Abstract
It is known that changes in gene expression within the nucleus accumbens (NAc) occur during cocaine dependence development. However, identification of specific genes involved in cocaine conditioning awaits further investigation. We conducted a high throughput gene expression profile analysis of the NAc, during different stages of the environment-elicited cocaine conditioning. Rats were assigned to two different environmental conditions. Cocaine conditioned group received a cocaine injection (10 mg/kg, i.p.) prior to being placed in the activity chambers. Control rats received saline injections before being exposed to their environment. Both groups received a saline injection in their home cage. Conditioning training lasted for 10 days. Animals were then re-exposed to their previously paired environments only on day 12 (test session). We found that the gene for arginine vasopressin (AVP) was differentially expressed on experimental subjects during all stages of environment-elicited cocaine conditioning. To further validate our molecular results, biochemical and immunolocalization experiments were conducted. We found the presence of AVP within accumbal fibers and changes in AVP protein levels following cocaine conditioning. Moreover, we tested the effects of accumbal microinfusions of either AVP receptor V1A agonist [pGlu4, Cyt6, Arg8] AVP 4-9 1.0 ng/0.5μl, or V1A antagonist (CH2) 5[Tyr (Me) 2] AVP, 1.0 ng/0.5μl or vehicle solution (0.9% saline solution) during different stages of the cocaine conditioning. Blockade of V1A receptors within the NAc during acquisition interrupted the expression of the conditioned response, while activation leads to an increase in this response. Our findings propose a new role for AVP in cocaine addiction.
Keywords: arginine vasopressin, cocaine, accumbens, rat, gene, microarrays
Introduction
The ability of cocaine to induce a complex pattern of immediate early gene (IEG) expression is thought to be an initial step to alter synaptic organization and produce persistent forms of neurobehavioral plasticity that contribute to addiction (Hyman and Malenka, 2001; Nestler, 2001, 2005, 2008). Cocaine induced alterations in gene expression were found using different drug schedules and behavioral paradigms (Toda et al. 2002; Freeman et al. 2001, 2002; Zhang et al. 2002 a, b). For example, changes in expression of several genes such as cAMP response element binding (CREB), or DeltaFosB, preprodynorphin, neurotensin, dynorphin, myocyte enhancer factor 2 and cdk5 within the dorsal and/or ventral striatum were detected for hours to weeks after cocaine exposure in different drug delivery schedules and conditioning paradigms (Daunais and McGinty 1996; Bibb et al., 2001; Nestler et al., 2001; Ramos-Ortoloza et al., 2009; Pulipparacharuvil et al., 2008). Moreover, previous studies have reported that the existing pool of proteins is susceptible to enzymatic modifications, such as phosphorylation, during cocaine conditioning (Tropea et al., 2008). Thus, these alterations may lead to the synaptic plasticity process observed in cocaine addiction in brain areas such as NAc (Carlezon et al, 1998) and hippocampus among others (Tropea et al., 2008). These enduring expression changes mediate the synaptic reorganization sub serving the psychostimulant-induced behavioral changes associated with addiction (Nestler, 2005). Cocaine can also induce changes in gene expression that are not altered immediately after withdrawal but show a late-developing change beginning a week or more after the last injection (Freeman et al., 2008). Moreover, other studies revealed that gene expression changes could begin as early as the first cocaine exposure (Zhang et al., 2002). Therefore, these diverse genetic findings led to the notion that gene expression profiles in cocaine addiction depended on the routes of administration, the animal species and the behavioral paradigm utilized.
The present experiments were aimed at examining what gene expression profile was present within the NAc during the acquisition and expression of cocaine conditioning. We used an environment-elicited cocaine-conditioned paradigm previously demonstrated to be a useful animal model to study the role of environmental cues in cocaine conditioned response (Rodríguez-Borrero et al., 2006). Interestingly, our molecular analysis revealed that AVP was differentially regulated during the environment-elicited cocaine-conditioning. AVP is a well-known neuropeptide produced by the hypothalamus, with well-characterized endocrine function (Verbalis, 2006). In addition, AVP is also involved in numerous processes including higher cognitive function such as learning and memory (for review see Engelmann et al. 1996), stress response (Ziegler and Herman, 2002), maternal behavior, pair bonding formation (Insel, Wang and Ferris, 1994; Pitcow et al., 2001; Kim, Murphy and Young, 2004), and neurodevelopment disorders such as autism (Kim et al., 2002). Behavioral studies also showed that blockade of V1a receptor within limbic structures inhibited learning in animals (Paban et al., 1998). Moreover, there is evidence that AVP receptors are widely distributed throughout the brain and in some peripheral tissue with different physiological functions (Thibonnier et al., 1998; Thibonnier et al., 2002). In addition, AVP was previously implicated in acquisition of cocaine seeking behavior (De Vry et al., 1988; van Ree et al., 1988; Sarnyai et al., 1992; Chui et al., 1998) and cocaine withdrawal (Zhou et al., 2005). However, previous studies did not address the effects of AVP on cocaine conditioning. Therefore, our present results are the first to describe a functional role of AVP within the NAc on the acquisition and expression of cocaine conditioning response.
Materials and Methods
Animals
Male Sprague Dawley rats (Charles River, Massachusetts, USA) weighing 275-300g were used in all experiments. Rats were housed in pairs using plastic cages in a controlled humidity and temperature (22°C) vivarium of the Department of Biology, University of Puerto Rico. Food and water was available at all times. Animals were kept on a 12-hour on-off light/dark cycle (lights on 7:00-19:00). Rats were handled immediately upon arrival in order to minimize stress during behavioral testing. All procedures were conducted in strict adherence to the National Institutes of Health Guide for the care and use of laboratory animals.
Conditioning methods
Activity chamber apparatus
Our conditioning protocol was previously described (Rodriguez-Borrero et al., 2006). Specifically, our protocol involves the introduction of olfactory and visuals cues specific for each study group (i.e. control and cocaine paired group). In particular, the olfactory cues were included in the protocol to enhance the saliency of the environment paired with the drug, as previous studies have shown that the use of these cues can elicit a similar conditioned response as the compound stimulus (Ferger and Kuschinsky, 1995). The experiments were conducted in Sixteen TruScan Photobeam Scanning System activity boxes designed by Coulbourn Instruments (PA, USA). The dimensions of the cages are 41 cm × 41cm × 41 cm inside while the base plate is 53 cm × 53 cm. The activity boxes have 16 photobeams that are 2.54 cm apart. These beams track the horizontal and vertical position of the animal thus allowing the experimenter to analyze many aspects of an animals’ general motor activity. The dependent measure for these studies was ambulatory distance traveled by the animals during the testing period. All chambers were located in a test room with distinctive environmental cues. Cocaine-paired chambers were surrounded by a 61 cm × 61 cm × 61 cm black plexi glass box (visual cues) and receive odor infusions of cold pressed California orange oil, (inner cues) (Sigma-Aldrich, MO, USA). These visual cues and the inner chambers’ cues became the environmental stimuli for the cocaine-paired animals. Control subject boxes were surrounded by a 61 cm × 61 cm × 61 cm black and white checkers square (visual cues) and received odor infusions of Nutmeg East Indian Oil (inner cues) (Sigma-Aldrich, MO, USA). These external visual cues and the inner chambers cues became the environmental stimuli for the saline-paired animals. The animals’ home cages were housed in a different room in the animal facility building distant from the testing room.
Training Sessions
Animals underwent one daily conditioning training session of 90 minutes, for 10 consecutive days. Rats were trained during the light phase of their cycle at approximately the same time each day (9:00 A.M.). Rats were assigned to one of three groups: cocaine paired or control and were placed in a specific activity chamber throughout all experimental sessions. The experimental groups received 10-mg/kg i.p. injection of cocaine-hydrochloride (Sigma-Aldrich, MO, USA) diluted in 0.9% sodium chloride solution prior to being placed in the chamber and a saline injection just after returning to their home cage. The control groups were exposed to saline i.p. injections prior to both training and home environments. In previous published experiments, we demonstrated that animals (unpaired group) injected with cocaine in their home cages after being exposed to the conditioning chambers showed no conditioning response when re-exposed to the chambers on the expression test session (Rodriguez-Borrero et al., 2006). Thus, these results suggested that the animals in the paired group established an association between the environment and the cocaine injections such that the presentation of the environmental cues alone induces behavioral response not observed in the control neither unpaired groups. This notion is supported by previous studies that showed that the psychostimulant reinforcing properties of conditioned stimuli elicit neural events that are similar to those produced by the drug itself (Martin-Iverson and McManus, 1990; Brown and Fibiger, 1992; Brown et al., 1992). Moreover, we propose that the conditioned response observed in the cocaine-paired group after exposure to the cocaine associated environment may be due to triggered memories related to the previous drug experience. Since only the paired group had a greater locomotor activity when tested, it appears that only this group formed an association between cocaine and the environmental cues during the conditioning phase. Therefore, we infer that all any changes in gene expression are due to the conditioning alone and not to cocaine exposure. Moreover, on the expression session we previously showed that cocaine blood levels of the experimental and cocaine control (unpaired) groups do not significantly differ from the saline controls (Rodríguez-Borrero et al., 2006).
Testing Session
Two days following the last training session (Day 10), trained animals were once again returned to the activity chambers and tested for 120 min. No cocaine or saline i.p injections were given on this test day. The behavioral parameters studied were: Ambulatory Distance, Ambulatory Move Time and Total Move Time.
Gene Expression Profile Analysis
A separate groups of animals (experimental and control groups) were conditioned as specified in the previous section. Microarray analyses were performed using rat 230A chips (Affymetrix, CA), which contain probes for over 14,000 rat genes expressed in 22,000 ESTs.
RNA collection and hybridization
Total RNA was extracted from NAc and mPFC tissue rich punches. The brain was extracted, frozen and placed in a rat brain matrix (Plastic One, VA.), and manually cut in 2mm sections beginning at stereotaxic coordinate AP+ 2.20 for the NAc and +3.70 for the medial prefrontal cortex. The punches were collected with a 10 gauge tissue puncher (Fine Science Tools, CA). The site collection of the tissue was confirmed by microscopic visualization following the coordinates on Paxinos and Watson, 1998. The integrity and purity of the RNA was confirmed by formaldehyde gel electrophoresis. Microarray analyses was performed essentially as described in the standard protocol outlined in the Gene Chip Expression Analysis Technical Manual (Affymetrix, CA) Probe values from CEL files were condensed to probe sets using both MAS5 and the gcRMA package from Bioconductor (www.bioconductor.org) and the R program (R Development Core Team (2004). The gcRMA dataset was unlogged and median scaled to a target intensity of 100. The MAS5 dataset was scaled to a target intensity of 200.
Microarrays: normalization, data analysis and clustering
Clustering analysis was performed with Spotfire software (2005, Spotfire Inc, MA) from gcRMA-processed data. Replicates were averaged, and then settings of maximum (value of 200) and max/median (values of greater than 2) were used to filter for differentially-expressed genes.
Validation of genes candidate from the microarrays
Radioimmunoassay
50μg of protein from the NAc obtained as in the previous section were acidified with 200μl of HCL 1M and the total volume is adjusted to 2ml. The sample was applied to an ODS-Silica Columns C18 Sep-Pak Columns (Waters Corp. Caguas, PR) previously equilibrated with 5ml of methanol and 10ml of distilled water. The peptides were eluted with 1ml of methanol (Sigma MO.) and evaporate to dryness in a speed vac centrifuge (Savant Instruments, NY, USA.). The sample was reconstituted with 500~ μl of 1% BSA-borate buffer, vortex and place at 37°C for ten minutes to ensure reconstitution of dried sample. 200μl were assayed in duplicate in the radioimmunoassay. The RIA assay was performed as recommended by the manufacturer (DIASORIN, MN, USA).
Enzyme-Linked ImmunoSorbent Assay
50μg of protein from the nucleus accumbens obtained as in the previous section were acidified with 200μl of HCL 1M and the total volume is adjusted to 2ml. The sample was applied to an ODS-Silica Columns C18 Sep-Pak Columns (Waters Corp. Caguas, PR) previously equilibrated with 5ml of methanol and 10ml of distilled water. The peptides were eluted with 1ml of methanol (Sigma MO.) and evaporate to dryness in a speed vac centrifuge (Savant Instruments, NY, USA). The sample was reconstituted in 200 μl of assay buffer (Tris Buffer Saline and sodium azide) and assayed in duplicates. The ELISA was carried out according to the manufacturer technical manual (Assay Designs, MI).
Indirect Immunofluorecence
For these experiments, animals (N=8) were conditioned as specified in the conditioning session. At day 12, drug expression session, the animals were removed from the locomotor activity box 30 minutes after the session had started. Animals were deeply anesthetized with an overdose of sodium pentobarbital (Sigma-Aldrich, MO) and were transcardially perfused with PB 0.1M followed by 4% paraformaldehyde (PFA, Fisher, Cayey, PR). Following this procedure, the brains were collected and stored in 4% PFA for several days before sectioning. Two days before cutting, brains were transferred to a 30% sucrose- 4% PFA solution. Brains were frozen and cut into 12 μm sections using a Leica Cryocut 1900 cryostat. Each session was placed on a ProbeOn™ microscope slide (Fisher Biotech, Cayey, PR). The tissue was dried and subject to IF using monoclonal antibodies to AVP (PS-41, Ben Barak et al., 1985). The PS-41 and the PS-38 antibodies against AVP and OT respectively (Ben-Barak et al.,1985, Whithall et al., 1985) were a gift from Dr. Hal Gainer (Chief, Laboratory of Neurochemistry, National Institute of Neurological Disorders and Stroke, National Institutes of Health).
The slides were placed on a platform shaker and washed three times with TBS (100mM Tris-HCl, pH7.5, 0.9% NaCl) to eliminate excess fixative. The tissue was blocked 1hr in NAS blocking solution (TBS, pH 7.2, 4% Normal Serum from species in which secondary antibody was made, 0.4% Triton X-100). Rinsed three times for 10 minutes and incubated 1hr in secondary antibody at a final concentration of 1/2000 (goat anti-mouse Cy3 conjugates, Jackson Lab, FL). Washed three times for 10 minutes and incubated in Hoechst dye for 15 minutes in a hood. After washing three times for ten minutes in a platform shaker the cover slide was placed using glycerol/0.1 M PBS.
Pharmacological Characterization of AVP within the NAc
Chronic cannulae implantation surgery
At the beginning of surgery, animals were anesthetized with sodium pentobarbital (i.p. 50 mg/kg, Sigma Chemical, MO, USA) and given atropine (0.54 mg/kg, s.c. Phoenix Pharmaceutical, MO, USA). Standard stereotaxic procedures, in conjunction with the atlas of Paxinos and Watson (1998), were used to implant bilateral indwelling 10 mm guide cannulae (23 gauge, Small Parts, FL, USA) aimed at the NAc shell and NAc core. The coordinates in mm for NAc were A-P + 3.5, M-L ± 1.4, D-V −5.0. The cannulae were secured with stainless steel screws (Plastic One, VA, USA) and dental cement and light-curable resin (Sepulveda Multidental, SJ, PR). In order to ensure clean and functional cannulae, wire stylets were inserted into the guides. Antibiotic ointment was placed on the incision and then sutured. Animals were allowed to recover for 7 days before the start of behavioral testing.
Microinfusions
For microinjections, wire stylets were removed and bilateral intracerebral microinjections of the drugs or vehicle were given using 12.5 mm injector cannulae (30 gauge, Small Parts, FL, USA). The injectors were then lowered until they reached the desired injection site. Usually, this site is between 1.0-3.0 mm below the tip of the guide cannula. Animals (n=8-10 in all groups) were hand-held during infusion. Before any experimental microinfusions were performed, all animals were given a preliminary saline infusion in order to adapt them to the injection procedure. Using a micro drive pump (Harvard Apparatus, CA, USA), drug microinfusions of, AVP agonist [pGlu4,Cyt6, Arg8]AVP 4-9, also know as AVP 4-9 1.0μg/0.5μl (Sigma-Aldrich, MO, USA), AVP antagonist (CH2) 5[Tyr (Me) 2] AVP), (Kruszynski et al.,1980), also know as manning compound-MC 1.0μg/0.5μl (gift of Dr. Maurice Manning) diluted in 0.9% sodium chloride solution (Abbott Laboratories, IL, USA), or vehicle solution (0.9% saline solution) 0.5 μl/site were administered to the site via polyethylene tubing (PE-10, Harvard Apparatus, CA, USA). The total time for 0.5μl infusion volumes was 1 min 33 s followed by a 1 min diffusion period. After the drug infusion, the injectors were removed and the stylets were replaced. The animals rested for 30 minutes prior to be placed in the activity chambers. Figure 1a describes the micro infusion delivery schedule for the pre-conditioning treatment, Figure 1b depicts the micro infusion delivery schedule for the post-conditioning treatment and Figure 1c describes the micro infusion delivery schedule for the drug expression (day 12).
Figure 1a.
This diagram represents the schedule of the pre-conditioning injection. All animals (n=8-10) in these experiments receive the NAc micro infusion before the 7, 8, 9, and 10 sessions. The effect was measured on the drug expression session (day 12).
Figure 1b.
This diagram represents the schedule of the post-conditioning injection. All animals (n=8-10) in these experiments receive the NAc micro infusion after the 7, 8, 9, and 10 sessions. The effect was measured on the drug expression session (day 12).
Figure 1c.
This diagram represents the schedule of the Drug Expression Session injection. All animals (n=8-10) in these experiments receive the NAc micro infusion before the drug expression. The effect was measured during this session (day 12).
Drugs
Cocaine Hydrochloride, Sodium Pentobarbital and [pGlu4,Cyt6, Arg8] AVP 4-9 were acquired from Sigma-Aldrich, St. Louis MO and diluted in 0.9% sodium chloride (Abbott Laboratories, IL). (CH2) 5[Tyr (Me) 2] AVP, Kruszynski et al., 1980) was a gift of Dr. Maurice Manning, Professor, The University of Toledo, OH. Atropine Sulfate was acquired from Phoenix Pharmaceutical, MO, USA.
Experimental Design and Statistical Analysis
Between-subjects design was used in the present experiments. With the between-subjects design, separate groups of animals receive different treatments. For this design, an N=8-10 animals was used. In the majority of pharmacological experiments, analysis of variance with multiple factors was used to analyze the data. When necessary, post hoc analysis of the data was used using Newman-Keuls tests.
Histological analysis
After the end of each experiment, animals were deeply anesthetized with an overdose of sodium pentobarbital (Sigma-Aldrich, MO) and were transcardially perfused with PB 0.01M followed by 4% paraformaldehyde (PFA, Fisher, Cayey, PR) . Following this procedure, the brains were collected and stored in 4% PFA for several days before sectioning. Two days before cutting, brains were transferred to a 30% sucrose- 4% PFA solution. Brains were frozen and cut into 60 μm sections using a Leica Cryocut 1900 cryostat. Each section was mounted to a gelatin-coated slide, defatted, and stained with cresyl violet stain (Sigma-Aldrich St. Louis). A projection microscope was utilized to verify the placements for each animal and for reconstruction of injection sites. Reconstructions are made using a hand-drawn atlas, originally derived from serial Nissl-stained sections of a normal rat brain (head orientation: nose bar 5 mm above interaural zero). Each section from the atlas is 0.12 mm apart. For those animals in which their placements were outside the selected brain region, scores were not included in the data analysis. Representative photomicrographs were collected.
Results
Gene Expression profile within the NAc during environment-elicited cocaine conditioning
In order to have an ordered analysis of the genes in the array we used a cluster analysis. The results were clustered using spotfire software. Gene expression profile analysis of the NAc during acquisition (Day, 1, 5 and 10) and expression (Day 12) in environment elicited cocaine conditioning revealed that among all the genes regulated by our experimental protocol, only the AVP gene showed a significant change in expression profile in all stages of the conditioning paradigm. Specifically, we found that AVP mRNA levels decreased in early and mid acquisition (see Table 1 and 2). In contrast, AVP mRNA levels increased during late acquisition (Table 3) and drug expression (Table 4). The fact that AVP gene regulation is consistently present in all stages of our conditioning protocol is a novel finding.
Table 1.
This table summarize results from the NAc early acquisition (session 1) analyzed by gcRMA.
| Gene Title | Fold Change |
|---|---|
| acidic (leucine-rich) nuclear phosphoprotein 32 family, member A | 3.1 |
| amyloid beta (A4) precursor-like protein 2 | −2.7 |
| arginine vasopressin | −8.5 |
| branched chain aminotransferase 1, cytosolic | −2.1 |
| calcium/calmodulin-dependent protein kinase II alpha subunit | 2.9 |
| cathepsin L | −2.8 |
| cold shock domain protein A | −2.5 |
| connective tissue growth factor | −2.4 |
| EST | 2.4 |
| eukaryotic translation initiation factor 4 gamma, 1 | −2.0 |
| microtubule-associated protein 2 | −2.1 |
| mitochondrial carrier triple repeat 1 (predicted) | −2.1 |
| Mitochondrial ribosomal protein L51 (predicted) | −2.2 |
| Muscleblind-like 2 (predicted) | 2.1 |
| nephroblastoma overexpressed gene | −3.9 |
| Phosphatidylinositol 3-kinase, regulatory subunit, polypeptide 1 | −2.2 |
| platelet-activating factor acetylhydrolase, isoform 1b, alpha2 subunit | −2.1 |
| pleckstrin homology domain containing, family B (evectins) member 2 (predicted) | −3.8 |
| PMF32 protein | −2.4 |
| potassium inwardly-rectifying channel, subfamily J, member 6 | −3.6 |
| programmed cell death 4 | −2.1 |
| RAB11B, member RAS oncogene family | −2.6 |
| Ras-induced senescence 1 | −9.7 |
| similar to hypothetical protein D4Ertd765e (predicted) | −2.0 |
| Similar to hypothetical protein MGC52110 | −4.2 |
| similar to Ras-related protein Rab-1B | −3.4 |
| similar to RIKEN cDNA 2010107G23 (predicted) | −2.1 |
| solute carrier family 17 (sodium-dependent inorganic phosphate cotransporter) | −2.5 |
| solute carrier family 17 (sodium-dependent inorganic phosphate cotransporter) | −2.2 |
| tenascin R | −2.1 |
| thyrotropin releasing hormone | −2.0 |
| Transcribed locus | −2.8 |
| Transcribed locus | 2.9 |
| Transcribed locus, weakly similar to XP_580018.1 | −3.7 |
| Trinucleotide repeat containing 6 (predicted) | −3.2 |
Table 2.
This table summarize results from the NAc mid acquisition (session 5) analyzed by gcRMA.
| Gene Title | Fold Change |
|---|---|
| arginine vasopressin | −14.7 |
| ATPase, Na+K+ transporting, alpha 3 subunit | −3.9 |
| calcium channel beta 1 subunit | −3.4 |
| Calreticulin | −2.3 |
| chondroitin sulfate proteoglycan 5 | −5.3 |
| cyclase-associated protein homologue | −2.2 |
| ecto-apyrase | 10 |
| Fyn proto-oncogene | −2.5 |
| glucocorticoid modulatory element binding protein 2 | 2.2 |
| Glutamate receptor, ionotropic, kainate 5 | −2.2 |
| glutamate receptor, metabotropic 5 | −2.5 |
| guanine nucleotide binding protein, alpha 12 | −2.5 |
| guanine nucleotide binding protein, gamma 7 | −2.6 |
| huntingtin-associated protein 1 | −2.2 |
| huntingtin-associated protein interacting protein (duo) | −2.5 |
| Myelin basic protein | −15.1 |
| Myomegalin | −2 |
| neural F box protein NFB42 | −2.5 |
| nuclear receptor subfamily 4, group A, member 3 | 2.4 |
| PAK-interacting exchange factor beta | −3.7 |
| phosphatidylinositol 4-kinase | −2.9 |
| pre-alpha-inhibitor, heavy chain 3 | −2 |
| protein kinase inhibitor, alpha | 2 |
| protein phospatase 3, regulatory subunit B, alpha isoform,type 1 | −3.6 |
| R.norvegicus mRNA for GABA-BR1a receptor. | −3.4 |
| Rattus norvegicus GIRK2A-3 gene for GIRK2A, complete cds. | 2 |
| SNRPN upstream reading frame | −2.2 |
| solute carrier family 4, member 3 | −2.4 |
| somatostatin receptor 2 | 3.4 |
| sphingosine 1-phosphate receptor | −2.5 |
| syntaxin 1a | 2.9 |
| v-akt murine thymoma viral oncogene homolog 1 | −3.2 |
| vesicular inhibitory amino acid transporter | −3.5 |
| Wolfram syndrome 1 | −2.2 |
Table 3.
This table summarize results from the NAc late acquisition (session 10) analyzed by gcRMA.
| Gene Title | Fold Change |
|---|---|
| arginine vasopressin | 2.8 |
| Biglycan | −4.1 |
| cadherin 22 | −2.8 |
| cytokine-induced neutrophil chemoattractant-2 | 5.1 |
| ESTs | 3.2 |
| ESTs | −2.4 |
| ESTs | 2.0 |
| ESTs | −3.6 |
| ESTs | −2.0 |
| ESTs | 2.0 |
| ESTs | 2.9 |
| ESTs, Moderately similar to centaurin delta 2, isoform b | 2.0 |
| ESTs, Moderately similar to RIKEN cDNA 2810411G23 [Mus musculus] | −2.7 |
| ESTs, Moderately similar to I49127 intracellular protein Mg11 - mouse | −4.7 |
| ESTs, similar to RAB8 interacting protein [Mus musculus] | 2.0 |
| ESTs, Weakly similar to hypoxia induced gene 1 [Rattus norvegicus] | 2.6 |
| ESTs, Weakly similar to S10471 cMG1 protein - rat [R.norvegicus] | −2.1 |
| ESTs, Weakly similar to S26689 hypothetical protein hc1 — mouse | −2.4 |
| G protein-coupled receptor, family C, group 1, member H | −2.1 |
| Orthodenticle (Drosophila) homolog 1 | 3.4 |
| RNA binding protein p45AUF1 | −2.4 |
| syntaxin 6 | 2.6 |
| Tamalin | 3.5 |
| Transthyretin (prealbumin, amyloidosis type I) | 15.6 |
Table 4.
This table summarize results from the NAc drug expression session (session 12) analyzed by gcRMA.
| Gene Title | Fold Change |
|---|---|
| Angiotensinogen | 10.2 |
| Arginine vasopressin | 100 |
| Arginosuccinate synthetase | 4.0 |
| Forkhead box P1 (predicted) | −2.4 |
| G protein-coupled receptor 6 | −1.7 |
| Glutathione peroxidase 3 | 2.7 |
| Neurofilament, heavy polypeptide | 3.2 |
| Oxcytocin | 100 |
| Phospholipase C , beta4 | 2.1 |
| Potassium voltage gated channel, Shaw-related subfamily, member 2 | 4.2 |
| Prostaglandin D2 synthase | 4.0 |
| Regulator of G-protein signaling 2 | −5.7 |
| Solute carrier family 17 (sodium-dependent inorganic phosphate co transporter) |
82.6 |
| Transcribed locus | 2.5 |
| Transcribed locus | −3.7 |
Gene Expression profile within the mPFC during environment-elicited cocaine conditioning
The mPFC brain region was also examined as a control site for our NAc studies. Tables 5 to 8 shows gene expression profile analysis of the mPFC during acquisition (Day, 1, 5 and 10) and expression (Day 12) of the environment elicited cocaine conditioning. In contrast to the NAc results, no common gene showing a differential expression profile between the stages of our conditioning paradigm. Moreover, AVP gene showed no changes in expression within the mPFC in any of the conditioning stages examined.
Table 5.
This table summarize results from the mPFC acute treatment (session 1) analyzed by gcRMA.
| Gene Title | FC |
|---|---|
| Rattus norvegicus activity regulated cytoskeletal-associated protein (Arc), mRNA. | 2.4 |
| Rat prostaglandin D synthetase mRNA, complete cds. | 2.2 |
| ESTs | −2.3 |
| ESTs | 2.2 |
| ESTs | −2.1 |
| Rattus norvegicus SMDF neuregulin beta 3 (Nrg1) mRNA, partial cds, alternatively spliced. |
−4.4 |
| Rattus norvegicus zinc finger protein 354A (Zfp354a), mRNA. | −6.1 |
| ESTs | −2 |
| ESTs | −2 |
| procollagen, type I, alpha 2 | 2.5 |
| ESTs, Weakly similar to nucleoporin 98 [Rattus norvegicus] [R.norvegicus] | −4.2 |
| ESTs, Highly similar to MOUSE Bone morphogenetic protein receptor type II precursor | −2.2 |
| ESTs | −2.1 |
| Rattus norvegicus Transthyretin (prealbumin, amyloidosis type I) (Ttr), mRNA. | −2.9 |
| ESTs, Weakly similar to FMOD_RAT Fibromodulin precursor | 2.7 |
Table 8.
This table summarize results from the mPFC expression (session 12) analyzed by gcRMA.
| Gene Title | Fold Change |
|---|---|
| protein phosphatase 3, catalytic subunit, beta isoform | −2 |
| adrenergic receptor, alpha 1d | 7.9 |
| dopamine receptor 1A | −4.5 |
| latent transforming growth factor beta binding protein 2 | 3.7 |
| rabphilin 3A-like (without C2 domains) | 2.8 |
| guanylate binding protein 2, interferon-inducible | 2.2 |
| enoyl-Coenzyme A, hydratase/3-hydroxyacyl Coenzyme A dehydrogenase | 2.1 |
| protein kinase C, beta 1 | −2.2 |
| renin 1 | −2.5 |
| solute carrier family 26, member 4 | 2.6 |
| CCAAT/enhancer binding protein (C/EBP), beta | 2 |
| regulator of G-protein signaling protein 2 | −2.4 |
| regulator of G-protein signaling 19 interacting protein 1 | −2.9 |
| profilin II | −3.2 |
| LL5 protein | −2.9 |
| vesicle docking protein, 115 kDa | −2.3 |
| v-crk-associated tyrosine kinase substrate | 6 |
| ankyrin-like repeat protein | 7.9 |
| Glutamine synthetase (glutamate-ammonia ligase) | −2.1 |
| lectin, galactose binding, soluble 1 | 3 |
| biglycan | 2.3 |
| ESTs, Weakly similar to B48013 proline-rich proteoglycan 2 precursor, parotid - rat | 5.3 |
| ubiquitin-conjugating enzyme E2D 3 (homologous to yeast UBC4/5) | −2 |
| EST, Moderately similar to T10_MOUSE Ser/Thr-rich protein T10 in DGCR region | −2.8 |
| ESTs, Highly similar to A38383 troponin C, fast skeletal muscle - mouse | −2.7 |
| EST, Highly similar to protocadherin 16 precursor; fibroblast cadherin FIB1; cadherin 19; fibroblast cadherin 1 |
−2.3 |
| ESTs | 4.5 |
| EST | −6.7 |
| Rn.54392 | 2.6 |
| ESTs | −4.8 |
| ESTs | −2.5 |
| a disintegrin and metalloprotease domain 23 | 3.7 |
| ESTs, Weakly similar to procollagen, type V, alpha 3 [Rattus norvegicus] | 2.7 |
| ESTs, Highly similar to A42772 mdm2 protein - rat (fragments) [R.norvegicus] | −3.4 |
| ESTs, Highly similar to ODPA_RAT Pyruvate dehydrogenase E1 component alpha sub-unit |
−2.2 |
| ESTs | −2.2 |
| ESTs | −2.1 |
| ESTs | 8.2 |
| ESTs | 3 |
| ESTs | −2.1 |
| ESTs | 14.1 |
| ESTs | −3.8 |
| ESTs | −8.6 |
| ESTs | −2.3 |
| ESTs | −6.9 |
| ESTs, Weakly similar to ISL2_RAT Insulin gene enhancer protein ISL-2 (Islet-2) [R.norvegicus] |
−2.1 |
| ESTs | 2.8 |
| ESTs, Weakly similar to Y18H1A.2.p [Caenorhabditis elegans] [C.elegans] | 2.4 |
| ESTs | −3.7 |
| ESTs | −2.2 |
| ESTs | 4.1 |
| ESTs, Highly similar to BTB (POZ) domain containing 1 [Homo sapiens] [H.sapiens] |
−2 |
| ESTs, Highly similar to Hus1 homolog (S. pombe) [Mus musculus] [M.musculus] | −2.2 |
| ESTs, Weakly similar to SYD_RAT ASPARTYL-TRNA SYNTHETASE (ASPARTATE--TRNA LIGASE) |
2 |
| ESTs, Weakly similar to RIKEN cDNA 5730493B19 [Mus musculus] [M.musculus] | −2.3 |
| ESTs, Weakly similar to Zinc finger, C3HC4 type (RING finger) [Caenorhabditis elegans] [C.elegans] |
−2.2 |
| ESTs, Weakly similar to B48013 proline-rich proteoglycan 2 precursor, parotid - rat [R.norvegicus] |
2 |
| phosphoinositol 3-phosphate-binding protein 2 | 2.4 |
| ESTs, Highly similar to NHPX_RAT NHP2-like protein 1 | −2.3 |
| ESTs | −3.7 |
| ESTs | −4.2 |
| ESTs | −2.2 |
| ESTs | −2.2 |
| ESTs, Highly similar to chromosome 20 open reading frame 163 [Homo sapiens] | 2.9 |
| ESTs, Moderately similar to TATA box binding protein (Tbp)-associated factor, RNA polymerase I |
−2.4 |
| ESTs, Highly similar to IKBA_RAT NF-kappaB inhibitor alpha (I-kappa-B-alpha) | 3.8 |
| ESTs | 2.2 |
| ESTs | 2.4 |
| ESTs | 2.5 |
| ESTs | −2 |
| ESTs, Moderately similar to KIAA0164 gene product [Homo sapiens] [H.sapiens] | −5.1 |
| ESTs, Moderately similar to SPS2_MOUSE Selenide,water dikinase 2 (Selenophosphate synthetase 2) |
−2.1 |
| ESTs, Weakly similar to transducin-like enhancer of split 3, homolog of Drosophila [Rattus norvegicus] |
2.2 |
| ESTs | −2.1 |
| ESTs | 2.3 |
| ESTs, Weakly similar to S41067 collagen alpha 1(III) chain - rat [R.norvegicus] | −3 |
| ESTs, Weakly similar to mitogen activated protein kinase kinase kinase kinase 2; RAB8 interacting protein |
−2.4 |
| ESTs | −2.1 |
| ESTs | 2.3 |
| ESTs, Weakly similar to ras-like protein [Rattus norvegicus] | −2.1 |
| ESTs, Weakly similar to carboxypeptidase Z [Rattus norvegicus] | 2.4 |
| ESTs, Weakly similar to B26423 serine proteinase inhibitor 2.2 - rat (fragment) [R.norvegicus] |
2.1 |
| ESTs, Highly similar to protein translocation complex beta; protein transport protein SEC61 |
−2.3 |
| ESTs, Moderately similar to PTD012 protein [Homo sapiens] | −2 |
| Hemoglobin, beta | −3 |
| hemoglobin beta chain complex | −2.1 |
| ADP-ribosylation factor domain protein 1, 64kD | −2.1 |
| secretory carrier membrane protein 1 | −2.8 |
| ER transmembrane protein Dri 42 | −2.6 |
| kinesin light chain 1 | −2.2 |
| Complement component 4 | 2.3 |
| G protein-coupled receptor 48 | −2.1 |
| Thyroglobulin | −3.3 |
| proline-rich proteoglycan 1 | 3.9 |
| solute carrier family 1, member 3 | −2.7 |
| mammary cancer associated protein RMT-1 | 2.2 |
| ether-a-go-go-like potassium channel 1 | 2 |
| dynamin 1-like | −2.3 |
| erythrocyte protein band 4.1-like 3 | −2.3 |
| crystallin, gamma E | 2.4 |
| plysia ras-related homolog A2 | −2.6 |
| Glycine receptor beta | −2.2 |
| leukotriene B4 receptor | −3 |
| RAB2, member RAS oncogene family | −2.2 |
| neurofilament, light polypeptide | −2.2 |
| Jun D proto-oncogene | −2.3 |
| gamma-aminobutyric acid receptor, subunit alpha 3 | −2.1 |
| small GTP-binding protein rab5 | −7.6 |
| trefoil factor 1 | −3.2 |
| adenosine kinase | −4.1 |
| non-histone chromosomal architectural protein HMGI-C | −3.2 |
| growth differentiation factor 8 | 2.8 |
| sialyltransferase 6 | −2 |
| protein tyrosine phosphatase, non-receptor type 12 | −3.4 |
| activity-dependent neuroprotective protein | −2.8 |
| synaptogyrin 1 | −2.6 |
| guanine nucleotide binding protein, alpha 12 | −2.1 |
| dynamin 1-like | −3 |
| synaptotagmin 7 | −7.9 |
| CD2 antigen | 2.1 |
| pancreatic polypeptide | 4.7 |
| Rattus norvegicus RAS guanyl releasing protein 1 (Rasgrp1), mRNA. | −2.2 |
| protein phospatase 3, regulatory subunit B, alpha isoform,type 1 | −6 |
| sec22 homolog | 2.1 |
| transient receptor potential-related protein, ChaK | −3.2 |
| CDC10 (cell division cycle 10, S.cerevisiae, homolog) | −2 |
| Eag-related gene member 2 | 3.4 |
| placental growth factor | −4.2 |
| SEC15 homolog (S. cerevisiae) | −2 |
Validation of genes candidate from the NAc microarrays
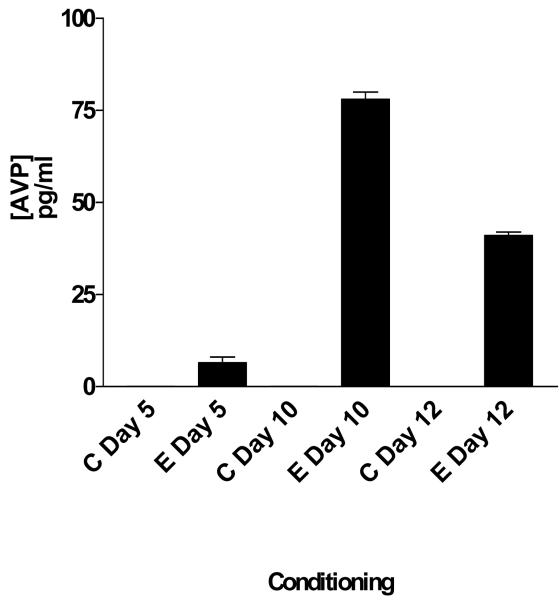
Using the criteria established for the selection of the genes candidate we decided to validate the presence of AVP and to confirm the presence of V1A receptor within the NAc. AVP levels were measured by means of RIA while IF confirmed the presence within the NAc. Figure 2 shows AVP concentration during acquisition and expression of the environment elicited cocaine conditioning. As depicted in this figure 2, AVP levels were detectable in cocaine-conditioned subjects but not within control subjects (F [5,6] = 857; p<0.001). No statistical difference was found between control subjects. Figure 3 shows the IF results that demonstrate that AVP immune reactivity is localized within the NAc Core (figure 3a) and Shell (figure 3b). These findings support our hypothesis that AVP is a cocaine-induced gene. Figure 4 shows IF of AVP within the Hypothalamus as a control site to demonstrate the specificity of the AVP labeling and to differentiate between soma and fiber labeling.
Figure 2. Radioimmunoassay of AVP.
AVP levels were measured during mid-acquisition (5 days), late conditioning (10 days) of cocaine conditioning and during the drug expression session (day12). Black bars represent cocaine-conditioned subjects. AVP was measured from NAc tissue rich punches and is expressed as pg/ml of total protein. The up regulation of AVP peptide during conditioning confirms the up regulation of the mRNA in the arrays—except for mid-acquisition. No AVP levels were found in control subjects. There were significant differences between C Mid Acquisition and E Mid Acquisition (* p<0.05), between Late Acquisition and E Late Acquisition (### p<0.001), between C Expression and E Expression (+++ p<0.001). C= Control, E= Experimental.
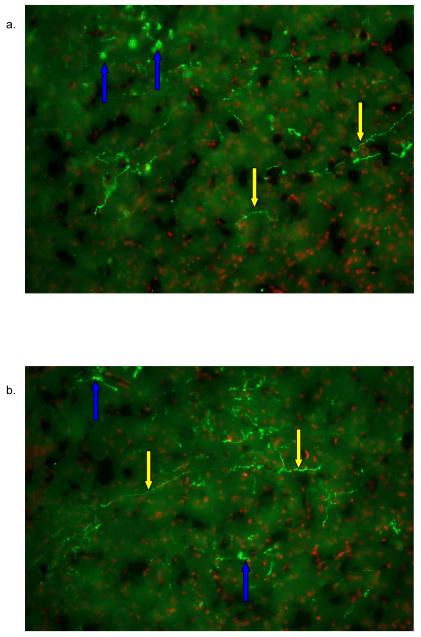
Figure 3. IF of AVP within the NAc core and shell.
AVP inmuno reactivity was detected with the NAc core (3a), and NAc shell (3b). AVP was detected with PS-41 monoclonal antibody and a goat anti mouse Cy3 (green) labeled secondary antibody. Nuclei were labeled with Hoescht dye (Red). Most of the label was found within the axons. Yellow arrows represent an axon fibers and blue arrows represent fibers that are outside the plane of focus thus showing a disperse immunofluorescence.
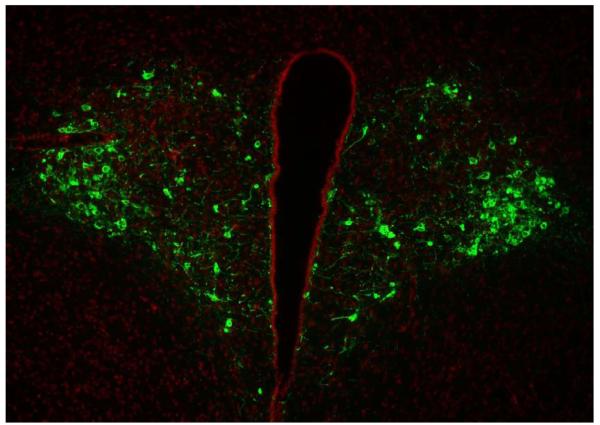
Figure 4. IF of AVP within the Hypothalamus.
Most of the AVP inmuno reactivity was detected within the PVN of the hypothalamus. AVP was detected with PS-41 monoclonal antibody and a goat anti mouse Cy3 (green) labeled secondary antibody. Nuclei were labeled with Hoescht dye (Red).
We also tested for the presence of V1A receptor within the NAc of rats exposed to our conditioning paradigm (data not shown). Other investigators previously reported the expression of V1A receptor within limbic structures (Dorsa et al 1984; Paban et al., 1999). Consistent with the previous published data, we were able to also detect V1A receptor protein levels within the NAc. However, we found that there were no significant differences (p> 0.05) between the controls or experimental animals for the V1A receptor protein levels during the test session (data not shown). These findings suggested that the presence of vasopressinergic actions within the NAc could play a role during the acquisition and expression of environment-elicited cocaine conditioning. This led us to further characterize the role of AVP within the NAc during the acquisition and expression of environment-elicited cocaine conditioned response.
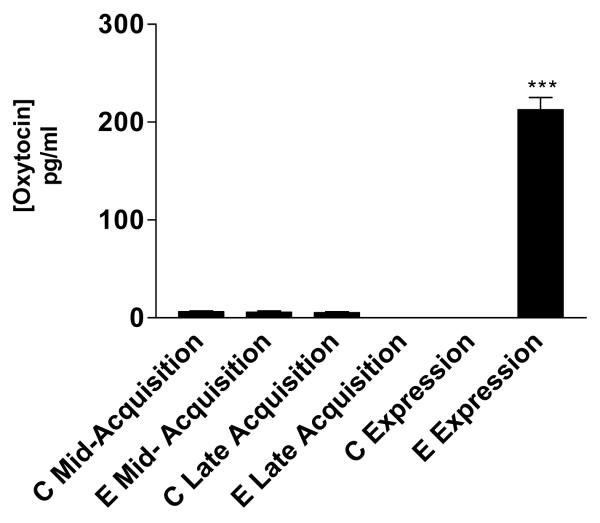
Furthermore, our array data showed that OT, a closely related peptide to AVP, was also up-regulated during the drug expression session (day 12). Therefore, we validated its presence in the NAc by means of EIA (figure 5) and IF (figure 6a and 6b).
Figure 5. EIA of Oxytocin.
OT levels were measured during mid-acquisition (5 days), late acquisition (10 days) of cocaine conditioning and during the drug expression session (day12). OT was measured from NAc tissue rich punches and is expressed as pg/ml of total protein. There was a significant difference between C Expression and E Expression (*** p<0.001). The up regulation of OT found in the expression session confirms the up regulation of the mRNA in the arrays. Also, OT levels were found in other conditioning phase but not comparable to the array results. C= control, E= Experimental.
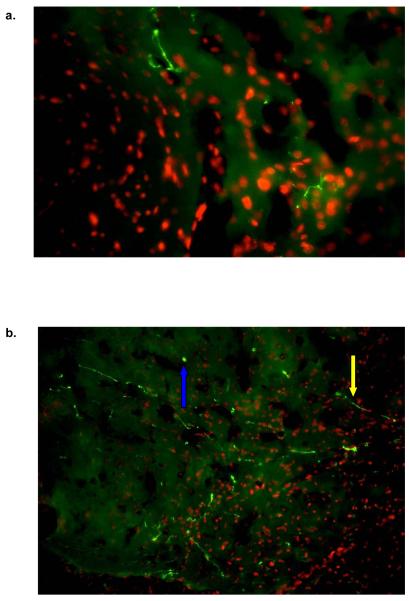
Figure 6. IF of OT Within the Nac core and shell.
OT inmuno reactivity was detected with the NAc core (5a), and NAc shell (5b). OT was detected with PS-38 monoclonal antibody and a goat anti mouse Cy3 (green) labeled secondary antibody. Nuclei were labeled with Hoescht dye (Red). Most of the label was found within the axons. Yellow arrows represent an axon fibers and blue arrows represent fibers that are outside the plane of focus thus showing a disperse immunofluorescence.
Characterization of the role of AVP within the NAc during the acquisition and expression of the environment-elicited cocaine conditioned response
The following set of experiments and their result were aimed to test the role of AVP within the NAc in the acquisition and expression of the environment elicited cocaine conditioning response. We studied the role of AVP before (pre-conditioning) and after (post-conditioning) the conditioning session and at the drug expression session.
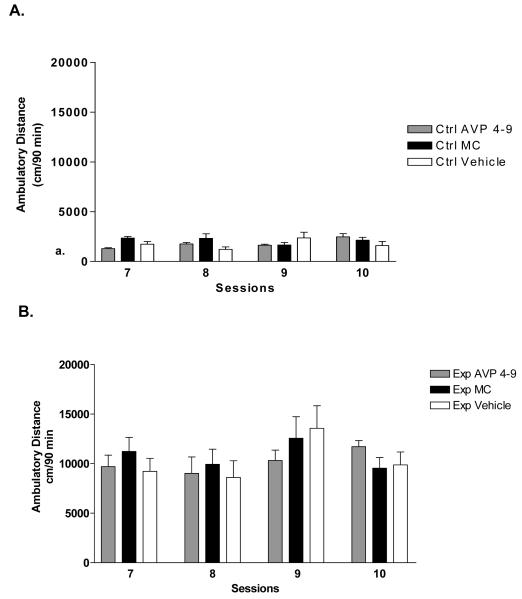
Effects of pre-conditioning microinjection of AVP 4-9 and MC in the expression of the environment-elicited cocaine conditioning
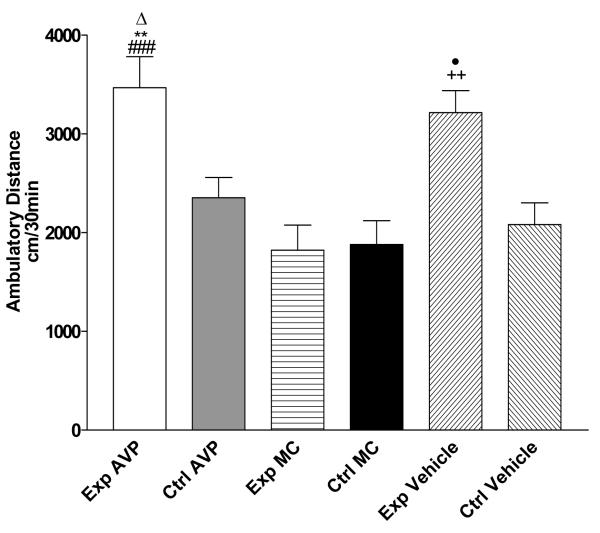
Figure 7a shows the effect of microinjection of AVP 4-9 and MC (1ng/0.5μl) within control subjects (n=8) before the sessions 7, 8, 9 and 10 (pre-conditioning). Two way ANOVA revealed that there is not a significant difference (p>0.5) between control subjects during acquisition of conditioning. Figure 7b shows the effects pre-conditioning microinjection of AVP 4-9 and MC (1ng/0.5μl) within cocaine paired subjects (n=10). A Two way ANOVA revealed that there is not a significant interaction (p>0.5) between cocaine paired groups during acquisition of conditioning. Therefore the pre-conditioning microinjection did not affect acquisition conditioning. Figure 8 shows the effect of this pre-conditioning microinjection on the drug expression session. A One way ANOVA showed that there is significant difference in treatment (F [5, 42] =9.43, p<0.0001). Specifically, a post hoc analysis (Tukey Multiple Comparison Test) revealed that there was a significant difference between experimental subjects injected with AVP 4-9 and control AVP 4-9 (p<0.05), experimental MC , control MC (p<0.001) and control vehicle (p<0.01). There was not a significant difference between experimental AVP 4-9 and experimental vehicle (p>0.05). Also, there was a significant difference between experimental vehicle and control AVP 4-9, control vehicle (p<0.05), and between control MC, experimental MC (p<0.01). This result showed that AVP 4-9 did not increase the conditioned response in experimental subjects when compared to experimental vehicle subjects. Also, MC decreased the conditioned response in experimental subjects. No difference in the conditioned response was observed within control subjects or MC subjects.
Figure 7. Effects of Pre-conditioning microinjections of AVP 4-9 and Manning Compound on acquisition of environment-elicited cocaine conditioning.
Microinjections were performed before placing the animals on the conditioning chamber on session 7, 8, 9 and 10. Top, panel a shows the effects on control subjects, bottom, panel b shows the effects on cocaine conditioned subjects.
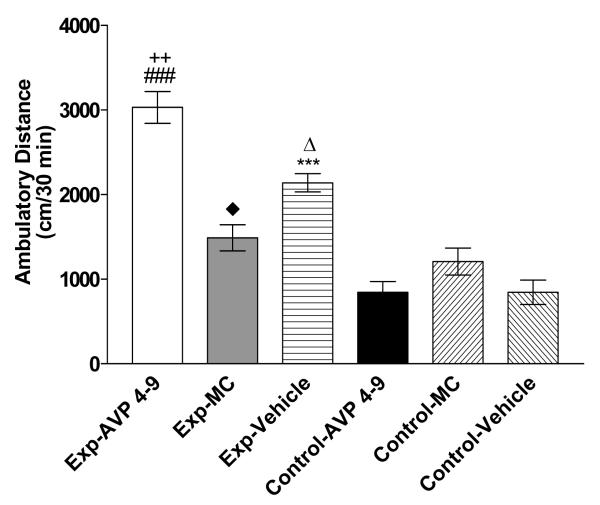
Figure 8. Effects of Pre-conditioning microinjections of AVP 4-9, Manning Compound and Vehicle on the drug expression session of environment-elicited cocaine conditioning.
Figure 8 shows ambulatory distance traveled within the first 30 minutes of the drug expression session. Experimental AVP 4-9 subjects traveled a greater ambulatory distance than control MC, experimental MC (###, p<0.001), control vehicle (**, p<0.01) and control AVP 4-9 (Δ, p<0.05). Experimental vehicle subjects traveled a greater ambulatory distance than control vehicle and control AVP 4-9 (•, p<0.05) control MC and experimental MC (++, p<0.01). No significant difference was found between the others groups (p>0.05). Exp AVP =Experimental AVP 4-9 treated subjects, Ctrl AVP= Control AVP 4-9 treated subjects, Exp MC= Experimental Manning Compound treated subjects, Ctrl MC= Control Manning Compound treated subjects, Exp and Ctrl Vehicle Experimental and Control Subjects treated with saline.
Effects of post-conditioning microinjections of AVP 4-9 and MC in expression of the environment-elicited cocaine conditioning
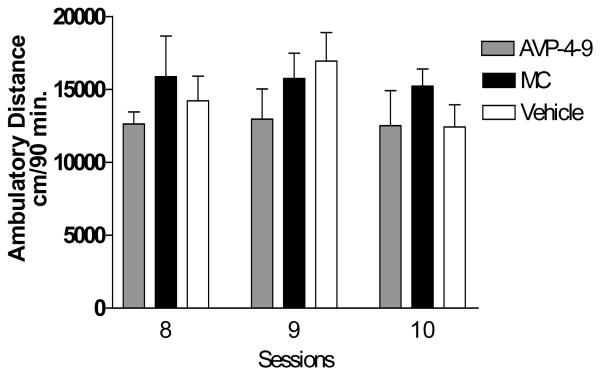
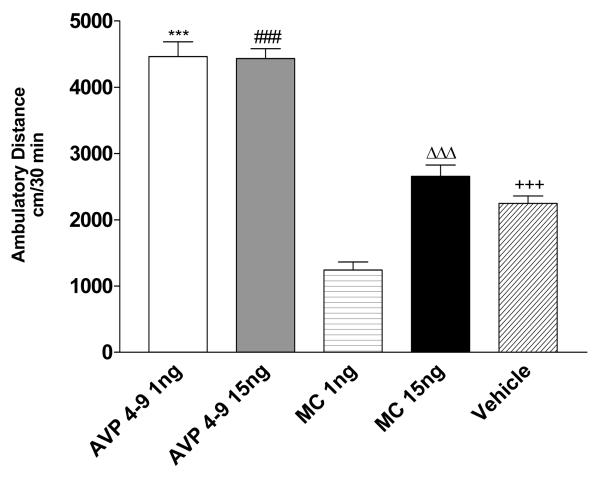
In order to asses the role of post-conditioning microinjections of AVP 4-9 and Manning Compound in the expression of environment-elicited cocaine conditioning, we microinjected these compounds after conditioning sessions 7, 8, 9 and 10. Figure 9 shows the effects of post-conditioning microinjections on the acquisition of conditioning. A Two-way ANOVA revealed that there was no significant difference between experimental subjects (p>0.05). These results confirmed that acquisition of cocaine conditioning was not affected by post-conditioning microinjections of either AVP 4-9 or MC. The effect of this treatment was then studied in drug expression. Figure 10 shows the effects of post-conditioning microinjections of AVP 4-9 and MC at doses of 1ng and 15ng/0.5μl per side on drug expression days. A One-way ANOVA revealed that there was a significant difference between treatments (F [5, 40] =56.75, p<0.001). Specifically, a post hoc analysis (Tukey Multiple Comparison Test) revealed that there was significant difference between Exp AVP 4-9 1ng and Exp vehicle, Exp MC 1ng, Exp MC 15ng subjects (p<0.001), between Exp AVP 4-9 15ng and Exp vehicle, Exp MC 1ng, Exp MC 15ng (p<0.001), and between Exp MC 1ng and Exp MC 15 ng (p<0.001). There was not a significant difference Exp AVP 4-9 1ng and Exp AVP 4-9 15ng (p>0.05) neither between Exp MC 15ng and Exp vehicle (p>0.05). These results showed that MC at a dose of 1ng/0.5μl produced significant decreases in the conditioned response to levels lower than the control but failed to do so at 15ng dose. Both the 1 and 15 ng/0.5μl doses of AVP 4-9 in experimental subjects produced an increased conditioned response when compared to experimental vehicle subjects. However, there was not a significant difference between both doses of AVP 4-9 studied suggesting that an over-activation of the receptor does not mean an over expression of the conditioned response. Neither AVP 4-9 nor MC at both doses studied affects the conditioned response in control subjects.
Figure 9. Effects of post-conditioning microinjections on acquisition of the cocaine conditioning.
The effect of post-conditioning microinjections on acquisition was assessed on experimental subjects during sessions 8, 9 and 10. There were not significant differences between subjects and groups. No data for the 7th day could be obtained.
Figure 10. Effects of AVP on the drug expression session on experimental subjects.
Figure 9 shows ambulatory distance traveled within the first 30 minutes of the drug expression session. Experimental subjects injected with AVP 4-9 at 1ng dose traveled greater ambulatory than MC 1ng, 15 ng and vehicle subjects (***, p<0.001). AVP 4-9 at a 15ng dose traveled a greater ambulatory distance than MC 1ng, MC 15ng and vehicle subjects (###, p<0.001). MC at 15ng dose traveled a greater ambulatory distance than MC 1ng dose subjects (ΔΔΔ, p<0.001). Vehicle treated subjects traveled a greater ambulatory distance than MC 1ng subjects (+++, p<0.001). No significant difference was found between AVP 4-9 1ng and 15ng and between MC 15ng and vehicle (p>0.05).
Effects of microinjection of AVP 4-9 and MC on the drug expression session of environment-elicited cocaine conditioning
In order to examine the role of AVP 4-9 and Manning Compound (1ng/0.5μl) in the expression of environment-elicited cocaine conditioning we microinjected 30 minutes before the drug expression session. Figure 11 shows the effects of the microinjection before the drug expression session on experimental and control subjects. A One Way ANOVA revealed that there was a significant difference between treatments (F [5, 47] =34.16, p<0.001). Specifically, a post hoc analysis (Tukey Multiple Comparison Test) revealed that there was a significant difference between Exp-AVP 4-9 and Exp-MC, Control AVP 4-9, Control MC and Control Vehicle subjects (p<0.001), between Exp-AVP 4-9 and Exp-Vehicle subjects (p<0.01), between Exp-MC and Control AVP 4-9, Control-Vehicle and Exp-Vehicle (p<0.05), between Exp-Vehicle and Control-AVP 4-9, Control-MC and Control-Vehicle (p<0.001). There was not a significant difference between Control-AVP 4-9 and Control-MC, Control-Vehicle (p>0.05), between Control-MC and Control-Vehicle and Exp-MC (p>0.05). Our results show that experimental subjects treated with MC have a decreased conditioned response toward the conditioning environment when compared to experimental vehicle and to experimental AVP 4-9. Also, we found that AVP 4-9 increased the conditioned response in experimental subjects to a higher level than experimental saline subjects. No significant effects were found between control subjects. This result demonstrated that AVP plays a role during expression of the cocaine conditioned response. Moreover, no effect was observed on control subjects.
Figure 11. Effects of microinjection of AVP 4-9, Manning Compound and Vehicle on the drug expression session of the environment-elicited cocaine conditioning.
Figure 11 shows ambulatory distance traveled within the first 30 minutes of the drug expression session. Experimental subjects injected with AVP 4-9 traveled grater ambulatory than Exp-MC, Control-AVP 4-9, Control MC and Control Vehicle subjects (###, p<0.001). Also, Exp-AVP 4-9 subjects traveled greater ambulatory distance than Exp-Vehicle subjects (++, p<0.01). Exp-Vehicle traveled greater ambulatory distance than Control-AVP 4-9, Control-MC and Control-Vehicle (***, p<0.001) and than Exp-MC (Δ, p<0.05). Exp-MC traveled greater ambulatory distance than Control-AVP 4-9 and Control-Vehicle (◆p<0.05). No significant difference was found between Exp-MC and Control-MC, Control-AVP 4-9 and Control-Vehicle neither Control-AVP 4-9 nor Control-MC (p>0.05).
Histology results
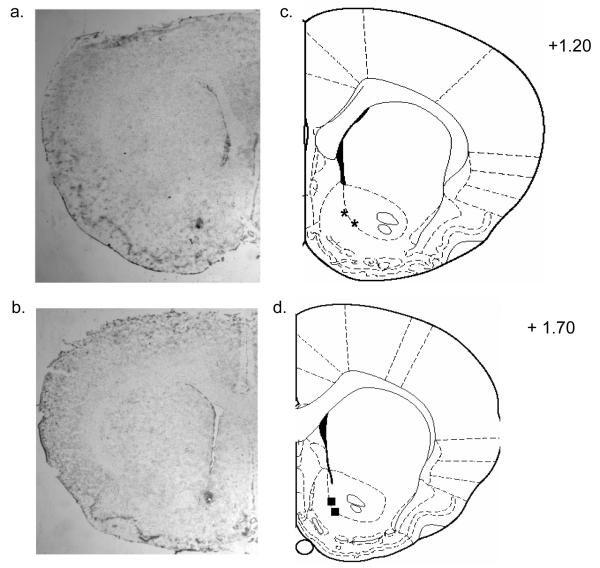
The brain sites of the microinjector tips of representative animals utilized in the study are shown in Figure 12. Figure 12a and b shows a representative photomicrograph of the injections site for the NAc. Figure 12c and d shows drawing of serial coronal sections from representative animals. Most placements were found between +1.70 to +1.20 from bregma. Placements for the NAc were considered accurate if they were located within the structure of interest in both hemispheres. Animals were also excluded if histological analyses revealed evidence of severe infection at the injection site or due to poor cannula placement.
Figure 12. Histological analysis of NAc microinjection.
Figure 12a and b shows representative photomicrograph of the injection site for the NAc. Figure 12c and d shows drawing of serial coronal sections from representative animals used in the experiments. Bilateral symbols show approximate cannulae placement for the NAc at +1.20 mm from bregma (asterisk) and NAc at +1.70 mm from bregma (squares).
Discussion
Our microarrays results demonstrated that there are different sets of genes expressed during the early, mid and late acquisition and during expression session of the environment-elicited cocaine conditioning. Specifically, our results from the gene expression analysis showed that AVP was the only gene showing either up or down regulation during the acquisition phase and expression session of environment-elicited cocaine conditioning (tables 1 to 4). AVP was down regulated during early and mid acquisition (FC −8.5, −14-7 respectively) and up regulated during late acquisition and drug expression session (+2.8, +100 respectively). Furthermore, AVP peptide levels during mid and late acquisition and drug expression session were confirmed by RIA validation studies. Although, AVP within the NAc could be detected in mid acquisition (±5.0pg), the array data suggested that there was a down-regulation of the mRNA (−14.7). Moreover, the results from the IF (figure 3a and 3b) confirmed that AVP is expressed within the NAc core and shell. We also validated several AVP related proteins like, PLCβ4, CaM 1 and V1A receptor within the NAc (data not shown). Our array results also demonstrated a significant OT mRNA up-regulation within the NAc on the drug expression session (day12). Validation studies by EIA and inmunoflourence revealed the presence of OT peptide within NAc fibers (Figure 5, 6a and 6b). Support evidence for our OT data comes from Butovsky et al., 2006 that showed OT mRNA and OT peptide presence within the NAc followed by chronic THC treatment. Unlike AVP expression found across all stages of cocaine conditioning, OT up regulation within the NAc was only detected on the Day 12. This demonstrated that between these two neuropeptides there are functional differences when it comes to cocaine addiction. Future OT studies will further explain this phenomenon and how it relates to possible neurochemical substrates subserving cocaine actions within the NAc. Taken together, these results suggest that there is a vasopressinergic system within the NAc that is activated by cocaine itself and its associated environmental cues.
These results raise the question of the cellular origin of AVP mRNA within the NAc. Our findings (Tables 1 to 4, figures 2 and 3) suggest that both the mRNA and the peptide were localized within NAc neurons and are produced in response to cocaine treatment. Previous findings fail to show that AVP is synthesized in the NAc at baseline levels. However, in our hands cocaine treatment induced a differential expression profile of AVP in rats (see table 1-4). Thus, our molecular evidence supports our interpretation that this gene is regulated by cocaine exposure and conditioning. Previous evidence supports these findings. For example, Zhou et al. 2005 reported that cocaine also increases AVP mRNA expression within limbic structures. However, in both the cited study and ours it is not known from where the AVP mRNA found was originating. Validation studies of the arrays data with inmunohistochemical experiments reported in our manuscript revealed AVP labeling within NAc fibers. Thus, perhaps our molecular data does support the notion that AVP is present in the NAc specifically in its fibers. Interestingly, AVP mRNA is mainly founded in the PVN and the SON of hypothalamus and the peptide in the pituitary gland. However, it was demonstrated that AVP mRNA could be transported to axons of the Median Eminence, Posterior pituitary (Trembleau, Morales and Bloom, 1994) and to the Bed Nucleus of the Striata Terminalis (Miller et al., 1992, Choi et al., 2007). Moreover, recent findings confirm the presence of a cis-acting element, called the denditric localizer sequence (DLS), within part of the coding and the 3′ untranslated region of the AVP mRNA (Mohr and Ritcher, 2004). These authors claim that this sequence can drive the AVP mRNA from the soma to the dendrite where it could be translated. Therefore, we can not rule out the possibility that AVP mRNA could be coming form the hypothalamus in response to cocaine treatment. As has been shown before, cocaine treatment activate the HPA axis (Goeders 2002 a, b) to an extent that all peptides of the HPA axis related to stress such as CRF, ACTH and corticosterone/cortisol are necessary for the cocaine seeking behavior, place preference and self administration to take place.
Previous studies showed that AVP is involved in mediating the acquisition of cocaine seeking behavior (De Vry et al., 1988; van Ree et al., 1988; Sarnyai et al., 1992) and cocaine withdrawal (Zhou et al., 2005). However, we are the first to report that AVP shows a change in expression profile in response to cocaine at least in our cocaine-conditioning paradigm. Therefore, we also expected that genes related to AVP signal transduction pathways such as PLCb4 and CaM 1 to be also affected by cocaine treatment. Previous evidence questioned the specific role of AVP in cocaine abuse (De Vry et al., 1988; van Ree et al., 1988). Based on our molecular data, our experiments seek to establish a link of AVP function within the NAc in cocaine conditioning via brain microinfusions. Our results demonstrated that AVP is not interfering with the acquisition of the cocaine conditioning but may be playing a role in the expression of the conditioned response. Recent review by Langraf and colleagues (2007) described that anxiety related behaviors in HAB rats correlate with an overexpression and over release of AVP within the paraventricular nucleus. Thus, it is implied that high and prolonged release levels of AVP elicit anxiety. In our study, we showed that upon exposure to the cocaine associated cues AVP levels rise. Therefore, it is possible to suggest that AVP actions are driving anxiety-like response that may contribute to craving and the conditioned response to the drug. Moreover, the fact that MC did not alter the response in control subjects suggests that the inhibitory effects of MC on experimental subjects are specific to cocaine conditioned subjects. This finding confirms our hypothesis that AVP could be mediating the expression of the cocaine conditioned response.
Our findings revealed that there was no a significant difference between groups (AVP 4-9, MC and vehicle) during the acquisition phase. These results suggested that post-conditioning microinjections do not interfere with the acquisition of cocaine conditioning nor the locomotor effect of cocaine. In addition, our data demonstrated that post-conditioning treatment was affecting expression of the conditioned response. This suggested that AVP may be playing a role in the events that take place within the NAc after the cocaine exposure. Moreover, our results also showed that experimental subjects treated with MC had a decreased response to the conditioning environment. Also, we found that AVP 4-9 increased the conditioned response in experimental subjects to a higher level than the experimental saline subjects while no significant effects were found between control subjects. This result demonstrated that AVP is playing a role during expression of the cocaine conditioned response. The fact that neither MC nor AVP 4-9 had an effect in control subjects confirmed our findings that AVP could be mediating cocaine conditioned responses and that cocaine effects are needed for AVP exert its effects.
Previous studies revealed that AVP and its related neuropeptides could reduce the acquisition of cocaine self-administration (SA) (van Ree et al., 1988). These authors demonstrate that DGAVP (des-glycinamide arginine vasopressin) and AVP 4-8 decreased heroin and cocaine intake of food deprived rats in a 6 hr session during five consecutive days. Interestingly, they found that AVP 4-9 increased heroin SA but decreased cocaine SA under these conditions. However, the authors failed to report the data of lever presses per hour of these animals, making it difficult to differentiate between cocaine reward parameters and cocaine seeking behavior. Interestingly, (Sarnyai et al., 1992), showed that repeated treatment with AVP did not affect cocaine induced locomotor activity. In contrast, AVP dose dependently attenuated the development of sensitization to the hypermotility-inducing effects of cocaine. However, the protocol used is different from our protocol because they injected cocaine (7.5 mg/kg, s.c.) 10 minutes before placing the animals in the locomotor activity box and measured the effects for 5 minutes. Our previous findings (Rodriguez-Borrero et al., 2006) showed that the major locomotor activating effects of cocaine came in the first 30-40 minutes of the injection. Moreover, the use of s.c. injections of AVP or its analogs in those study raised the question of multiple receptor activation effects on drug SA or behavioral responses.
Our results of the stimulatory action of AVP in environment-elicited cocaine-conditioning seem to be in contrast to the data from the previous studies where AVP and its analogs decreased the cocaine response. In our study, we measured the effects of AVP on the acquisition and expression of the environment-elicited cocaine-conditioning response using a paradigm that allowed us to measure the response to cocaine injection before any other pharmacological manipulation. Also, we used rats instead of mice, different pharmacological doses of AVP and a different treatment schedule. Neither MC nor AVP 4-9 within the NAc affected the locomotor stimulating effects of cocaine on pre or post-conditioning injected experimental subjects. Therefore, the use of specific brain site microinjection allowed us to isolate specific interactions between AVP, cocaine and the NAc. Moreover, the fact that AVP gene was regulated in different stages of our cocaine conditioning paradigm suggested that AVP may play a role within the NAc in the acquisition and expression of the environment elicited cocaine conditioning. Interestingly, it was demonstrated that cocaine increased aggression behavior in adolescent Syrian Hamsters in an AVP dependant manner within the hypothalamus (Jackson et al., 2005). Furthermore, they demonstrated that cocaine treatment increases AVP release within the hypothalamus. This finding suggested that cocaine could indeed affect the expression of AVP on brain specific sites as shown in our results. This evidence supports our findings that specific brain region manipulations are necessary to establish a correlation between cocaine-AVP interactions in the paradigm under study.
Our study also revealed that the AVP antagonist, MC, decreased the cocaine conditioned response and its effects were more pronounced in the post-conditioning treatment group. We speculated that MC is blocking the synaptic events that take place after the cocaine-conditioning session that are necessary for expression of this conditioning. Furthermore, the agonist, AVP 4-9, increased the expression of the conditioning response. It was previously shown that rats receiving a post-conditioning injection of AVP found a water tube faster than saline injected in a novel open field environment (Ettenberg et al., 1983a), an effect that is reversed by the use of an AVP antagonist (Ettenberg et al 1983b). Recently, it was also demonstrated that NC-1900, a novel AVP 4-9 fragment analog, facilitated memory retention and retrieval in a step-through-type passive avoidance test (Sato et al., 2004). These authors reported that post-conditioning injection of NC-1900 facilitated memory retention. Moreover, an injection of NC-1900 administered 10 minutes before the testing session facilitated the memory retrieval of this behavioral test. These data support our findings that post-conditioning microinjection seemed to be more effective on the expression of the cocaine conditioned response.
More recent findings (Zhou et al., 2005), showed that AVP mRNA increased in acute (3hr) and sub-acute (24hr) cocaine withdrawal within the amygdala in an opioid dependent manner. In this study, no AVP mRNA was found on chronic (10 days) withdrawal or when naloxone was administered after the cocaine injection. Thus, these findings proposed a potential role for amygdalar AVP in the aversive consequences of early cocaine withdrawal. Interestingly, opioids mechanism has been related to cocaine withdrawal. Specifically, there is evidence that showed that expression of Dyn A, a kappa opioid receptor endogenous ligand, within the NAc shell enhanced the aversive effects of cocaine injection by increasing the aversive valence of cocaine exposure (Carlezon Jr. et al., 1998). Therefore, we can speculate that AVP within the NAc could also be involved in the learning of the aversive effects during drug withdrawal such as hyperlocomotion and drug seeking behavior. It has long been recognized that the cocaine abuser avoids this aversive state (Kampman et al., 2004) and increases drug intake, thus becoming more susceptive developing addiction. The fact that MC reduced the locomotor activity in cocaine conditioned subjects suggested that we maybe have blocked this aversive effect driven by cocaine within the NAc. Therefore, our pharmacological findings suggest that AVP may be mediating learning of the aversive effects during drug withdrawal. This is of particular interest because AVP could be proposed as a pharmacological target for treating the aversive effects of cocaine withdrawal and conditioning.
Table 6.
This table summarize results from the mPFC acquisition treatment (session 5) analyzed by gcRMA
| Gene Title | Fold Change |
|---|---|
| latent transforming growth factor beta binding protein 2 | 3.2 |
| carbonic anhydrase 3 | 2 |
| Glutamine synthetase (glutamate-ammonia ligase) | 2.3 |
| ESTs, Highly similar to stomatin-like protein 2 [Mus musculus] [M.musculus] | −2.1 |
| ESTs, Weakly similar to FMOD_RAT Fibromodulin precursor (FM) [R.norvegicus] | −2 |
| ESTs | 2.9 |
| ESTs | 2.6 |
| ESTs | −2.9 |
| ESTs | −2.2 |
| ESTs | −2.8 |
| synaptic vesicle glycoprotein 2 b | 2.8 |
| ESTs | 2.1 |
| ESTs | −3.8 |
| ESTs, Weakly similar to AJ18 protein [Rattus norvegicus] [R.norvegicus] | −2.3 |
| ESTs | 3.6 |
| ESTs | 2.2 |
| ESTs, Weakly similar to T03096 CDO protein - rat [R.norvegicus] | −2.9 |
| ESTs | 2.5 |
| ESTs, Highly similar to Notch gene homolog 2, (Drosophila) [Rattus norvegicus] [R.norvegicus] |
−2.1 |
| ESTs | −2.4 |
| ESTs | −3.3 |
| ESTs | 2.9 |
| ESTs, Highly similar to; protein transport protein SEC61 beta subunit [Homo sapiens] | −2.4 |
| ESTs, Highly similar to RETB_RAT Plasma retinol-binding protein precursor (PRBP) (RBP) | −2.1 |
| myelin-associated oligodendrocytic basic protein | 2.1 |
| N-ethylmaleimide sensitive factor | 2.3 |
| growth differentiation factor 8 | −2.1 |
| solute carrier family 17 (sodium-dependent inorganic phosphate cotransporter), member 7 | 2.6 |
| cadherin 22 | 2.5 |
| Myelin basic protein | 2.8 |
Table 7.
This table summarize results from the mPFC chronic treatment (session 10) analyzed by gcRMA.
| Gene Title | Fold Change |
|---|---|
| Rattus norvegicus Transthyretin (prealbumin, amyloidosis type I) (Ttr), mRNA. | 7.6 |
| ESTs | 4.9 |
| ESTs, Highly similar to gene rich cluster, C9 gene [Mus musculus] [M.musculus] | 4.2 |
| ESTs, Highly similar to RPB5_HUMAN DNA-directed RNA polymerase II 23 kDa polypeptide |
2.1 |
| Rattus norvegicus macrophage-derived chemokine mRNA, partial cds. | −3.4 |
| Rattus norvegicus zinc finger protein HIT-4 mRNA, complete cds. | 3.1 |
| ESTs, Moderately similar to protein tyrosine phosphatase 4a1 [Rattus norvegicus] | 3.1 |
| Rattus norvegicus smooth muscle cell LIM protein (SmLIM) mRNA, complete cds. | 4 |
| ESTs | −3.3 |
| Rattus norvegicus mRNA for phosphatidylinositol 3-kinase p45 subunit, complete cds. | 2.6 |
| ESTs, Highly similar to BAG2_HUMAN BAG-family molecular chaperone regulator-2 | −3.4 |
| ESTs, Moderately similar to RIKEN cDNA 0610009O03 [Mus musculus] | 2.6 |
| ESTs, Moderately similar to tyrosyl-DNA phosphodiesterase [Homo sapiens] | 2.2 |
| Rattus norvegicus leukotriene C4 synthase (Ltc4s), mRNA. | 2.1 |
Acknowledgements
This research was supported by SCoRE S06GM 5S06M08102 to CSMV, ERB. JM was supported by RISE R25 GM6-1151-01. No financial involvement of any of the authors has biased the present investigation. We also would like to thank Dr. Harold Gainer for PS-38 and the PS41 antibodies and Dr. Maurice Manning for the AVP receptor antagonist.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ben-Barak Y, Russell J, Whitnall M, Ozato K, Gainer H. Neurophysin in the hypothalamo-neurohypophysial system. I. Production and characterization of monoclonal antibodies. J Neuroscience. 1985;5:81–97. doi: 10.1523/JNEUROSCI.05-01-00081.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb J, Nishi A, O’Callaghan J, Ule J, Lan M, Snyder G, Horiuchi A, Saito T, Hisanaga S, Andrew J, Czernik A, Nairn A, Greengard P. Phosphorylation of Protein Phosphatase Inhibitor-1 by Cdk5. J. Biol. Chem. 2001;276:14490–97. doi: 10.1074/jbc.M007197200. [DOI] [PubMed] [Google Scholar]
- Carlezon WJ, Jr., Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, Duman RS, Neve RL, Nestler E. Regulation of cocaine reward by CREB. Science. 1998;282:2272–75. doi: 10.1126/science.282.5397.2272. [DOI] [PubMed] [Google Scholar]
- Choi DC, Furay AR, Evanson NK, Ostrander MM, Ulrich-Lai YM, Herman JP. Bed Nucleus of the Stria Terminalis Subregions Differentially Regulate Hypothalamic—Pituitary—Adrenal Axis Activity: Implications for the Integration of Limbic Inputs. Journal Neuroscience. 2007;27:2025–2034. doi: 10.1523/JNEUROSCI.4301-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chui J, Kalant H, Le DA. Vasopressin opposes locomotor stimulation by etanol, cocaine and amphetamine in mice. Eur J Pharmacology. 1998;355:11–17. doi: 10.1016/s0014-2999(98)00465-8. [DOI] [PubMed] [Google Scholar]
- Daunais J, McGinty J. The effects of D1 or D2 dopamine receptor blockade on zif/268 and preprodynorphin gene expression in rat forebrain following a short-term cocaine binge. Brain Res Mol Brain Res. 1996;35(12):237–48. doi: 10.1016/0169-328x(95)00226-i. [DOI] [PubMed] [Google Scholar]
- De Vry J, Donselaar I, van Ree JJM. Effects of desglycinamide9, (Arg8) vasopressin and vasopressin antiserum on the acquisition of intravenous cocaine self-administration in the rat. Life Sci. 1988;42(26):2709–15. doi: 10.1016/0024-3205(88)90247-0. [DOI] [PubMed] [Google Scholar]
- Dorsa D, Petracca F, Baskin D, Cornett L. Localization and characterization of vasopressin-binding sites in the amygdala of the rat brain. J Neuroscience. 1984;4:1764–70. doi: 10.1523/JNEUROSCI.04-07-01764.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann M, Wotjak CT, Neumann I, Ludwig M, Landgraf R. Behavioral consequences of intracerebral vasopressin and oxytocin: focus on learning and memory. Neurosci Biobehav Rev. 1996;20(3):341–58. doi: 10.1016/0149-7634(95)00059-3. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Le Moal M, Koob G, Bloom F. Vasopressin potentiation in the performance of a learned appetitive task: reversal by a pressor antagonist analog of vasopressin. Pharmacol Biochem Behav. 1983;18(4):645–7. doi: 10.1016/0091-3057(83)90294-0. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, van der Kooy D, Le Moal M, Koob G, Bloom F. Can aversive properties of (peripherally-injected) vasopressin account for its putative role in memory? Behav Brain Res. 1983;7(3):331–50. doi: 10.1016/0166-4328(83)90024-4. [DOI] [PubMed] [Google Scholar]
- Ferger B, Kuschinsky K. A comparison of different sensory stimuli in producing conditioning apomorphine effects. Behav Pharmacol. 1995;6:40–45. [PubMed] [Google Scholar]
- Freeman WM, Brebner K, Lynch W, Patel KM, Robertson DJ, Roberts DCS, Vrana KE. Changes in rat frontal cortex gene expression following chronic cocaine. Molecular Brain Research. 2002;104:11–20. doi: 10.1016/s0169-328x(02)00197-3. [DOI] [PubMed] [Google Scholar]
- Freeman WM, Nader MA, Nader SH, Robertson DJ, Gioia L, Mitchell SM, Daunais JB, Porrino LJ, Friedman DP, Vrana KE. Chronic cocaine-mediated changes in non-human primate NA gene expression. J Neurochemistry. 2001;77:542–549. doi: 10.1046/j.1471-4159.2001.00252.x. [DOI] [PubMed] [Google Scholar]
- Goeders N. Stress and Cocaine Addiction. Journal Pharmacology and Experimental Therapeutics. 2002a;301:785–789. doi: 10.1124/jpet.301.3.785. [DOI] [PubMed] [Google Scholar]
- Goeders N. The HPA axis and Cocaine Reinforcement. Psychoneuroendocrinology. 2002b;27(12):13–33. doi: 10.1016/s0306-4530(01)00034-8. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- Insel T, Wang Z, Ferris C. Patterns of brain vasopressin receptor distribution associated with social organization in microtine rodents. J. Neuroscience. 1994;14:5381–92. doi: 10.1523/JNEUROSCI.14-09-05381.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D, Burns R, Trksak G, Simeone B, DeLeon K, Connor D, Harrison RJ, Melloni R. Anterior hypothalamic vasopressin modulates the aggression-stimulating effects of adolescent cocaine exposure in Syrian hamsters. Neuroscience. 2005;133(3):635–46. doi: 10.1016/j.neuroscience.2005.02.047. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Pettinati HM, Volpicelli JR, Oslin DM, Lipkin C, Sparkman T, O’Brien CP. Cocaine dependence severity predicts outcome in outpatient detoxification from cocaine and alcohol. Am J Addict. 2004;13(1):74–82. doi: 10.1080/10550490490265389. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Young LJ, Gonen D, Veenstra-VanderWeele J, Courchesne R, Courchesne E, Lord C, Leventhal BL, Cook EH, Jr, Insel TR. Transmission disequilibrium testing of arginine vasopressin receptor 1A (AVPR1A) polymorphisms in autism. Mol Psychiatry. 2002;7(5):503–7. doi: 10.1038/sj.mp.4001125. [DOI] [PubMed] [Google Scholar]
- Kruszynski M, Lammek B, Manning M, Seto J, Haldar J, Sawyer WH. Two highly potent antagonist of the vasopressor response to Arginine Vasopressin. J.Med. Chem. 1980;23:364–368. doi: 10.1021/jm00178a003. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Kessler MS, Bunck M, Murgatroyd C, Spengler D, Zimbelmann M, Nussbaumer M, Czibere L, Turck CW, Singewald N, Rujescu D, Frank E. Candidate genes of anxiety-related behavior in HAB/LAB rats and mice: focus on vasopressin and glycoxalase-I. Neuroscience Biobehavior Review. 2007;31(1):89–102. doi: 10.1016/j.neubiorev.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Lim MM, Murphy AZ, Young LJ. Ventral striatopallidal oxytocin and vasopressin V1a receptors in the monogamous prairie vole (Microtus ochrogaster) J Comp Neurol. 2004;468(4):555–70. doi: 10.1002/cne.10973. [DOI] [PubMed] [Google Scholar]
- Miller MA, DeVries GJ, al-Shamma HA, Dorsa DM. Decline of vasopressin immunoreactivity and mRNA levels in the bed nucleus of the stria terminalis following castration. J. Neuroscience. 1992;12:2881–2887. doi: 10.1523/JNEUROSCI.12-08-02881.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr E, Richter D. Subcellular vasopressin mRNA trafficking and local translation in dendrites. Journal Neuroendocrinology. 2004;16(4):333–339. doi: 10.1111/j.0953-8194.2004.01176.x. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular Basis of Long Term Plasticity Underlying Addiction. Nat. Rev Neuroscience. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Nestler E, Barrot M, Self D. ΔFosB: A sustained molecular switch for addiction. PNAS. 2001;98:11042–46. doi: 10.1073/pnas.191352698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. The neurobiology of cocaine addiction. Sci Pract Perspect. 2005;3(1):4–10. doi: 10.1151/spp05314. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Transcriptional mechanisms of addiction: role of DeltaFosB. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3245–55. doi: 10.1098/rstb.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paban V, Alescio-Lautier B, Devigne C, Soumireu-Mourat B. The behavioral effect of vasopressin in the ventral hippocampus is antagonized by an oxytocin receptor antagonist. Eur J Pharmacol. 1998;361(23):165–73. doi: 10.1016/s0014-2999(98)00704-3. [DOI] [PubMed] [Google Scholar]
- Paban V, Alescio-Lautier B, Devigne C, Soumireu-Mourat B. Fos protein expression induced by intracerebroventricular injection of vasopressin in unconditioned and conditioned mice. Brain Res. 1999;17:115–131. doi: 10.1016/s0006-8993(99)01232-9. [DOI] [PubMed] [Google Scholar]
- Pulipparacharuvil S, Renthal W, Hale CF, Taniguchi M, Xiao G, Kumar A, Russo SJ, Sikder D, Dewey CM, Davis MM, Greengard P, Nairn AC, Nestler EJ, Cowan CW. Cocaine regulates MEF2 to control synaptic and behavioral plasticity. Neuron. 2008;59(4):621–33. doi: 10.1016/j.neuron.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego: 1998. [DOI] [PubMed] [Google Scholar]
- Pitkow L, Sharer C, Ren J, Insel T, Terwilliger E, Young L. Facilitation of Affiliation and Pair-Bond Formation by Vasopressin Receptor Gene Transfer into the Ventral Forebrain of a Monogamous Vole. J. Neuroscience. 2001;21(18):7392–96. doi: 10.1523/JNEUROSCI.21-18-07392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Ortolaza DL, Negron A, Cruz D, Falcon E, Iturbe MC, Hernandez-Cajigas M, Maldonado-Vlaar CS. Intra-accumbens injections of Neurotensin receptors antagonist SR48692 enhanced cocaine self-administration intake in rats exposed to an environmentally elicited reinstatement paradigm. Brain Research. 2009;1280:124–136. doi: 10.1016/j.brainres.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Borrero E, Bernardo-Colon A, Burgos-Martir M, Alvarez-Carrillo JE, Del Campo-Estrada Y, Abella-Ramirez C, Maldonado-Vlaar CS. NMDA antagonist AP-5 increase environmentally induced cocaine-conditioned locomotion within the nucleus accumbens. Pharmacology, Biochemistry and Behavior. 2006;85(1):178–184. doi: 10.1016/j.pbb.2006.07.034. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Szabo G, Kovacs G, Telegdy G. Opposite actions of oxytocin and vasopressin in the development of cocaine-induced behavioral sensitization in mice. Pharmacol Biochem Behav. 1992;43(2):491–4. doi: 10.1016/0091-3057(92)90182-f. [DOI] [PubMed] [Google Scholar]
- Sato T, Tanaka K, Teramoto T, Ohnishi Y, Hirate K, Irifune M, Nishikawa T. Facilitative effect of a novel AVP fragment analog, NC-1900, on memory retention and recall in mice. Peptides. 2004;25(7):1139–46. doi: 10.1016/j.peptides.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Thibonnier M, Conarty D, Preston J, Wilkins P, Berti-Mattera L, Mattera R. Molecular pharmacology of human vasopressin receptors. Adv Exp Med Biol. 1998;449:251–76. doi: 10.1007/978-1-4615-4871-3_34. [DOI] [PubMed] [Google Scholar]
- Toda S, McGinty JF, Kalivas PW. Repeated cocaine administration alters the expression of genes in corticolimbic circuitry after a 3-week withdrawal: a DNA macroarray study. J. Neurochemistry. 2002;82:1290–1299. doi: 10.1046/j.1471-4159.2002.01083.x. [DOI] [PubMed] [Google Scholar]
- Trembleau A, Morales M, Bloom FE. Aggregation of vasopressin mRNA in a subset of axonal swellings of the median eminence and posterior pituitary: light and electron microscopic evidence. Journal Neuroscience. 1994;14:39–53. doi: 10.1523/JNEUROSCI.14-01-00039.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropea TF, Kosofsky BE, Rajadhyaksha AM. Enhanced CREB and DARPP-32 phosphorylation in the nucleus accumbens and CREB, ERK, and GluR1 phosphorylation in the dorsal hippocampus is associated with cocaine-conditioned place preference behavior. Journal Neurochemistry. 2008 Aug;106(4):1780–90. doi: 10.1111/j.1471-4159.2008.05518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ree J, Burbach-Bloemarts E, Wallace M. Vasopressin neuropeptides and acquisition of heroin and cocaine self-administration in rats. Life Sci. 1988;42(10):1091–9. doi: 10.1016/0024-3205(88)90565-6. [DOI] [PubMed] [Google Scholar]
- Verbalis JG. Whole-body volume regulation and escape from antidiuresis. Am J Med. 2006;119(7 Suppl 1):S21–9. doi: 10.1016/j.amjmed.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Whitnall MH, Key S, Ben-Barak Y, Ozato K, Gainer H. Neurophysin in the hypothalamo-neurohypophysial system. II. Immunocytochemical studies of the ontogeny of oxytocinergic and vasopressinergic neurons. J. Neurosciense. 1985;5:98–109. doi: 10.1523/JNEUROSCI.05-01-00098.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Zhang L, Lou D, Nakabeppu Y, Zhang J, Xu M. The Dopamine D1 receptor is a critical mediator for cocaine-induced gene expression. Journal of Neurochemistry. 2002a;82:1453–1464. doi: 10.1046/j.1471-4159.2002.01089.x. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhang D, Xu M. Identification of Chronic Cocaine-Induced Gene Expression Through Dopamine D1 Receptors by Using cDNA Microarrays. Ann. N.Y. Acad. Sci. 2002b;965:1–9. doi: 10.1111/j.1749-6632.2002.tb04146.x. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Bendor JT, Yuferov V, Schlussman SD, Ho A, Kreek MJ. Amygdalar vasopressin mRNA increases in acute cocaine withdrawal: evidence for opioid receptor modulation. Neuroscience. 2005;134(4):1391–7. doi: 10.1016/j.neuroscience.2005.05.032. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Leri F, Cummins E, Hoeschele M, Kreek MJ. Involvement of arginine vasopressin and V1b receptor in heroin withdrawal and heroin seeking precipitated by stress and by heroin. Neuropsychopharmacology. 2008;33(2):226–36. doi: 10.1038/sj.npp.1301419. [DOI] [PubMed] [Google Scholar]
- Ziegler D, Herman J. Neurocircuitry of Stress Integration: Anatomical Pathways Regulating the Hypothalamo-Pituitary-Adrenocortical Axis of the Rat. Integr. Comp. Biol. 2002;42:541–551. doi: 10.1093/icb/42.3.541. [DOI] [PubMed] [Google Scholar]