Abstract
Cancer susceptibility results from interactions between sensitivity and resistance alleles. We employed murine chromosome substitution strains to study how resistance alleles affected sensitive alleles during chemically-induced lung carcinogenesis. The C57BL/6J-Chr#A/J strains, constructed by selectively breeding sensitive A/J and resistant C57BL/6J (B6) mice, each contain one pair of A/J chromosomes within an otherwise B6 genome. Pas1, the major locus responsible for this differential strain response to urethane carcinogenesis, resides on Chr 6, but C57BL/6J-Chr6A/J mice (hereafter CSS-6) developed few tumors following a single urethane injection, which demonstrates epistatic interactions with other B6 alleles. CSS6 mice developed dozens of lung tumors after chronic urethane exposure, however, indicating that these epistatic interactions could be overcome by repeated carcinogen administration. Unlike A/J, but similar to B6 mice, CSS6 mice were resistant to lung carcinogenesis induced by 3-methylcholanthrene (MCA). Tumor multiplicity increased if BHT administration followed urethane exposure, showing that a Chr 6 gene(s) regulates sensitivity to chemically-induced tumor promotion. Unlike A/J tumors (predominantly codon 61 A→ T transversions), Kras mutations in tumors induced by urethane in CSS-6 mice were similar to B6 tumors (codon 61 A→G transitions). DNA repair genes not located on Chr 6 may determine the nature of Kras mutations. CSS-6 mice are a valuable resource for testing the ability of candidate genes to modulate lung carcinogenesis.
Introduction
Lung cancer is the leading cause of cancer death in both men and women in the USA, with 200,000 new cases of lung cancer estimated to be diagnosed this year. The dismal 15% survival rate over 5 years indicates that only 30,000 of these patients will be alive in 2014(1). Smoking contributes to 85–90% of all lung cancers, but non-smoking-related lung cancer, which is more common in women than men, is the 7th leading cause of cancer death(2). Since only ~15% of smokers develop lung cancer, genetic factors presumably contribute to disease susceptibility.
Recent lung cancer familial linkage studies delineated regions on human Chromosomes 6 and 12 that contributed to the risk of lung cancer, chronic obstructive pulmonary disease (COPD), and diminished lung function (3;4). Chronic pulmonary inflammation predisposes toward lung cancer, and several chromosomal sites in mice that determine responsiveness to pro-inflammatory pneumotoxicants correspond to loci containing lung cancer susceptibility genes(5).
Mice develop pulmonary adenocarcinoma (AC) that is histologically and genetically similar to human AC within a relatively short time span (6–42 wks) in response to chemical carcinogens(6) and within 2–36 wks in response to genetic induction with oncogenes such as Kras(7–9). A/J mice are particularly sensitive to carcinogen-induced lung tumorigenesis, and develop spontaneous lung tumors during their lifespan(10). Conversely, C57BL/6J (B6) mice are resistant to both carcinogen-induced and spontaneous lung tumor formation(10;11). Mapping studies involving Mendelian crosses(12), recombinant inbred strains(13;14), and advanced intercross lines(15) using these two strains have yielded much information about lung cancer susceptibility. Among the many susceptibility genes mapped using A/J and B6, the Pas1 (Pulmonary adenoma susceptibility 1) locus on Chr 6 that contains six genes is responsible for > 60% of the differential susceptibility to lung carcinogenesis in these two strains (16). Polymorphisms in two candidate genes in this locus, Kras and Casc1(17), contribute directly to this difference in susceptibility, while polymorphisms in the Lrmp gene that maps to this site in mice are associated with survival in human lung cancer patients(18). Genome-wide association studies, linkage disequilibrium analysis, new mouse genetic models, high throughput screening, in silico mapping, and expression genetics are additional tools for identifying the responsible genes at these susceptibility loci.
Chromosome substitution strains (CSS mice) have been constructed by selectively breeding mice such that a pair of chromosomes from one strain is substituted into the genome of a second strain(19). For A/J and B6 mice, a panel of 22 strains of C57BL/6J-Chr#A/J mice (each of the 19 autosomes, X and Y chromosomes, and mitochondrial DNA, hereafter designated CSS#) contain a single pair of A/J chromosomes within the B6 background. Each strain is thus consomic for all A/J alleles on one chromosome, and any effects of B6 alleles that reside on other chromosomes on those A/J alleles can be assessed. We used these mice to examine functional aspects of chromosomes harboring Pas1 and other susceptibility loci to mouse lung tumorigenesis.
Materials and Methods
Mice
CSS breeding pairs were obtained from the breeding colony at Case Western Reserve University (CSS4, 6, 9, 10, 11, 14, 15, 17, 18, 19, Y) or from Jackson Laboratory (6, 11). These mice and (B6 × CSS6) F1 mice were bred at the Center for Laboratory Animal Control at the University of Colorado Denver. B6 and A/J mice were obtained from Jackson Laboratory. All mice were maintained on hardwood bedding with a 12 h dark/light cycle, and given water and Harlan Teklad 2018 global diet ad libitum according to protocols approved by the University of Colorado Institution of Animal Care and Use Committee. Pups were weaned at 3 wks of age and subjected to experimental procedures 3–7 wks later. Some CSS strains (CSS15, CSS18) were difficult to breed, so these neonates were placed in cages with CXB4/J or FVB/J surrogate mothers immediately after birth.
Carcinogenesis protocols
Single urethane carcinogenesis
Male and female mice were injected once IP with 1 mg urethane/g body weight dissolved in saline vehicle. Sixteen wks later, mice were sacrificed by lethal pentobarbital injection, and their lungs removed. Tumors were dissected from surrounding tissue using a dissecting microscope equipped with fiberoptic lighting and counted, and their diameters measured with digital calipers prior to freezing for subsequent Kras mutational analysis. In tumor progression studies, additional B6, A/J, and CSS6 mice were harvested 20, 32, and 42 wks after a single urethane injection.
Multiple urethane carcinogenesis
Male and female B6, A/J, CSS6, CSS11, and (B6 × CSS6) F1 mice were injected once weekly with 1 mg urethane/g body wt urethane for 6 wks, and tumors harvested as above 20 wks after the initial urethane injection.
Two-stage carcinogenesis
Male and female and CSS6 mice were subjected to either a single 1 mg/g urethane or 10 mg/kg MCA IP injection, followed by 6 weekly IP BHT injections (150 mg/kg week one, 200 mg/kg, thereafter). Mice serving as controls for 2-stage carcinogenesis mice received a single MCA dose followed by 6 weekly vehicle injections.
Male B6 mice were subjected to 6 weekly urethane injections (a regimen yielding 100% incidence and 5 tumors/mouse), followed by 6 weekly BHT injections (as above) to determine whether BHT treatment enhanced tumor number or tumor size, and tumors were harvested as described above.
Tumor histology
A/J and CSS6 were sacrificed 42 wks after a single urethane injection. Lungs were perfused with saline via the pulmonary artery and inflated with 10% buffered formalin for 1 hr prior to fixation in 10% buffered formalin for 24 hr. Fixed lungs were paraffin embedded, cut into 4 μm sections, affixed to glass slides, and stained with hematoxylin/eosin to visualize lung structure using an Olympus BX-41 microscope equipped with a digital camera.
Kras mutational analyses
Tumor DNA was prepared using the DNeasy (Qiagen) or Nextec (Express Biotech) kits for tissues according to the manufacturer's instructions. Mutational analysis was performed using PCR to amplify Kras exon 2 which contains codon 61, as described previously. Resulting PCR products were treated with ExoSapIT (USB) to remove unreacted dNTPs and oligos, and submitted to the University of Colorado Cancer Center DNA Core facility for sequencing. Urethane causes either an A→T transversion or A→G transition in the second position of the CAA codon 61 sequence, resulting in a GLN → LEU or GLN → ARG substitution, respectively.
Statistical Analysis
Data was analyzed using GraphPad Prism 4 software. Interactions between gender and strain were analyzed by 2-way ANOVA with a Bonferoni correction. Differences in multiplicity and diameter were determined using ANOVA with a Dunnett's post-hoc analysis; B6 values were used as controls. Incidence was analyzed by Chi square with a Bonferoni correction.
Results
Survey of selected B6.A strains for their susceptibility to urethane-induced lung carcinogenesis
Comparisons of lung tumor multiplicity, incidence, and size following a single urethane injection among eleven CSS strains, B6, and A/J mice are shown in Table 1. Three strains, CSS6, CSS11, and CSS17, developed significantly more tumors than B6, although substantially less than A/J mice. These three strains also had a higher incidence of tumor formation than B6. CSS11 mice were unique among these CSS strains in developing significantly larger tumors than B6, although tumors in CSS17, CSS19, and A/J mice exhibited a trend toward larger tumors. Gender differences and interactions between gender and strain were analyzed by 2-way ANOVA, and no interactions were evident. Gender differences were observed in A/J mice, with females developing 30% fewer tumors than males, but this was not seen in the B6 or CSS strains. Lung tumor susceptibility sites on Chrs 6 (Pas1) (20), 11 (Par1)(21), and 17 (Pas2)(16) have been described previously. Since Chr 6 contains the Pas1 site, which accounts for 2/3 of the variation in tumor multiplicity between A/J and B6 progenitors(16), perhaps the most remarkable data in Table 1 is the low tumor multiplicity in CSS6 mice (1.8 ± 0.3) relative to A/J (36 ± 2.6). This indicates considerable dilution of the effects of A/J Pas1 susceptibility alleles by B6 genes on other chromosomes.
Table I.
Urethane-induced lung tumor formation in B6, CSS, and A/J mice.
| Strain | N (male, female) | Multiplicity* | Fold-increase over B6 | Incidence† (%) | Diameter (mm)** | % Kras mutational frequency (N) |
|---|---|---|---|---|---|---|
| B6 | 26 (13, 13) | 0.5 ± 0.1 | -- | 27 | 0.5 ± 0.03 | 55 (11) |
| CSS4 | 19 (10, 9) | 0.3 ± 0.2 | 0.6 | 24 | 0.4 ± 0.04 | 60 (5) |
| CSS6 | 26 (14, 12) | 1.8 ± 0.3a | 3.9 | 8b | 0.6 ± 0.04 | 73 (51)c |
| CSS9 | 25 (13, 12) | 0.6 ± 0.1 | 1.3 | 48 | 0.5 ± 0.03 | 45 (11) |
| CSS10 | 27 (14, 13) | 0.4 ± 0.1 | 0.9 | 37 | 0.4 ± 0.03 | 60 (10) |
| CSS11 | 26 (16, 10) | 1.6 ± 0.2a | 3.5 | 88b | 0.7 ± 0.03a | 75 (36)c |
| CSS14 | 27 (15, 12) | 0.3 ± 0.1 | 0.6 | 22b | 0.5 ± 0.04 | 67 (3) |
| CSS15 | 26 (14, 12) | 0.8 ± 0.2 | 1.7 | 58 | 0.5 ± 0.05 | 45 (22) |
| CSS17 | 23 (11, 12) | 1.2 ± 0.3a | 2.6 | 62b | 0.7 ± 0.03 | 87 (20)c |
| CSS18 | 21 (11, 10) | 0.8 ± 0.2 | 1.7 | 50 | 0.6 ± 0.04 | 59 (14) |
| CSS19 | 24 (12, 12) | 0.9 ± 0.2 | 2.0 | 64b | 0.7 ± 0.04 | 89 (17)c |
| CSSY | 23 (23, 0) | 0.7 ± 0.2 | 1.5 | 52 | 0.5 ± 0.03 | 75 (3) |
| A/J | 23 (10, 13) | 36 ± 3 | 78 | 100b | 0.7 ± 0.0a | 82 (78)c |
Average tumor number/mouse ± SEM
No. mice with tumors/No. mice injected
Average tumor diameter ± SEM
p < 0.05 vs. B6
p < 0.005 vs. B6
p < 0.001 vs. B6 by Chi-square analysis
Susceptibility to repeated carcinogen exposure
The relatively low number of tumors in urethane-treated CSS6 mice was surprising since this same protocol administered to (B6 × A/J) F1 mice produced an average of 15 tumors/mouse(22). The background, B6 for all chromosomes except Chr 6, thus attenuated CSS6 lung tumor susceptibility more than initially hypothesized. Strains with intermediate lung tumor susceptibility, such as BALB/cByJ, develop 2–3 tumors/mouse in response to a single urethane administration, but increase their tumor number substantially with increased urethane exposure (up to 25 tumors/mouse)(22). We therefore investigated whether escalating the number of urethane injections would amplify the lung tumor multiplicities in B6, CSS6, CSS11, and A/J mice. Each of these strains developed significantly more tumors, with 100% incidence, upon enhancing urethane exposure (Figure 1). Classically resistant B6 mice exhibited a tumor multiplicity of 6.2 tumors/mouse, a 12-fold increase over a single urethane treatment. CSS11 tumor multiplicity also increased but not as robustly (2.3-fold). CSS6 multiplicity was 11.4-fold higher with this chronic urethane regimen than with a single urethane injection, an increase similar to that displayed by B6 mice, resulting in a tumor multiplicity approaching that of A/J mice receiving a single urethane injection. The sizes of tumors induced with this increased urethane exposure did not rise above that seen after a single urethane injection, and were similar to each other (Figure 1B). Thus, the number of initiated clones that grow to macroscopic size increased with more urethane, but growth rate was unaffected.
Fig. 1. Lung tumor development following multiple injections of urethane.
Average tumor multiplicity (A) and tumor diameter (B) in B6, CSS6, CSS11, and A/J mice treated with either a single 1mg/g dose of urethane (white bars) or 6 weekly 1 mg/g urethane doses (black bars). Mice were sacrificed 20 wks after the first urethane injection and tumors evaluated. Error bars represent SEM. (A) 6 urethane vs. 1 urethane: all strains have significant differences in multiplicity with chronic dosing (*p< 0.001). (B) The diameters of lung tumors from A/J mice treated with 6 urethanes are significantly larger than those from A/J mice treated with a single urethane injection as well as similarly treated B6, CSS6, and CSS11 mice (*p < 0.001). Tumors in CSS11 mice are larger than B6 (#p < 0.05. (C) (B6 × CSS6) F1, B6, and CSS6 mice were subjected to 6 wkly urethane injections, and tumors counted 20 wks after the first injection. **p < 0.01 vs. B6 and CSS6.
To determine whether the enhanced tumor formation in CSS6 mice following multiple urethane injections displayed dominant or recessive inheritance, we generated (B6 × CSS6) F1 mice, and treated them with 6 weekly urethane injections. These F1 mice developed more tumors than either parent (Figure 1C), an example of transgressive variation. This suggests the presence of a recessive resistance locus on A/J Chr 6, since the only difference among these mice resides in heterozygosity of the alleles on Chr 6. This is supported by the fact that CSS-6 mice develop fewer tumors than do similarly treated Pas1 congenic mice (4.1 vs. 5.5 tumors/mouse respectively; (23)), since these mice would have 2 copies of this putative A/J recessive resistance allele while the Pas1 congenics would not.
Latency of tumor development
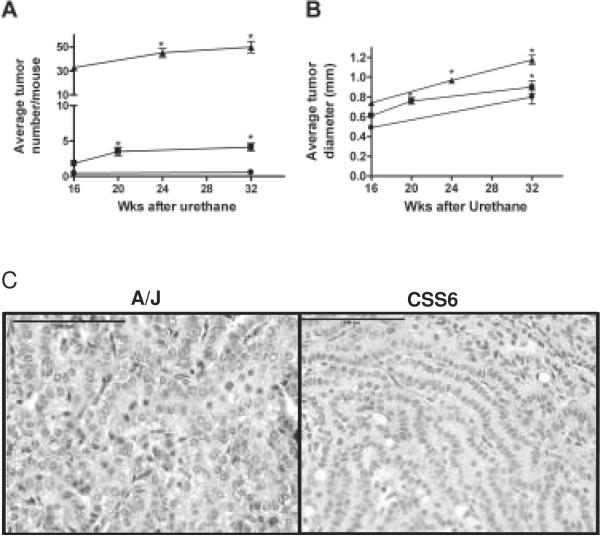
A/J mice exhibit visible lung tumors (> 0.3 mm diameter) within 6 wks of urethane treatment(24), and by 42 wks some of these tumors have reached 2–3 mm diameter(25). Tumor multiplicity in A/J mice increases steadily until about 20 wks after urethane treatment and then levels off, while the number of microscopic tumors observed in B6 mice six wks after urethane does not change with time(24). To determine whether CSS6 lung tumors grow more slowly than A/J, possibly accounting for their reduced tumor number, tumor multiplicity and diameter were examined as a function of time after a single urethane injection (Figure 2). A/J and CSS6 tumor multiplicities increased slightly after 20wk while tumor number in B6 mice remained unchanged (Figure 2A). Average tumor diameter increased over time in all three strains, although to slightly lesser extents in CSS6 and B6 mice (Figure 2B). Lung tumor incidence reached 100% twenty wks after urethane administration to CSS6 mice, but remained at only 40% in B6 mice, even by 32 wks. Histological examination of urethane-induced lung tumors from A/J and CSS6 mice 42 wks after urethane treatment displayed differences in size and degree of malignancy, even though tumor sizes were similar 20 wks after urethane (Figure 1B). A/J tumors exhibited nuclear dysmorphology (Figure 2C), nuclear: cytoplasmic ratio variability, extensive vascularization, and increased invasiveness as evidenced by satellite tumor cells entering into the surrounding lung tissue. Tumors in CSS6 mice were smaller and displayed more benign characteristics. A/J Chr 6 alleles (common to both A/J and CSS6 mice) thus affect multiplicity and incidence with little effect on growth rate and progression (Table I).
Fig. 2. Time course of lung tumor development.
Time courses of tumor multiplicity (A) and diameter (B) after a single urethane exposure. A/J (▲), CSS6 (■), and B6 (●). * p < 0.01 vs. 16 wks. (C) H&E stain of A/J and CSS6 lung tumors 42 wks after urethane treatment. Bar is 100 μm.
Susceptibility is carcinogen dependent in CSS6 mice
The extent of BHT-induced tumor promotion differs when urethane or MCA are employed as initiating agents in A/J mice. If BHT treatment follows urethane in A/J mice, tumor number increases by about 50%(26), while tumor number increased 5-fold in A/J mice treated first with MCA and then followed by BHT (Figure 3A). B6 mice are resistant to MCA and MCA/BHT carcinogenesis(11;27). To determine if the presence of A/J Chr 6 alleles within a resistant B6 background (i.e., CSS6 mice) affects their susceptibility to MCA and/or MCA/BHT lung tumor formation, CSS6 mice were subjected to both MCA/BHT (using the 7.5 mg/kg MCA dose determined to produce the highest fold-change in tumor multiplicity in response to BHT in A/J mice; see Supporting Material Figure 1) and urethane/BHT 2-stage protocols (Figure 3A). CSS6 mice were resistant to MCA carcinogenesis, analogous to the B6 progenitor strain(11), suggesting that the ability of MCA to induce initiating mutations is not due to polymorphisms between A/J and B6 genes residing on Chr 6. However, CSS6 mice subjected to urethane followed by BHT treatment developed 44% more tumors than their urethane/vehicle-treated controls (Figure 3A), analogous to the A/J progenitor(28). There were no differences in tumor diameter or incidence between carcinogen and carcinogen/BHT treatment groups (data not shown). BHT treatment following urethane or MCA does not increase tumor number in B6 mice(11). Since B6 mice are resistant to tumor formation with either of these carcinogens, it is not clear whether this lack of promotability is due to resistance to the promoting effects of BHT or a lack of initiating mutations. Treatment of B6 mice with 6 weekly urethane injections increased incidence to 100%, indicating that initiation occurred with this urethane regimen. To test whether BHT treatment could now promote lung tumor formation in these initiated animals, we subjected B6 mice to a 6 urethane/6 BHT protocol (Figure 3B). A slight increase in tumor multiplicity in the BHT-treated animals was observed that was not statistically significant, confirming earlier results suggesting that BHT does not promote lung tumors in B6 mice. These results indicate that not only is susceptibility dependent on the nature of the carcinogen, but that tumor promotion by BHT is due at least in part to genes located on Chr 6.
Fig. 3. BHT-induced lung tumor promotion and pulmonary inflammation.
(C) CSS6 mice were treated with MCA + vehicle (white bars) or MCA + BHT (black bars), and tumors counted 20 wks after MCA injection. *p < 0.03 vs. vehicle. (D) BAL macrophage (gray bars) and lymphocyte (black bars) contents in mice treated with 4 weekly BHT or corn oil vehicle (control) injections. *p < 0.05 vs. control. (E) B6 mice were given 6 wkly urethane injections followed by either 6 wkly BHT or vehicle injections. Tumors were harvested 20 wks after the initial urethane injection.
Kras mutational analyses
Urethane induces Kras codon 61 mutations that lead to constitutively-activated (GTP-bound) Kras protein(29). Tumors from A/J, B6, and CSS strains subjected to urethane and urethane/BHT tumorigenesis protocols for various lengths of time were analyzed for their Kras codon 61 mutations. Typically, ~90% of lung tumors induced by urethane in A/J mice contain a codon 61 A→T transversion resulting in a GLN→ LEU amino acid substitution(13;29). Kras mutations are observed in approximately half of the B6 lung tumors(13), but are predominantly a codon 61 A→ G transition (GLN→ ARG amino acid substitution). We examined the frequency and type of codon 61 mutation in the CSS, A/J, and B6 mice, as well as the Kras mutational spectrum in CSS6 mice subjected to different urethane protocols. Kras mutations in most CSS strains were identical to that of the B6 progenitor (A → G), while most mutations in A/J mice were A → T as reported previously (Fig. 4A, Supporting Material Figure 2). The frequency of GLN → LEU mutations in CSS6 mice increased as a function of both time and increased carcinogen exposure but never to the frequency observed in A/J mice (Figure 4B,C). The percentage of mice with Kras mutations was significantly higher in tumors from CSS6, CSS11, CSS17, and CSS19 compared to the 55% rate observed in B6 tumors (Table I), suggesting that DNA repair may not be as efficient in these strains. Except for CSS19, those strains with the highest rate of Kras mutations were also those with the highest tumor multiplicity.
Fig. 4. Kras mutational analysis in A/J, B6, and CSS strains.
(A) Strain survey of Kras codon 61 sequence in urethane-induced lung tumors from B6, CSS, and A/J mice. Kras codon 61 sequence: Wild type CAA, (white bars); CGA, (gray bars); CTA, (black bars). (B) Kras mutations as a function of time after mice received a single uethane injection in CSS6 and A/J mice. (C) Kras mutations as a function of number of urethane injections, one vs.6, in B6, CSS6, and A/J mice.
Discussion
Tumor susceptibility analyses in CSS mice yielded results consistent with loci previously mapped by conventional methods on Chrs 6, 11, and 17, but the increases in tumor multiplicity above that of B6 mice were modest at best. A similarly low urethane-induced tumor multiplicity of 5.6 tumors/mouse 32 wks after urethane was observed in a Pas1A/J congenic on the B6 background(23), consistent with our 4.1 tumors/mouse in CSS-6 mice at the same time point and confirming this epistatic effect of B6 alleles. These epistatic interactions prevented tumor formation and also altered the nature of which activating Kras mutations appeared in, and presumably generated, the arising tumors. Negative as well as positive epistatic interactions have been observed among Sluc (Susceptibility to lung cancer) loci that affect the overall lung tumor susceptibility phenotype(31), and we previously reported epistatic interactions in A/J and BALB/c mouse lung tumor susceptibility(12).
To characterize these interactions further, we examined different tumorigenesis protocols and latency of tumor formation in CSS6, B6, and A/J mice. Surprisingly, chronic urethane treatment over 6 wks increased tumor number and incidence in resistant B6 mice, resulting in lung tumor multiplicities higher than had been previously reported(11). This increased lung tumor susceptibility could reflect the removal of pathogens from the Jackson Laboratory facilities, which has changed susceptibilities to diseases such as gallstones(32), atherosclerosis(33), colon carcinogenesis(34), and asthma(35) in some strains. This increased sensitivity to lung carcinogenesis induced by multiple urethane exposures will aid in evaluating the contribution of expression of specific proteins on lung carcinogenesis, since knock-out mice are often generated on the B6 background. When CSS6 mice were subjected to chronic urethane administration, they developed 21 tumors/mouse, similar to BALB/cByJ mice which develop 2–3 tumors/mouse in response to a single urethane injection and 25 tumors/mouse following chronic urethane exposure(22). If a gene investigated for its effects on lung tumorigenesis is not located on Chr 6, CSS6 mice can effectively be used as the background strain in studies with transgenic or knockout mice. A/J and CSS11 mice chronically exposed to urethane also showed enhanced tumor multiplicities above those occurring with a single urethane administration, although the fold increases were not as dramatic as with CSS6 mice.
Differences in tumor number between CSS6 mice and A/J mice were not due to a slower tumor growth rate in CSS6 mice, as determined upon examining tumor multiplicity and size in B6, CSS6, and A/J mice at various times after urethane administration (Figure 2). The number of tumors in CSS6 mice doubled with time following carcinogen application, but never approached the multiplicity of A/J mice. Tumor multiplicity remained unchanged in B6 mice at all intervals following urethane, emphasizing that their resistance is not due to a delay in tumor formation and suggesting that B6 mice remove or repair initiated cells more efficiently than A/J and CSS6 mice. Initiated lung epithelial cells in B6 mice do not retain a capacity to overcome growth controls at later times after carcinogen exposure; older studies by Heston(36) reached similar conclusions.
B6 and CSS6 mice were also subjected to two-stage initiation/promotion carcinogenesis protocols (Figure 3). Since B6 mice have previously been shown to be resistant to both single urethane and MCA-induced carcinogenesis(11), we subjected them to a multiple urethane/BHT protocol to ensure that initiation occurred. As demonstrated previously(11;26), no matter which carcinogenesis protocol was employed, B6 mice do not increase their lung tumor number in response to BHT treatment. However, CSS6 mice developed a significant and reproducible increase in tumor number following BHT treatment with urethane as the initiating carcinogen similar to that observed previously in A/J mice(26). As with B6 mice, CSS6 mice do not develop tumors in response to MCA. These studies indicate that while susceptibility to BHT-induced lung tumor promotion lies at least in part on Chr 6, genes regulating susceptibility to MCA-induced lung tumors reside elsewhere.
A major difference between A/J and CSS6 tumors was the nature and frequency of the Kras initiating mutations. Other studies have indicated that tumors expressing the GLN → ARG substitution are more aggressive than tumors in which Kras has a GLN → LEU substitution(29). This GLN→ ARG mutation predominated in the relatively resistant CSS and B6 strains, possibly because it is more aggressive and can overcome growth inhibitory signals from normal cells. If CSS6 mice are repeatedly exposed to carcinogen or allowed more time for tumor development, more tumors harbor the less aggressive GLN→ LEU substitution. Mechanisms by which the GLN → ARG substitutions cause more aggressive tumors than GLN → LEU substitutions have not been demonstrated. The presence or absence of a particular mutation may be due to mechanisms mice use to repair transversions vs. transitions. B6 mice may be more efficient at repairing A → T transversions than A/J mice, and/or A/J mice may repair A → G transitions more readily. Previous studies indicated that the susceptibility of A/J mice to urethane-induced lung tumor development was due to the absence of one copy of a 37 base pair tandem repeat in intron 2 of the Kras structural gene(37). This polymorphism is not responsible for the difference in Kras initiating mutations between A/J and B6 mice since CSS6 mice have the A/J intron 2 sequence but exhibit B6-like Kras mutations.
In humans, KRAS mutations were detected in 25–40% of precancerous atypical adenomatous hyperplasia (AAH), suggesting that it is an early event in lung tumorigenesis(38). Kras is mutated in about 25%(39) of lung tumors from smokers but only in 15% of non-smokers(40). Interestingly, Kras mutations in smokers were predominantly transversions while those in never smokers were more likely to be transitions(40). In both cases, activating mutations were predominantly in codon 12 with few in codons 13 and 61. Seventy-one percent of Kras mutations in human lung tumors were G→T transversions, while G→A transitions and G→C transversions were found in equal numbers(41). The type of Kras mutation was influenced by race, geographical location(42), and organ site. In human pancreatic tumors, Kras mutations are predominantly G→T transversions(43) while those in colon tumors are predominantly G→A transitions(44). Lung tumors in African-Americans exhibit a higher rate of Kras mutations and statistically more G→T transversions leading to codon 12 CYS substitutions in their lung tumors compared to other races(42). Although there is controversy over whether lung cancer survival differs with the presence/absence of a KRAS mutation(41;45), the substitution of a hydrophobic amino acid at codon 12 yields a better prognosis while substitution of CYS, ARG, or ASP has grimmer consequences(41).
The results described herein indicate that the presence of susceptibility alleles may not result in increased neoplasia. CSS6 mice contain the locus previously determined to be responsible for 2/3 of the difference in susceptibility between A/J and B6 mice, yet display a tumor multiplicity more similar to resistant B6 mice. Epistatic interactions decrease susceptibility in these mice, cautioning that genetic testing at one or two loci may have little predictive power in whether a patient will develop lung cancer.
Supplementary Material
Acknowledgements
This work was supported by USPHS grant CA33497 (Dwyer-Nield and Malkinson) and NCRR grant RR12305 (Nadeau).
Reference List
- (1).Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008 Mar;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- (2).Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers--a different disease. Nat Rev Cancer. 2007 Oct;7(10):778–90. doi: 10.1038/nrc2190. [DOI] [PubMed] [Google Scholar]
- (3).Schwartz AG, Ruckdeschel JC. Familial lung cancer: genetic susceptibility and relationship to chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006 Jan 1;173(1):16–22. doi: 10.1164/rccm.200502-235PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Bailey-Wilson JE, Amos CI, Pinney SM, Petersen GM, de Andrade M, Wiest JS, Fain P, Schwartz AG, You M, Franklin W, Klein C, Gazdar A, et al. A major lung cancer susceptibility locus maps to chromosome 6q23-25. Am J Hum Genet. 2004 Sep;75(3):460–74. doi: 10.1086/423857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Bauer AK, Malkinson AM, Kleeberger SR. Susceptibility to neoplastic and non-neoplastic pulmonary diseases in mice: genetic similarities. Am J Physiol Lung Cell Mol Physiol. 2004 Oct;287(4):L685–L703. doi: 10.1152/ajplung.00223.2003. [DOI] [PubMed] [Google Scholar]
- (6).Malkinson AM. Primary lung tumors in mice as an aid for understanding, preventing, and treating human adenocarcinoma of the lung. Lung Cancer. 2001 Jun;32(3):265–79. doi: 10.1016/s0169-5002(00)00232-4. [DOI] [PubMed] [Google Scholar]
- (7).Fisher GH, Wellen SL, Klimstra D, Lenczowski JM, Tichelaar JW, Lizak MJ, Whitsett JA, Koretsky A, Varmus HE. Induction and apoptotic regression of lung adenocarcinomas by regulation of a K-Ras transgene in the presence and absence of tumor suppressor genes. Genes Dev. 2001 Dec 15;15(24):3249–62. doi: 10.1101/gad.947701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Jackson EL, Willis N, Mercer K, Bronson RT, Crowley D, Montoya R, Jacks T, Tuveson DA. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001 Dec 15;15(24):3243–8. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Floyd HS, Farnsworth CL, Kock ND, Mizesko MC, Little JL, Dance ST, Everitt J, Tichelaar J, Whitsett JA, Miller MS. Conditional expression of the mutant Ki-rasG12C allele results in formation of benign lung adenomas: development of a novel mouse lung tumor model. Carcinogenesis. 2005 Dec;26(12):2196–206. doi: 10.1093/carcin/bgi190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Shimkin MB, Stoner GD. Lung tumors in mice: application to carcinogenesis bioassay. Adv Cancer Res. 1975;21:1–58. doi: 10.1016/s0065-230x(08)60970-7. [DOI] [PubMed] [Google Scholar]
- (11).Miller YE, Dwyer-Nield LD, Keith RL, Le M, Franklin WA, Malkinson AM. Induction of a high incidence of lung tumors in C57BL/6 mice with multiple ethyl carbamate injections. Cancer Lett. 2003 Aug 20;198(2):139–44. doi: 10.1016/s0304-3835(03)00309-4. [DOI] [PubMed] [Google Scholar]
- (12).Festing MF, Lin L, Devereux TR, Gao F, Yang A, Anna CH, White CM, Malkinson AM, You M. At least four loci and gender are associated with susceptibility to the chemical induction of lung adenomas in A/J x BALB/c mice. Genomics. 1998 Oct 15;53(2):129–36. doi: 10.1006/geno.1998.5450. [DOI] [PubMed] [Google Scholar]
- (13).Lin L, Festing MF, Devereux TR, Crist KA, Christiansen SC, Wang Y, Yang A, Svenson K, Paigen B, Malkinson AM, You M. Additional evidence that the K-ras protooncogene is a candidate for the major mouse pulmonary adenoma susceptibility (Pas-1) gene. Exp Lung Res. 1998 Jul;24(4):481–97. doi: 10.3109/01902149809087382. [DOI] [PubMed] [Google Scholar]
- (14).Malkinson AM, Radcliffe RA, Bauer AK. Quantitative trait locus mapping of susceptibilities to butylated hydroxytoluene-induced lung tumor promotion and pulmonary inflammation in CXB mice. Carcinogenesis. 2002 Mar;23(3):411–7. doi: 10.1093/carcin/23.3.411. [DOI] [PubMed] [Google Scholar]
- (15).Wang M, Lemon WJ, Liu G, Wang Y, Iraqi FA, Malkinson AM, You M. Fine mapping and identification of candidate pulmonary adenoma susceptibility 1 genes using advanced intercross lines. Cancer Res. 2003 Jun 15;63(12):3317–24. [PubMed] [Google Scholar]
- (16).Festing MF, Yang A, Malkinson AM. At least four genes and sex are associated with susceptibility to urethane-induced pulmonary adenomas in mice. Genet Res. 1994 Oct;64(2):99–106. doi: 10.1017/s0016672300032705. [DOI] [PubMed] [Google Scholar]
- (17).Liu P, Wang Y, Vikis H, Maciag A, Wang D, Lu Y, Liu Y, You M. Candidate lung tumor susceptibility genes identified through whole-genome association analyses in inbred mice. Nat Genet. 2006 Aug;38(8):888–95. doi: 10.1038/ng1849. [DOI] [PubMed] [Google Scholar]
- (18).Manenti G, Galbiati F, Pettinicchio A, Spinola M, Piconese S, Leoni VP, Conti B, Ravagnani F, Incarbone M, Pastorino U, Dragani TA. A V141L polymorphism of the human LRMP gene is associated with survival of lung cancer patients. Carcinogenesis. 2006 Jul;27(7):1386–90. doi: 10.1093/carcin/bgi332. [DOI] [PubMed] [Google Scholar]
- (19).Nadeau JH, Singer JB, Matin A, Lander ES. Analysing complex genetic traits with chromosome substitution strains. Nat Genet. 2000 Mar;24(3):221–5. doi: 10.1038/73427. [DOI] [PubMed] [Google Scholar]
- (20).Gariboldi M, Manenti G, Canzian F, Falvella FS, Radice MT, Pierotti MA, Della PG, Binelli G, Dragani TA. A major susceptibility locus to murine lung carcinogenesis maps on chromosome 6. Nat Genet. 1993 Feb;3(2):132–6. doi: 10.1038/ng0293-132. [DOI] [PubMed] [Google Scholar]
- (21).Manenti G, Gariboldi M, Elango R, Fiorino A, De Gregorio L, Falvella FS, Hunter K, Housman D, Pierotti MA, Dragani TA. Genetic mapping of a pulmonary adenoma resistance (Par1) in mouse. Nat Genet. 1996 Apr;12(4):455–7. doi: 10.1038/ng0496-455. [DOI] [PubMed] [Google Scholar]
- (22).Malkinson AM, Beer DS. Major effect on susceptibility to urethan-induced pulmonary adenoma by a single gene in BALB/cBy mice. J Natl Cancer Inst. 1983 May;70(5):931–6. [PubMed] [Google Scholar]
- (23).Zhang Z, Futamura M, Vikis HG, Wang M, Li J, Wang Y, Guan KL, You M. Positional cloning of the major quantitative trait locus underlying lung tumor susceptibility in mice. Proc Natl Acad Sci U S A. 2003 Oct 28;100(22):12642–7. doi: 10.1073/pnas.2133947100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).O'Donnell EP, Zerbe LK, Dwyer-Nield LD, Kisley LR, Malkinson AM. Quantitative analysis of early chemically-induced pulmonary lesions in mice of varying susceptibilities to lung tumorigenesis. Cancer Lett. 2006 Sep 28;241(2):197–202. doi: 10.1016/j.canlet.2005.10.012. [DOI] [PubMed] [Google Scholar]
- (25).Redente EF, Orlicky DJ, Bouchard RJ, Malkinson AM. Tumor signaling to the bone marrow changes the phenotype of monocytes and pulmonary macrophages during urethane-induced primary lung tumorigenesis in A/J mice. Am J Pathol. 2007 Feb;170(2):693–708. doi: 10.2353/ajpath.2007.060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Malkinson AM, Beer DS. Pharmacologic and genetic studies on the modulatory effects of butylated hydroxytoluene on mouse lung adenoma formation. J Natl Cancer Inst. 1984 Oct;73(4):925–33. [PubMed] [Google Scholar]
- (27).Bauer AK, Dwyer-Nield LD, Keil K, Koski K, Malkinson AM. Butylated hydroxytoluene (BHT) induction of pulmonary inflammation: a role in tumor promotion. Exp Lung Res. 2001 Apr;27(3):197–216. doi: 10.1080/019021401300053948. [DOI] [PubMed] [Google Scholar]
- (28).Witschi H, Williamson D, Lock S. Enhancement of urethan tumorigenesis in mouse lung by butylated hydroxytoluene. J Natl Cancer Inst. 1977 Feb;58(2):301–5. doi: 10.1093/jnci/58.2.301. [DOI] [PubMed] [Google Scholar]
- (29).Nuzum EO, Malkinson AM, Beer DG. Specific Ki-ras codon 61 mutations may determine the development of urethan-induced mouse lung adenomas or adenocarcinomas. Mol Carcinog. 1990;3(5):287–95. doi: 10.1002/mc.2940030509. [DOI] [PubMed] [Google Scholar]
- (30).You M, Candrian U, Maronpot RR, Stoner GD, Anderson MW. Activation of the Ki-ras protooncogene in spontaneously occurring and chemically induced lung tumors of the strain A mouse. Proc Natl Acad Sci U S A. 1989 May;86(9):3070–4. doi: 10.1073/pnas.86.9.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Fijneman RJ, de Vries SS, Jansen RC, Demant P. Complex interactions of new quantitative trait loci, Sluc1, Sluc2, Sluc3, and Sluc4, that influence the susceptibility to lung cancer in the mouse. Nat Genet. 1996 Dec;14(4):465–7. doi: 10.1038/ng1296-465. [DOI] [PubMed] [Google Scholar]
- (32).Svenson KL, Von SR, Magnani PA, Suetin HR, Paigen B, Naggert JK, Li R, Churchill GA, Peters LL. Multiple trait measurements in 43 inbred mouse strains capture the phenotypic diversity characteristic of human populations. J Appl Physiol. 2007 Jun;102(6):2369–78. doi: 10.1152/japplphysiol.01077.2006. [DOI] [PubMed] [Google Scholar]
- (33).Jackson Laboratories Mouse Phenome Database. Mouse Genome Informatics. 2008 Available from: URL: www.informatics.jax.org.
- (34).Radiloff DR, Rinella ES, Threadgill DW. Modeling cancer patient populations in mice: Complex genetic and environmental factors. Drug Discovery Today: Disease Models. 2007;4(2):83–8. doi: 10.1016/j.ddmod.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Pinto LH, Eaton E, Chen B, Fleisher J, Shuster D, McCauley J, Kedainis D, Siepka SM, Shimomura K, Song EJ, Husain A, Lakser OJ, et al. Gene-environment interactions in a mutant mouse kindred with native airway constrictor hyperresponsiveness. Mamm Genome. 2008 Jan;19(1):2–14. doi: 10.1007/s00335-007-9082-9. [DOI] [PubMed] [Google Scholar]
- (36).Heston WE. Genetic analysis of susceptibility to induced pulmonary tumors in mice. J Natl Cancer Inst. 1942;3:69–78. [Google Scholar]
- (37).Chen B, Johanson L, Wiest JS, Anderson MW, You M. The second intron of the K-ras gene contains regulatory elements associated with mouse lung tumor susceptibility. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1589–93. doi: 10.1073/pnas.91.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Cooper CA, Carby FA, Bubb VJ, Lamb D, Kerr KM, Wyllie AH. The pattern of K-ras mutation in pulmonary adenocarcinoma defines a new pathway of tumour development in the human lung. J Pathol. 1997 Apr;181(4):401–4. doi: 10.1002/(SICI)1096-9896(199704)181:4<401::AID-PATH799>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- (39).Suzuki M, Shigematsu H, Iizasa T, Hiroshima K, Nakatani Y, Minna JD, Gazdar AF, Fujisawa T. Exclusive mutation in epidermal growth factor receptor gene, HER-2, and KRAS, and synchronous methylation of nonsmall cell lung cancer. Cancer. 2006 May 15;106(10):2200–7. doi: 10.1002/cncr.21853. [DOI] [PubMed] [Google Scholar]
- (40).Riely GJ, Kris MG, Rosenbaum D, Marks J, Li A, Chitale DA, Nafa K, Riedel ER, Hsu M, Pao W, Miller VA, Ladanyi M. Frequency and distinctive spectrum of KRAS mutations in never smokers with lung adenocarcinoma. Clin Cancer Res. 2008 Sep 15;14(18):5731–4. doi: 10.1158/1078-0432.CCR-08-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Siegfried JM, Gillespie AT, Mera R, Casey TJ, Keohavong P, Testa JR, Hunt JD. Prognostic value of specific KRAS mutations in lung adenocarcinomas. Cancer Epidemiol Biomarkers Prev. 1997 Oct;6(10):841–7. [PubMed] [Google Scholar]
- (42).Hunt JD, Strimas A, Martin JE, Eyer M, Haddican M, Luckett BG, Ruiz B, Axelrad TW, Backes WL, Fontham ET. Differences in KRAS mutation spectrum in lung cancer cases between African Americans and Caucasians after occupational or environmental exposure to known carcinogens. Cancer Epidemiol Biomarkers Prev. 2002 Nov;11(11):1405–12. [PubMed] [Google Scholar]
- (43).Smit VT, Boot AJ, Smits AM, Fleuren GJ, Cornelisse CJ, Bos JL. KRAS codon 12 mutations occur very frequently in pancreatic adenocarcinomas. Nucleic Acids Res. 1988 Aug 25;16(16):7773–82. doi: 10.1093/nar/16.16.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Fearon ER, Kohn DB, Winkelstein JA, Vogelstein B, Blaese RM. Carrier detection in the Wiskott Aldrich syndrome. Blood. 1988 Nov;72(5):1735–9. [PubMed] [Google Scholar]
- (45).Slebos RJ, Kibbelaar RE, Dalesio O, Kooistra A, Stam J, Meijer CJ, Wagenaar SS, Vanderschueren RG, van Zandwijk N, Mooi WJ. K-ras oncogene activation as a prognostic marker in adenocarcinoma of the lung. N Engl J Med. 1990 Aug 30;323(9):561–5. doi: 10.1056/NEJM199008303230902. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.