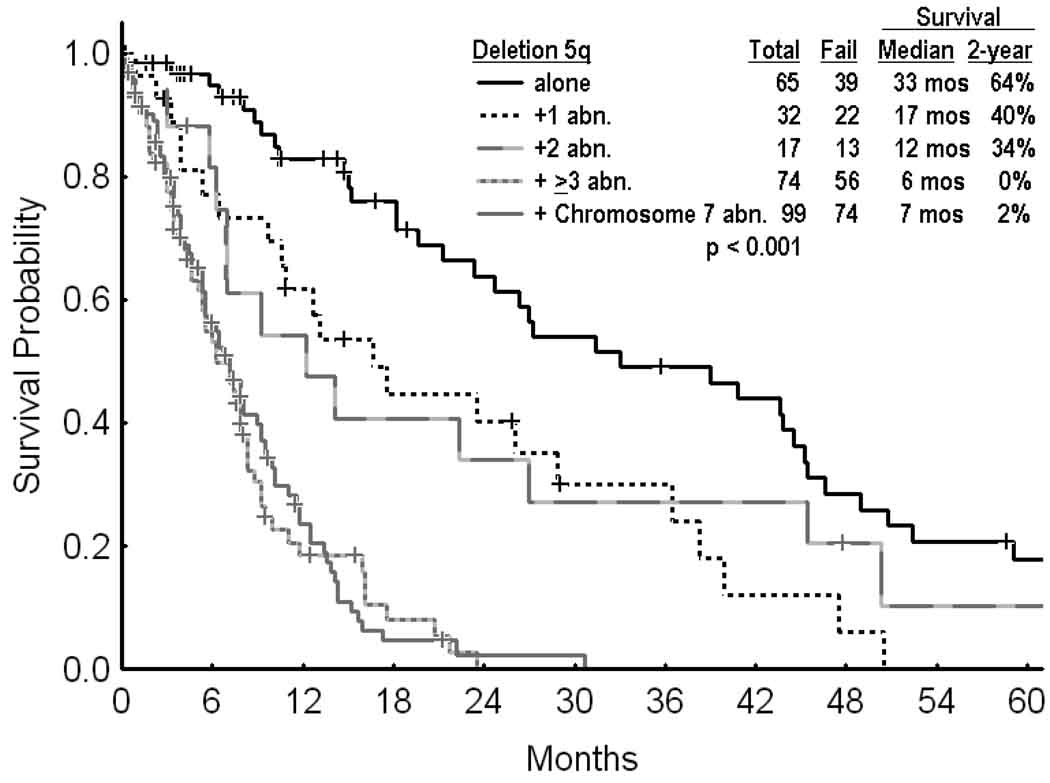
The median survival was 33 months in patients with deletion 5q alone, 17 months in patients with the presence of one additional abnormality, and 6–12 months in patients with ≥ 2 additional abnormalities or with chromosome 7 abnormalities (Figure 2, p<0.001).
Figure 2.
Survival in MDS and deletion 5q by the presence of additional chromosomal abnormalities.
The distribution of patients with MDS and deletion 5q without or with additional cytogenetic abnormalities by FAB and IPSS categories are detailed in Table 2: 93% of patients with low risk IPSS had only deletion 5q (this is because the IPSS low risk would not allow for any additional chromosomal abnormalities except for loss of chromosome Y; this would recategorize the karyotype as intermediate, (i.e. Score 0.5); 57% of patients with refractory anemia and 49% of patients with < 5% blasts had deletion 5q alone or with one additional abnormality. However most patients with deletion 5q and ≥ 2 abnormalities or additional chromosome 7 abnormality had advanced MDS by IPSS (as expected per IPSS definition) or FAB.
Table 2.
Distribution of Patients with Deletion 5q by FAB, IPSS, and percent of marrow blasts
| No. (%) of Row | ||||||
|---|---|---|---|---|---|---|
| A. FAB Group | Alone (n=65) |
+1 Abn (n=32) |
+2 Abn (n=17) |
+ ≥3 Abn (n=74) |
+ 7 Chromosome Abn (n=99) |
Total (n=287) |
| RA | 33 (45) | 9 (12) | 7 (10) | 12 (16) | 13 (18) | 74 (26) |
| RA – RS | 6 (24) | 4 (16) | 3 (12) | 3 (12) | 9 (36) | 25 (9) |
| RAEB | 23 (16) | 17 (12) | 5 (4) | 47 (33) | 49 (35) | 141 (49) |
| RAEBT | 1 (3) | 1 (3) | 1 (3) | 11 (30) | 23 (62) | 37 (13) |
| CMML | 2 (20) | 1 (10) | 1 (10) | 1 (10) | 5 (50) | 10 (4) |
| B. IPSS Risk * | ||||||
| Low | 25 (93) | 2 (7) | 0 | 0 | 0 | 27 (9) |
| Intermediate 1 | 30 (46) | 17 (26) | 7 (11) | 6 (9) | 6 (9) | 66 (23) |
| Intermediate 2 | 10 (8) | 11 (8) | 9 (7) | 42 (32) | 61 (46) | 133 (46) |
| High | 0 | 2 (3) | 1 (2) | 26 (43) | 32 (53) | 61 (21) |
| C. Percent Marrow Blasts | ||||||
| 0–4 | 41 (36) | 15 (13) | 11 (10) | 21 (28) | 27 (24) | 115 (40) |
| 5–9 | 12 (15) | 9 (11) | 3 (4) | 22 (27) | 37 (45) | 83 (29) |
| 10–19 | 12 (14) | 8 (9) | 3 (3) | 31 (35) | 35 (39) | 89 (31) |
Patients with prior therapy were classified by their IPSS risk group at the time of referral; abn=abnormality
Outcome of patients with “lower risk” MDS and deletion 5q
The lenalidomide experience involved mostly patients with “lower risk” MDS, i.e. IPSS low-intermediate 1 risk. To provide the comparative experience, two analyses were performed.
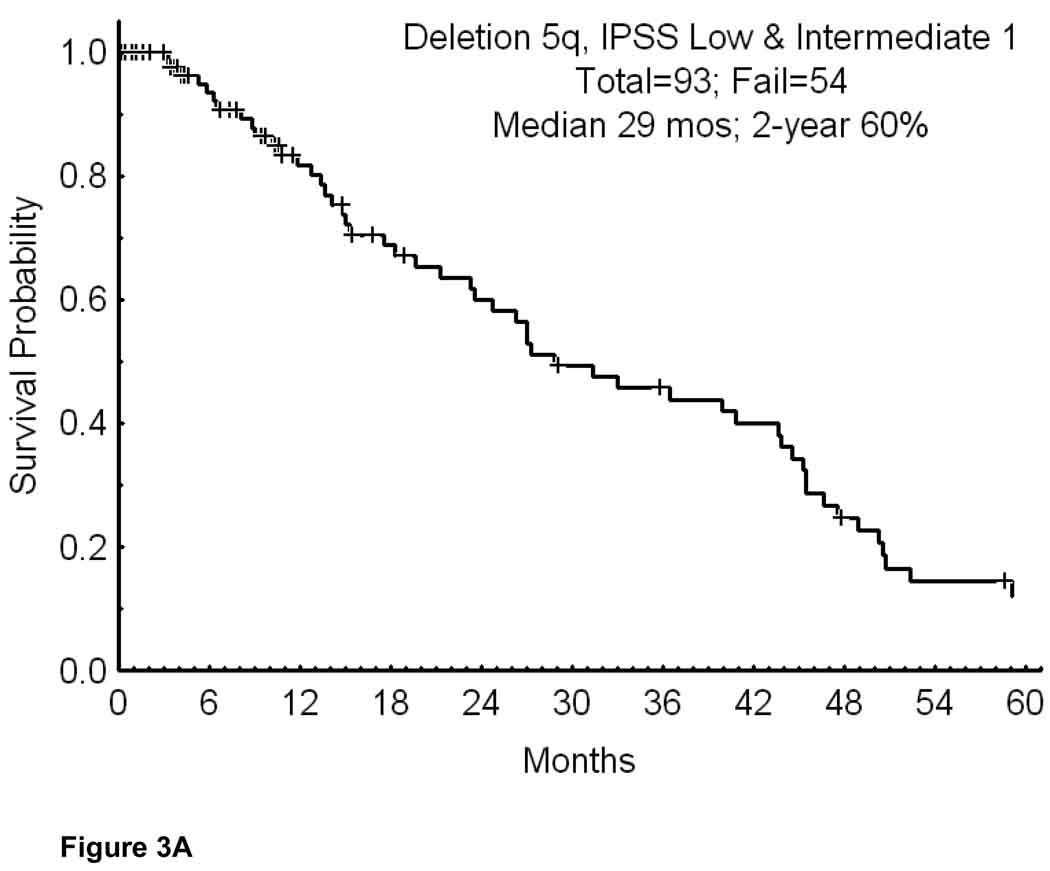
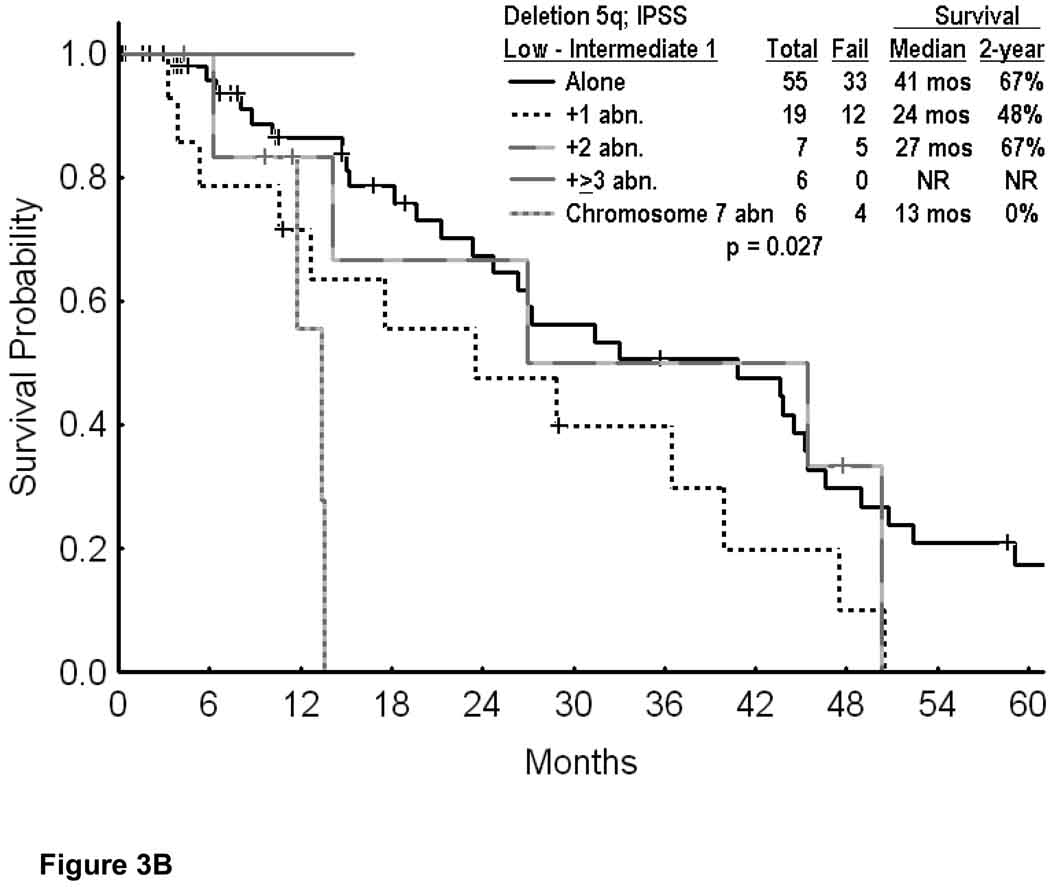
In the first analysis we included only patients with IPSS low-intermediate 1 risk disease. This reduced the number of patients to 93 (Appendix I; Table1). Their median survival was 29 months; the 2-year survival rate was 60% (Figure 3A). Figure 3B shows the survival by cytogenetic category. While the numbers were small in some subsets, the 2-year survival rates were significantly poorer with the presence of additional cytogenetic abnormalities or with chromosome 7 abnormalities (p=0.027). Considering patients with the same entry criteria as in the original lenalidomide study (i.e. platelets > 50×109/L) the number of patients was 89. It decreased to 58 (2.1% of total MDS group) if secondary MDS and proliferative chronic myelomonocytic leukemia were also excluded (i.e. similar to the entry criteria on the pivotal lenalidomide study); Only 5 of the 58 patients received lenalidomide. Their median survival was 44 months and the 2-year survival rate 80%. Thirty-eight of the 58 patients had deletion 5q alone; their 2-year survival rate was 85%.
Appendix I Table 1.
Characteristics of Patients with deletion 5q Who Presented with Lower Risk MDS
| No. (%) | |||
|---|---|---|---|
| Parameter | Category | IPSS Low- Intermediate 1 (n=93) |
Less Than 5 % Blasts (n=115) |
| Age (years) | ≥ 60 | 74 (80) | 85 (74) |
| Gender | Female | 47 (51) | 53 (46) |
| Hemoglobin (g/dl) | < 10 | 65 (70) | 92 (80) |
| Platelets (×109/L) | < 100 | 16 (17) | 53 (46) |
| WBC (×109/L) | < 1 | 0 | 1 (1) |
| Absolute granulocyte count (×109/L) | < 1 | 7 (8) | 24 (21) |
| Marrow blasts % | < 5 | 73 (79) | 115 (100) |
| 5–10 | 18 (19) | 0 | |
| 10–19 | 2 (2) | 0 | |
| FAB morphology | RA-RAS | 67 (72) | 98 (85) |
| RAEB | 25 (27) | 14 (12) | |
| RAEBT | 1 (1) | 1 (1) | |
| CMML | 0 | 2 (2) | |
| IPSS risk | Low | 27 (29) | 27 (24) |
| Intermediate 1 | 66 (71) | 46 (40) | |
| Prior therapy | No | 41 (44) | 65 (57) |
| Yes | 52 (56) | 50 (44) | |
| Duration of MDS (mos) | < 3 | 16 917) | 34 (30) |
| 3–12 | 35 (37) | 43 (37) | |
| > 12 | 42 (45) | 38 (33) | |
| Cytogenetic abnormalities in addition | + 1 | 19 (20) | 15 (13) |
| to chromosome 5 | + 2 | 7 (8) | 11 (10) |
| + ≥ 3 | 6 (7) | 21 (18) | |
| + chromosome 7 abnormality |
6 (7) | 27 (23) | |
Figure 3.
Survival in MDS IPSS Low-Intermediate 1 and deletion 5q (A; n=108); and survival in MDS IPSS Low-Intermediate 1 risk by the specific abnormalities associated with deletion 5q (B).
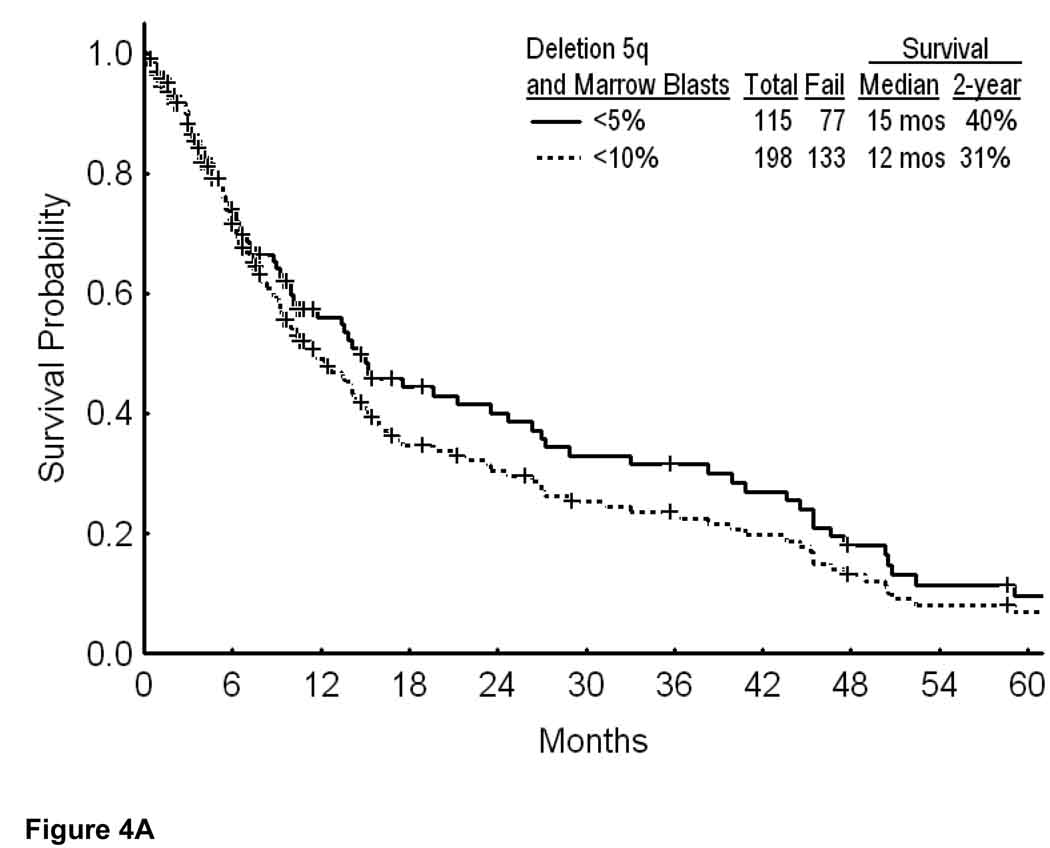
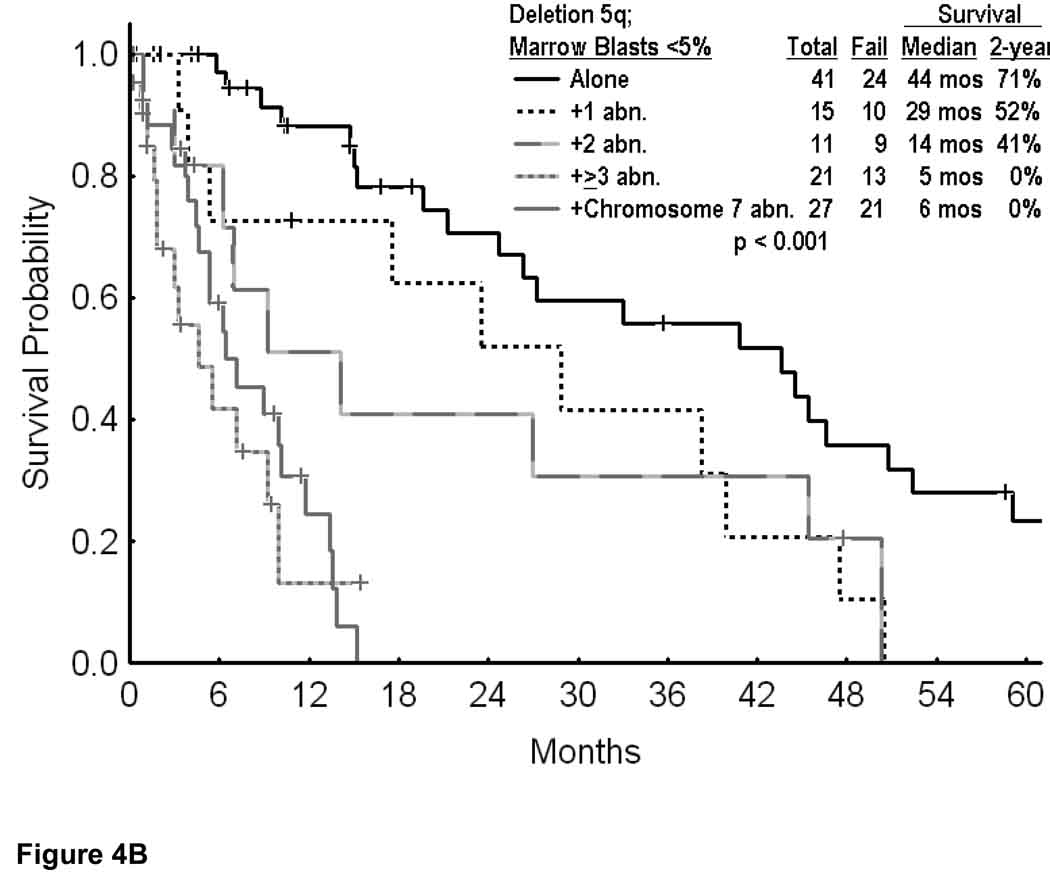
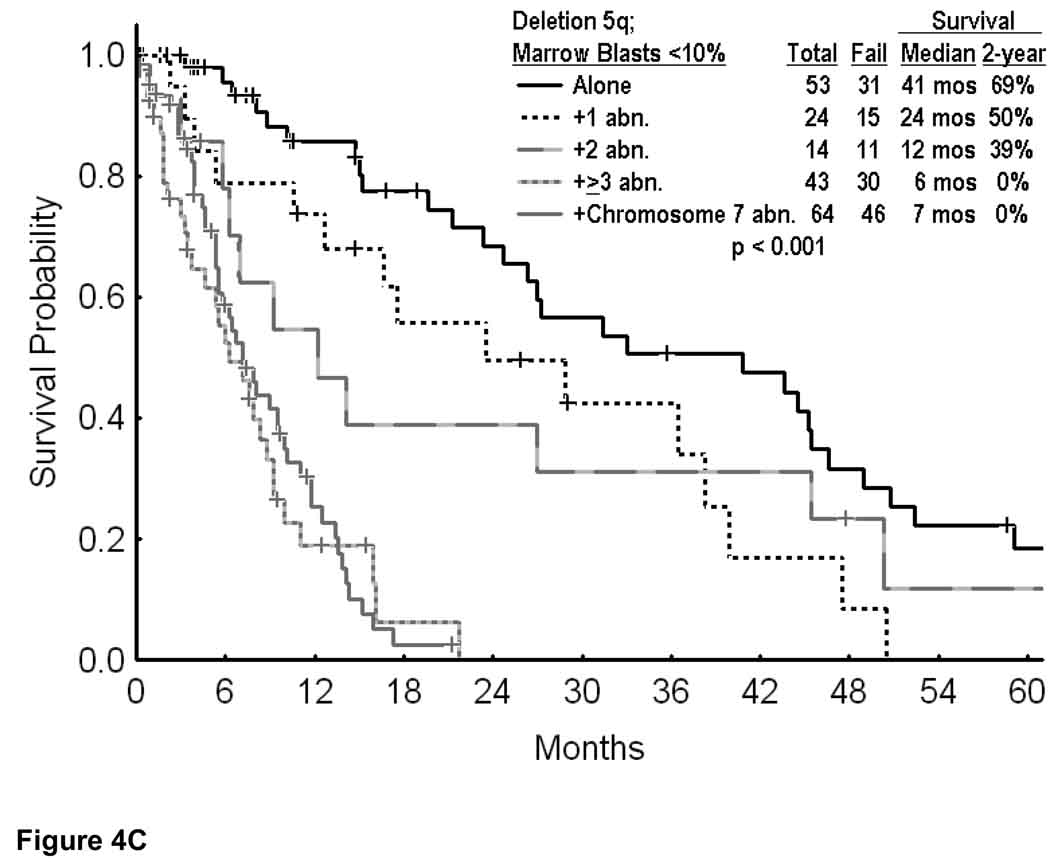
The second analysis focused on patients with deletion 5q and low percent of marrow blasts, since these are presently sometimes offered lenalidomide therapy. Overall, 198 patients had blasts < 10%, and 115 patients had blasts < 5%. In these two categories, overall survival is shown in Figure 4A. The median survival was 15 months if marrow blasts were < 5%, and 12 months if marrow blasts were < 10%. Survival by cytogenetic category for blasts < 5% is shown in Figure 4B, and for blasts < 10% in Figure 4C. Regardless of whether the marrow blasts were < 5% or < 10%, the estimated 2-year survival rates were 69% to 71% with deletion 5q alone, 50% to 52% with deletion 5q and one additional abnormality, but 0% with ≥ 3 additional cytogenetic abnormalities or a chromosome 7 abnormality (Figures 4B and C; p values < 0.001). Again, these figures illustrate the short survival of patients with deletion 5q and additional abnormalities.
Figure 4.
Survival in MDS and deletion 5q by the percent of bone marrow blasts (A); with marrow blasts <5% by the specific additional cytogenetic abnormalities (B); and with marrow blasts <10% by the specific additional cytogenetic abnormalities (C).
Outcome of patients with monosomy 5
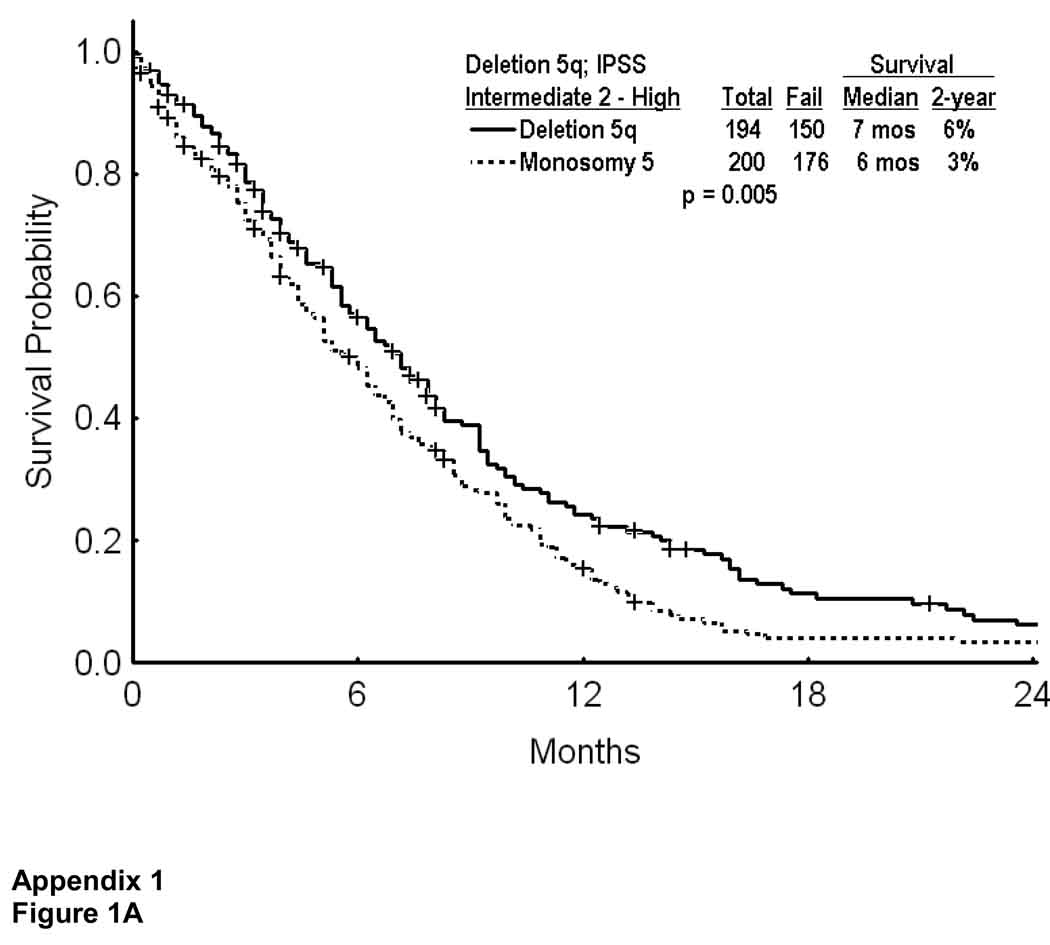
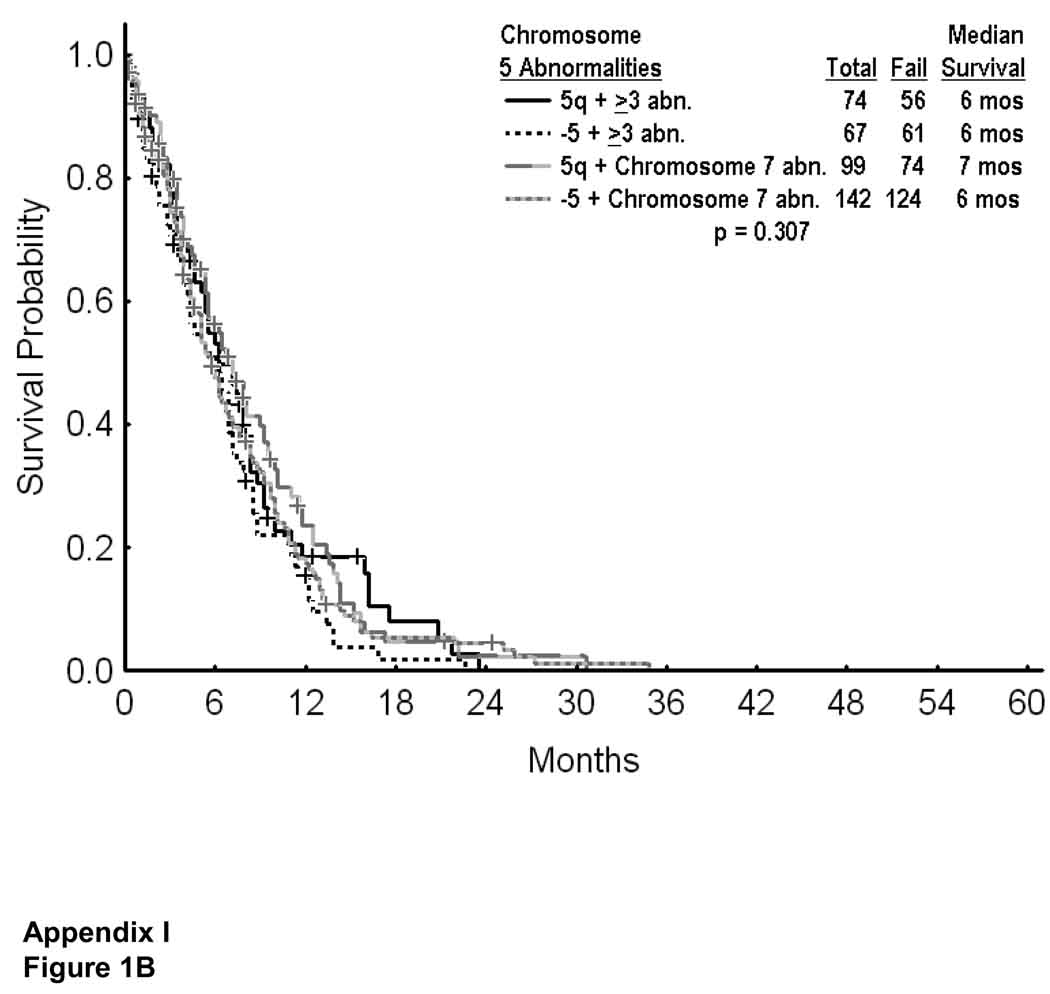
Unlike patients with deletion 5q, most patients with monosomy 5 had higher risk MDS (IPSS intermediate 2 or high in 93%, Table 1). They also had a significantly higher incidence of additional cytogenetic abnormalities, and of additional chromosome 7 abnormalities, both being adverse (Table 1). Survival of MDS with monosomy 5 was worse than with deletion 5q in patients with IPSS intermediate 2 or high risk (Appendix I; Figure 1A). Survival was however similar with monosomy 5 versus deletion 5q in patients with ≥ 3 abnormalities or additional chromosome 7 abnormality (Appendix I, Figure 1B; p=0.307)
APPENDIX I Figure 1.
Survival in MDS with deletion 5q versus monosomy 5 in patients with IPSS intermediate 2 or high risk (A), and by presence of additional cytogenetic abnormalities (B).
DISCUSSION
The experience with lenalidomide in patients with lower risk MDS and deletion 5q showed cytogenetic responses, independent of the presence or absence of additional cytogenetic abnormalities. The multivariate analysis did not select the percent of marrow blasts or the French-American British (FAB) subtype as prognostic.(11) However, only 6 of 148 patients (4%) had a “complex” karyotype i.e. chromosome 5 and ≥ 2 additional abnormalities (total ≥ 3 abnormalities), and 31 (21%) has “excess blasts”, i.e. ≥ 5 marrow blasts.(11) This suggested the need to establish a historical experience against which the lenalidomide experience could be evaluated.
Our study highlights some important points.
The first is that considering patients with MDS and deletion 5q as one group, as might have been inferred by practicing oncologists from the lenalidomide experience, may not be appropriate. For example 34% of our patients with deletion 5q also had chromosome 7 abnormalities, which conferred a very poor prognosis. This would also be true if only patients with < 5% blasts were considered: 27/115 (23%) of patients had deletion 5q and 7 abnormalities; their median survival was only 6 months, and the 2-year survival 0%. This subset comprised at most 7% of patients treated in the pivotal lenalidomide study.(11) The potential survival benefit with lenalidomide therapy would be easily and rapidly identifiable if a substantial group of such patients is treated with lenalidomide.
A second important point is the generally poor survival of patients who have deletion 5q with other abnormalities. Among such groups, even considering “lower risk MDS” (i.e. blasts < 5%) the median survivals ranged from 6 to 29 months and the 2-year survival rates from 0% to 52%. Again, among such groups, the potential benefit of lenalidomide would be easily identifiable. These findings have also been noted by others in smaller study groups.(8–10,16, 17) Giagounidis et. al. compared 66 patients with MDS and isolated deletion 5q with 10 patients with deletion 5q and one additional abnormality. The median survivals were 146 months versus 45 months (p=0.0085).(8) Cermak reviewed 29 patients with MDS and deletion 5q; 12 had additional chromosomal abnormalities and had a worse survival (median 35 months) compared with the others (median 45 months).(10) del Mar Mallo, et al reviewed their experience from Spain in 305 patients with deletion 5q. Survival in their study was strongly associated with the presence of additional chromosomal abnormalities; the median survival was 69 months among 204 patients with deletion 5q alone, 55 months among 52 patients with deletion 5q and 1 additional abnormality but only 8 months, among 49 patients with deletion 5q and 2 to 5 additional abnormalities. (16) Holtan et. al. reviewed the Mayo Clinic experience over a 15-year period in 130 patients with MDS and deletion 5q. (17) Similar to our study, and in contrast to the experience of del Mar Mallo et. al (16), the median survival of patients in their study was only 9.5 months. This suggests a difference in the profile of patients referred to tertiary centers (as opposed to patients seen in the oncology community), who had a high incidence of complex karyotypes (≥ 3 abnormalities; incidence 58% in the series from the Mayo Clinic but only 16% in the Spanish series). Holtman et al also found the presence of deletion 5q and 2 or more additional chromosomal abnormalities to be significant dominant independent adverse prognostic factor (17).
A third issue concerns the use of lenalidomide in current oncology practice. The incidence of deletion 5q in MDS is about 10%; the incidence of any chromosome 5 abnormality is about 20%. From our analysis, the percent of patients who are candidates for lenalidomide, based on the entry criteria of the original study of List et. al (11) (IPSS low or intermediate 1, deletion 5q, platelets more than 50×109/L) would be 2% to 3%. Many oncologists may be offering lenalidomide to patients with MDS subsets in which lenalidomide is largely untested in controlled trials, and where lenalidomide may or may not be effective.
The final issue concerns patients with MDS and deletion 5q versus other chromosome 5 abnormalities, essentially monosomy 5. The MDS literature is not very clear about such distinctions, often categorizing both groups as “chromosome 5 abnormalities”. The lenalidomide experience emphasized the need to clarify whether the two groups, deletion 5q and monosomy 5, had different presentations or prognoses. Our analysis showed that monosomy 5 is more often associated with advanced MDS (IPSS intermediate 2 and high risk) as well as with other adverse cytogenetic findings (multiple additional abnormalities; chromosome 7 abnormalities), and was uncommon in lower risk MDS and as a single abnormality. However, within the context of the more adverse groups (where both deletion 5q and monosomy 5 occur at a reasonable frequency), outcomes with deletion 5q versus monosomy 5 were similar. (Figure 1B)
In summary, this analysis established baseline expectations for patients with MDS and deletion 5q (as well as monosomy 5) by different cytogenetic subsets, and among patients with “lower risk” MDS. This database could be used for comparative purposes with the lenalidomide experience.
Acknowledgments
Supported in part by Leukemia SPORE Grant 1P50CA100632-04, and PO1CA108631, NIH/NCI
REFERENCES
- 1.Bennett JM, Catovsky D, Daniel MT, et al. Proposals for the classification of the myelodysplastic syndromes. Br J Heamatol. 1982;51:189–199. [PubMed] [Google Scholar]
- 2.Harris NJ, Jaffe ES, Diebold J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting. J Clin Oncol; Airlie House; November 1977; Virginia. 1999. pp. 3835–3849. [DOI] [PubMed] [Google Scholar]
- 3.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088. [PubMed] [Google Scholar]
- 4.Kantarjian H, O’Brien S, Ravandi F, et al. Proposal for a new risk model in myelodysplastic syndrome that accounts for events not considered in the original international prognostic scoring system. Cancer. 2008;113:1351–1361. doi: 10.1002/cncr.23697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faderl S, Kantarjian H. Myelodysplastic Syndromes. In: DeVita V, Lawrence T, Rosenberg S, editors. Cancer: Principles and Practice of Oncology. 8th Edition. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. pp. 2292–2303. [Google Scholar]
- 6.Nimer S, Golde D. The 5q–abnormality. Blood. 1987;70:1705–1712. [PubMed] [Google Scholar]
- 7.Nimer S. Clinical management of myelodysplastic syndromes with interstitial deletion of chromosome 5q. JCO. 2006;24:2576–2582. doi: 10.1200/JCO.2005.03.6715. [DOI] [PubMed] [Google Scholar]
- 8.Giagounidis AAN, Germing U, Haase S, et al. Clinical, morphological, cytogenetic, and prognostic features of patients with myelodysplastic syndromes and del(5q) including band q31. Leukemia. 2004;18:113–119. doi: 10.1038/sj.leu.2403189. [DOI] [PubMed] [Google Scholar]
- 9.Mathew P, Tefferi A, Dewald GW, et al. The 5q- syndrome: A single-institution study of 43 consecutive patients. Blood. 1993;81:1040–1045. [PubMed] [Google Scholar]
- 10.Cermák J, Michalová K, Brezinová J, et al. A prognostic impact of separation of refractory cytopenia with multilineage dysplasia and 5q-syndrome from refractory anemia in primary myelodysplastic syndrome. Leuk Res. 2003;27:221–229. doi: 10.1016/s0145-2126(02)00096-6. [DOI] [PubMed] [Google Scholar]
- 11.List A, Dewald G, Bennett J, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355:1456–1465. doi: 10.1056/NEJMoa061292. [DOI] [PubMed] [Google Scholar]
- 12.European Medicines Agency. Assessment Report for Lenalidomide Celgene Europe. London: 2008. May 30, Doc. Ref: EMEA/CHMP/249329/2008. [Google Scholar]
- 13.Göhring G, Giagounidis A, Aul C, Büsche G, Kreipe H, Hellström-Lindberg E, Knight RD, Schlegelberger B. Long-Term Cytogenetic Follow-up of MDS Patients with 5q- Treated within the MDS-003 (CC-5013-MDS-003) Study: Evolution to Complex Clones and Progression to AML. Blood. 2008;112 abst 1647. [Google Scholar]
- 14.Kantarjian H, Talpaz M, O'Brien S, et al. Survival benefit with imatinib mesylate versus interferon-α-based regimens in newly diagnosed chronic-phase chronic myelogenous leukemia. Blood. 2006;108:1835–1840. doi: 10.1182/blood-2006-02-004325. [DOI] [PubMed] [Google Scholar]
- 15.Roy L, Guilhot J, Krahnke T, et al. Survival advantage from Imatinib compared to the combination Interferon-α plus Cytarabine in chronic phase CML: historical comparison between two phase III trials. Blood. 2006;108:1478–1484. doi: 10.1182/blood-2006-02-001495. [DOI] [PubMed] [Google Scholar]
- 16.del Mar Mallo M, Cervera J, Schanz J, et al. Prognostic Impact of Additional Chromosomal Aberrations (ACA) to 5q- in Patients with primary Myelodysplastic Syndrome. Blood. 2008;112(S1) abst 1649. [Google Scholar]
- 17.Holtan S, Santana-Davila R, DeWald G, et al. Myelodysplastic syndromes associated with interstitial deletion of chromosome 5q: Clinicopathologic correlations and new insights from the prelenalidomide era. Am. J. Hematol. 2008;83:708–713. doi: 10.1002/ajh.21245. [DOI] [PubMed] [Google Scholar]