Abstract
We propose that the normal immunocompetent B cell repertoire is replete with B cells making antibodies that recognize brain antigens. Although B cells that are reactive with self antigen are normally silenced during B cell maturation, the blood–brain barrier (BBB) prevents many brain antigens from participating in this process. This enables the generation of a B cell repertoire that is sufficiently diverse to cope with numerous environmental challenges. It requires, however, that the integrity of the BBBs is uninterrupted throughout life to protect the brain from antibodies that crossreact with microorganisms and brain antigens. Under conditions of BBB compromise, and during fetal development, we think that these antibodies can alter brain function in otherwise healthy individuals.
It has become increasingly clear that the brain maintains a constant dialogue with the immune system through communication networks that are just now being revealed at the molecular level. We now know that the brain helps to control immune activation. For example, in the cholinergic anti-inflammatory pathway, the vagus nerve has been shown to excite sympathetic nerves that innervate the spleen and form direct synapses with cells of the immune system1. In this manner, signalling through the vagus nerve can modulate effector mechanisms of both the innate and adaptive immune systems1. It is also clear that many substances produced in the brain modulate the function not only of neurons but also of cells of the immune system. Elegant studies have shown that immune cells express receptors for pituitary hormones (such as prolactin, growth hormone, insulin-like growth factor 1 and thyroid-stimulating hormone) and neuro-transmitters (such as acetylcholine, glutamate, noradrenaline and endorphins) and that immune function can be controlled through these pathways2. Less is perhaps known about potential homeostatic effects of the immune system on the brain. Recent studies have shown that MHC class I molecules modulate neural synapse formation during brain development and can regulate the function of these synapses in the adult brain3. Cytokines can also have homeostatic functions in the brain; for example, tumour necrosis factor (TNF) regulates the recycling of the α-amino-3-hydroxy-5-methyl-4-isoazoleproprionic acid (AMPA) class of glutamatergic receptors, which bind the neurotransmitter glutamate and initiate excitatory activity in neurons4.
However, the immune system can also cause brain pathology, one aspect of which is the focus of this Opinion article. Some of these pathologies have been extensively studied. For example, in systemic lupus erythematosus (SLE), antibody-mediated activation of endothelial cells and initiation of the clotting cascade in the vasculature of the brain can lead to vasculitis or thrombosis and ensuing ischaemic and inflammatory brain pathology5. In multiple sclerosis, there is large-scale infiltration of cells of the immune system into the brain parenchyma as well as activation of resident inflammatory cells, astrocytes and microglial cells (see Glossary), which results in nerve damage6. In addition to such clinically obvious autoimmune and inflammatory diseases of the brain involving invasion of immune cells into the brain parenchyma, high-resolution neuroimaging studies show that many more individuals have structural lesions in the brain or functional alterations in network connectivity that have not been attributed to the immune response and are not associated with immune cell infiltrates. Although it has been assumed that these changes are the result of neurodegenerative diseases or otherwise asymptomatic vascular disease in adults, or unexplained developmental abnormalities in children, we suggest that immune-mediated damage to the central nervous system (CNS) might occur more commonly than we currently recognize. Furthermore, we propose that this type of disease might arise in apparently healthy individuals who are not genetically predisposed to autoimmunity and do not have a defect in immunological tolerance, in the absence of infiltration of immune cells into the brain and in the absence of clinical, perhaps even subclinical, brain inflammation.
The focus of this Opinion article is the potential role of serum antibodies in modulating adult and fetal brain function. We propose that many acquired changes or congenital impairments in cognition and behaviour might be the consequence of common, circulating brain-specific antibodies that can alter brain function if they gain access to brain tissue.
Brain-reactive antibodies
In recent years, numerous brain-reactive antibodies have been identified in human sera and have been proposed to associate with neurological or neuropsychiatric symptoms (TABLE 1). These antibodies can be divided into three categories: antibodies that have a causal relationship with the development of symptoms; antibodies that are generated as a secondary symptom during brain disease, perhaps as a result of brain injury; and antibodies that will turn out to not be associated with disease as more careful studies are carried out (false-positive cases).
Table 1.
Antibody-related disorders of the peripheral and central nervous systems
| Disorder | Defined antigen | Autoantibody present in CSF | Antibody useful in diagnosis | Antibody binding relevant to disease mechanism | Passive transfer model | Clinical response to immune modulation | maternal transmission |
|---|---|---|---|---|---|---|---|
| Peripheral nervous system | |||||||
| Myasthenia gravis | AchR and MuSK | ND | Yes64 | Yes | Yes | Yes | Yes65 |
| Lambert–eaton syndrome and acquired neuromyotonia | VGCC and VGKC | ND | Yes | Yes66 | Yes | Yes | ND |
| Arthrogryposis multiplex congenital | Fetal AchR | ND | Yes | Yes | Yes67 | ND | Yes |
| Post-infectious polyradiculopathy (Guillain–Barré syndrome) | Gangliosides* and microbial glycan‡ | ND | Yes17 | Yes | Yes68 | Yes | ND |
| Central nervous system: spinal cord | |||||||
| HAM and tropical spastic paraparesis | hnRNP A1 (REF. 69) | ND | ND | ND | ND | ND | ND |
| Central nervous system: brain | |||||||
| Neuromyelitis optica | Aquaporin 4 | Yes9 | Yes | Yes70 | ND | Yes71 | ND |
| Paraneoplastic syndromes | Many72 | Yes72 | Yes | ND | ND | ND | ND |
| NMDAR73 | Yes73 | Yes | ND | ND | ND | ND | |
| Limbic encephalitis | NMDAR7, NR1 and NR2B NMDAR subunits and VGKC74 | Yes | Yes | Yes | ND | Yes | ND |
| Rasmussen encephalitis | GRIA3 (REFS 75,76) | Yes | Yes | ND | ND | ND | ND |
| Hashimoto’s encephalitis | AKR1A1 (REF. 77) | Yes | Yes | Yes | ND | Yes | ND |
| Encephalitis lethargica | ND | Yes78 | ND | ND | ND | ND | ND |
| Stiff person syndrome | GAD and gephyrin§ | Yes79 | Yes | Yes | ND | Yes80 | ND |
| Post-streptococcal movement disorders (rheumatic fever) | β-tubulin14,81, lysoganglioside and GlcNAc14 | Yes | Yes | Yes15 | ND | ND | ND |
| SLE | NR2A and NR2B | Yes24–26 | Yes24–26 | Yes19 | Yes31 | Yes26 | Yes51 |
| NSPA | ND | ND | Yes11 | ND | ND | ND | |
| PANDAs | ND82 | ND | ND | ND | ND | ND | ND |
| Gluten enteropathy | Synapsin 1 | ND | ND | Yes83 | Yes84 | ND | ND |
| Autism | ND | ND | ND | ND | Yes55,56 | ND | ND |
| Dyslexia | ND | ND | ND | ND | Yes56 | ND | ND |
Ganglioside specifically refers to GM1, GD1a, GM1b and GalNAcGD1a. Also, in variant Guillain–Barré syndrome, the defined antigens are GQ1b, GT1a, GD3 and GD1b.
Microbial glycan refers to Campylobacter jejuni lipooligosaccharide.
Gephyrin is a cytoplasmic protein that associates with glycine and γ-aminobutyric acid receptors. AchR, acetylcholine receptor; AKR1A1, aldo-keto reductase 1; CSF, cerebrospinal fluid; GAD, glutamate decarboxylase; GalNAc, N-acetyl galactosamine; GlcNAc, N-acetyl-β-D-glucosamine; GRIA3, glutamate receptor, ionotrophic, AMPA 3; HAM, HTLv type 1-associated myelopathy; hnRNP A1, heterogeneous ribonucleoprotein A1; MuSK, muscle-specific kinase; ND, not determined; NMDAR, N-methyl-D-aspartate glutamatergic receptor; NR1, NMDAR subunit 1; NSPA, neuronal surface P antigen; PANDAs, paediatric autoimmune neuropsychiatric disorders associated with streptococcal infections; SLE, systemic lupus erythematosus; VGCC, voltage-gated calcium channel; VGKC, voltage-gated potassium channel.
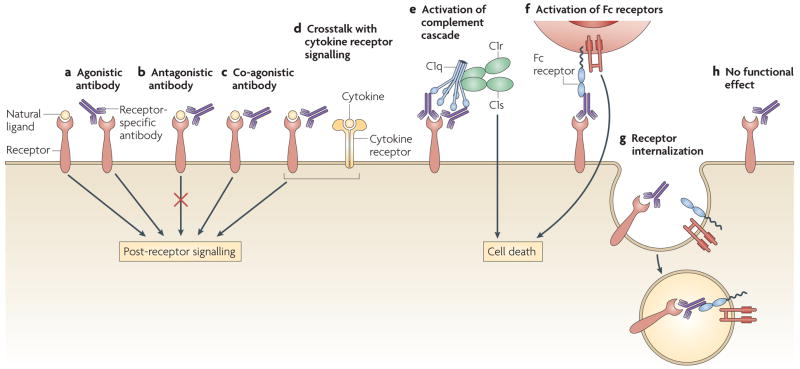
At present, few of these antibodies have clearly delineated mechanisms of neuro-toxicity, but three main mechanisms of antibody function are possible (FIG. 1). Some antibodies might act as receptor agonists (by either mimicking ligand binding or acting through allosteric modulation) or antagonists. Some antibodies might cause antigenic modulation, thereby altering the density of the target antigen on the cell surface (for example, altering the density of a receptor through internalization). Other antibodies might require interaction with diverse components of the immune system to mediate their effects. For example, some antibodies might activate complement, whereas others might engage Fc receptors on resident cells in the brain or on infiltrating inflammatory cells. It is also possible that some antibodies bind brain antigens but have no effect.
Figure 1. Antibodies can have a range of effector functions.
Binding of a brain-reactive antibody to its target antigen (in this case a receptor) can have various effects. a. Antibodies can function as receptor agonists, either through ligand mimicry or allosteric modulation. b. Antibodies can function as receptor antagonists. c. Antibodies can function as receptor co-agonists. d. cytokine signalling pathways can crosstalk with antibody-mediated receptor signalling. e. Interactions between antibodies and receptors can activate complement cascades. f. Antibody binding can activate Fc receptors. g. Antibodies can cause receptor internalization (antigenic modulation), thereby altering antigen density on the cell surface. h. Antibodies can bind to the receptor without altering its function and without having any effect. c1, complement component 1.
Brain-reactive antibodies have been observed in patients with several neurological malignancies, autoimmune diseases, seizures, movement disorders and ischaemic brain syndromes. Individuals with altered behaviour, abnormal cognition and neurodegenerative diseases also have these antibodies (TABLE 1). For example, antibodies specific for several different glutamate receptors have been reported in seizure disorder, malignancy-associated encephalopathy and neurodegenerative disease7. It is not yet clear which of these antibodies cause brain pathology or by what mechanism, although some of the antibodies have been shown to act as agonists for AMPA receptors and kainate receptors in vitro8. Antibodies specific for aquaporin 4, a water channel that is expressed on astrocytes, are useful for the diagnosis of neuromyelitis optica. These antibodies initiate complement activation at the site of deposition. Expression of the astrocytic glutamate transporter depends on the presence of aquaporin 4; so, antibody-mediated modulation of the density of aquaporin 4 might affect the cell surface expression of the glutamate transporter and in turn expose surrounding cells to the excito-toxic effects of increased levels of glutamate9. Limbic encephalitis, both paraneoplastic and idiopathic, is often associated with antibodies that are specific for voltage-gated potassium channels10. It is not clearly established whether and how these antibodies contribute to symptoms of this disease, but they might cause autonomic hyperexcitability. Antibodies specific for ribosomal P protein are present almost exclusively in patients with SLE. These antibodies have recently been shown to crossreact with a 331 kDa membrane protein on neurons and to stimulate calcium influx that results in cell death11. Clinical data indicate that these antibodies might be associated with psychosis12,13.
Antibodies that are generated as part of a protective response to infection have also been shown to bind brain antigens through molecular mimicry. Elegant studies have shown that antibodies specific for an N-acetyl-β-D-glucosamine epitope on polysaccharide from streptococcal bacteria crossreact with a lysoganglioside expressed on neurons14,15. These antibodies have been shown to trigger the phosphorylation of calcium/calmodulin protein kinase II, which is found in neurons throughout the brain and is a component of many activation pathways. In patients with rheumatic fever14, the lysoganglioside-specific antibodies target an antigen that is preferentially expressed or is particularly accessible on neurons in the basal ganglia, a group of brain structures that have a role in movement control. Because titres of these antimicrobial and brain-specific cross-reactive antibodies are high in the cerebrospinal fluid (CSF) of patients with rheumatic fever and chorea and decrease as the involuntary movements become less frequent, it is presumed that these antibodies are responsible for the effects of rheumatic fever on the CNS. Staining of brain sections from patients with rheumatic fever using a lysoganglioside-specific antibody shows that there is regional specificity of the antigen within the basal ganglia, the brain region from which the abnormal movements are initiated14. This observation further supports the causal role of antibodies in the chorea of patients with rheumatic fever14,15. It remains unclear whether antibodies specific for streptococci have a role in other movement disorders and in neuropsychiatric syndromes16.
Another example of brain-reactive antibodies that crossreact with microbial antigen is provided by the ganglioside-specific antibodies that are present in patients with Guillain– Barré syndrome that crossreact with a lipooligosaccharide on the surface of Campylobacter jejuni. These antibodies impair schwann cell function through a complement-dependent process. This example differs from the other cases described in this Opinion article, as the blood–brain barrier (BBB; BOX 1) does not isolate the target antigen, which is present on perisynaptic Schwann cells encasing the nerve root. These cells are located outside the BBB and are exposed to circulating antibodies and complement proteins17,18.
Box 1. The blood–brain barrier.
The blood–brain barrier (BBB) is composed of a network of endothelial cells, pericytes and astrocytes, and functions to limit the entry of soluble molecules and cells into the brain parenchyma58. The BBB is tightest in capillaries, in which solute diffusion is controlled, and is weaker in postcapillary venules, where leukocyte recruitment takes place59. Indeed, areas of leukocyte infiltration do not frequently correspond to capillary sites, where classical markers such as tagged dextran or tagged albumin permeate the BBB60. The BBB is robust in capillaries owing to specialized endothelial cells that express proteins forming tight junctions between cells. In addition to the adhesion molecules that are expressed by endothelial cells in other tissues61, endothelial cells in the brain also express a unique adhesion molecule, integrin cytoplasmic domain-associated protein (ICAP)62, which recruits blood-borne mononuclear cells into the brain.
The BBB is fully formed by the end of gestation (the precise timing during gestation is not known), but its integrity can still be modulated. The regulatory mechanisms include alterations in paracellular permeability (by affecting the strength of tight junctions) and changes in transcellular permeability (by affecting the capacity of endothelial cells to internalize molecules through endocytosis)58. It has been assumed that molecules present in plasma cross the BBB mainly through paracellular routes; however, recent reports show that certain cytokines and chemokines are transported through transcellular routes using receptor-mediated endocytosis63.
It is not known how antibodies cross the BBB during moments of compromise to barrier integrity. Capillary and postcapillary sites in the BBB might be transfer sites depending on whether antibodies are transported by transcellular endocytosis or by paracellular routes (tight junction dysfunction), or are produced by lymphocytes undergoing transendothelial migration (FIG. 2). It is probable that cytokines are crucial for modulating antibody influx. Cytokines might have different effects on capillary and postcapillary compartments of the BBB. Structural differences in the BBB in different regions of the brain and structural differences in different compartments of the BBB might help to explain the diversity of antibody-mediated brain pathologies. Alternatively, in some cases antibodies might be synthesized within brain tissue by B cells penetrating the BBB, and the effects of antibodies will be proximal to the B cell infiltration.
In general, the antibodies that are associated with CNS disease seem to be generated in secondary lymphoid organs and thereafter gain access to the CNS from the circulation. The molecular pathways that are triggered or blocked by presumed pathogenic brain-reactive antibodies are not well understood. The identification of antibodies that are specific for neurons or glial cells should allow the molecular identification of the target antigen and an understanding of the pathways that are activated after antibody binding. Indeed, antibodies arising in pathological conditions that bind to brain antigens could teach us a great deal about differences in the phenotype and function of neurons and glial cells in different regions of the brain.
Antibodies specific for NMDAR
Our own studies have focused on a subset of DNA-specific antibodies that are present in the serum of 30–60% of patients with SLE19. These antibodies crossreact with a consensus pentapeptide, DWEYS, in theNR2A (also known as GRIN2A) and NR2B (also known as GRIN2B) subunits of the N-methyl-D-aspartate receptor (NMDAR)19. NMDAR is highly expressed by excitatory neurons throughout the brain and it binds the neurotransmitter glutamate20,21. When activated, NMDAR functions as a conduit for calcium influx from the extracellular milieu, resulting in large calcium increases intracellularly, which might lead to excitotoxic effects. Antibodies that bind both DNA and the consensus DWEYS peptide also bind native NMDAR, activate NMDAR in brain slices ex vivo and mediate excitotoxic death of neurons when injected directly into the brain parenchyma19. The finding that Fab′2 fragments of these antibodies can also cause excitotoxicity indicates that the antibodies do not need to trigger immune cell-mediated cytotoxic mechanisms or cause inflammation to result in neuronal death, and they probably function as receptor agonists or positive modulators of NMDAR. This conclusion is further supported by two observations: first, NMDAR antagonists can inhibit this antibody-mediated excitotoxicity; and second, the endogenous neurotransmitter glutamate must be present for the antibody to have a toxic effect. In our preferred model, glutamate opens the NMDAR pore and the NMDAR-specific antibody increases the duration of the open state, thus augmenting calcium influx into the cell. This model shows how antibodies can work differently from conventional pharmacological agonists and suggests a strategy for new therapies.
Our current studies using monoclonal antibodies cloned from B cells from the peripheral blood of patients with SLE that crossreact with NMDAR and DNA show that individual antibodies can have different effects on NMDAR activation, with some behaving as strong co-agonists and some having little effect on pore permeability22. We predict, therefore, that the physiological effect of a polyclonal antibody response on the brain in vivo will depend on the particular combination of the functional antibodies that are present. Furthermore, the target antigen of a brain-reactive antibody might be expressed on more than one cell type in the brain and might have a slightly different subunit composition or post-translational modification on each cell type. In this way, a particular antibody might preferentially bind and modulate a specific cell type. Finally, it should be noted that antibodies targeting the same antigen could differ with respect to isotype and, therefore, could have varying effector functions.
Many clinical studies have shown that neuropsychiatric symptoms occur in 35–75% of patients with SLE (a condition known as NPSLE) worldwide23. Some of the most frequent symptoms are cognitive impairment, in particular memory defects, and mood disorder. As it is well known that NMDAR has a crucial role in memory function21, it seems that antibodies targeting NMDAR might account for at least the memory impairment in NPSLE.
We and others have detected NMDAR-specific antibodies not just in the serum of patients with SLE but also in their CSF19,24–26. Several groups have attempted to determine whether patients who have serum antibodies with crossreactivity to DNA and NMDAR have impairments in cognitive function that are greater, or occur more frequently, than those in patients who lack these antibodies in the serum; these studies have generated inconsistent results27–29. By contrast, a small number of studies has shown a correlation between the presence of these antibodies in the CSF and increased manifestations of NPSLE24–26. Importantly, CSF titres of these antibodies correlate with CNS manifestations of NPSLE but not with peripheral nerve manifestations of the disease. This is consistent with the expression of NMDAR in the CNS but not by peripheral nerves. It also underscores the need to study antibodies in both serum and CSF when investigating a causal connection between antibody activity and neuropsychiatric symptoms.
The injection of CSF from patients with SLE containing these crossreactive DNA- and NMDAR-specific antibodies into the brains of mice causes excitotoxic neuronal death, which shows that the antibodies bathing the brains of these patients are at sufficient concentrations to cause neuronal death30. In addition, antibodies that are specific for NMDAR have been eluted from the brain of a patient with SLE and retain their excitotoxic activity when injected into mouse brain31. So, neurotoxic antibodies can be present in both the CSF and the brain of a patient. These results strongly indicate that antibodies could mediate brain dysfunction in patients with SLE.
The BBB and brain pathology
Mice can be induced to express high titres of antibodies that are crossreactive with DNA and NMDAR through immunization with a multimeric form of the consensus DWEYS peptide. When these antibodies are present in the circulation of mice, there is no brain pathology. However, when the mice are given agents that breach the BBB, there is an influx of antibodies into the brain tissue and an ensuing neuropsychiatric syndrome19. Mice expressing NMDAR-specific antibodies that are treated with lipopolysaccharide (LPS) to disrupt the BBB have a loss of neurons in the hippocampus with no apparent influx of inflammatory cells from the circulation and no prolonged activation of resident inflammatory cells32. The mice have a memory disorder that is consistent with the loss of hippocampal neurons32. When adrenaline is given systemically to mice with high titres of DNA- and NMDAR-specific antibodies, there is also a breach in the BBB33. However, this breach is not followed by loss of hippocampal neurons, but instead by damage to cells in the lateral amygdala. Moreover, the mice fail to perform normally in a fear-conditioning task that is known to depend on the intact function of the amygdala33. These observations demonstrate three important points. First, they explain why the presence of antibodies with potential brain reactivity in the serum does not necessarily correlate with CNS disease (in the presence of an intact BBB), as shown by the lack of a consistent correlation between cognitive impairment and serum levels of NMDAR-specific antibodies in patients with SLE27–29. Second, agents that breach the integrity of the BBB can do so with regional specificity. Third, the same antibodies can cause more than one neuropsychiatric symptom depending on the region of the brain that is exposed to the antibodies.
Modulating the BBB
Brain-reactive antibodies in the circulation do not have pathological consequences until there is a breach of BBB integrity. It is therefore crucial to understand the factors that decrease or increase the integrity of the BBB with respect to the transport of antibodies (FIG. 2 and Supplementary information S1 (table)). It is important to note that studies of the BBB have focused on the penetration of marker molecules such as tagged dextrans or tagged albumin into brain tissue, and not specifically of IgG; therefore, the mechanisms of antibody penetration across the BBB still await elucidation.
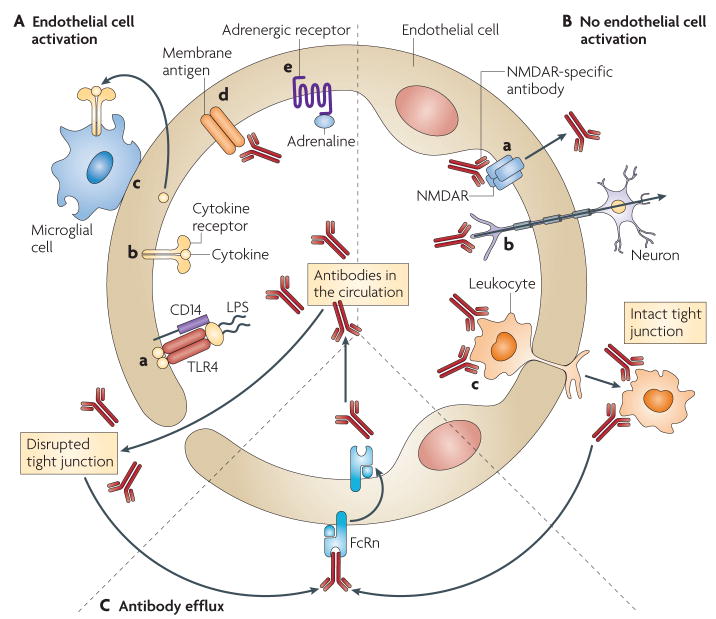
Figure 2. Schematic representation of the possible mechanisms regulating the influx and efflux of antibodies through the blood–brain barrier.
A. Antibody transfer can be induced by endothelial cell activation. During infection, microbial substances (such as lipopolysaccharide (LPs)) cause a breach in the blood–brain barrier (BBB) by binding receptors (such as Toll-like receptor 4 (TLr4)) on endothelial cells and inducing their activation, and by activating perivascular macrophages at circumventricular areas (a). cytokines that are secreted systemically in response to LPs or other pro-inflammatory molecules bind to their receptors on endothelial cells, inducing their activation and altering the architecture of tight junctions (b). cytokines and chemokines can be transported directly through the BBB by transcellular receptor-dependent mechanisms, which might lead to the activation of immune cells inside the brain that initiate and/or potentiate BBB dysfunction (c). Antibodies in the circulation might bind and activate endothelial cells to alter barrier integrity (d). During conditions of stress, trauma or extreme exercise, binding of adrenaline to adrenergic receptors on endothelial cells alters the BBB by unknown mechanisms that might include alterations in blood flow. B. Antibody transfer can also be induced in the absence of endothelial cell activation. Antibodies recognizing molecules on endothelial cells (for example, transferrin receptor) are transported into the brain by receptor-mediated endocytosis. Antibodies that are specific for N-methyl-d-aspartate receptor (NMDAr) can access the brain in a similar manner, as depicted (a). retrograde axonal transport of IgG has been proposed to occur in neurons with axons that protrude towards the lumen of BBB capillaries (b). In postcapillary venules, transendothelial migration of leukocytes can import antibodies into the brain. B cells migrating into the brain could constitute a permanent source of antibody (c). C. Antibodies that have been transported into the brain are effluxed back to the circulation by the neonatal Fc receptor (Fcrn), and potentially by other Fcrs in the circumventricular organs.
The BBB is highly responsive to cytokines34. Indeed, direct activation of endothelial cells by circulating cytokines can result in increased barrier permeability35. Furthermore, an ‘inside out’ signalling process can be triggered by cytokines that have already accessed the brain or by molecules that are synthesized within the brain. TNF, for example, activates brain-resident cells that then secrete BBB-modulating substances, such as prostaglandins. Importantly, the choroid plexus and the leptomeninges, which are collectively known as the circum-ventricular organs, lack barrier properties and facilitate the inside out signalling process that modulates the BBB34,35. So, regions that are naturally lacking BBB properties might serve as sensory areas to initiate a cascade of events that result in breaches of the BBB elsewhere35.
Many modulators of the integrity of the BBB are effector molecules of the innate immune system. It has been established through in vitro models and in vivo studies that TNF, interleukin-1 (IL-1), the C5a component of the complement cascade and IL-6 can all disrupt the BBB34,36–38. Molecules derived from other sources can also modulate the integrity of the BBB. For example, adrenaline can cause a breach in BBB integrity under conditions of stress, extreme exercise and trauma39. Substances such as cocaine and nicotine also disrupt the BBB, either through a direct effect on endothelial cells or through indirect effects that have been incompletely characterized40,41. In addition, chronic hypertension leads to a long-term decrease in the integrity of the BBB42. Some studies suggest that brain endothelial cells express NMDAR, and that binding of glutamate to these receptors results in the breach of BBB integrity43. This indicates that some antibodies specific for NMDAR could facilitate their own transport into the brain by acting as agonists or co-agonists for NMDAR (FIG. 2). Importantly, the brain microvascula-ture exhibits regional expression of receptors for molecules that modulate BBB integrity, which probably accounts for the regional specificity of agents that breach the BBB.
Some agents, such as corticosteroids and type I interferons (IFNα and IFNβ), preserve or increase BBB function44,45. This property of IFNβ is presumed to account for its effectiveness as a therapeutic agent for multiple sclerosis44. In addition, although some studies suggest that oestrogen has a protective effect on BBB integrity46,47, the literature on this subject is inconclusive.
The brain not only has a specialized architecture to prevent the large-scale transport of antibodies and other molecules into the brain, but also actively transports immunoglobulins out of the brain48. The neonatal Fc receptor (FcRn) is expressed on the lumenal aspect of endothelial cells throughout the body and allows antibodies to exit the circulation and penetrate tissue49. Remarkably, FcRn has the opposite polarity on endothelial cells in the brain, and therefore it functions to remove immunoglobulins from brain tissue48. It has been proposed that other FcRs expressed in the circumventricular organs might have a role in the protective efflux of antibodies from the brain into the blood50, but this hypothesis requires further investigation.
Fetal brain development
It is reasonable to speculate that some maternal brain-reactive antibodies might cause fetal brain pathology because the BBB does not begin to form until the second trimester of fetal development; the exact time of full functionality is not established and might differ between species. Therefore, maternal antibodies have direct access to fetal brain during gestation, and brain-reactive antibodies could affect fetal brain development even in an uncomplicated pregnancy.
Our studies show that pregnant female mice harbouring DNA- and NMDAR-specific crossreactive antibodies have pups with abnormal fetal brain development51. When the pups are born, they have a delay in reflex acquisition and, as adults, they have impairments in some behavioural tasks that depend on the function of the neocortex. Studies looking at the offspring of mice with high or low antibody titres show a dose-dependent effect of antibody exposure. Similarly, pups born to mice that have been immunized with a peptide present in both serotonin 5HT4 receptor and Ro52 ribonucleoprotein have sensorimotor and cardiac conduction defects (relating to the ability of the heart to conduct electrical impulses) that are consistent with neonatal lupus52. So, in mice, maternal antibody can alter fetal brain development and cause long-term impairment of brain function, which indicates that maternal antibodies might contribute to the increased incidence of learning disabilities in the children of mothers with SLE.
In another example, antibodies present in the serum of mothers of autistic children have been suggested to contribute to autism53,54. Some mothers of autistic children have antibodies that bind brain tissue; when these antibodies were administered to gestating mice and monkeys, they caused abnormal behaviour in their offspring55,56.
We suggest that as more studies of the effects of maternal antibodies on the fetal brain are carried out, it will be important to keep in mind that the same antibody might have different effects on fetal and adult neurons, either because of differences in antigen expression and accessibility or because of differences in antibody-induced signalling cascades. Also, there might be antibodies that are specific only for adult neurons or, alternatively, only for fetal neurons. Moreover, studies in rodents indicate that the effects of fetal brain exposure to toxins might not be evident in young pups or even in adults until the animal experiences a stressor, such as ischaemia, to the CNS, at which time the neuropsychiatric effects of the toxin exposure might be revealed57. So, antibodies that affect fetal brain development could result in no clinical symptoms until there is an insult to the CNS. These potential effects of maternal antibodies on fetal brain development might be difficult to diagnose because of the variable time delay before the effects are manifested and the possibility that they never become clinically evident in some individuals.
Implications and therapeutic prospects
In this Opinion article, we propose a new model of antibody-mediated brain dysfunction that stems from the following premises: first, B cells making antibodies that react with the brain are not systematically removed from the B cell repertoire; second, immune-mediated alterations in brain structure and function can occur in the absence of overt inflammation and immune cell infiltration; third, insults to the BBB that allow antibodies to access adult brain tissue can occur in the absence of brain pathology and B cell infiltration into the brain; and fourth, maternal antibodies have unrestricted access to the developing fetal brain, which is not protected by the BBB for at least part of the gestation period.
The diversity of B cell receptors for antigen in the B2 cell compartment (where conventional B cells reside) does not begin to be generated until after the BBB is thought to form. Because brain antigens are sequestered from developing B2 cells, there is no selection process to remove self-reactive B cells that are specific for brain antigens (known as negative selection). This allows for a more diverse B cell repertoire, but the consequence is that when B cells respond to microbial challenge, the antibodies that are produced have the potential to be crossreactive with the brain. An intact BBB is therefore essential early in the development of the immune system for the generation of a more diverse B cell repertoire because it limits the antigens against which negative selection operates. At the same time, the BBB is crucial for protecting the brain from elicited antibodies that crossreact with brain antigens.
The findings we discuss in this Opinion article indicate that antibodies which protect against microbial infection might mediate various changes in brain function. We speculate that there are many as-yet-unidentified antibodies that have the potential to alter brain function in adults, during a compromise to the integrity of the BBB, or in fetuses before the BBB is fully formed (BOX 2). Similarly, the numerous insults that could compromise the integrity of the BBB need to be characterized, as well as the specific brain regions that are thereby exposed to antibodies. We speculate, for example, that physiological stress (which is known to compromise the BBB) and the presence of neurotoxic antibodies in the serum could together cause a proportion of cases of post-traumatic stress disorder through damage to the amygdala.
Box 2. Unanswered questions regarding brain-reactive antibodies.
What are the antigenic specificities of brain-reactive antibodies?
What are the effects of brain-reactive antibodies in adult and/or fetal brain? Clearly, antibodies can destroy cells, but presumably they might modulate function in more subtle ways as well. Those antibodies that modulate function might have irreversible or reversible effects.
What determines synergy or antagonism between the effects of antibodies and cytokines in the brain?
Can antibodies protect brain cells against brain damage? Antibodies might antagonize the neurotoxic effects of cytokines or other substances, or they might initiate regenerative neurogenesis.
What are the effects of genetic or hormonal differences on the response of neurons or glial cells to antibody-mediated modulation?
This model expands our understanding of the influence of antibodies in human patho-biology and suggests that antibody-mediated brain disorders could occur in all individuals if increased serum titres of antimicrobial and brain-reactive antibodies coincide with a breach in the integrity of the BBB. These disorders could therefore occur in individuals who do not have intrinsic defects in immune tolerance. Nevertheless, the implications of the model are optimistic regarding therapy. It is promising to consider that diseases that were previously attributed to ageing of the brain might instead be mediated by antibodies and therefore might be preventable. This might also apply to conditions affecting brain development in children that were previously not understood. Our studies show that small peptides that engage the antigen-binding site of NMDAR-specific antibodies can prevent cellular damage in both adult and fetal brain19,51. This therapeutic approach is particularly attractive, as it involves no immunosuppression. A daunting challenge will be to determine exactly when a loss of BBB integrity occurs. Current assessments of this using gadolinium scans are not practical for repeated evaluations because of both their toxicity and cost. A non-invasive test of barrier integrity will be essential for an effective therapeutic regimen.
We are at the beginning of a learning curve to understand how frequently antibodies cause brain disease, the mechanisms by which they enter brain tissue, how they affect brain function and whether blocking molecules can be synthesized to inhibit the binding of brain-reactive antibodies to their target antigens. This endeavour will need to be multidisciplinary, but the path is clear and the medical implications are enormous.
Supplementary Material
Acknowledgments
We are grateful to O. Bloom, E. Chang, T. Faust, M. Scharff and K. Tracey for suggestions. These studies are supported by grants from the Alliance for Lupus Research and the National Institutes of Health to B.D., P.T.H. and B.T.V.; P.M.-O. is a fellow of the Arthritis Foundation.
Glossary
- Amygdala
An almond-shaped brain region, located deep in the temporal lobe of the brain, which is involved in the neural processing of emotions.
- Astrocyte
A star-shaped glial cell that is the most abundant cell type in the brain. Astrocytes regulate the external chemical environment of neurons by removing excess ions, notably potassium, and by recycling neurotransmitter molecules.
- Basal ganglia
A group of brain structures (striatum, subthalamic nucleus and substantia nigra) that is located deep in the centre of the brain and is involved in the neural processing of motor function and cognition.
- Chorea
Any of several neurological disorders associated with rheumatic fever and marked by involuntary, jerky movements, especially of the arms, legs and face, and by lack of coordination.
- Choroid plexus
A vascular extension of the ventricles in the brain that regulates the intraventricular pressure by secreting or absorbing cerebrospinal fluid.
- Excitotoxic effect
A pathological process by which neurons are destroyed as a result of excessive levels of the excitatory neurotransmitter glutamate, which overactivates the NMDA receptor and the AMPA receptor, allowing for unusually high levels of calcium to enter the cell and trigger enzymatic cascades that lead to cell death.
- Fear-conditioning task
A behavioural method that is used to teach an animal to fear a stimulus that is neutral in nature by associating it with an aversive stimulus (such as a shock, a loud noise or an unpleasant odour).
- Glial cell
A non-neuronal cell of the nervous system that is essential for maintaining the health of neurons. According to size, glial cells are divided into microglia and macroglia (astrocytes, oligodendrocytes and others).
- Hippocampus
A banana-shaped brain region that is located in the medial temporal lobe of the brain and is involved in the neural processing of memory and spatial navigation.
- Leptomeninges
The arachnoid mater and pia mater of the meninges, which is a system of three layers (dura mater, arachnoid mater and pia mater) that encloses the brain.
- Limbic encephalitis
An inflammation of the central nervous system in which the pathological signs are localized to the medial temporal lobes.
- Microglial cell
A small glial cell that is a specialized type of macrophage. Microglial cells are mobile within the brain, multiply when the brain is damaged and have a protective role.
- Neocortex
The outer region of the cerebrum, consisting of superficial grey matter (neurons grouped in several layers) and deeper white matter (myelinated axons). It is essential for the sensory, motor and cognitive organization of behaviour.
- Neuromyelitis optica
An autoimmune inflammatory disorder in which the pathological signs are focused on the optic nerves.
- Paraneoplastic
A symptom complex that co-occurs with cancer and is mediated by antibodies that recognize antigens in the tumour cells. The antibodies crossreact with antigens in the central nervous system or the peripheral nervous system.
- Schwann cell
A glial cell that is filled with myelin and that surrounds the axons of neurons.
Footnotes
DATABASES
Entrez Gene: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=gene
IL-1|IL-6|NR2A|NR2B|TNF
OMIM: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=OMIM
Guillain–Barré syndrome | SLE
FURTHER INFORMATION
Betty Diamond’s homepage: http://www.northshorelij.com/body.cfm?id=10371
SUPPLEMENTARY INFORMATION
See online article: S1 (table)
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.Pavlov VA, et al. Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain Behav Immun. 2009;23:41–45. doi: 10.1016/j.bbi.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jara LJ, Navarro C, Medina G, Vera-Lastra O, Blanco F. Immune–neuroendocrine interactions and autoimmune diseases. Clin Dev Immunol. 2006;13:109–123. doi: 10.1080/17402520600877059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goddard CA, Butts DA, Shatz CJ. Regulation of CNS synapses by neuronal MHC class I. Proc Natl Acad Sci USA. 2007;104:6828–6833. doi: 10.1073/pnas.0702023104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-α. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- 5.Meroni PL, et al. Endothelium and the brain in CNS lupus. Lupus. 2003;12:919–928. doi: 10.1191/0961203303lu503oa. [DOI] [PubMed] [Google Scholar]
- 6.Minagar A, Carpenter A, Alexander JS. The destructive alliance: interactions of leukocytes, cerebral endothelial cells, and the immune cascade in pathogenesis of multiple sclerosis. Int Rev Neurobiol. 2007;79:1–11. doi: 10.1016/S0074-7742(07)79001-3. [DOI] [PubMed] [Google Scholar]
- 7.Pleasure D. Diagnostic and pathogenic significance of glutamate receptor autoantibodies. Arch Neurol. 2008;65:589–592. doi: 10.1001/archneur.65.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Selak S, Paternain AV, Fritzler MJ, Lerma J. Human autoantibodies against early endosome antigen-1 enhance excitatory synaptic transmission. Neuroscience. 2006;143:953–964. doi: 10.1016/j.neuroscience.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Jarius S, et al. Mechanisms of disease: aquaporin-4 antibodies in neuromyelitis optica. Nature Clin Pract Neurol. 2008;4:202–214. doi: 10.1038/ncpneuro0764. [DOI] [PubMed] [Google Scholar]
- 10.Jacob S, et al. Hypothermia in VGKC antibody-associated limbic encephalitis. J Neurol Neurosurg Psychiatry. 2008;79:202–204. doi: 10.1136/jnnp.2007.130039. [DOI] [PubMed] [Google Scholar]
- 11.Matus S, et al. Antiribosomal-P autoantibodies from psychiatric lupus target a novel neuronal surface protein causing calcium influx and apoptosis. J Exp Med. 2007;204:3221–3234. doi: 10.1084/jem.20071285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdel-Nasser AM, Ghaleb RM, Mahmoud JA, Khairy W, Mahmoud RM. Association of anti-ribosomal P protein antibodies with neuropsychiatric and other manifestations of systemic lupus erythematosus. Clin Rheumatol. 2008;27:1377–1385. doi: 10.1007/s10067-008-0921-1. [DOI] [PubMed] [Google Scholar]
- 13.Bonfa E, et al. Association between lupus psychosis and anti-ribosomal P protein antibodies. N Engl J Med. 1987;317:265–271. doi: 10.1056/NEJM198707303170503. [DOI] [PubMed] [Google Scholar]
- 14.Kirvan CA, Swedo SE, Kurahara D, Cunningham MW. Streptococcal mimicry and antibody-mediated cell signaling in the pathogenesis of Sydenham’s chorea. Autoimmunity. 2006;39:21–29. doi: 10.1080/08916930500484757. [DOI] [PubMed] [Google Scholar]
- 15.Kirvan CA, Swedo SE, Snider LA, Cunningham MW. Antibody-mediated neuronal cell signaling in behavior and movement disorders. J Neuroimmunol. 2006;179:173–179. doi: 10.1016/j.jneuroim.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 16.Singer HS, et al. Serial immune markers do not correlate with clinical exacerbations in pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. Paediatrics. 2008;121:1198–1205. doi: 10.1542/peds.2007-2658. [DOI] [PubMed] [Google Scholar]
- 17.Willison HJ. The immunobiology of Guillain–Barré syndromes. J Peripher Nerv Syst. 2005;10:94–112. doi: 10.1111/j.1085-9489.2005.0010202.x. [DOI] [PubMed] [Google Scholar]
- 18.Overell JR, Willison HJ. Recent developments in Miller Fisher syndrome and related disorders. Curr Opin Neurol. 2005;18:562–566. doi: 10.1097/01.wco.0000173284.25581.2f. [DOI] [PubMed] [Google Scholar]
- 19.Diamond B, et al. Immunity and acquired alterations in cognition and emotion: lessons from SLE. Advances Immunol. 2006;89:289–320. doi: 10.1016/S0065-2776(05)89007-8. [DOI] [PubMed] [Google Scholar]
- 20.Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Science STKE. 2004:re16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- 21.Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87:1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J, et al. Identification of DNA-reactive B cells in patients with systemic lupus erythematosus. J Immunol Methods. 2008;338:79–84. doi: 10.1016/j.jim.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanly JG. New insights into central nervous system lupus: a clinical perspective. Curr Rheumatol Rep. 2007;9:116–124. doi: 10.1007/s11926-007-0005-2. [DOI] [PubMed] [Google Scholar]
- 24.Arinuma Y, Yanagida T, Hirohata S. Association of cerebrospinal fluid anti-NR2 glutamate receptor antibodies with diffuse neuropsychiatric systemic lupus erythematosus. Arthritis Rheum. 2008;58:1130–1135. doi: 10.1002/art.23399. [DOI] [PubMed] [Google Scholar]
- 25.Yoshio T, Onda K, Nara H, Minota S. Association of IgG anti-NR2 glutamate receptor antibodies in cerebrospinal fluid with neuropsychiatric systemic lupus erythematosus. Arthritis Rheum. 2006;54:675–678. doi: 10.1002/art.21547. [DOI] [PubMed] [Google Scholar]
- 26.Fragoso-Loyo HF, et al. Serum and cerebrospinal fluid autoantibodies in patients with neuropsychiatric lupus erythematosus. Implications for diagnosis and pathogenesis. PloS Med. 2008;3:e3347. doi: 10.1371/journal.pone.0003347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanly JG, Robichaud J, Fisk JD. Anti-NR2 glutamate receptor antibodies and cognitive function in systemic lupus erythematosus. J Rheumatol. 2006;33:1553–1558. [PubMed] [Google Scholar]
- 28.Lapteva L, et al. Anti-N-methyl-D-aspartate receptor antibodies, cognitive dysfunction, and depression in systemic lupus erythematosus. Arthritis Rheum. 2006;54:2505–2514. doi: 10.1002/art.22031. [DOI] [PubMed] [Google Scholar]
- 29.Omdal R, et al. Neuropsychiatric disturbances in SLE are associated with antibodies against NMDA receptors. Eur J Neurol. 2005;12:392–398. doi: 10.1111/j.1468-1331.2004.00976.x. [DOI] [PubMed] [Google Scholar]
- 30.DeGiorgio LA, et al. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nature Med. 2001;7:1189–1193. doi: 10.1038/nm1101-1189. [DOI] [PubMed] [Google Scholar]
- 31.Kowal C, et al. Human lupus autoantibodies against NMDA receptors mediate cognitive impairment. Proc Natl Acad Sci USA. 2006;103:19854–19859. doi: 10.1073/pnas.0608397104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kowal C, et al. Cognition and immunity; antibody impairs memory. Immunity. 2004;21:179–188. doi: 10.1016/j.immuni.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 33.Huerta PT, Kowal C, DeGiorgio LA, Volpe BT, Diamond B. Immunity and behavior: antibodies alter emotion. Proc Natl Acad Sci USA. 2006;103:678–683. doi: 10.1073/pnas.0510055103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Banks WA. Blood–brain barrier transport of cytokines: a mechanism for neuropathology. Curr Pharm Des. 2005;11:973–984. doi: 10.2174/1381612053381684. [DOI] [PubMed] [Google Scholar]
- 35.Roth J, Harre EM, Rummel C, Gerstberger R, Hubschle T. Signaling the brain in systemic inflammation: role of sensory circumventricular organs. Front Biosci. 2004;9:290–300. doi: 10.2741/1241. [DOI] [PubMed] [Google Scholar]
- 36.Bauer B, Hartz AM, Miller DS. Tumor necrosis factor α and endothelin-1 increase P-glycoprotein expression and transport activity at the blood–brain barrier. Mol Pharmacol. 2007;71:667–675. doi: 10.1124/mol.106.029512. [DOI] [PubMed] [Google Scholar]
- 37.Argaw AT, et al. IL-1β regulates blood–brain barrier permeability via reactivation of the hypoxia-angiogenesis program. J Immunol. 2006;177:5574–5584. doi: 10.4049/jimmunol.177.8.5574. [DOI] [PubMed] [Google Scholar]
- 38.Paul R, et al. Lack of IL-6 augments inflammatory response but decreases vascular permeability in bacterial meningitis. Brain. 2003;126:1873–1882. doi: 10.1093/brain/awg171. [DOI] [PubMed] [Google Scholar]
- 39.Kuang F, et al. Extravasation of blood-borne immunoglobulin G through blood–brain barrier during adrenaline-induced transient hypertension in the rat. Int J Neurosci. 2004;114:575–591. doi: 10.1080/00207450490422731. [DOI] [PubMed] [Google Scholar]
- 40.Dhillon NK, et al. Cocaine-mediated alteration in tight junction protein expression and modulation of CCL2/CCR2 axis across the blood–brain barrier: implications for HIV-dementia. J Neuroimmune Pharmacol. 2008;3:52–56. doi: 10.1007/s11481-007-9091-1. [DOI] [PubMed] [Google Scholar]
- 41.Hawkins BT, et al. Nicotine increases in vivo blood–brain barrier permeability and alters cerebral microvascular tight junction protein distribution. Brain Res. 2004;1027:48–58. doi: 10.1016/j.brainres.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 42.Nag S, Harik SI. Cerebrovascular permeability to horseradish peroxidase in hypertensive rats: effects of unilateral locus ceruleus lesion. Acta Neuropathol. 1987;73:247–253. doi: 10.1007/BF00686618. [DOI] [PubMed] [Google Scholar]
- 43.Kuhlmann CR, et al. MK801 blocks hypoxic blood–brain-barrier disruption and leukocyte adhesion. Neurosci Lett. 2009;449:168–172. doi: 10.1016/j.neulet.2008.10.096. [DOI] [PubMed] [Google Scholar]
- 44.Kraus J, et al. Interferon-β stabilizes barrier characteristics of the blood–brain barrier in four different species in vitro. Mult Scler. 2008;14:843–852. doi: 10.1177/1352458508088940. [DOI] [PubMed] [Google Scholar]
- 45.Kim H, et al. Dexamethasone coordinately regulates angiopoietin-1 and VEGF: a mechanism of glucocorticoid-induced stabilization of blood–brain barrier. Biochem Biophys Res Commun. 2008;372:243–248. doi: 10.1016/j.bbrc.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 46.Liu R, et al. 17β-estradiol attenuates blood–brain barrier disruption induced by cerebral ischemia-reperfusion injury in female rats. Brain Res. 2005;1060:55–61. doi: 10.1016/j.brainres.2005.08.048. [DOI] [PubMed] [Google Scholar]
- 47.Sohrabji F. Guarding the blood–brain barrier: a role for estrogen in the etiology of neurodegenerative disease. Gene Expr. 2007;13:311–319. doi: 10.3727/000000006781510723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y, Pardridge WM. Mediated efflux of IgG molecules from brain to blood across the blood–brain barrier. J Neuroimmunol. 2001;114:168–172. doi: 10.1016/s0165-5728(01)00242-9. [DOI] [PubMed] [Google Scholar]
- 49.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nature Rev Immunol. 2007;7:715–725. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 50.Siegelman J, Fleit HB, Peress NS. Characterization of immunoglobulinG–Fc receptor activity in the outflow system of the CSF. Cell Tissue Res. 1987;248:599–605. doi: 10.1007/BF00216489. [DOI] [PubMed] [Google Scholar]
- 51.Lee JY, et al. Maternal lupus and congenital cortical impairment. Nature Med. 2009;15:91–96. doi: 10.1038/nm.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eftekhari P, et al. Induction of neonatal lupus in pups of mice immunized with synthetic peptides derived from amino acid sequences of the serotoninergic 5HT4 receptor. Eur J Immunol. 2001;31:573–579. doi: 10.1002/1521-4141(200102)31:2<573::aid-immu573>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 53.Braunschweig D, et al. Autism: maternally derived antibodies specific for fetal brain proteins. Neurotoxicology. 2008;29:226–231. doi: 10.1016/j.neuro.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singer HS, et al. Antibodies against fetal brain in sera of mothers with autistic children. J Neuroimmunol. 2008;194:165–172. doi: 10.1016/j.jneuroim.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 55.Dalton P, et al. Maternal neuronal antibodies associated with autism and a language disorder. Ann Neurol. 2003;53:533–537. doi: 10.1002/ana.10557. [DOI] [PubMed] [Google Scholar]
- 56.Martin LA, et al. Stereotypies and hyperactivity in rhesus monkeys exposed to IgG from mothers of children with autism. Brain Behav Immun. 2008;22:806–816. doi: 10.1016/j.bbi.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adde-Michel C, Hennebert O, Laudenbach V, Marret S, Leroux P. Effect of perinatal alcohol exposure on ibotenic acid-induced excitotoxic cortical lesions in newborn hamsters. Pediatr Res. 2005;57:287–293. doi: 10.1203/01.PDR.0000148712.30716.9D. [DOI] [PubMed] [Google Scholar]
- 58.Hawkins BT, Davis TP. The blood–brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 59.Bechmann I, Galea I, Perry VH. What is the blood–brain barrier (not)? Trends Immunol. 2007;28:5–11. doi: 10.1016/j.it.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 60.Engelhardt B, Wolburg H. Transendothelial migration of leukocytes: through the front door or around the side of the house? Eur J Immunol. 2004;34:2955–2963. doi: 10.1002/eji.200425327. [DOI] [PubMed] [Google Scholar]
- 61.Chang DD, Wong C, Smith H, Liu J. ICAP-1, a novel β1 integrin cytoplasmic domain-associated protein, binds to a conserved and functionally important NPXY sequence motif of a β1 integrin. J Cell Biol. 1997;138:1149–1157. doi: 10.1083/jcb.138.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cayrol R, et al. Activated leukocyte cell adhesion molecule promotes leukocyte trafficking into the central nervous system. Nature Immunol. 2008;9:137–145. doi: 10.1038/ni1551. [DOI] [PubMed] [Google Scholar]
- 63.Ge S, Song L, Serwanski DR, Kuziel WA, Pachter JS. Transcellular transport of CCL2 across brain microvascular endothelial cells. J Neurochem. 2008;104:1219–1232. doi: 10.1111/j.1471-4159.2007.05056.x. [DOI] [PubMed] [Google Scholar]
- 64.Newsom-Davis J. The emerging diversity of neuromuscular junction disorders. Acta Myol. 2007;26:5–10. [PMC free article] [PubMed] [Google Scholar]
- 65.Riemersma S, et al. Association of arthrogryposis multiplex congenita with maternal antibodies inhibiting fetal acetylcholine receptor function. J Clin Invest. 1996;98:2358–2363. doi: 10.1172/JCI119048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lang B, Vincent A. Autoantibodies to ion channels at the neuromuscular junction. Autoimmun Rev. 2003;2:94–100. doi: 10.1016/s1568-9972(02)00146-5. [DOI] [PubMed] [Google Scholar]
- 67.Dalton P, et al. Fetal arthrogryposis and maternal serum antibodies. Neuromuscul Disord. 2006;16:481–491. doi: 10.1016/j.nmd.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 68.Halstead SK, et al. Anti-disialoside antibodies kill perisynaptic Schwann cells and damage motor nerve terminals via membrane attack complex in a murine model of neuropathy. Brain. 2004;127:2109–2123. doi: 10.1093/brain/awh231. [DOI] [PubMed] [Google Scholar]
- 69.Lee SM, Dunnavant FD, Jang H, Zunt J, Levin MC. Autoantibodies that recognize functional domains of hnRNPA1 implicate molecular mimicry in the pathogenesis of neurological disease. Neurosci Lett. 2006;401:188–193. doi: 10.1016/j.neulet.2006.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hinson SR, et al. Pathogenic potential of IgG binding to water channel extracellular domain in neuromyelitis optica. Neurology. 2007;69:2221–2231. doi: 10.1212/01.WNL.0000289761.64862.ce. [DOI] [PubMed] [Google Scholar]
- 71.Jarius S, et al. Antibody to aquaporin-4 in the long-term course of neuromyelitis optica. Brain. 2008;131:3072–3080. doi: 10.1093/brain/awn240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med. 2003;349:1543–1554. doi: 10.1056/NEJMra023009. [DOI] [PubMed] [Google Scholar]
- 73.Dalmau J, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25–36. doi: 10.1002/ana.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vincent A, et al. Potassium channel antibody-associated encephalopathy: a potentially immunotherapy-responsive form of limbic encephalitis. Brain. 2004;127:701–712. doi: 10.1093/brain/awh077. [DOI] [PubMed] [Google Scholar]
- 75.Whitney KD, McNamara JO. GluR3 autoantibodies destroy neural cells in a complement-dependent manner modulated by complement regulatory proteins. J Neurosci. 2000;20:7307–7316. doi: 10.1523/JNEUROSCI.20-19-07307.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cohen-Kashi Malina K, Ganor Y, Levite M, Teichberg VI. Autoantibodies against an extracellular peptide of the GluR3 subtype of AMPA receptors activate both homomeric and heteromeric AMPA receptor channels. Neurochem Res. 2006;31:1181–1190. doi: 10.1007/s11064-006-9143-6. [DOI] [PubMed] [Google Scholar]
- 77.Gini B, et al. Novel autoantigens recognized by CSF IgG from Hashimoto’s encephalitis revealed by a proteomic approach. J Neuroimmunol. 2008;196:153–158. doi: 10.1016/j.jneuroim.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 78.Dale RC, et al. Encephalitis lethargica syndrome: 20 new cases and evidence of basal ganglia autoimmunity. Brain. 2004;127:21–33. doi: 10.1093/brain/awh008. [DOI] [PubMed] [Google Scholar]
- 79.Butler MH, et al. Autoimmunity to gephyrin in stiff-man syndrome. Neuron. 2000;26:307–312. doi: 10.1016/s0896-6273(00)81165-4. [DOI] [PubMed] [Google Scholar]
- 80.Dalakas MC, et al. High-dose intravenous immune globulin for stiff-person syndrome. N Engl J Med. 2001;345:1870–1876. doi: 10.1056/NEJMoa01167. [DOI] [PubMed] [Google Scholar]
- 81.Kirvan CA, Cox CJ, Swedo SE, Cunningham MW. Tubulin is a neuronal target of autoantibodies in Sydenham’s chorea. J Immunol. 2007;178:7412–7421. doi: 10.4049/jimmunol.178.11.7412. [DOI] [PubMed] [Google Scholar]
- 82.Snider LA, Swedo SE. PANDAS: current status and directions for research. Mol Psychiatry. 2004;9:900–907. doi: 10.1038/sj.mp.4001542. [DOI] [PubMed] [Google Scholar]
- 83.Alaedini A, et al. Immune cross-reactivity in celiac disease: anti-gliadin antibodies bind to neuronal synapsin I. J Immunol. 2007;178:6590–6595. doi: 10.4049/jimmunol.178.10.6590. [DOI] [PubMed] [Google Scholar]
- 84.Boscolo S, et al. Gluten ataxia: passive transfer in a mouse model. Ann NY Acad Sci. 2007;1107:319–328. doi: 10.1196/annals.1381.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.