Abstract
Neurophysiological, biochemical, and anatomical evidence implicates glutamatergic mechanisms in epileptic seizures. Until recently, however, longitudinal characterization of in vivo glutamate dynamics was not possible. Here, we present data using in vivo magnetic resonance spectroscopy (MRS) optimized for the detection of glutamate to identify changes that evolve following kainic acid (KA)-induced status epilepticus. Wild-type male Wistar rats underwent whole brain MR imaging and single voxel MRS on a clinical 3T scanner equipped with a high-strength insert gradient coil. Scanning took place before and then 3 days, 28 – 32 days, and 42 – 50 days after induction of status epilepticus. Analyses compared 5 seizure (Sz), 5 no-seizure (NoSz; received KA but did not exhibit seizures), and 6 control (Con) animals. This longitudinal study demonstrated reduced glutamate levels in vivo in the dorsal hippocampus 3 days and 1 month following status epilepticus in Sz animals compared with Con animals. Additionally, previous results were replicated: in the Sz group, computed T2 was higher in the ventral hippocampus and limbic cortex 3 days after seizure activity compared with baseline but resolved in both regions at the 1 month scan, suggesting a transient edema. Three days following seizure activity, N-acetylaspartate (NAA) declined and lactate increased in the dorsal hippocampus of the Sz group compared with the Con and NoSz group; both metabolites approached baseline levels by the third scan. Taken together, these results support the conclusion that seizure activity following KA infusion causes loss of glutamatergic neurons.
Keywords: glutamate, hippocampus, cerebellum, longitudinal, rat, status epilepticus
1. Introduction
Ample data support a role for glutamate in epilepsy. In vivo microdialysis studies report elevated extracellular glutamate levels before or during seizures in hyperexcitable brain regions in animal models of epilepsy (Liu et al., 1997; Sierra-Paredes et al., 1998; but see Tanaka et al., 1996; Wade et al., 1987) and in patients with medial temporal lobe epilepsy (Carlson et al., 1992; During and Spencer, 1993; Wilson et al., 1996). A sustained elevation in basal release of glutamate (Zhang et al., 1991), in addition to initiating and maintaining epileptic seizures, may be excitotoxic and contribute to cell death (e.g., Magloczky and Freund, 1993; Meldrum, 1993). In the interictal stage, in vitro studies report low glutamate content in hippocampal tissue from both animals (Koyama, 1972) and humans (Peeling and Sutherland, 1993) potentially reflecting the loss of glutamatergic neurons of the hippocampus. Recent in vitro magnetic resonance spectroscopy (MRS) studies demonstrate a reduction in the amount of 13C labeling of glutamate in the hippocampal formation of systemically treated kainic acid (KA) rats (Alvestad et al., 2008) and biopsied hippocampal tissue from patients with medial temporal lobe epilepsy (Petroff et al., 2002). However, our understanding of the role of altered glutamate homeostasis in epilepsy is incomplete, especially in vivo, and techniques such as in vivo microdialysis have limited applicability to longitudinal studies (Plock and Kloft, 2005).
Temporal lobe epilepsy can be modeled in rodents by administration of KA (Williams et al., 2007). As an excitatory amino acid agonist (Ferkany et al., 1982), KA can provoke prolonged seizures (i.e., status epilepticus), characterized by salivation, rearing, bilateral upper extremity clonus, and falling (Lothman and Collins, 1981), and set in motion a process of epileptogenesis that may lead to spontaneous seizures (Williams et al., 2007). Postmortem histological evaluation demonstrates a specific pattern of neuronal loss largely restricted to cells of the hippocampus, amygdala, and related parts of the thalamus and cortex (e.g., piriform and entorhinal cortices, Nadler and Cuthbertson, 1980; Schwob et al., 1980; Sperk et al., 1983; Sperk, 1994). This specific regional profile of brain damage in rodents following KA-induced status epilepticus resembles Ammon’s horn sclerosis (Sagar and Oxbury, 1987). Neuronal loss as a consequence of KA-induced status epilepticus involves, at last in part, an apoptotic mechanism dependent on activation of caspase-3 protease activity (Faherty et al., 1999; Kondratyev and Gale, 2000; Kondratyev and Gale, 2004; Puig and Ferrer, 2002; Tokuhara et al., 2007).
In vivo studies using magnetic resonance imaging (MRI) protocols sensitive to mobile water protons (e.g., T2-weighted images or computed T2 maps) have enabled tracking of the evolution of brain changes associated with KA-induced seizures. T2-weighted images typically detect hyperintensities at 24h (e.g., TE=272ms, Ebisu et al., 1996) that persist for 3 days after systemic KA injection (Ebisu et al., 1994), and for 10 days following local intracerebral KA injections (e.g., Bouilleret et al., 2000; Luna-Medina et al., 2007; Tanaka et al., 1993). Postmortem analyses suggest that regions of hyperintense T2-weighted signals in the acute period following seizure activity reflect edema (e.g., Hantraye et al., 1992; Tanaka et al., 1993).
Along with gross morphology and tissue quality measurements from structural MRI, chemical constituents of tissue are detectable with MRS (cf., Adalsteinsson et al., 2002; Zahr et al., 2009). N-acetylaspartate (NAA), a presumed neuronal marker, is lower in the rat hippocampus (Najm et al., 1997; Najm et al., 1998) and piriform cortex (Ebisu et al., 1996) 24h to at least 7 days (Najm et al., 1997) after systemically administered KA; NAA reductions persist for up to 84 days following local intracerebral stereotaxic injections of KA (Luna-Medina et al., 2007; Tokumitsu et al., 1997). KA administration is also associated with an increase in hippocampal lactate that occurs within 3h of seizure induction (Meric et al., 1994) and persists for 7 days after systemic injection (Najm et al., 1997; Najm et al., 1998) and upwards of 9 days after local hippocampal injection of KA (Luna-Medina et al., 2007).
The present in vivo longitudinal study was undertaken to replicate previous MRI and MRS results, and to extend earlier work by quantifying glutamate levels at baseline and as long as 1 month following KA-induced status epilepticus. We hypothesized that MRI would detect a transient increase in computed T2, indicative of edema in response to systemic KA-induced status epilepticus, and that structural evidence for neuronal loss in limbic structures would be paralleled by ventricular enlargement. We also predicted that NAA levels would decrease and lactate levels would increase in the dorsal hippocampus as a result of KA-induced seizure activity. Notably, based on recent in vitro studies using 13C labeled glucose, we expected levels of glutamate to decline in the dorsal hippocampus in the interictal period following status epilepticus. Confirmation of previous results (reduced NAA and increased T2 and lactate) supports the novel in vivo finding of decreased glutamate following KA-induced status epilepticus. Radiological results were verified with postmortem immunohistochemical staining for caspase-3.
2. Results
Between-group analyses were conducted on computed T2 data from scans 1 and 2 and on MRS data from scans 1, 2, and 3. Only the seizure (Sz) group was scanned 4 times and formed the basis for within-subject analyses of ventricular volume (Table 1). Body weight data for the control (Con) and no seizure (NoSz) groups is absent at the 42 – 50 day time point since these groups did not receive a 4th scan. For the first 3 scans, there were no group differences in body weights; within each group, however, rats were significantly larger at scan 3 compared with scans 1 and 2.
Table 1.
Scan Schedule and Weights (g) ± SE
| N | Scan 1 baseline | Scan 2 3 days after KA | Scan 3 28–32 days after KA | Scan 4 42–50 days after KA | |
|---|---|---|---|---|---|
| Control | 6 | 391.8±6.8a,b | 397.2±7.5a,b | 456.7±9.4b | NA |
| KA-no seizure | 5 | 383.4±6.3a,b | 374.6±7.4a,b | 441.6±6.0b | NA |
| KA-seizure | 5 | 396.4±6.7a,b,c | 396.6±19.6a,b,c | 452.8±17.5b,c | 473.8±18.4b,c |
T2 data analyzed
MRS data analyzed
ventricular volume analyzed
NA=not acquired
2.1 Changes in Computed Tissue T2
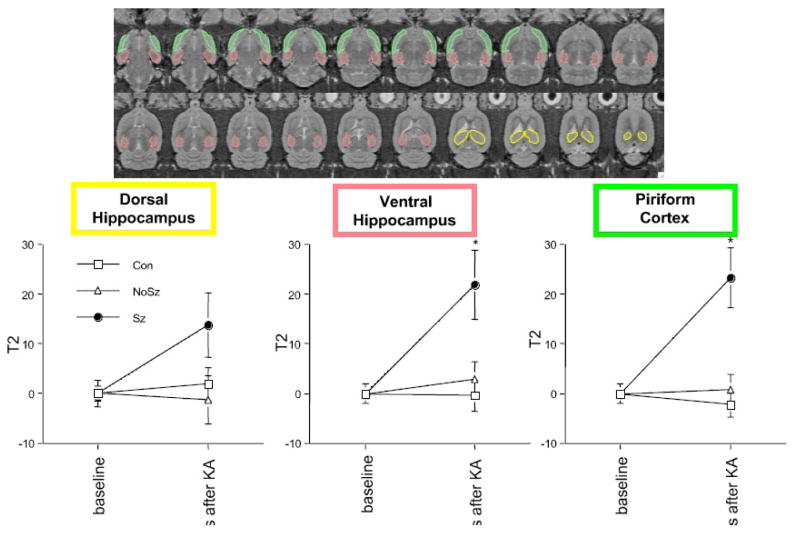
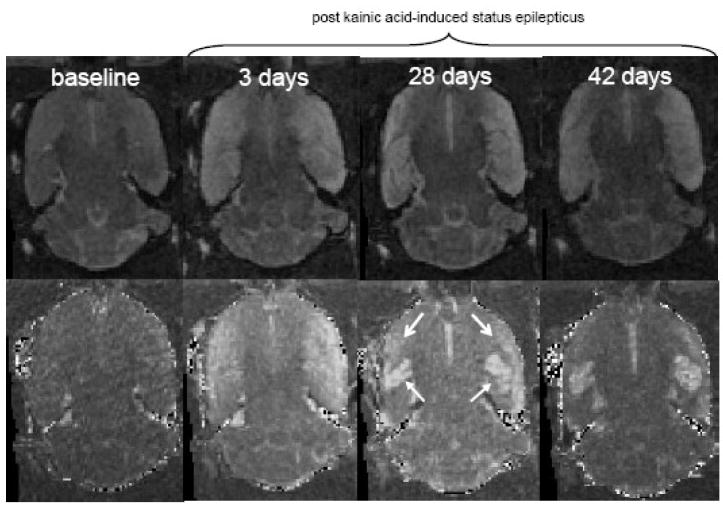
Regions of interest (ROI) were manually drawn on late-echo images in the dorsal and ventral hippocampus, and incortical regions (starting at ~ −7.00 Bregma) lateral to the hippocampus extending from the entorhinal cortex to the piriform cortex (referred to as the limbic cortex, Figure 1). For statistical analysis, tissue T2 values were computed using the average ROI values for the early- and late-echo images. A 3-group (Con, Sz, NoSz) by 2-time (baseline and 3 days after KA) analysis of variance (ANOVA) examining computed T2 changes in the dorsal hippocampus did not reveal any group, time, or interaction effects (Figure 1). In the ventral hippocampus, a similar ANOVA yielded a group-by-time interaction (F(2,13)=6.01, p=.0142, GG=.0142; nonparametric Kruskal-Wallis (KW) results: H=6.38, p=.041), indicating that the Sz group had a significant computed T2 increase between scan 1 and scan 2 relative to the Con and NoSz groups, neither of which changed between scans. Follow-up t-tests confirmed a greater computed T2 change (percent increase from baseline) in the ventral hippocampus 3 days after KA-induced status epilepticus in the Sz group relative to the Con group (t(9)=3.09, p=.0129) and the NoSz group (t(8)=2.43, p=.0412, Figure 1). The limbic cortex showed a similar pattern, where an ANOVA yielded a group-by-time interaction (F(2,13)=11.85, p=.0012, GG=.0012; KW results: H=9.97, p−.0069). The Sz group had increased computed T2 in the limbic cortex 3 days after KA-induced status epilepticus compared with the Con (t(9)=4.26, p=.0021) and NoSz (t(8)=3.38, p=.0097) groups (Figure 1). One month (28 – 32 days) following status epilepticus, the computed T2 increase in the limbic cortex had resolved; in the ventral hippocampus, fluid-filled cysts were clearly evident precluding meaningful computation of T2 values at the third scan (Figure 2).
Figure 1.
top: manually drawn regions of interest (ROIs) on late-echo axial slices for computation of T2. bottom: Percent computed T2 increase in the 3 regions, 3 days after kainic acid-induced status epilepticus. * p≤.05 between Sz group and Con group, and Sz group and NoSz group.
Figure 2.
Late-echo (top panel) and computed T2 (bottom panel) MR images of an axial brain slice of a single rat. Closed arrows point to the ventral hippocampus, open arrows to the limbic cortex. Note the regions of hyperintensity on computed T2 images at 3 days, and the presence of fluid-filled cysts in the ventral hippocampus at 28 and 42 days following status epilepticus.
2.2 Ventricular Enlargement
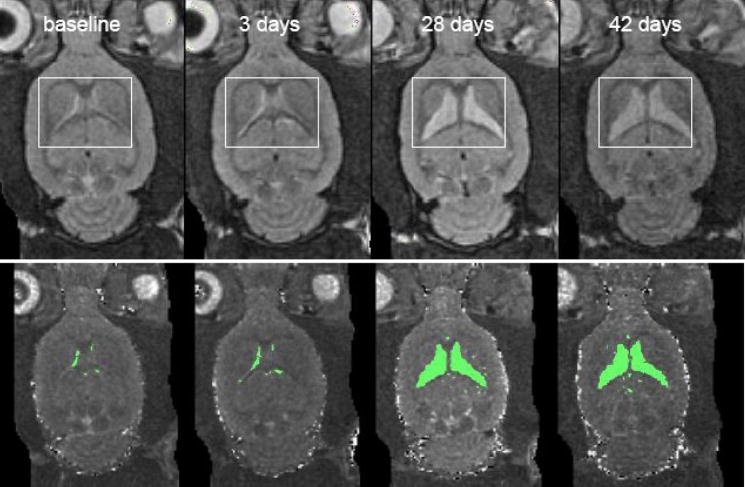
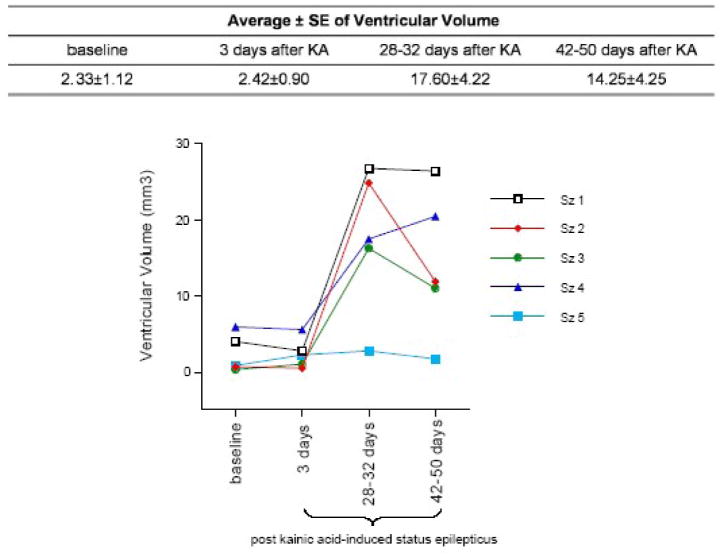
Within the Sz group, a repeated-measures ANOVA examining ventricular volume across the 4 MRI sessions revealed a significant effect of time (F(3,12)=10.68, p=.001, nonparametric Friedman test results: χ2=10.68, p=.0136), indicating an increase in ventricular volume (Figure 3). Three days after KA-induced status epilepticus, the Sz group showed no significant increase in ventricular volume (p=.8573). One month after status epilepticus, however, ventricular volume in the 5 Sz rats was 7.6 fold higher than baseline (p=.0195) and 7.3 fold higher than it was 3 days after KA administration (p=.027). Between 1 month (28 – 32 days) and 42 – 50 days after status epilepticus, 2 of the 5 seizure animals showed ventricular shrinkage, although individual variability among seizure animals was large (Figure 4).
Figure 3.
top: Early-echo MR images of an axial brain slice of a single seizure rat demonstrating enlargement of ventricular volume. bottom: Thresholded ventricles on T2 computed images from the same animal.
Figure 4.
Quantified ventricular volume of each seizure animal at the 3 time points following kainic acid- induced status epilepticus.
2.3 Metabolite Changes
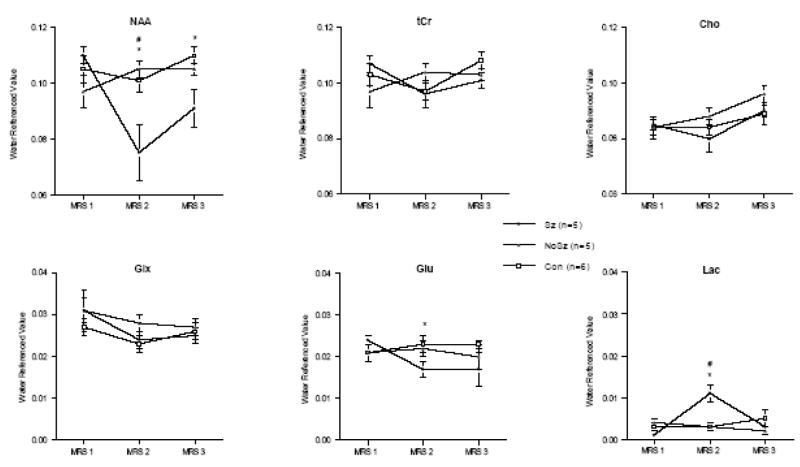
MRS data were collected in the dorsal hippocampus to evaluate the effects of the KA-induced status epilepticus and in the cerebellum as a control brain structure thought to be spared by seizure activity (Figures 5). In the dorsal hippocampus, a 3-group (Con, NoSz, Sz) repeated-measures (3 scan sessions, 6 metabolite signals) ANOVA revealed group-by-metabolite (F(10,65)=2.84, p=.006, GG=.02) and group-by-metabolite-by-time (F(20,130)=3.5, p=.0001, GG=.005) interactions, indicating a significant effect of status epilepticus on metabolite levels. Three days after seizure induction, NAA in the Sz group was significantly lower than in the Con (t(9)=2.62, p=.014) and NoSz (t(8)=2.94, p=.0093) groups (Figure 6). At the 1 month scan there was 50% recovery of NAA, with levels which had dropped to 69% of baseline up to 83% of baseline; however, NAA levels in the Sz group were still significantly lower than the Con group (t(9)=2.66, p=.0129; Figure 7). Within the Sz group, the decrease in NAA between baseline and 3 days following seizure activity was significant (t(4)=2.99, p=.0401), as was the increase in NAA between 3 and 28 – 32 days following seizures (t(4)=3.66, p=.0215).
Figure 5.
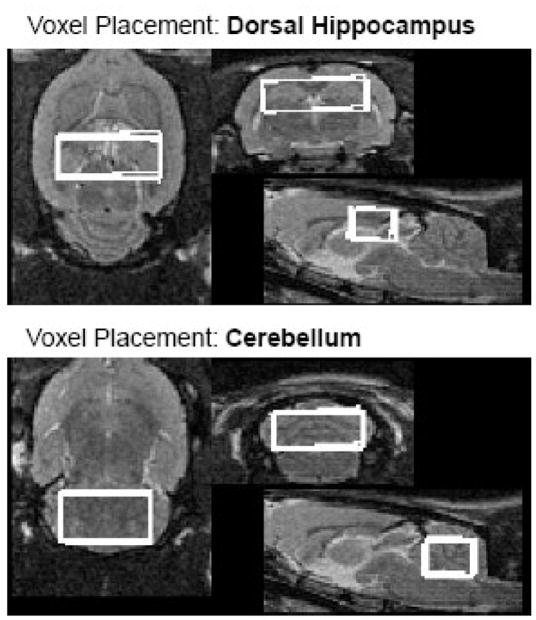
Voxel placement in the dorsal hippocampus and cerebellum including reproducibility of voxel placement across the first 3 scan time points.
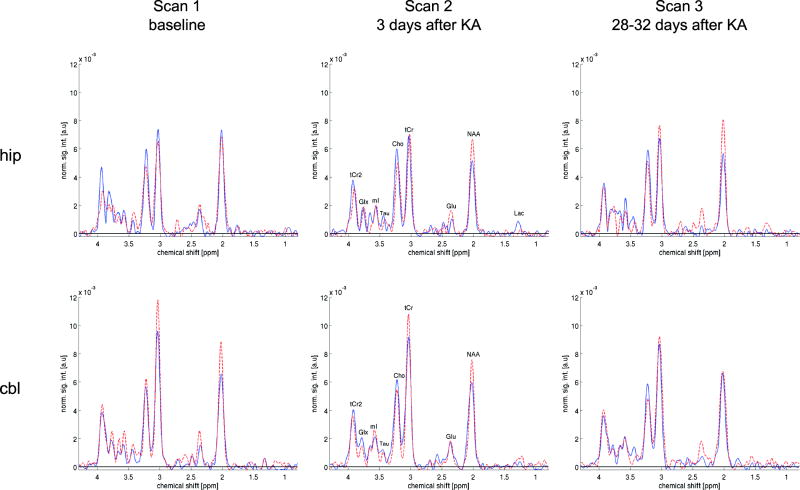
Figure 6.
Sample spectra from a single seizure animal (dashed, red) and a single control animal (solid, blue) for voxels in the dorsal hippocampus (top) and cerebellum (bottom) across the first 3 scan sessions.
Figure 7.
Water-referenced metabolite levels in the dorsal hippocampus at baseline (MRS 1), 3 days (MRS 2), and 28–32 days (MRS 3) after kainic acid (Sz+NoSz) or water (Con) injection as quantified using peak integration.
* p<.05 between Sz and Con, # p<.05 between Sz and NoSz.
Lactate increased 3 days after status epilepticus in the dorsal hippocampus in the Sz group compared with both the Con (t(9)=3.55, p=.0031) and NoSz (t(8)=2.96, p=.0091) groups. By the third scan, 1 month after status epilepticus, lactate levels in the dorsal hippocampus were indistinguishable among the 3 groups (Figure 7). Within the Sz group, the significant increase in lactate 3 days following status epilepticus (t(4)=3.93, p=.0171) was followed by a significant decrease in lactate at the scan performed 1 month following seizure activity (t(4)=3.01, p=.0397).
MRS-detectable glutamate in the dorsal hippocampus showed modest but predicted (1-tailed t-tests) changes. Specifically, glutamate tended to be lower in the Sz group compared with the Con group at 3 days and 1 month (3 days: t(9)=1.93, p=.0427, 1 month: t(9)=1.80, p=.0528) and lower than the NoSz group at 3 days (t(8)=1.84, p=.0515) but not at 1 month following seizure activity. Within the Sz group, the decrease in glutamate observed between baseline and 3 days following seizures was significant (t(4)=3.07, p=.0186); at the third scan, 1 month following seizures, glutamate levels in the Sz group remained lower than at baseline (t(4)=1.97, p=.0603). Status epilepticus did not significantly affect any of the other MRS-measured metabolites in the dorsal hippocampus.
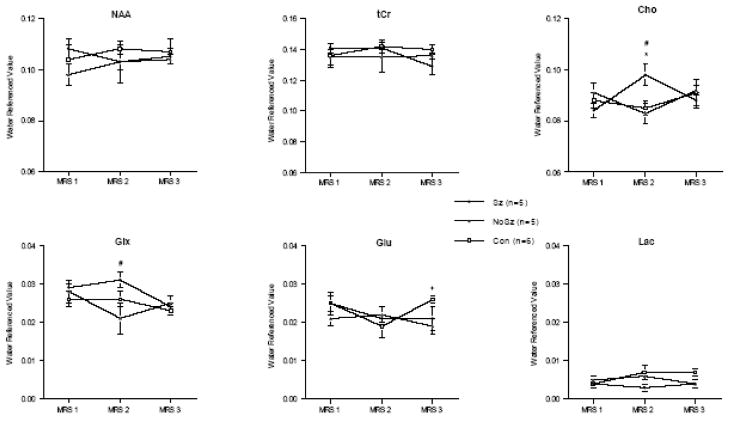
In the cerebellum, a similar 3-group repeated-measures ANOVA yielded a group-by-metabolite-by-time interaction (F(20,130)=2.36, p=.002, GG=.03), likewise indicating a significant seizure effect on metabolite levels. Three days after seizure induction, there was a significant increase in Cho in the cerebellum of the Sz group compared with the Con (t(9)=2.47, p=.0353) and NoSz (t(8)=2.49, p=.0374) groups that resolved 1 month later (Figure 8). The Glx peak was also higher in the cerebellum of the Sz group 3 days after seizures compared with the NoSz group (t(8)=2.56 p=.0337), but this was in part due to a drop in Glx in the NoSz group (Figure 8). A significant difference in Glu was observed between the Con and NoSz groups (t(9)= 2.95, p=.0163) 1 month after KA administration, but the significance was carried by an inexplicable increase in Glu in the Con group.
Figure 8.
Water-referenced metabolite levels in the cerebellum at baseline (MRS 1), 3 days (MRS 2), and 28–32 days (MRS 3) after kainic acid (Sz+NoSz) or water (Con) injection as quantified using peak integration.
* p<.05 between Sz and Con, # p<.05 between Sz and NoSz, + p<.05 between Con and NoSz.
2.4 Immunohistochemistry
A 3 group-by-3 region (CA1, CA3, DG) repeated-measures ANOVA revealed group effects (F(2,12)=15.83, p=.0004), indicating a disproportionately higher number of caspase-3 staining cells in the Sz group than in the Con or NoSz groups. Follow-up t-tests revealed that the Sz group had a higher number of caspase-3 staining cells compared with the Con and NoSz groups in the CA1 (Con t(9)=4.18, p=.0024; NoSz t(7)= 5.65, p=.0008) and CA3 (Con t(9)=2.58, p=.0296; NoSz t(7)= 3.23, p=.0144) regions of the hippocampus. Total caspase-3 staining in the hippocampus was likewise higher in the Sz group than the Con (t(9)=4.92, p=.0008) and NoSz (t(7)=5.38, p=.001) groups.
2.5 Correlations
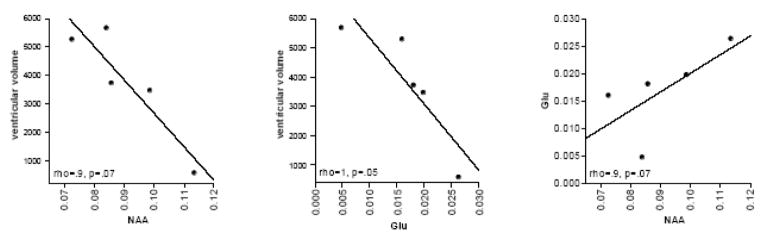
The relationships between ventricular volume, NAA, and glutamate at the third scan were explored using simple regression analysis including only the Sz group. A significant correlation between ventricular volume and glutamate levels (rho=1, p=.05) revealed that lower glutamate levels were associated with larger ventricular volumes (Figure 9). Similarly, lower NAA levels were associated with larger ventricular volumes (rho=.9, p=.07; Figure 9). Glutamate and NAA were related such that lower glutamate correlated with lower NAA (rho=.9, p=.07; Figure 9).
Figure 9.
Correlations (non parametric Spearman Rank) between ventricular volume and NAA or glutamate, and NAA and glutamate at the third scan.
3. Discussion
KA per se was ineffective in producing in vivo evidence of brain changes; by contrast, status epilepticus produced both local and global changes (O’Shaughnessy and Gerber, 1986). At 3 days following status epilepticus, edematous tissue localized bilaterally to the ventral hippocampus and limbic cortex, as evidenced by the increase in computed T2. One month following status epilepticus, the T2 increase in the limbic cortex resolved, cavitations were present in the ventral hippocampus, and signs of ex vacuo ventricular dilation were evident. Partial recovery of ventricular volume was observed 42 – 50 days following seizure induction in 2 of 5 seizure rats. At the acute, 3 day time-point, the dorsal hippocampus revealed significant declines in NAA and glutamate and increases in lactate selective to rats that exhibited status epilepticus. While a fall in lactate to baseline levels was observed, NAA and glutamate levels remained lower in the Sz compared with the Con group at the third scan, 1 month following status epilepticus. At postmortem, 50 days after status epilepticus, immunohistochemistry revealed a higher number of caspase-3 stained cells in CA1 and CA3 regions of the hippocampus in the Sz group compared with the NoSz and Con groups, indicative of cell death and consistent with the enduring depression of NAA and glutamate in the Sz group.
3.1 Changes in Computed T2
Late-echo fast spin-echo (FSE) images revealed hyperintensities quantified as increased computed T2 values in the ventral hippocampus and limbic cortex of the Sz group 3 days after status epilepticus in agreement with previous MR studies carried out 1 – 3 days following systemic KA-induced seizures (Ebisu et al., 1994; Ebisu et al., 1996; Righini et al., 1994) and upwards of 9 days following local KA injections into the hippocampus (Bouilleret et al., 2000; Luna-Medina et al., 2007; Tanaka et al., 1993). Brain edema begins 2 – 24h after KA injection (Lassmann et al., 1984; Schwob et al., 1980; Sperk et al., 1983), and postmortem evidence suggests that the hyperintense T2-weighted signal observed at acute times following KA-induced seizure activity reflects acute edema (Bouilleret et al., 2000; Norman et al., 1990; Tanaka et al., 1993). In humans with temporal lobe epilepsy, focal regions with high signal intensity on T2-weighted images have been noted and ascribed to transient focal edema when the MR scan is performed within hours of a seizure (Kramer et al., 1987; Stone et al., 1986).
At the third scan, 1 month after status epilepticus, areas with high computed T2 in the ventral hippocampus and limbic cortex had resolved: in the ventral hippocampus, hyperintensity was replaced with fluid-filled cysts. This contrasts with studies demonstrating persisting high signal intensity on T2-weighted images at 1 month (Tanaka et al., 1993) and 4 months (Bouilleret et al., 2000) following seizure activity. These inconsistent results may be due to the injection method used (previous studies used local stereotaxic injections of KA into limbic regions) or because in previous studies, epileptogenesis resulted in repetitive spontaneous motor seizures acutely associated with an increase in T2-weighted signal intensity and indicative of edema.
3.2 Ventricular Enlargement
One month after seizure induction, lateral ventricular volume in the Sz group was significantly larger than at baseline and 3 days after KA administration, in agreement with MRI studies performed 9 – 10 days following KA treatment (Righini et al., 1994; Wolf et al., 2002). Previous postmortem histology performed 1 – 7 days post systemic KA treatment reveals widespread neural damage in the hippocampal formation, amygdaloid complex, limbic cortex (piriform and entorhinal cortices), and midline thalamus (Bates et al., 1988; Sperk, 1994). Thus, enlargement of the lateral ventricles is assumed to result from atrophy of the surrounding brain tissue, namely the hippocampus, amygdala, piriform cortex, and thalamus (Roland and Savage, 2007). Although considered a marker for hippocampal atrophy related to Alzheimer’s disease (Camara et al., 2008), caution must be used when interpreting increases in ventricular volume, i.e., dysmorphology of a non-tissue, as a surrogate, to draw conclusions about the target structure (Pfefferbaum et al., 2004b).
3.3 Metabolite Changes
The decrease in NAA observed in the dorsal hippocampus 3 days following status epilepticus comports with studies in which a reduced NAA signal was observed in the hippocampus 12h to 7 days following systemic KA-induced seizures (Ebisu et al., 1994; Ebisu et al., 1996; Najm et al., 1997; Najm et al., 1998) and 1 to 84 days following local stereotaxic KA injections (Luna-Medina et al., 2007; Mao et al., 2007; Tokumitsu et al., 1997). In the current study, the reduction in NAA in the dorsal hippocampus was not associated with changes in computed T2 (Ebisu et al., 1996; Tokumitsu et al., 1997) suggesting that MRS may detect a subtler or different form of tissue damage than is possible with T2-weighted imaging or regionally computed T2.
A 50% recovery of NAA levels was observed in the Sz group at 1 month compared with levels at 3 days following status epilepticus. Because it is unlikely that neurogenesis accounts for the recovery in NAA levels (Nixon, 2006), and because previous studies suggest that NAA levels can decline in response to KA-induced seizures even with little histological evidence for neuronal loss (cf., Ebisu et al., 1996), it is presumed that the interpretation of NAA as solely a marker of neuronal viability is insufficient (Moffett et al., 2007). The profound but transient decrease in NAA observed at 3 days following seizure activity may reflect mitochondrial dysfunction or compromised ATP production at the acute time point (Moffett et al., 2007; Retz and Coyle, 1982). By contrast, the fraction of NAA that remained attenuated in the Sz group at the 1 month scan may indeed represent a loss of neurons, an interpretation underscored by the relationship between NAA and ventricular volume (Figure 9).
Levels of lactate were increased 3 days following KA-induced seizures (Luna-Medina et al., 2007; Najm et al., 1998) but returned to baseline levels 1 month later (Najm et al., 1997). KA administration is associated with energy failure: a dramatic increase in glucose metabolism (Lothman and Collins, 1981) and a rapid and significant reduction in ATP and glucose (Retz and Coyle, 1982) may lead to accumulation of lactate. Concurrently, mitochondrial dysfunction related to impending neuronal loss may also increase lactate production (Ebisu et al., 1996). Evidence for an increase in lactate following KA-induced seizure activity has been provided by several studies (e.g., Luna-Medina et al., 2007; Meric et al., 1994; Najm et al., 1997) and interpreted as reflecting an increase in cellular activity and metabolism leading to energy failure; the mismatch between oxidative metabolism and glycolysis may produce lactate.
Comporting with in vitro results (Alvestad et al., 2008; Petroff et al., 2002), the current in vivo study revealed a statistically significant reduction in the levels of glutamate in the dorsal hippocampus at 3 days following status epilepticus in the Sz group compared with the NoSz and Con groups. At the 1-month scan, the levels of glutamate tended to be lower in the Sz group compared with the Con group. In human studies, levels of glutamate were described as lowest in gliotic regions of the epileptic hippocampus (Peeling and Sutherland, 1993; Petroff et al., 2002) suggesting that hippocampal glutamate levels in epilepsy may reflect severity of neuronal loss and the associated gliosis. The hippocampal formation of rats following KA-induced status epilepticus demonstrates reduced 13C-labeling of glutamate, implying reduced glutamate content and variously interpreted as reflecting loss of glutamatergic neurons of the hippocampus or mitochondrial dysfunction of the remaining neurons (Alvestad et al., 2008). The correlation observed in the current study between reduced glutamate levels and enlarged ventricular volume (suggestive of neuronal loss), however, supports the former interpretation (Figure 9).
For the most part, metabolite levels in the cerebellum did not change as a function of KA administration or status epilepticus. An exception was an increase in Cho 3 days following KA-induced status epilepticus. This increase in Cho is a novel finding that, if not a chance occurrence, may reflect generalized membrane breakdown, demyelination, or inflammation (Govindaraju et al., 2000a) accompanying status epilepticus.
Because the CT-PRESS data were acquired at TEs ranging from 36.6 ms to 241.4ms (average TE = 139ms), the signal intensities in the spectrum could be significantly affected by T2. However, if the metabolites showed a similar trend to longer T2 as water, this would lead to higher signals from all metabolites and, hence, could not explain the observed signal decreases from NAA and glutamate. Although a longer T2 could plausibly explain the increase in the lactate signal, we believe a change in lactate concentration was the dominant cause, in agreement with previous findings and interpretations (e.g., Luna-Medina et al., 2007; Meric et al., 1994; Najm et al., 1997; Najm et al., 1998).
3.4 Immunohistochemistry
Some histological studies implicate apoptotic processes in the neuronal death that occurs in brain regions targeted by KA-induced status epilepticus (Faherty et al., 1999; Kondratyev and Gale, 2000). As caspase-3 proteases play a crucial role in apoptosis, immunohistochemistry for the caspase-3 protein was carried out to evaluate apoptotic cells in the CA1, CA3, and DG regions of the entire hippocampus (both dorsal and ventral regions). A significantly higher number of caspase-3 staining cells were observed in the Sz group than in the Con and NoSz groups, consistent with previous studies (Faherty et al., 1999; Kondratyev and Gale, 2000). Status epilepticus induced brain damage is pronounced in CA1 and CA3 regions of the hippocampus (Bouilleret et al., 2000; Sperk et al., 1983), while the DG is relatively spared (Ben-Ari et al., 1981; Lothman and Collins, 1981; Olney et al., 1986). This pattern of insult and sparing is consistent with the current findings where caspase-3 staining was pronounced in the CA1 and CA3 regions of the Sz group but indistinguishable among the three groups in the DG.
3.5 Limitations
Animals receiving KA and exhibiting status epilepticus are prone to the development of spontaneous seizures following a latent period. The length of the latent period, and the frequency of seizure occurrence, is, however, a contentious area of research (e.g., Williams et al., 2007). In this study, although animals were regularly observed during the first week following KA administration when no spontaneous seizure activity was perceived, the animals were not monitored by video or electroencephalographic recordings 24h a day for accurate detection of the first spontaneous motor seizure (Williams et al., 2007). Thus, it is possible that at least some of the animals in the Sz group had spontaneous seizures by scan 4 that may explain the high variability in ventricular volume enlargement and recovery.
The peak integration technique is potentially more prone to be affected by changes or differences in T2′ (i.e., the time constants for the signal decay due to B0 inhomogeneities) than are line-fitting routines such as the LCModel. However, this quantitation technique was used to quantify MRS data because one-dimensional (1D) linear least squares fitting methods such as the LCModel are in general not applicable to quantifying two-dimensional (2D) constant time point resolved spectroscopy (CT-PRESS) data. However, efforts are underway to develop an LCModel-type quantification method suitable for CT-PRESS data that will take advantage of the full 2D spectral information.
4. Conclusions
Unlike postmortem analyses where observations are limited to specific time points following insult, in vivo MR imaging and spectroscopy provide the opportunity to track the evolution and resolution of brain compromise. Three days following KA-induced status epilepticus, increases in computed T2 in the ventral hippocampus and limbic cortex indicated edema, whereas decreases in NAA and glutamate, and increases in lactate in the dorsal hippocampus indicated energy failure and the onset of tissue damage. One month following status epilepticus, the computed T2 increase had resolved, indicating that the edema was transient, and lactate levels normalized, indicating a return to normal glucose utilization or mitochondrial function. Some cavitation of ventral hippocampal tissue limited tissue volume recovery. NAA and glutamate levels remained significantly lower than baseline levels, and together with correlations with ventricular volume and the significant presence of caspase-3 staining indicate that a fraction of cells in the dorsal hippocampus were irrecoverably damaged. At the final scan, 42 – 50 days after status epilepticus onset, shrinkage of the enlarged ventricles occurred in some animals that may be indicative of tissue recovery. Detection of transient and permanent changes in the brain highlights the utility of MRI and MRS as tools to characterize the structural and neurometabolic profile of disease processes and their potential for evaluating the effects of medical and surgical treatments for diseases such as epilepsy.
5. Experimental Procedures
5.1 Animals
The study group comprised 16 male, wild-type Wistar rats (Charles River Laboratories) singly housed with free access to food (vitamin- and mineral- enriched mouse and rat diet, #7001; Teklad, Madison, WI) and water. Rats were acclimated for 4 weeks before the first imaging session (baseline scan), at which time their average weight was 390.6±15.3g. The Institutional Animal Care and Use Committees at SRI International and Stanford University approved all procedures.
5.2 Kainic Acid-Induced Status Epilepticus
Kainic acid (Cayman Chemical, Ann Arbor MI), purchased as a crystalline solid, was diluted in phosphate-buffered saline (PBS) to a final concentration of 2mg/ml (pH adjusted to 7.2). After the baseline scan, 10 of 16 rats were administered intraperitoneally (i.p.) a starting dose of 10mg/kg KA, then 10mg/kg KA i.p. each hour for 4h (Hellier et al., 1998; Hellier and Dudek, 2006) and monitored for seizure activity according to a modified Racine scale: 0=no changes, .5=wet dog shakes, 1=mouth and facial movements, 2=head nodding, 3=forelimb clonus, 4=rearing, 5=rearing and falling (Racine, 1972). Five of the ten animals that received KA exhibited seizure activity with a minimum score of 3 for at least 3h (total dose: 10–50mg/kg); this behavioral pattern was classified as status epilepticus and these animals were included in the seizure (Sz) group. The other 5 of 10 rats that received KA (total KA dose = 50mg/kg) did not exhibit seizure activity and were classified as the no seizure (NoSz) animals. The remaining 6 animals were injected i.p. with equivalent volumes of saline and were designated the control (Con) group. Seizure animals were monitored daily until they consumed food and water, groomed, and defecated as normal (~one week). Until this point of recovery, animals were kept hydrated with subcutaneous injections of saline (10ml) and fed Critical Care (Oxproline, Murdock, NE) intragastrically.
5.3 MR Scanning Procedures
Schedule
All animals were scanned at baseline (scan 1), and then 3 days (scan 2) and 1 month (28 – 32 days, scan 3) after KA administration (Table 1). To track local brain damage progress or recovery, the Sz group had an additional scanning session 42 – 50 days (scan 4) after status epilepticus induction.
Anesthesia and Monitoring
Animals were held in an MR-invisible structure providing support for a radio frequency (RF) coil and a nose cone for delivery of isoflurane anesthesia (1.5 to 3.5%) and oxygen (1.5L/min) (Adalsteinsson et al., 2004; Pfefferbaum et al., 2004a). Blood oxygen saturation, pulse rate, rectal temperature, and respiration were monitored throughout the experiment. Heating was provided by pre-warmed bags of saline solution placed under the animals. Scanning sessions were ~2h for each rat.
MRI Acquisition
The experiments were conducted on a clinical 3T GE Signa MR scanner equipped with a high-strength insert gradient coil (peak strength=600mT/m, peak slew rate=3200T/m/s, Chronik et al., 2000a; Chronik et al., 2000b). The gradient system was operated at an amplitude of 500mT/m with a slew rate of 1800mT/m/ms. A custom-made rat brain quadrature head coil (Ø=44mm) was used for both RF excitation and signal reception. A gradient-recalled echo (GRE) localizer scan (echo time (TE)/repetition time (TR)=1.9/7.7ms, field of view (FOV)=80×80mm2, 256×128 matrix, 5mm slice per plane) was used to position the animals in the scanner and for graphical prescription of the subsequent scans. High-resolution-, dual-echo-, FSE images were acquired in the coronal plane, transaxial to the magnet system bore (TE1/TE2/TR=12/60/5000ms, FOV=64×48mm2, 256×192 matrix, echo train length=8, 50 slices, .3mm thick, 0mm skip (no slice gap), 4 acquisitions, NEX=3). Nominal acquisition resolution was .25mm × .25mm in plane. Herein, “early echo” refers to TE1=12ms and “late echo” refers to TE2=60ms.
Image Postprocessing
MRI data from scans 1, 2, and 3 were quantified after registration to the baseline images of one untreated, reference animal (Pfefferbaum et al., 2008). For registration, to support more finely sampled deformation fields, the data were up-sampled to near-isotropic voxels (.117 × .117 × .125mm) using cubic spline interpolation. Each animal’s scan 1 image was registered to the reference animal’s scan 1 data with rigid followed by affine followed by nonrigid registration (Rohlfing and Maurer, 2003; Rohlfing et al., 2006; Rohlfing et al., 2008). For longitudinal alignment, each animal’s scan 2 and scan 3 images were similarly registered to its own scan 1 data. For the Sz group, each animal’s scan 4 images were also registered to its own scan 1 data.
By concatenating inter-animal and within-animal transformations for scan 2 and 3, rather than registering scan 2 and scan 3 images to the reference animal independently, it was ensured that the unavoidable residual misalignments between animals were consistent for all three scans within each animal. Rigid registrations, preserving relative within-animal size, were retained for individual and group average display. T2 maps (computed voxel-by-voxel: T2=[TE2−TE1]/[log(IntensityTE1)−log(IntensityTE2)]) were constructed for display and are herein referred to as computed T2. The three regions of interest for tissue T2 computation were drawn on the time 2 data for each rat. After rigid alignment (preserving all size information) of all sessions to the same image space, the ROIs were projected onto times 1 and 3 images and manually edited to ensure that no ventricular enlargement or cavitation was included in the ROI. A 5 slice ROI encompassing the lateral ventricle was defined on a group average time 1 brain. Rigid inter-subject alignment for all animals for all times preserved ventricular size and allowed for projection of the ventricular ROI to each animal for each observation time. Thresholding for ventricular volume was accomplished by constructing histograms for all computed T2 voxels in the ROI, normalizing the histograms to time 1, and defining all voxels greater than 110ms as cerebral spinal fluid (Figure 3).
MRS Acquisition
FSE images were used to prescribe voxels in the dorsal hippocampus to determine seizure effects and in the cerebellum to provide a control structure. Optimization of voxel size for high signal-to-noise precluded MRS measurements in the smaller ventral hippocampus. The dorsal hippocampal voxel (~.16cm3 = 9.8×4×4mm3) was centered at approximately −4.00mm Bregma (and extended 2mm anterior and posterior to this central point), extended 4.9mm to the right and left of midline, and 4mm inferior to −3.10mm Bregma, according to the atlas of Paxinos & Watson (2005). The cerebellar voxel (~.19cm3 = 8.5×5×4.6mm3) extended 2.5mm anterior and posterior to a central position at approximately −11.80mm Bregma, 4.25mm to the right and left of midline, and 4.6mm inferior to −3.60mm Bregma, according to the atlas of Paxinos & Watson (2005).
Spectroscopy was performed with a constant time point resolved spectroscopy (CT-PRESS) sequence (Dreher and Leibfritz, 1999; Mayer and Spielman, 2005; Mayer et al., 2007), which allows for the detection of J-coupled resonances (e.g., glutamate) with increased signal to noise ratio (SNR) and spectral resolution by using effective homonuclear decoupling. CT-PRESS consists of a modified PRESS module in which the position of the last refocusing pulse is shifted to encode the chemical shift (CS) in the second time dimension (t1). The pulse was shifted in 129 steps with increment Δt1/2 = .8ms corresponding to a spectral width (SW1) of 625Hz in f1. The average TE of the sequence was 139ms, optimized for the detection of glutamate. To increase signal-to-noise ratio, data acquisition (2048 complex points at SW2 = 5000Hz) started immediately after the last crusher gradient of the second refocusing pulse. A three-pulse CS-selective sequence for water suppression and an outer volume suppression module using selective saturation pulses preceded CT-PRESS. TR was 2s, and 4 excitations were performed without data acquisition to establish a steady state. With 6 averages, the acquisition time per spectrum was 26:36min. An additional acquisition without water suppression was carried out (17 CS encoding steps, Δt1/2 = 6.4ms, 2 averages, Tacq = 1:16min) to measure tissue water content used to normalize the metabolite signal intensities.
The amount of cerebral spinal fluid and tissue water in the spectroscopic voxel was estimated by fitting the data acquired without water suppression across the 17 TEs to a bi-exponential model, as described in Mayer et al (2007). Apodization of the water-suppressed data involved multiplication with sine-bell functions in both time dimensions and zero filling up to 4096×1024 data points. A t1-dependent shift was applied in t2 to correct for the different start of data acquisition. After performing a two-dimensional fast Fourier transform, effectively decoupled one-dimensional CT-PRESS spectra were obtained by integrating the signal along f2 within a ±13Hz interval around the spectral diagonal. Metabolite signals in the one-dimensional spectra were evaluated by peak integration with an interval of ±6Hz. The quality of the spectra allowed evaluation of signals from N-acteylaspartate (NAA, 2.02ppm), total creatine (tCr, 3.03ppm and 3.93ppm), choline-containing compounds (Cho, 3.24ppm), glutamate (Glu, 2.36ppm), the combined resonances of glutamate and glutamine (Glx, 3.78ppm), and lactate (1.31ppm) (cf., Govindaraju et al., 2000b).
To investigate spatial consistency of voxel prescriptions (i.e., their reproducibility), the voxel ROIs were generated as binary masks for each acquisition, i.e., for each animal and each scan time. For each animal, the ROIs from the follow-up scans were reformatted into the baseline scan coordinates of the same animal via the rigid coordinate transformations previously determined by image-to-image registration. A Dice overlap measure (Dice, 1945) was then computed between the baseline and each of the reformatted follow-up ROIs, again for each animal separately. The resulting overlap scores were as follows: for the hippocampal ROIs, .868±.011; for the cerebellar ROIs, .883±.008. To put these values into perspective, we refer to previous results (Rohlfing et al., 2004) that quantified the dependence of the Dice measure on the shape and size of the region of interest. In short, the Dice measure between discretely sampled regions is more sensitive for smaller structures and for structures with larger surface areas relative to their volumes. In particular, given the surface-to-volume ratio of an ROI, one can compute the Dice overlap between that ROI and the same ROI after eroding it by one pixel. The result represents the Dice overlap that corresponds to under-segmentation of the ROI by one pixel everywhere along its surface. For the ROIs under consideration here, the mean surface-to-volume ratio was .301 for the hippocampal ROIs and .277 for the cerebellum ROIs. For a surface-to-volume ratio of .3, a previously derived formula (Rohlfing et al., 2004) for missing one pixel along the entire surface of the ROI yields a Dice overlap of .82. The reproducibility of our ROIs is thus substantially better.
5.4 Immunohistochemistry
After MR scanning session 4, 50 days following status epilepticus induction, all animals were anesthetized and perfused transcardially with 4% paraformaldehyde (PF) solution. Brains were removed, postfixed in 4% PF (4°C overnight), and then transferred for storage and shipment into a 30% sucrose phosphate buffer solution (PBS). The histologist (ELFC) sectioned brains into serial coronal 40μm sections using an American Optical freezing microtome. Sections were saved in strict anatomical order, collected in a 1/8 series, and stored in .1% NaN3 in .1M PBS at 4°C until processing for immunohistochemistry (IHC).
Every 7th section of the hippocampus, Bregma −1.8mm to −6.3mm (Paxinos and Watson, 1997), was mounted on a glass slide (SuperFrost Plus: Fisher Scientific, Hampton, NH) and allowed to air-dry overnight. Prior to antibody incubation, sections were pretreated as follows, antigen unmasking (.01M citric acid, pH 6.0, 95°C, 10min), membrane permeabilization (.1% trypsin in .1M Tris and .1% CaCl2, 10min.), and DNA denaturing (2N HCl in .1M PBS, 30min). Sections were then incubated in 1% hydrogen peroxide in .1M PBS (20 min) to remove any endogenous peroxide activity. Non-specific binding was blocked by incubating sections in 5% normal horse serum (NHS) and .5% TritonX 100 in PBS for 60min. Sections were incubated in rabbit polyclonal cleaved caspase-3 (Asp 175) antibody (Cell Signaling Technologies, Danvers, MA) diluted 1:500 in 5% NHS, .5% Tween 20 in PBS (24h) and then in undiluted Rabbit ImmPRESS (Vector Labs, Burlingame, CA, 60min). DABIMPACT (Vector Labs) was used to visualize the staining reaction. Sections were counterstained with Fast Red (Vector Labs), dehydrated through a graded series of ethanols, and coverslipped with Permount. Omission of the primary antibody resulted in a lack of specific staining.
Cell counts were performed in the neuronal cell layers of the hippocampus using 200X (20X objective, .40NA) and 400X magnification (40X objective, .65NA) under brightfield illumination on a Nikon SBR-KT microscope. All caspase-3 positive pyramidal cells in CA1, CA3, and the granule cell layer of the dentate gyrus were counted in the right hemisphere of every 7th hippocampal section throughout the anterior to posterior extent of the hippocampal formation.
5.5 Statistical Analysis
Group differences were analyzed using repeated-measures ANOVA and confirmed, in certain instances, by the Kruskal-Wallis (KW) one-way analysis of variance by ranks. Only group effects and their interactions were of interest to this analysis. Follow-up between group differences were conducted with t-tests (1-tailed for predicted directions).
Acknowledgments
This work was supported by the National Institute on Alcohol Abuse and Alcoholism (AA005965, AA013521-INIA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature References
- Adalsteinsson E, Sullivan EV, Pfefferbaum A. Biochemical, Functional and Microstructural Magnetic Resonance Imaging (MRI) In: Liu Y, Lovinger DM, editors. Methods in Alcohol-Related Neuroscience Research. CRC Press; Boca Raton, FL: 2002. pp. 345–372. [Google Scholar]
- Adalsteinsson E, Hurd RE, Mayer D, Sailasuta N, Sullivan EV, Pfefferbaum A. In vivo 2D J-resolved magnetic resonance spectroscopy of rat brain with a 3-T clinical human scanner. Neuroimage. 2004;22:381–6. doi: 10.1016/j.neuroimage.2003.12.046. [DOI] [PubMed] [Google Scholar]
- Alvestad S, Hammer J, Eyjolfsson E, Qu H, Ottersen OP, Sonnewald U. Limbic structures show altered glial-neuronal metabolism in the chronic phase of kainate induced epilepsy. Neurochem Res. 2008;33:257–66. doi: 10.1007/s11064-007-9435-5. [DOI] [PubMed] [Google Scholar]
- Bates JF, Peake L, Swearengen ES, Hall TW, Standish LJ. CL 218–872 pretreatment and intervention block kainate-induced convulsions and neuropathology. Behav Neurosci. 1988;102:84–92. doi: 10.1037//0735-7044.102.1.84. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Tremblay E, Riche D, Ghilini G, Naquet R. Electrographic, clinical and pathological alterations following systemic administration of kainic acid, bicuculline or pentetrazole: metabolic mapping using the deoxyglucose method with special reference to the pathology of epilepsy. Neuroscience. 1981;6:1361–91. doi: 10.1016/0306-4522(81)90193-7. [DOI] [PubMed] [Google Scholar]
- Bouilleret V, Nehlig A, Marescaux C, Namer IJ. Magnetic resonance imaging follow-up of progressive hippocampal changes in a mouse model of mesial temporal lobe epilepsy. Epilepsia. 2000;41:642–50. doi: 10.1111/j.1528-1157.2000.tb00223.x. [DOI] [PubMed] [Google Scholar]
- Camara O, Schnabel JA, Ridgway GR, Crum WR, Douiri A, Scahill RI, Hill DL, Fox NC. Accuracy assessment of global and local atrophy measurement techniques with realistic simulated longitudinal Alzheimer’s disease images. Neuroimage. 2008;42:696–709. doi: 10.1016/j.neuroimage.2008.04.259. [DOI] [PubMed] [Google Scholar]
- Carlson H, Ronne-Engstrom E, Ungerstedt U, Hillered L. Seizure related elevations of extracellular amino acids in human focal epilepsy. Neurosci Lett. 1992;140:30–2. doi: 10.1016/0304-3940(92)90674-v. [DOI] [PubMed] [Google Scholar]
- Chronik B, Alejski A, Rutt BK. Design and fabrication of a three-axis multilayer gradient coil for magnetic resonance microscopy of mice. Magma. 2000a;10:131–146. doi: 10.1007/BF02601848. [DOI] [PubMed] [Google Scholar]
- Chronik B, Alejski A, Rutt BK. Design and fabrication of a three-axis multilayer gradient coil for magnetic resonance microscopy of mice. Magma. 2000b;10:131–46. doi: 10.1007/BF02601848. [DOI] [PubMed] [Google Scholar]
- Dice L. Measures of the amount of ecologic association between species. Ecology. 1945;26:297–302. [Google Scholar]
- Dreher W, Leibfritz D. Detection of homonuclear decoupled in vivo proton NMR spectra using constant time chemical shift encoding: CT-PRESS. Magn Reson Imaging. 1999;17:141–50. doi: 10.1016/s0730-725x(98)00156-8. [DOI] [PubMed] [Google Scholar]
- During MJ, Spencer DD. Extracellular hippocampal glutamate and spontaneous seizure in the conscious human brain. Lancet. 1993;341:1607–10. doi: 10.1016/0140-6736(93)90754-5. [DOI] [PubMed] [Google Scholar]
- Ebisu T, Rooney WD, Graham SH, Weiner MW, Maudsley AA. N-Acetylaspartate as an in vivo marker of neuronal viability on kainate-induced status epilepticus: 1H magnetic resonance spectroscopic imaging. Journal of Cerebral Blood Flow and Metabolism. 1994;14:373–382. doi: 10.1038/jcbfm.1994.48. [DOI] [PubMed] [Google Scholar]
- Ebisu T, Rooney WD, Graham SH, Mancuso A, Weiner MW, Maudsley AA. MR spectroscopic imaging and diffusion-weighted MRI for early detection of kainate-induced status epilepticus in the rat. Magn Reson Med. 1996;36:821–8. doi: 10.1002/mrm.1910360604. [DOI] [PubMed] [Google Scholar]
- Faherty CJ, Xanthoudakis S, Smeyne RJ. Caspase-3-dependent neuronal death in the hippocampus following kainic acid treatment. Brain Res Mol Brain Res. 1999;70:159–63. doi: 10.1016/s0169-328x(99)00143-6. [DOI] [PubMed] [Google Scholar]
- Ferkany JW, Zaczek R, Coyle JT. Kainic acid stimulates excitatory amino acid neurotransmitter release at presynaptic receptors. Nature. 1982;298:757–9. doi: 10.1038/298757a0. [DOI] [PubMed] [Google Scholar]
- Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR in Biomedicine. 2000a;13:129–153. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000b;13:129–53. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Hantraye P, Leroy-Willig A, Denys A, Riche D, Isacson O, Maziere M, Syrota A. Magnetic resonance imaging to monitor pathology of caudate-putamen after excitotoxin-induced neuronal loss in the nonhuman primate brain. Exp Neurol. 1992;118:18–23. doi: 10.1016/0014-4886(92)90018-l. [DOI] [PubMed] [Google Scholar]
- Hellier JL, Patrylo PR, Buckmaster PS, Dudek FE. Recurrent spontaneous motor seizures after repeated low-dose systemic treatment with kainate: assessment of a rat model of temporal lobe epilepsy. Epilepsy Res. 1998;31:73–84. doi: 10.1016/s0920-1211(98)00017-5. [DOI] [PubMed] [Google Scholar]
- Hellier JL, Dudek FE. Chemoconvulsant Model of Chronic Spontaneous Seizures. In: Skolnick P, editor. Short Protocols in Neuroscience: Systems and Behavioral Method. John Wiley and Sons Ltd; 2006. [DOI] [PubMed] [Google Scholar]
- Kondratyev A, Gale K. Intracerebral injection of caspase-3 inhibitor prevents neuronal apoptosis after kainic acid-evoked status epilepticus. Brain Res Mol Brain Res. 2000;75:216–24. doi: 10.1016/s0169-328x(99)00292-2. [DOI] [PubMed] [Google Scholar]
- Kondratyev A, Gale K. Latency to onset of status epilepticus determines molecular mechanisms of seizure-induced cell death. Brain Res Mol Brain Res. 2004;121:86–94. doi: 10.1016/j.molbrainres.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Koyama I. Amino acids in the cobalt-induced epileptogenic and nonepileptogenic cat’s cortex. Can J Physiol Pharmacol. 1972;50:740–52. doi: 10.1139/y72-109. [DOI] [PubMed] [Google Scholar]
- Kramer RE, Luders H, Lesser RP, Weinstein MR, Dinner DS, Morris HH, Wyllie E. Transient focal abnormalities of neuroimaging studies during focal status epilepticus. Epilepsia. 1987;28:528–32. doi: 10.1111/j.1528-1157.1987.tb03683.x. [DOI] [PubMed] [Google Scholar]
- Lassmann H, Petsche U, Kitz K, Baran H, Sperk G, Seitelberger F, Hornykiewicz O. The role of brain edema in epileptic brain damage induced by systemic kainic acid injection. Neuroscience. 1984;13:691–704. doi: 10.1016/0306-4522(84)90089-7. [DOI] [PubMed] [Google Scholar]
- Liu Z, Stafstrom CE, Sarkisian MR, Yang Y, Hori A, Tandon P, Holmes GL. Seizure-induced glutamate release in mature and immature animals: an in vivo microdialysis study. Neuroreport. 1997;8:2019–23. doi: 10.1097/00001756-199705260-00043. [DOI] [PubMed] [Google Scholar]
- Lothman EW, Collins RC. Kainic acid induced limbic seizures: metabolic, behavioral, electroencephalographic and neuropathological correlates. Brain Res. 1981;218:299–318. doi: 10.1016/0006-8993(81)91308-1. [DOI] [PubMed] [Google Scholar]
- Luna-Medina R, Cortes-Canteli M, Sanchez-Galiano S, Morales-Garcia JA, Martinez A, Santos A, Perez-Castillo A. NP031112, a thiadiazolidinone compound, prevents inflammation and neurodegeneration under excitotoxic conditions: potential therapeutic role in brain disorders. J Neurosci. 2007;27:5766–76. doi: 10.1523/JNEUROSCI.1004-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magloczky Z, Freund TF. Selective neuronal death in the contralateral hippocampus following unilateral kainate injections into the CA3 subfield. Neuroscience. 1993;56:317–35. doi: 10.1016/0306-4522(93)90334-c. [DOI] [PubMed] [Google Scholar]
- Mao H, Toufexis D, Wang X, Lacreuse A, Wu S. Changes of metabolite profile in kainic acid induced hippocampal injury in rats measured by HRMAS NMR. Exp Brain Res. 2007;183:477–85. doi: 10.1007/s00221-007-1061-6. [DOI] [PubMed] [Google Scholar]
- Mayer D, Spielman DM. Detection of glutamate in the human brain at 3 T using optimized constant time point resolved spectroscopy. Magnetic Resonance in Medicine. 2005;54:439–442. doi: 10.1002/mrm.20571. [DOI] [PubMed] [Google Scholar]
- Mayer D, Zahr NM, Sullivan EV, Pfefferbaum A. In vivo metabolite differences between the basal ganglia and cerebellum of the rat brain detected with proton MRS at 3T. Psychiatry Res. 2007;154:267–73. doi: 10.1016/j.pscychresns.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum BS. Excitotoxicity and selective neuronal loss in epilepsy. Brain Pathol. 1993;3:405–12. doi: 10.1111/j.1750-3639.1993.tb00768.x. [DOI] [PubMed] [Google Scholar]
- Meric P, Barrere B, Peres M, Gillet B, Berenger G, Beloeil JC, Seylaz J. Effects of kainate-Induced seizures on cerebral metabolism: a combined H-1 and P-31 NMR study in rat. Brain Research. 1994;638:53–60. doi: 10.1016/0006-8993(94)90632-7. [DOI] [PubMed] [Google Scholar]
- Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol. 2007;81:89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler JV, Cuthbertson GJ. Kainic acid neurotoxicity toward hippocampal formation: dependence on specific excitatory pathways. Brain Res. 1980;195:47–56. doi: 10.1016/0006-8993(80)90865-3. [DOI] [PubMed] [Google Scholar]
- Najm IM, Wang Y, Hong SC, Luders HO, Ng TC, Comair YG. Temporal changes in proton MRS metabolites after kainic acid-induced seizures in rat brain. Epilepsia. 1997;38:87–94. doi: 10.1111/j.1528-1157.1997.tb01082.x. [DOI] [PubMed] [Google Scholar]
- Najm IM, Wang Y, Shedid D, Luders HO, Ng TC, Comair YG. MRS metabolic markers of seizures and seizure-induced neuronal damage. Epilepsia. 1998;39:244–50. doi: 10.1111/j.1528-1157.1998.tb01368.x. [DOI] [PubMed] [Google Scholar]
- Nixon K. Alcohol and adult neurogenesis: roles in neurodegeneration and recovery in chronic alcoholism. Hippocampus. 2006;16:287–95. doi: 10.1002/hipo.20162. [DOI] [PubMed] [Google Scholar]
- Norman AB, Thomas SR, Pratt RG, Samaratunga RC, Sanberg PR. T1 and T2 weighted magnetic resonance imaging of excitotoxin lesions and neural transplants in rat brain in vivo. Exp Neurol. 1990;109:164–70. doi: 10.1016/0014-4886(90)90070-9. [DOI] [PubMed] [Google Scholar]
- O’Shaughnessy D, Gerber GJ. Damage induced by systemic kainic acid in rats is dependent upon seizure activity--a behavioral and morphological study. Neurotoxicology. 1986;7:187–202. [PubMed] [Google Scholar]
- Olney JW, Collins RC, Sloviter RS. Excitotoxic mechanisms of epileptic brain damage. Adv Neurol. 1986;44:857–77. [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Elsevier Academic Press; London: 2005. [Google Scholar]
- Peeling J, Sutherland G. 1H magnetic resonance spectroscopy of extracts of human epileptic neocortex and hippocampus. Neurology. 1993;43:589–94. doi: 10.1212/wnl.43.3_part_1.589. [DOI] [PubMed] [Google Scholar]
- Petroff OA, Errante LD, Rothman DL, Kim JH, Spencer DD. Glutamate-glutamine cycling in the epileptic human hippocampus. Epilepsia. 2002;43:703–10. doi: 10.1046/j.1528-1157.2002.38901.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. In vivo structural imaging of the rat brain with a 3-T clinical human scanner. J Magn Reson Imaging. 2004a;20:779–85. doi: 10.1002/jmri.20181. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Carmelli D. Morphological changes in aging brain structures are differentially affected by time-linked environmental influences despite strong genetic stability. Neurobiol Aging. 2004b;25:175–83. doi: 10.1016/s0197-4580(03)00045-9. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Zahr NM, Mayer D, Vinco S, Orduna J, Rohlfing T, Sullivan EV. Ventricular Expansion in Wild-Type Wistar Rats after Alcohol Exposure by Vapor Chamber. Alcoholism, Clinical and Experimental Research. 2008;32:1459–1467. doi: 10.1111/j.1530-0277.2008.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plock N, Kloft C. Microdialysis--theoretical background and recent implementation in applied life-sciences. Eur J Pharm Sci. 2005;25:1–24. doi: 10.1016/j.ejps.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Puig B, Ferrer I. Caspase-3-associated apoptotic cell death in excitotoxic necrosis of the entorhinal cortex following intraperitoneal injection of kainic acid in the rat. Neurosci Lett. 2002;321:182–6. doi: 10.1016/s0304-3940(01)02518-6. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–94. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Retz KC, Coyle JT. Effects of kainic acid on high-energy metabolites in the mouse striatum. J Neurochem. 1982;38:196–203. doi: 10.1111/j.1471-4159.1982.tb10872.x. [DOI] [PubMed] [Google Scholar]
- Righini A, Pierpaoli C, Alger JR, Di Chiro G. Brain parenchyma apparent diffusion coefficient alterations associated with experimental complex partial status epilepticus. Magn Reson Imaging. 1994;12:865–71. doi: 10.1016/0730-725x(94)92027-3. [DOI] [PubMed] [Google Scholar]
- Rohlfing T, Maurer CR. Nonrigid image registration in shared-memory multiprocessor environments with application to brains, breasts, and bees. IEEE Transactions on Information Technology in Biomedicine. 2003;7:16–25. doi: 10.1109/titb.2003.808506. [DOI] [PubMed] [Google Scholar]
- Rohlfing T, Brandt R, Menzel R, Maurer CR., Jr Evaluation of atlas selection strategies for atlas-based image segmentation with application to confocal microscopy images of bee brains. Neuroimage. 2004;21:1428–1442. doi: 10.1016/j.neuroimage.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Rohlfing T, Sullivan EV, Pfefferbaum A. Deformation-based brain morphometry to track the course of alcoholism: Differences between intra-subject and inter-subject analysis. Psychiatry Research: NeuroImaging. 2006;146:157–170. doi: 10.1016/j.pscychresns.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Rohlfing T, Zahr NM, Sullivan EV, Pfefferbaum A. The SRI24 multi-channel brain atlas: Construction and applications. Medical Imaging 2008: Image Processing. Proceedings of SPIE. 2008:6914. doi: 10.1117/12.770441. EID 691409 (12 pages) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland JJ, Savage LM. Blunted hippocampal, but not striatal, acetylcholine efflux parallels learning impairment in diencephalic-lesioned rats. Neurobiol Learn Mem. 2007;87:123–32. doi: 10.1016/j.nlm.2006.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar HJ, Oxbury JM. Hippocampal neuron loss in temporal lobe epilepsy: correlation with early childhood convulsions. Ann Neurol. 1987;22:334–40. doi: 10.1002/ana.410220309. [DOI] [PubMed] [Google Scholar]
- Schwob JE, Fuller T, Price JL, Olney JW. Widespread patterns of neuronal damage following systemic or intracerebral injections of kainic acid: a histological study. Neuroscience. 1980;5:991–1014. doi: 10.1016/0306-4522(80)90181-5. [DOI] [PubMed] [Google Scholar]
- Sierra-Paredes G, Galan-Valiente J, Vazquez-Illanes MD, Aguilar-Veiga E, Soto-Otero R, Mendez-Alvarez E, Sierra-Marcuno G. Extracellular amino acids in the rat hippocampus during picrotoxin threshold seizures in chronic microdialysis experiments. Neurosci Lett. 1998;248:53–6. doi: 10.1016/s0304-3940(98)00332-2. [DOI] [PubMed] [Google Scholar]
- Sperk G, Lassmann H, Baran H, Kish SJ, Seitelberger F, Hornykiewicz O. Kainic acid induced seizures: neurochemical and histopathological changes. Neuroscience. 1983;10:1301–15. doi: 10.1016/0306-4522(83)90113-6. [DOI] [PubMed] [Google Scholar]
- Sperk G. Kainic acid seizures in the rat. Prog Neurobiol. 1994;42:1–32. doi: 10.1016/0301-0082(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Stone JL, Hughes JR, Barr A, Tan W, Russell E, Crowell RM. Neuroradiological and electroencephalographic features in a case of temporal lobe status epilepticus. Neurosurgery. 1986;18:212–6. doi: 10.1227/00006123-198602000-00019. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Graham SH, Simon RP. The role of excitatory neurotransmitters in seizure-induced neuronal injury in rats. Brain Res. 1996;737:59–63. doi: 10.1016/0006-8993(96)00658-0. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Tanaka T, Kondo S, Hori T, Fukuda H, Yonemasu Y, Tanaka M, Shindo K. Magnetic resonance imaging in kainic acid-induced limbic seizure status in cats. Neurol Med Chir (Tokyo) 1993;33:285–9. doi: 10.2176/nmc.33.285. [DOI] [PubMed] [Google Scholar]
- Tokuhara D, Sakuma S, Hattori H, Matsuoka O, Yamano T. Kainic acid dose affects delayed cell death mechanism after status epilepticus. Brain Dev. 2007;29:2–8. doi: 10.1016/j.braindev.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Tokumitsu T, Mancuso A, Weinstein PR, Weiner MW, Naruse S, Maudsley AA. Metabolic and pathological effects of temporal lobe epilepsy in rat brain detected by proton spectroscopy and imaging. Brain Res. 1997;744:57–67. doi: 10.1016/s0006-8993(96)01071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade JV, Samson FE, Nelson SR, Pazdernik TL. Changes in extracellular amino acids during soman- and kainic acid-induced seizures. J Neurochem. 1987;49:645–50. doi: 10.1111/j.1471-4159.1987.tb02912.x. [DOI] [PubMed] [Google Scholar]
- Williams PA, Hellier JL, White AM, Staley KJ, Dudek FE. Development of spontaneous seizures after experimental status epilepticus: implications for understanding epileptogenesis. Epilepsia. 2007;48(Suppl 5):157–63. doi: 10.1111/j.1528-1167.2007.01304.x. [DOI] [PubMed] [Google Scholar]
- Wilson CL, Maidment NT, Shomer MH, Behnke EJ, Ackerson L, Fried I, Engel J., Jr Comparison of seizure related amino acid release in human epileptic hippocampus versus a chronic, kainate rat model of hippocampal epilepsy. Epilepsy Res. 1996;26:245–54. doi: 10.1016/s0920-1211(96)00057-5. [DOI] [PubMed] [Google Scholar]
- Wolf OT, Dyakin V, Patel A, Vadasz C, de Leon MJ, McEwen BS, Bulloch K. Volumetric structural magnetic resonance imaging (MRI) of the rat hippocampus following kainic acid (KA) treatment. Brain Res. 2002;934:87–96. doi: 10.1016/s0006-8993(02)02363-6. [DOI] [PubMed] [Google Scholar]
- Zahr NM, Mayer D, Hsu O, Vinco S, Orduna J, Sullivan EV, Pfefferbaum A. In vivo neurochemical evidence for alcohol binge induced neural compromise and recovery in alcohol-preferring rats. Research Society on Alcoholism; San Diego, CA: 2009. [Google Scholar]
- Zhang WQ, Hudson PM, Sobotka TJ, Hong JS, Tilson HA. Extracellular concentrations of amino acid transmitters in ventral hippocampus during and after the development of kindling. Brain Res. 1991;540:315–8. doi: 10.1016/0006-8993(91)90527-3. [DOI] [PubMed] [Google Scholar]