Abstract
Objective
To assess the efficacy and safety of low dose adrenaline injected subcutaneously to prevent acute adverse reactions to polyspecific antivenom serum in patients admitted to hospital after snake bite.
Design
Prospective, double blind, randomised, placebo controlled trial.
Setting
District general hospital in Sri Lanka.
Subjects
105 patients with signs of envenomation after snake bite, randomised to receive either adrenaline (cases) or placebo (controls) immediately before infusion of antivenom serum.
Interventions
Adrenaline 0.25 ml (1:1000).
Main outcome measures
Development of acute adverse reactions to serum and side effects attributable to adrenaline.
Results
56 patients (cases) received adrenaline and 49 (controls) received placebo as pretreatment. Six (11%) adrenaline patients and 21 (43%) control patients developed acute adverse reactions to antivenom serum (P=0.0002). Significant reductions in acute adverse reactions to serum were also seen in the adrenaline patients for each category of mild, moderate, and severe reactions. There were no significant adverse effects attributable to adrenaline.
Conclusions
Use of 0.25 ml of 1:1000 adrenaline given subcutaneously immediately before administration of antivenom serum to patients with envenomation after snake bite reduces the incidence of acute adverse reactions to serum.
Key messages
Antivenom serum is the only effective treatment for envenomation after snake bite
Acute adverse reactions to the serum are common and include anaphylactic shock
The prophylactic use of 1:1000 adrenaline in a dose of 0.25 ml given subcutaneously immediately before infusion of antivenom serum significantly reduces the risk of acute adverse reactions
Introduction
Antivenom serum is the most effective, if not the only effective, treatment available for management of snake bite envenomation. Adverse effects of the serum are common,1 and anaphylaxis can be fatal. Increasing the safety of treatment with antivenom serum for snake bite victims is, therefore, a matter of high priority.
Several methods have been used to reduce acute adverse reactions to the serum. The most widely used serum is polyvalent, but there is now a trend towards production of monovalent serum. There is no evidence, however, that this would result in fewer adverse effects than polyvalent antivenom serum. Immunotherapy, another possible treatment option, is still at an early experimental stage. A small test dose of antivenom serum can be used to detect patients who may develop acute adverse reactions to antivenom. This method, however, is insensitive and can give rise to anaphylaxis by itself.2
Prophylactic use of hydrocortisone and antihistamines before infusion with antivenom serum is also practised. Antihistamines, however, counter only the effects of histamine release. Hydrocortisone takes time to act, and hence it will not be effective against acute adverse reactions that can develop almost immediately after serum treatment, which is very often administered urgently to snake bite victims. For these reasons it is not established practice to give hydrocortisone or antihistamines routinely to patients with snake bite before antivenom serum treatment.
Adrenaline is the drug of choice in the treatment of anaphylaxis, and it is likely to be useful in the prevention of such acute reactions to serum. There is a general reluctance to use adrenaline because of potential side effects and lack of clear guidelines on use. The only available data on use of adrenaline before treatment with antivenom serum are from a few uncontrolled retrospective studies from Australia.3 These data suggest that adrenaline is safe and effective in reducing acute adverse reactions.
Methods
We assessed the efficacy and safety of low dose, subcutaneous adrenaline as prophylaxis against acute adverse reactions to antivenom serum. The study was a prospective, randomised, double blind, placebo controlled trial conducted in north eastern Sri Lanka. It was carried out during the rice paddy harvesting season, when there is a high admission rate for snake bite. Consecutive patients who were admitted after a snake bite and required antivenom serum because of systemic envenomation or severe local envenomation were randomised to receive either adrenaline (cases) or identical appearing placebo (controls) as pretreatment. The randomisation unit was the individual and the schedule determined by computer generated random numbers. The schedule was known only to one author (SBG), who did not participate in the treatment of patients or data collection. Patients were excluded from the study if they were pregnant; aged under 12 or over 70 years; said they had received antisera, including antivenom serum, in the past; had known adverse reactions to adrenaline, atopy, wheezing, hypertension, ischaemic heart disease, transient ischaemic attacks, strokes, or unexplained focal neurological signs; or had received any treatment other than first aid before admission. Patients were also excluded if electrocardiography on admission showed ischaemic changes or arrhythmias. Written informed consent was obtained from all participating patients.
Treatment and follow up
All patients were given 0.25 ml of 1:1000 adrenaline or placebo injected subcutaneously into the forearm immediately before infusion with antivenom was started. Placebo consisted of 0.9% sodium chloride. Adrenaline and placebo were drawn into 1 ml “insulin” syringes which were stored in closed rigiform containers and refrigerated at 4°C. No test dose of antivenom was used, and neither hydrocortisone nor promethazine were given as pretreatment. Patients were monitored for development of acute adverse reactions to the serum and the test drug. Pulse and blood pressure (every 15 minutes) and an electrocardiogram (at least once within 30 minutes of being given the test drug and whenever clinically indicated) were monitored during infusion of antivenom and for 1 hour thereafter.
Neurological evaluations were done at the beginning and end of the infusion and whenever relevant during the observation period. Patients who received antivenom serum were kept in hospital for at least 96 hours after the infusion. Acute adverse reactions were graded as mild, moderate, or severe according to the following criteria: mild—pruritus, skin rash without pruritus, mild urticaria, fever, rigors, nausea, vomiting; moderate—extensive urticaria, facial oedema, bronchospasm without cyanosis; severe—pulmonary oedema, bronchospasm with cyanosis, stridor, shock. If a reaction developed while the patient was receiving the serum or if the patient developed arrhythmias, ischaemic changes on the electrocardiogram, or a measurable rise in blood pressure (systolic >20 mm Hg or diastolic >10 mm Hg from level before test drug was given) after the test drug and the serum, he or she was given appropriate treatment and did not take further part in the trial. Reactions to antivenom serum were initially treated by stopping the infusion and administering 0.5 ml of 1:1000 adrenaline intramuscularly, 200 mg hydrocortisone intravenously, and 25 mg promethazine intramuscularly.
Statistical analysis
In a pilot study in which we retrospectively analysed records of snake bite victims admitted to Base Hospital, Polonnaruwa, between June and December 1997 we found adverse reactions to antivenom documented in 87 (38.2%) of 228 patients. We estimated that acute adverse reactions occur in about 40% of patients who receive antivenom serum and therefore calculated (by using epi Info 6.03) that a sample size of 210 would be needed to detect a relative risk ratio of 1.5 with 80% power and 95% confidence in a prospective, randomised cohort study. Differences between cases and controls were analysed with χ2 test and Fisher’s exact test for dichotomous variables and Student’s t test for continuous variables.
Ethics
Ethical clearance was obtained from the ethics committee, Faculty of Medicine, University of Kelaniya. Although we planned to recruit 210 patients, ethical approval was given on condition that randomisation schedules would be decoded and a preliminary analysis of data performed at a half way point in the study.
Results
The study started in March 1998 and ended in July 1998, when 105 patients had been recruited. This was because analysis of results at the half way point in the study showed significant differences in the incidence of acute adverse reactions between cases and controls.
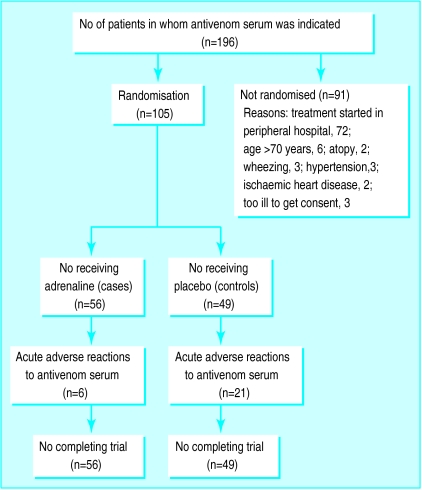
Within this 4 month period a total of 196 patients (134 males) were admitted to Polonnaruwa Hospital with envenomation after snake bite for whom treatment with antivenom serum was indicated. Only 105 could be recruited to the study as the other 91 had one or more exclusion criteria (figure). Of the 105 patients recruited, 56 received adrenaline and 49 received placebo. Patients’ sociodemographic characteristics and degree of envenomation on admission are shown in table 1. There was no significant difference between the two groups for any variable. Ten vials of Haffkine polyspecific antivenom serum (Haffkine Laboratories, Mumbai, India), an equine serum product, were used for all patients. The serum was administered intravenously as a 200 ml infusion (reconstituted with sterile distilled water and diluted in 0.9% sodium chloride) at 50 drops per minute (with an infusion set in which 15 drops deliver 1 ml) over 1 hour. All serum used during the study was from the same batch supplied to the hospital.
Table 1.
Characteristics and degree of envenomation on admission of patients according to allocation to pretreatment with adrenaline or placebo. Values are numbers of patients unless stated otherwise
| Detail | Adrenaline (n=56) | Placebo (n=49) |
|---|---|---|
| Male | 42 | 36 |
| Mean (SD; range) age (years) | 38.39 (13.09; 14-65) | 34.61 (13.11; 17-65) |
| Mean (SD; range) weight (kg) | 52.8 (12.4; 36.5-71) | 55.7 (14.1; 42-68) |
| Species of snake*: | ||
| Uncertain | 24 | 23 |
| Russell’s viper (Daboia russelli) | 22 | 17 |
| Cobra (Naja naja) | 5 | 6 |
| Sri Lankan krait (Bungarus ceylonicus) | 5 | 3 |
| First aid before admission: | ||
| Received | 4 | 4 |
| Not received | 52 | 45 |
| Mean (SD) delay in admission to hospital (min)† | 165 (89.4) | 136 (97.6) |
| Systemic envenomation‡: | 53 | 45 |
| Coagulopathy | 10 | 5 |
| Neuromuscular complications | 24 | 22 |
| Renal impairmant | 1 | 0 |
| More than one feature of envenomation: | 18 | 18 |
| Coagulopathy and neuromuscular | 14 | 12 |
| Coagulopathy and renal | 2 | 2 |
| Neuromuscular and renal | 2 | 2 |
| Coagulopathy, neuromuscular, and renal | 0 | 2 |
| Significant local envenomation only§ | 3 | 4 |
Type of snake mentioned only when dead snake was brought to hospital.
According to patient or accompanying person.
30 patients in adrenaline group and 27 in placebo group also had severe local swelling.
Swelling of more than half of bitten limb (none had necrosis).
None of the study patients died. Table 2 shows the incidence of acute adverse reactions to antivenom serum. There was a highly significant reduction of acute adverse events in the adrenaline group. Significant reductions in acute adverse reactions to the serum were also seen in the adrenaline group for each category of mild, moderate, and severe reaction.
Table 2.
Acute adverse reactions to antivenom serum in patients bitten by snakes, according to pretreatment (adrenaline or placebo)
| Detail | Adrenaline (n=56) | Placebo (n=49) | Relative risk (95% CI) | P value |
|---|---|---|---|---|
| No (%) of reactions to serum | 6 (11) | 21 (43) | 0.25 (0.11 to 0.57) | 0.0002 |
| Timing of adverse reactions to serum (min): | ||||
| <15 | 5 | 19 | ||
| 15-30 | 1 | 2 | ||
| >30 | 0 | 0 | ||
| No (%) of reactions according to severity: | ||||
| Mild | 2 (4) | 7 (14) | 0.25 (0.05 to 1.15) | 0.05 |
| Moderate* | 4 (7) | 10 (20) | 0.35 (0.12 to 1.05) | 0.04 |
| Severe* | 0 | 4 (8) | 0.00 (0.00 to 1.3) | 0.04 |
9 patients with moderate reactions and 2 with severe reactions in the placebo group, and all 4 patients with moderate reactions in the adrenaline group in addition had one or more features that were classified under mild reactions.
In none of the patients who received adrenaline or placebo did blood pressure rise over 160/100 mm Hg nor did any experience neurological deficit suggestive of a cerebrovascular accident during monitoring. Two patients who received adrenaline and five who received placebo developed sinus tachycardia. No patient developed any other arrhythmia. Transient drowsiness was observed in one patient in the adrenaline group and three in the placebo group. These were probably effects of envenomation or antivenom serum rather than effects of the premedication (all four patients had other features of a moderate or severe acute adverse reaction to the serum).
Discussion
There is no clear scientific evidence or uniform policy for the use of premedication to prevent acute adverse reactions to antivenom serum. Among the drugs used hydrocortisone and antihistamines, although probably of little value, seem to be the most preferred. Adrenaline is used in only a few centres.4 In a retrospective analysis of the use of premedication before treatment with antivenom serum for snake bite in Australia, adrenaline, steroids, and antihistamines used in different combinations were found to reduce adverse reactions to the serum from 12.5% to 3%.3 Although the data did not differentiate between effects of individual drugs, the most effective premedication in the combinations seemed to be adrenaline.
A major concern regarding the use of adrenaline as premedication is the potential risk of intracerebral haemorrhage; a result of the combination of the ability of certain snake venoms to cause coagulopathy and the risk of hypertension with use of adrenaline. This has led to a reluctance to use adrenaline, and in Sri Lanka it is hardly, if ever, used. Few studies have analysed the risk of cerebral haemorrhage after use of adrenaline. Of seven cases of fatal intracerebral haemorrhage after snake bite documented in Australia, only three patients had received adrenaline as premedication, making the evidence incriminating adrenaline as the cause of cerebral haemorrhage weak.5 Fears of development of hypertension after low dose adrenaline also seem unfounded. In a study by Heilborn et al, eight patients who received 0.5 mg adrenaline subcutaneously showed only transient and probably insignificant rises in systolic blood pressure, while diastolic blood pressure decreased.6
Our study is the first prospective, randomised, placebo controlled trial to investigate the efficacy and safety of adrenaline as prophylaxis against acute adverse reactions to antivenom. Pretreatment with a small dose of subcutaneous adrenaline significantly reduced the incidence of acute adverse reactions to polyvalent antivenom serum. Adrenaline is cheap and easy to use. We did not encounter significant adverse effects attributable to it; there were no cases of acute neurological deficit suggestive of cerebrovascular accidents or patients in whom blood pressure rose significantly. It must be pointed out, however, that patients who were potentially at high risk for use of adrenaline were excluded from the study, and results regarding its safety should be viewed in this context. Nevertheless, the benefits of this treatment seem to outweigh potential risks. The use of low dose subcutaneous adrenaline as a prophylactic measure to prevent acute adverse reactions to antivenom can be recommended in patients who have no contraindications for its use.
Figure.
Progress of patients who were bitten by snakes through trial of adrenaline in prevention of acute reactions to antivenom serum
Acknowledgments
We thank Drs R Seneviratne, P Atapattu, L Wickremasinghe, T Gajanayake, M Mushin, R Niyas, and H Sathischandra and the nursing staff of the medical wards of the Base Hospital Polonnaruwa for their help with this study and Dr Pushpa Jayawardena for help with statistics.
Footnotes
Funding: No additional funding.
Competing interests: None declared.
References
- 1.Karunarathne KE de S, Anandadas JA. The use of antivenom in snakebite poisoning. Ceylon Med J. 1973;1:37–43. [PubMed] [Google Scholar]
- 2.Weerasinghe WMT. Treatment of snakebite. Ceylon Med J. 1983;28:182–185. [PubMed] [Google Scholar]
- 3.Sutherland SK. Antivenom use in Australia: premedication, adverse reactions and the use of venom detection kits. Med J Aust. 1992;157:734–735. [PubMed] [Google Scholar]
- 4.Sutherland SK. Treatment of snake bite in Australia and Papua New Guinea. Aust Fam Physician. 1976;5:272–288. [PubMed] [Google Scholar]
- 5.Tibballs J. Premedication for snake antivenom. Med J Aust. 1994;160:4–6. [PubMed] [Google Scholar]
- 6.Heilborn H, Hjemdahl P, Daleskog M, Adamsson U. Comparison of subcutaneous injection and high dose inhalation of epinephrine—implications for self treatment to prevent anaphylaxis. J Allergy Clin Immunol. 1986;78:1174–1179. doi: 10.1016/0091-6749(86)90268-x. [DOI] [PubMed] [Google Scholar]