Abstract
Background
Lens cataract is associated with protein oxidation and aggregation. Two proteins that cause cataract when deleted from the lens are methionine sulfoxide reductase A (MsrA) that repairs protein methionine sulfoxide (PMSO) oxidized proteins and α-crystallin which is a two subunit (αA and αB) chaperone. Here, we tested whether PMSO formation damages α-crystallin chaperone function and whether MsrA could repair PMSO-α-crystallin.
Methods
Total α-crystallin was oxidized to PMSO and evaluated by CNBr-cleavage and mass spectrometry. Chaperone activity was measured by light scattering using lysozyme as target. PMSO-α-crystallin was treated with MsrA, and repair was assessed by CNBr cleavage, mass spectrometry and recovery of chaperone function. The levels of α-crystallin-PMSO in the lenses of MsrA-knockout relative to wild-type mice were determined.
Results
PMSO oxidation of total α-crystallin (met 138 of αA and met 68 of αB) resulted in loss of α-crystallin chaperone activity. MsrA treatment of PMSO-α-crystallin repaired its chaperone activity through reduction of PMSO. Deletion of MsrA in mice resulted in increased levels of PMSO-α-crystallin.
Conclusions
Methionine oxidation damages α-crystallin chaperone function and MsrA can repair PMSO-α-crystallin restoring its chaperone function. MsrA is required for maintaining the reduced state of α-crystallin methionines in the lens.
Significance
Methionine oxidation of α-crystallin in combination with loss of MsrA repair causes loss of α-crystallin chaperone function. Since increased PMSO levels and loss of α-crystallin function are hallmarks of cataract, these results provide insight into the mechanisms of cataract development and likely those of other age-related diseases.
Keywords: Methionine sulfoxide reductase, α-crystallin, Cataract, Chaperone activity, Oxidative stress
Introduction
α-crystallin is a major structural eye lens protein representing as much as 50% of total soluble lens protein [1]. In addition to its structural and refractive role in the lens, α-crystallin is a molecular chaperone [2–3] that functions to prevent protein aggregation [1]. α-crystallin has also been implicated in apoptotic control and cell survival [4]. In its native state in the lens, α-crystallin consists of two subunits called αA- and αB-crystallin that consist of 173 and 175 amino acids respectively. αA- and αB-crystallin share as much as 57% homology and exist in the lens in a 3:1 ratio [5]. Importantly, deletion of αA-crystallin results in cataract formation in mice [6] and mutation of αA-crystallin (R116C) causes cataract in humans [7]. Mutation of αB-crystallin (R120G) causes cataract and desmin-related myopathy in humans [8].
A key oxidation that is also associated with a multitude of age-related diseases is oxidation of protein methionines to protein methionine sulfoxide (PMSO). Methionine and cysteine residues are some of the most susceptible protein amino acids to oxidation and are the first to be oxidized by almost all forms of reactive oxygen species (ROS) under oxidative stress conditions. Formation of PMSO can lead to significant changes in protein structure, loss of regulatory function and loss of or in some cases increased activity [9]. Since the chaperone function of α-crystallin is lost upon oxidative conditions [10–11], specific oxidation of α-crystallin methionines to PMSO could result in loss of chaperone function thereby contributing to protein aggregation and ultimately the development of cataract and other α-crystallin-associated diseases. One unique feature of methionine oxidation is that, unlike many irreversible protein oxidations, PMSO formation can be repaired by a novel class of enzymes called methionine sulfoxide reductases (Msrs) [12]. The Msr family consists of two specific enzyme activities and four separate enzymes called MsrA and MsrB1, B2 and B3. MsrA is believed to be selective for the reduction of S-epimers of PMSO while the MsrBs are believed to be selective for the R-epimers that arise from random symmetrical oxidation of methionine [12]. MsrA acts as a powerful antioxidant by catalyzing the thioredoxin-dependent reduction of free and protein bound methionine sulfoxide back to reduced methionine. Thioredoxin is recycled by NADPH and thioredoxin reductase [13].
In the eye lens, MsrA has been localized to the lens epithelium and fiber cells [14]. Its over-expression protects lens epithelial cells against oxidative stress [14]. Conversely, its deletion makes lens cells more sensitive to oxidative stress [14], results in increased lens ROS levels, loss of mitochondrial function [15] and lens cataract in hyperbaric oxygen-treated mice [16].
Accumulation of methionine oxidized lens proteins increases in the human lens with age and is a key characteristic of age-related cataract where as much as 60% of total protein methionine is found as PMSO [17–18]. These results suggest that loss of Msr activity plays a major role in lens aging and cataract formation.
Several studies have directly tied α-crystallin methionine oxidation to age-related cataract formation. It has been demonstrated that methionine and tryptophan residues of α-crystallin become oxidized following incubation with 3-hydroxykynurenine, a lens pigment and UV filter, which increases with age [19]. It has also been shown that methionines of αA- and αB-crystallins are oxidized in clear middle-aged (45–65) human lenses [20–21]. Importantly, αA-crystallin is found to be oxidized to methionine sulfoxide in rat hereditary cataracts [22] and interestingly, substitution of methionine 68 in αB-crystallin with a less hydrophobic residue (Thr) leads to loss of chaperone activity [23]. These data provide evidence that methionine oxidation plays a key role in cataract formation through loss of α-crystallin chaperone function. Msr repair of methionine oxidized α-crystallin would therefore be expected to restore α-crystallin chaperone function thereby maintaining lens clarity. Lens cataract could occur as a result of protein aggregation caused by loss of α-crystallin chaperone function arising from age-dependent methionine oxidation with concomitant loss of MsrA repair.
Based on these previous studies we hypothesized that methionine oxidation of α-crystallin could lead to loss of chaperone activity and that MsrA could restore the chaperone activity of methionine oxidized α-crystallin. To test this hypothesis we oxidized α-crystallin at specific methionines, we tested the chaperone activity of the methionine oxidized α-crystallin and we evaluated the ability of MsrA to repair the methionine oxidized α-crystallin and restore its lost chaperone activity. We also evaluated whether MsrA and α-crystallin d interact in lens cells in vivo and we examined the presence of methionine oxidized α-crystallin in the lenses of MsrA knockout relative to wild-type mice. Our data provide evidence that methionine oxidation damages α-crystallin chaperone activity and that MsrA can repair oxidized α-crystallin methionines and restore the chaperone function of α-crystallin. We also provide evidence that MsrA and α-crystallin interact in lens cells and that at least methionine 68 of αB-crystallin is oxidized to PMSO in the actual lens, upon deletion of MsrA in mice. These results are consistent with an essential role for MsrA in the maintenance of lens α-crystallin chaperone function. Loss of MsrA activity upon aging or environmental insult could, therefore, result in loss of α-crystallin chaperone function and contribute to the development of cataract and other age-related oxidative stress associated disorders involving α-crystallin including desmin-related myopathy [8], age-related macular degeneration [24], Lewy body disease [25–26], Parkinson’s disease [27] and Alzheimer’s disease [28].
Methods
HLE B3 cell culture
Human lens epithelial cells (HLE B3) were grown and cultured in DMEM (Invitrogen) supplemented with 15% FBS (Invitrogen), gentamicin (50 units/ml; Invitrogen), penicillin-streptomycin antibiotic mix (50 units/ml; Invitrogen) and fungizone (5ul/ml; Invitrogen) at 37 °C in the presence of 5% CO2.
Immunoprecipitations
An MsrA specific antibody (Abcam, Cambridge, MA) was conjugated to NHS activated beads (Covance Research Products, Princeton, NJ). 500 μg of mitochondrial fraction from HLE cells was incubated overnight at 4 °C in a test tube rotator at a concentration of 1 mg total protein per 100 μg antibody. Beads were collected by centrifugation at 1,000 × g for 1 minute and washed 4 x with 1 x PBS 1 mM n-dodecyl-β-D-maltopyranoside (nDMP). Protein was eluted by incubating for 10 minutes in 0.2 M Glycine 1 mM n-DMP buffer pH 2.5 with frequent agitation. Elution was repeated 3 times prior to washing the beads and storing them in PBS 0.05% sodium azide for future use. Eluted protein was analyzed by SDS-PAGE and Western blotting with an MsrA-specific antibody at 1:2,000 and an α-crystallin-specific antibody at 1:20,000.
500 μg of HLE protein mitochondrial fraction was incubated with 20 μg anti-αAB-crystallin antibody (Stressgen) for 1 h at 4 °C while rotating. The mixture was then added to 25 μl packed Protein A-agarose beads (Sigma-Aldrich, St Louis, MO) pre-equilibrated with RIPA buffer (50mM Tris-HCl pH 7.4, 150mM NaCl, 1mM EDTA, 1% Triton X-100, 1% Sodium deoxycholate, 0.1% SDS) and mixed by rotation at 4 °C overnight. The beads were then collected by centrifugation at 8000 rpm for 30 seconds in a microcentrifuge. The supernatant was saved for further analysis. The beads were washed 3 times in 1 ml 1x PBS 1mM n-DMP (Sigma-Aldrich). Protein was eluted by boiling for 10 minutes in 40 μl 2x SDS loading buffer. Eluted protein was analyzed by SDS-PAGE and western blotting as described above.
Identification of methionine oxidations of α-crystallin
Cyanogen bromide (CNBr) hydrolyzes peptide bonds at the C-terminus of methionine residues; it does not recognize methionine sulfoxide, the oxidized form of methionine, resulting in lack of cleavage at methionine sulfoxide residues. Thus, CNBr cleavage is a sensitive detection method to identify methionine sulfoxide in proteins [29]. Bovine total α-crystallin (Sigma-Aldrich) (800 μg) was incubated with hypochlorous acid (HOCl; Sigma-Aldrich) (1.2 mM) at room temperature for 15 minutes to convert methionines to methionine sulfoxide. Where indicated, methionine sulfoxide was repaired by MsrA using DTT as a reducing agent for the enzyme. Briefly, DTT (15 mM) was added to the oxidized α-crystallin for 15 min followed by MsrA (1.2 mM) for 1 h at 37 °C. Samples were dried on a vacuum centrifuge and then subjected or not to CNBr cleavage. Incubation with DTT alone at these conditions did not reduce oxidized methionines of α-crystallin (data not shown). Treated or untreated α-crystallin protein was then diluted in 70% formic acid to 10–20 mg/ml as required by the standard CNBr protocol [29]. CNBr (Sigma-Aldrich) was added in a 2:1 w/w ratio and the reaction mixture incubated at room temperature for 20 h in the dark. The reaction was terminated by addition of 5 volumes ddH2O and 5 volumes 1 M ammonium bicarbonate. Samples were then concentrated using an Amicon stirred ultrafiltration cell. 5 μg of treated or untreated α-crystallin protein was run on a 17% SDS-PAGE gel. The gels were stained with Colloidal blue for 4 h and de-stained in ddH2O overnight.
Chaperone function of oxidized and repaired α-crystallin
Chaperone activity was assayed by aggregation of lysozyme (Sigma-Aldrich) in the presence or absence of wild type, oxidized and oxidized and repaired α-crystallin. The ability of α-crystallin to prevent DTT-induced (20 mM) aggregation of lysozyme at 37 °C was monitored by measuring light scattering at 360 nm as a function of time in a Shimadzu UV 1700 spectrophotometer (Columbia, MD) equipped with a temperature regulated cell holder. Lysozyme is destabilized by reduction of its disulphide bonds using DTT [30]. The α-crystallin target protein ratio was 1:1 for all samples. For oxidation of α-crystallin the protein was incubated with HOCl in a 1:6 molar ratio at room temperature for 15 minutes. To terminate the reaction, 5 volumes of 50 mM sodium phosphate buffer pH 7.3 were added and the samples concentrated on an Amicon stirred ultrafiltration cell (Amicon, Millipore, Bedford, MA) to remove HOCl. We compared pre and post ultrafiltration samples using the chaperone assays and results indicate that post ultrafiltration most of the HOCl is removed although quantization of HOCl levels was not possible. Oxidized α-crystallin (3.6 μM) was repaired using 60 nM MsrA for 2 h at 37 °C. Oxidation and repair of α-crystallin was confirmed by mass spectrometry by separating 2 μg of incubated α-crystallins on a C4 column, on-line analysis by electrospray ionization mass spectrometry, and deconvolution of the resulting spectra was carried out as previously described [31], except using 0.1% formic acid in the mobile phase and a 20 μl/min flow rate.
Detection of oxidized methionines in α-crystallin in vivo
MsrA knockout (C57 Black 6, C57BL/6) and wild type mice (B6129SF2/J strain) were sacrificed at 6 months and the eyes enucleated. Lenses were microdissected and the cortical and nuclear fractions frozen on liquid nitrogen prior to lyphilization. Lens proteins from each fraction were isolated by reconstitution in PBS, fractions were centrifuged at 10,000 g for 30 min at 4 °C and the supernatant collected. Soluble lens protein extracted in this way was subjected to CNBr cleavage and α-crystallin analyzed by western blotting. Lens protein was diluted in 70% formic acid to 10–20 mg/ml as required by the standard CNBr protocol [29]. CNBr (Sigma-Aldrich) was added in a 2:1 w/w ratio and the reaction mix incubated at room temperature for 20 hours in the dark. The reaction was terminated by addition of 5 volumes ddH2O and 5 volumes 1 M ammonium bicarbonate. Samples were then concentrated using an Amicon stirred ultrafiltration cell. 5 μg of treated or untreated α-crystallin protein was run on a 17% SDS-PAGE gel. SDS-PAGE and western blotting were carried out as previously described [16]. Western blots of CNBr cleaved lens proteins were analyzed using a 1:20,000 dilution of anti-αAB -crystallin antibody (Stressgen).
Results
MsrA repairs methionine oxidized α-crystallin
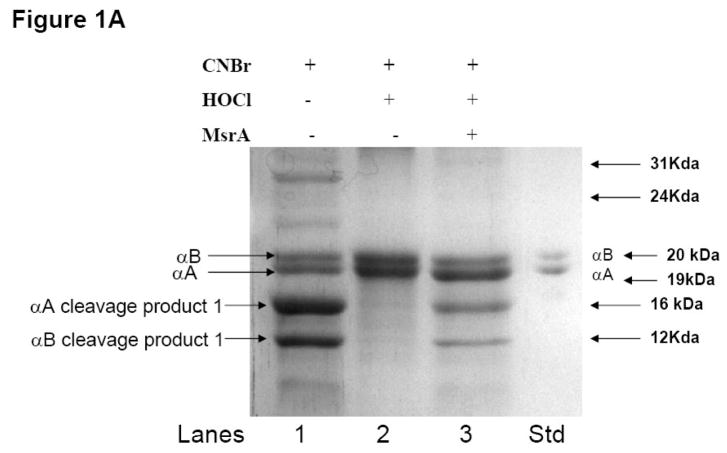
Hypochlorous acid (HOCl) is an oxidant that preferentially targets methionine residues in proteins [32]. HOCl has previously been shown to oxidize key methionine residues in cytochrome c [16, 33] as well as methionines of actin [34], myoglobin [35] and cathepsin G [36]. To detect methionine sulfoxide residues in total α-crystallin, we used CNBr to specifically cleave methionines and visualized the cleavage products by SDS-PAGE and colloidal blue staining. CNBr hydrolyses peptide bonds at the COOH-terminus of methionine, but does not cleave the oxidized methionine sulfoxide product of HOCl-treatment. To determine if HOCl-treated α-crystallin could be repaired by MsrA, the samples were subsequently incubated in the presence or absence of MsrA and DTT as a reducing system.
SDS-PAGE analysis of CNBr cleaved untreated α-crystallin (Figure 1, lane 1) produced 4 bands, the original parent αA-crystallin (19 kDa) and αB-crystallin (20 kDa) bands and two CNBr cleavage products with molecular weights of approximately 16 kDa and 12 kDa. These bands are consistent with CNBr cleavage at methionines 138 of αA- and 68 of αB-crystallin, respectively. The two smaller predicted cleavage products of 3.7 kDa (αA-crystallin) and 7.8 kDa (αB-crystallin) were not detectable on the 17% SDS-PAGE gel as they were likely lost during ultrafiltration on the 10 kDa cut off membrane. Oxidation of α-crystallin with HOCl (1.2 mM for 15 min at room temperature) led to loss of cleavage by CNBr indicating the presence of oxidized methionines and resulting in the detection of only αA-crystallin and αB-crystallin parent bands (Figure 1, lane 2). Incubation of the oxidized α-crystallin with MsrA (1.2 mM for 2 h at 37 °C) repaired the oxidized methionines as evidenced by the detection of cleavage products missing in the oxidized form (Figure 1, lane 3). Based on the intensity of the bands in the repaired samples (compare Figure 1, lanes 1 and 3), the repair of methionine oxidized α-crystallin was not 100%. This is to be expected since at most 50% of oxidized methionines would be expected to be in the S-form of PMSO that MsrA is believed to selectively recognize, and the reaction is unlikely to be 100% efficient. No effect was detected with DTT alone as shown in Figure 1B indicating that DTT at the concentration employed can not reduce PMSO in the absence of MsrA.
Figure 1.
Figure 1A. MsrA repairs α-crystallin oxidized by HOCl in vitro.
Colloidal blue staining of SDS-PAGE of α-crystallin (5 μg) following CNBr cleavage. Lane 1 – untreated α-crystallin cleaved with CNBr. Lane 2 – oxidized α-crystallin (800 μg oxidized with 1.2 mM HOCl) cleaved with CNBr. Lane 3 - oxidized α-crystallin incubated with MsrA (1.2 mM) and DTT (5 mM) for 1 hr at 37 °C and cleaved with CNBr. Lane 4 - Total α-crystallin standard.
Figure 1B. DTT alone has no effect on HOCl-induced oxidation of α-crystallin.
Colloidal blue staining of SDS-PAGE of α-crystallin (5 μg) following CNBr cleavage. Lane 1 – untreated α-crystallin cleaved with CNBr. Lane 2 – oxidized α-crystallin (800 μg oxidized with 1.2 mM HOCl) cleaved with CNBr. Lane 3 - oxidized α-crystallin incubated with DTT (5 mM) alone for 1 hr at 37 °C and cleaved with CNBr.
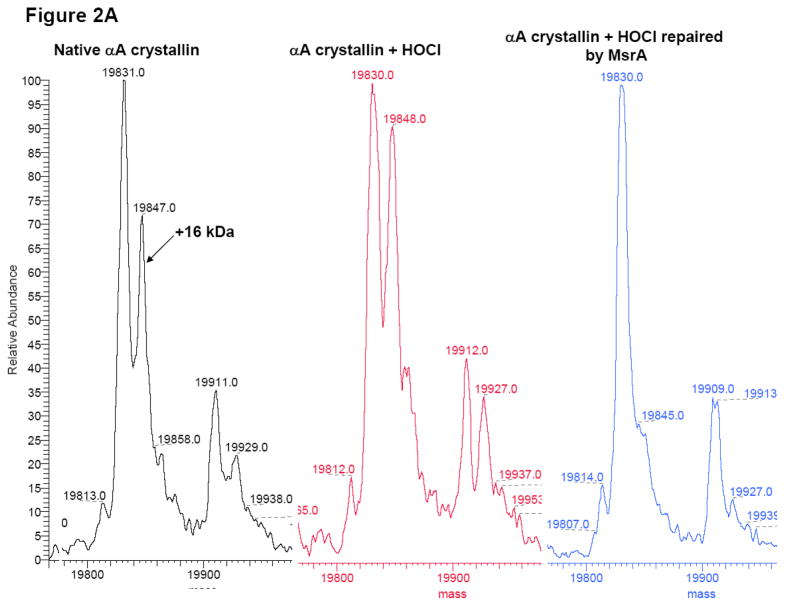
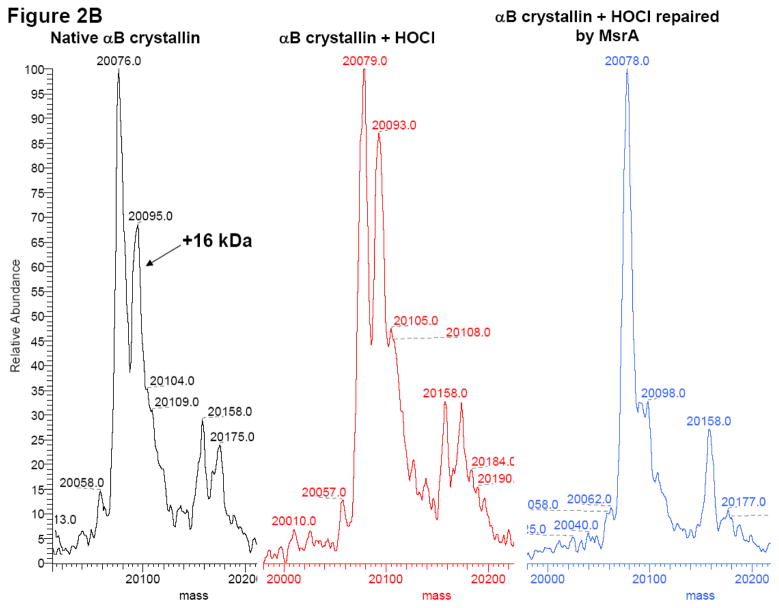
As a secondary conformation, oxidation and repair of α-crystallin was further analyzed by mass spectrometry (Figure 2A and Figure 2B). Total α-crystallin was separated into αA-crystallin and αB-crystallin by reverse-phase chromatography. The deconvoluted electrospray mass spectra of untreated bovine αA-crystallin contained proteins of average molecular weight 19,831 and 19,911, corresponding to the normal and phosphorylated forms of αA-crystallin, respectively (Figure 2A, black spectra). The addition of one oxygen molecule to these species yields molecular weights of 19,847 and 19,929 respectively. This indicated that in the native untreated protein, some endogenously oxidized αA-crystallin occurs. Oxidation of α-crystallin with a 1:6 molar ratio of HOCl for 15 min led to increased levels of methionine oxidation, as evidenced by the increases in the peaks at 19,848 and 19,927 (Figure 2A, red spectra). Incubation of the oxidized α-crystallin (3.6 μM) with 60 nM MsrA for 2 h at 37 °C resulted in a reduction in the oxidized αA-crystallin peaks at 19,845 and 19,927 (Figure 2A, blue spectra). Similar results were observed for αB-crystallin as shown in Figure 2B where the non-phosphorylated and phosphorylated masses of bovine αB-crystallin were 20,076 and 20,158, respectively. The addition of one oxygen molecule to these species yielded molecular weights of 20,095 and 20,175, (Figure 2B, black spectra) respectively. Oxidation with HOCl increased the abundance of these oxidized forms at 20,093 and 20,174 (Figure 2B, red spectra), and treatment MsrA resulted in their near absence (Figure 2B, blue spectra). In contrast, to the approximately 50% repair detected following CNBr cleavage of MsrA repaired α-crystallin (Figure 1A), almost 100% α-crystallin repair was detected for the mono-oxidized form of both αA-crystallin and αB-crystallin repaired by MsrA. This is not predicted on the basis of the previously reported S-PMSO preference of MsrA and we do not know the reason for this disparity. It is possible that the specificity of mouse MsrA used in these studies is different then E. coli or yeast MsrA specificity [12], alternatively this difference could be accounted for by separation and/or detection differences inherent in the mass spec analysis between the two enantiomers of α-crystallin-PMSO. The small differences in molecular weight reported for the various α-crystallin species was due to instrumental error, which was approximately 0.01% of the measured mass.
Figure 2. Deconvoluted ESI mass spectra of HOCl-oxidized α-crystallin.
- Native αA-crystallin (black spectra), αA-crystallin oxidized by HOCl (1:6 total HOCl: α-crystallin for 15 min) (red spectra), oxidized αA-crystallin repaired with MsrA (blue spectra).
- Native αB crystallin (black spectra), αB-crystallin oxidized by HOCl (1:6 total HOCl: α-crystallin for 15 min) (red spectra), oxidized αB-crystallin repaired with MsrA (blue spectra).
MsrA restores the chaperone activity of α-crystallin lost upon methionine oxidation
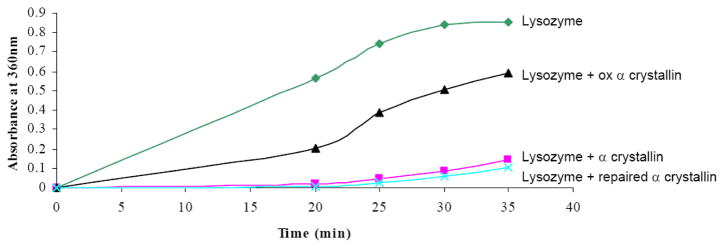
To examine the chaperone activity of total α-crystallin, we tested its ability to prevent chemical denaturation of lysozyme as previously described [1]. Incubation of lysozyme with α-crystallin in a 1:1 ratio protected lysozyme from chemically induced denaturation (Figure 3). Oxidation of α-crystallin led to loss of chaperone activity as evidenced by an approximately 70% decrease in lysozyme protection. Methionine-oxidized α-crystallin (the same 1:6 HOCl: α-crystallin ratio preparations confirmed for methionine oxidation shown in Figure 2A and 2B) treated with MsrA and DTT as a reducing system, exhibited restored chaperone activity to a level approximately the same as untreated α-crystallin (Figure 3). This is consistent with the mass spec data Figure 2A and 2B (black spectra) showing some percent endogenously oxidized αA-crystallin and αB-crystallin in the untreated sample, indicating as predicted less than 100% repair. These results indicate that oxidation of methionines in α-crystallin leads to loss of chaperone activity and that MsrA can repair and restore the chaperone activity of methionine oxidized α-crystallin.
Figure 3. MsrA restores the chaperone activity of total α-crystallin lost upon oxidation with HOCl.
Chaperone activity of α-crystallin using lysozyme as a target. Lysozyme was chemically denatured using 20 mM DTT. Incubation of lysozyme with α-crystallin in a 1:1 ratio protected lysozyme from denaturation. Incubation of lysozyme with HOCl-oxidized α-crystallin in a 1:1 ratio did not protect lysozyme from denaturation. Incubation of HOCl-oxidized α-crystallin with MsrA for 2 h at 37 °C restored the chaperone function of α-crystallin.
MsrA interacts with α-crystallin in lens cells
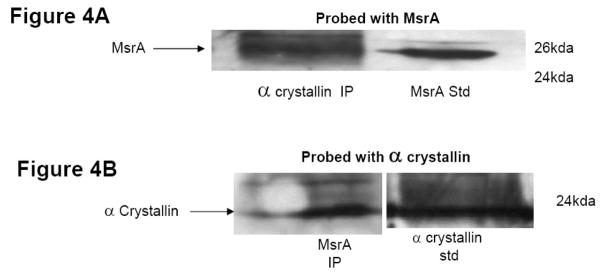
To determine if a physical interaction between MsrA and α-crystallin takes place in lens epithelial cells (HLEs), protein extracts were prepared from cultured human lens HLEB3 cells and co-immunoprecipitation with α-crystallin-specific and MsrA-specific antibodies were carried out. Western blot analysis of HLE cytosolic extracts immunoprecipitated with an α-crystallin antibody and probed with an MsrA-specific antibody detected MsrA (Figure 4A). Conversely, western analysis of HLE extracts immunoprecipitated with MsrA antibody and probed with an α-crystallin-specific antibody detected α-crystallin (Figure 4B). It is likely that MsrA preferentially binds oxidized α-crystallin because oxidation could cause a change in exposed hydrophobic regions of the protein. Some endogenous α-crystallin oxidation is likely under normal conditions facilitating MsrA binding detected here. These results provide evidence that α-crystallin and MsrA interact in lens epithelial cells.
Figure 4. MsrA and α-crystallin co-immunoprecipitate in human lens epithelial cell extracts.
Mitochondrial extracts where prepared from cultured human lens epithelial cells and incubated with either α-crystallin antibody and protein G agarose or MsrA antibodies conjugated to NHS-activated beads. Both methods result in antibody complexes. After thorough washing to remove un-conjugated proteins, conjugated protein complexes were eluted, run on SDS-PAGE gels and probed with either crystallin antibody or MsrA antibodies. Thus MsrA specific antibodies detect MsrA isolated using α-crystallin antibodies and conversely α-crystallin specific antibodies detect α-crystallin using MsrA specific antibodies. Both confirm in vivo complexes between MsrA and α-crystallin.
- Immunoblotting of HLE extracts immunoprecipitated using an α-crystallin-specific antibody and probed with MsrA-specific antibody. Lane 1 α-crystallin IP = immunoprecipitate carried out using the α-crystallin-specific antibody.
- Immunoblotting of HLE extracts immunoprecipitated using an MsrA-specific antibody and probed with α-crystallin-specific antibody. Lane 1 MsrA IP = immunoprecipitate carried out using the MsrA-specific antibody.
αB-crystallin is methionine-oxidized in the lenses of MsrA-knockout mice relative to wild-type mice
To determine if MsrA is required for maintaining the reduced state of α-crystallin in vivo, lenses from wild-type and MsrA-knockout mice were microdissected and proteins extracted from the lens cortex. These were subjected to CNBr cleavage and the resulting proteins separated by SDS-PAGE and visualized by western blotting with an anti-α-crystallin antibody. Two lenses from each group were examined. In the wild-type mice, 4 bands were detected including the parent αA- and αB-crystallin bands (Figure 5, lanes 1 and 2) and an αA-crystallin cleavage product of about 16 kDa consistent with CNBr cleavage at methionine 138. Also detected was an αB-crystallin CNBr cleavage product at approximately 12 kDa indicative of methionine 68 cleavage (Figure 5, lanes 1 and 2). By contrast, the 12 kDa αB-crystallin cleavage product was not detected in the MsrA-knockout preparation (Figure 5, lanes 3 and 4) suggesting that αB-crystallin methionine 68 was oxidized to methionine sulfoxide in the lenses of the MsrA-knockout mice. The 16 kDa band resulting from cleavage of methionine 138 of αA-crystallin was unaffected in MsrA-knockout mice, therefore no oxidation was found at this methionine. Increased non-reducible HMW aggregates of α-crystallin were also detected in the one of the knockout mice (Figure 5, lane 3).
Figure 5. Oxidation of α-crystallin in MsrA knockout mouse lens.
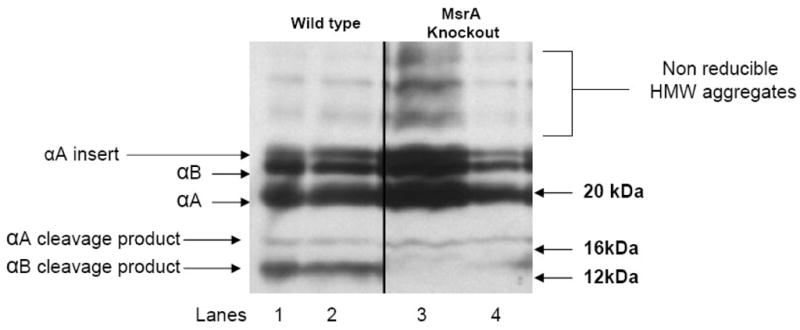
Immunoblots of total lens protein from two wild type and two MsrA knockout lenses cleaved with CNBr and probed with an α-crystallin specific antibody. Lanes 1–2 5 μg protein from wild type untreated mouse lenses. Lanes 3–4 5 μg protein from untreated MsrA knockout lenses.
Discussion
It is well accepted that protein oxidation plays a major role in cataract of the eye lens. The two most prominent oxidations associated with lens aging and cataracts are oxidation of cysteine to cysteine disulfide and oxidation of protein methionine to methionine sulfoxide [17–18]. Methionine sulfoxide formation leads to changes in protein structure, loss of regulatory function and loss of enzyme activity [12], which could disrupt the lens homeostasis and result in cataract formation [16].
Unlike many age-related protein oxidations, PMSO formation can be uniquely repaired by a family of enzymes called the methionine sulfoxide reductases (Msrs) [13]. MsrA is specific for the S-epimer of PMSO while three separate enzymes called MsrB1, B2 and B3 are specific for the R-epimer [13]. All four of these enzymes have been shown to be important for resistance of lens cells to oxidative stress [14–15, 37].
In the lens, the best studied of these is MsrA. MsrA has been shown to be localized throughout the human lens, particularly in the lens epithelium and posterior fiber cells [14]. Increased expression of MsrA protects lens cells against oxidative stress while deletion of MsrA renders lens cells more sensitive to oxidative stress [14]. Importantly, deletion of MsrA in mice exposed to hyperbaric oxygen stress results in cataract formation [16] suggesting an essential role for MsrA in lens cell protection and repair of oxidized protein methionines. To date, the only target for MsrA action identified in the lens is cytochrome c, which was found to be oxidized at methionine in the MsrA-knockout mouse lens and repairable by MsrA in vitro [16].
Consistent with a critical role for MsrA in defense of the human lens against aging and cataract, it has been shown that levels of PMSO increase in the human lens with age [17] and that over 60% of total lens protein methionines are oxidized upon human cataract formation [17–18]. These data indicate that high abundance lens proteins are oxidized at methionines upon aging and in cataract.
α-crystallin represents up to 50% of the soluble protein in the lens [1] making it a major candidate for methionine oxidation. Moreover, it is a molecular chaperone believed to protect other lens proteins by preventing aggregation and subsequent light scattering [1]. α-crystallin is also important for a wide range of lens functions including cytoskeleton remodeling [38], inhibition of apoptosis, and resistance of lens cells to stress [4]. Oxidation of α-crystallin has been previously shown to decrease its chaperone function [39] and lead to its aggregation [39–40]. There are two oxidizable methionines in each α-crystallin subunit that are conserved between a multitude of species including humans. Methionine 68 of αB-crystallin (identified here as oxidized in MsrA-deficient mouse lenses) is located in the putative substrate recognition sequence [41]. These collective properties of α-crystallin make it a likely target for MsrA repair. To date, the possible consequence of methionine oxidation on the function of α-crystallin remains relatively unexplored. In the present report, we hypothesized that methionine oxidation could reduce the chaperone function of α-crystallin and that MsrA could restore this function through the repair of oxidized methionines.
To test this hypothesis we specifically oxidized α-crystallin at methionines, confirmed this oxidation by CNBr cleavage and mass spectroscopy analysis and tested the resulting methionine sulfoxide α-crystallin for its chaperone activity using chemical denaturing conditions. We then tested the ability of purified MsrA to repair the oxidized α-crystallin and restore its chaperone function. Finally, we examined the possible interaction between α-crystallin and MsrA in lens cells and examined α-crystallin for methionine oxidation in the lenses of MsrA-knockout relative to wild-type mice.
Oxidation of α-crystallin with HOCl oxidized methionine 138 of αA-crystallin and methionine 68 of αB-crystallin as shown by CNBr cleavage (Figure 1). MsrA was able to reduce the methionine sulfoxide at these sites back to methionine. Oxidation of α-crystallin was confirmed using mass spectroscopy analysis (Figures 2A and 2B). Consistent with the hypothesis that MsrA repairs and restores the chaperone function of α-crystallin in the lens, we found that methionine oxidation reduced the ability of α-crystallin to protect lysozyme against chemical denaturation (Figure 3). These data provide strong evidence that the oxidation of methionines in α-crystallin leads to loss of chaperone function that can be restored by MsrA. All data are consistent with the fact that MsrA repairs α-crystallin PMSO and that this repair is independent of the DTT reducing system. However, each method indicated a different level of MsrA repair, ranging from approximately 50% by CNBr cleavage, to approximately 60% by chaperone measurement to greater than 90% by mass spec analysis. Although the reason for this disparity is not known these differences are most likely the result of inherent differences in sensitivity between the three methods and all three methods confirm MsrA repair. MsrA and α-crystallin were found to interact in human lens cells (Figure 4A and 4B), this interaction is not surprising since α-crystallin acts as a molecular chaperone and therefore interacts with many proteins in the cell but it nevertheless provides evidence that it interacts in vivo. The interaction does suggest that α-crystallin could be a major target for MsrA in the lens. Finally we examined the lenses of wild-type and MsrA knockout mice and found that αB-crystallin is oxidized at methionine 68 in vivo (Figure 5, the 12 kDa band), suggesting that MsrA is required to maintain the reduced and functioning state of α-crystallin in vivo. Further work is required to examine the negative result with respect to αA-crystallin (Figure 5, the 16 kDa band). αA-crystallin may be oxidized with further aging or oxidative stress treatment of the MsrA-knockout mice. Loss of chaperone function in conjunction with loss of MsrA repair is likely to result in lens cataract and be involved in other diseases associated with α-crystallin expression and oxidative stress. Point mutations in both αA- and αB-crystallins have been shown to cause disease in humans. For instance, the arginine 116 to cysteine conversion of αA-crystallin causes congenital cataract [7] while an arginine 120 to glycine point mutation in αB-crystallin has been shown to cause desmin-related myopathy as well as cataracts [8]. The R116C mutation leads to a highly oligomerized αA-crystallin that is defective in chaperone function [42–43]. The R120G mutation also results in irregular structure and decreased chaperone function of αB-crystallin [8, 43]. In addition, the G98R mutation in αA-crystallin leads to altered chaperone function and changes in the secondary, tertiary and quaternary structure result in an aggregation prone protein [44]. Oxidation by Fenton-type reaction of rat recombinant αA- and αB-crystallin was shown to result in higher molecular weight proteins that lacked chaperone function [39]. This same Fenton-type reaction was shown to result in modification of methionines with no other oxidative modification [40]. This evidence suggests that single site mutations or methionine oxidations of either αA- or αB-crystallin adversely alter protein structure leading to higher molecular weight aggregates and decreased chaperone function resulting in a predisposition to cataract formation.
The location of methionines in α-crystallin is consistent with their having major consequences in structure and chaperone function. Recognition site 2 of αB-crystallin [41] contains the methionine 68 of this protein and this site was shown to be important in the oligomerization and exchange dynamics of αB-crystallin as well as the sequence specific interaction of the αA- and αB-crystallin subunits. Although site directed mutagenesis of three residues in this sequence showed some effect on structure, no effect was seen on chaperone function. Mutation of methionine 68 has yet to be studied and may provide insight into the role of methionines in α-crystallin structure and chaperone function.
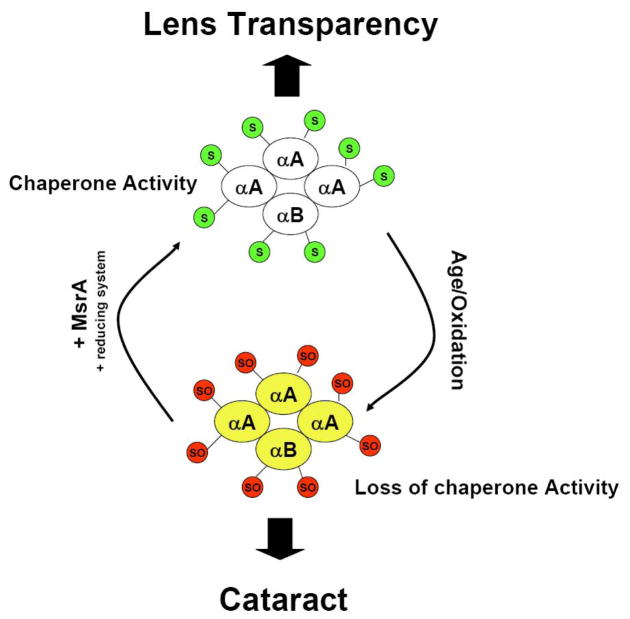
In summary this report examined the ability of MsrA to repair and restore the chaperone function of α-crystallin lost upon methionine oxidation. We demonstrated that oxidation of methionine leads to loss of chaperone function that can be restored by MsrA repair. In addition, we determined that lenses of MsrA knockout mice contain higher levels of oxidized αB-crystallin relative to wild-type. Our conclusion, that repair of oxidized methionines in α-crystallin is necessary for the maintenance of chaperone function and therefore lens transparency is summarized in Figure 6. The data collectively suggest that at least one requirement for MsrA in lens and other tissues is likely maintenance and repair of α-crystallin chaperone activity which is required for a multitude of important lens functions ranging from refraction through cytoskeletal remodeling and apoptotic control. Loss of α-crystallin chaperone function likely results in lens protein aggregation which may account for cataract formation found in the absence of MsrA in mice. Since PMSO levels increase in the human lens with age and upon cataract formation [17–18] it is likely that α-crystallin is oxidized in the human lens as a consequence of decreased MsrA levels and/or activity with age. Indeed, MsrA levels and activities have been shown to decrease with age in multiple rat tissues [45]. Such a decrease in the lens could result in α-crystallin oxidation, protein aggregation and ultimately cataract formation. Given the role of MsrA and α-crystallin in the lens and other tissues and diseases, these results are likely applicable to our understanding of these oxidative stress associated and aging disease mechanisms and they may provide a basis for the development of intelligently designed interventions for these conditions.
Figure 6. Possible mechanism of MsrA repair of α-crystallin and potential effect of chaperone activity and cataract.
Reduced α-crystallin (3:1 ratio αA-crystallin: αB-crystallin) may become oxidized upon aging and other oxidative stress conditions, in this Figure reduced methionines (S) are shown as green circles and oxidized methionines (SO) are shown as red circles. This may lead to loss of chaperone function and potentially cataract in the absence of MsrA repair. If repaired by MsrA the α-crystallin chaperone function would be restored and cataract formation would be averted.
Acknowledgments
This work was supported by Grants RO1 EY13022 from the National Institute of Health (MK), EY02027 (FJG), EY014803 (FJG) and EY010572 (LD). We thank Dr. Rodney Levine for the gift of purified mouse MsrA and the MsrA knockout mice.
Abbreviations
- MsrA
methionine sulfoxide reductase A
- PMSO
protein methionine sulfoxide
- HLE B3
Human lens epithelial cell
- nDMP
n-dodecyl-β-D-maltopyranoside
- HOCl
hypochlorous acid
- CNBr
Cyanogen Bromide
- ROS
reactive oxygen species
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Horwitz J. Alpha-crystallin. Exp Eye Res. 2003;76:145–153. doi: 10.1016/s0014-4835(02)00278-6. [DOI] [PubMed] [Google Scholar]
- 2.Horwitz J. α–crystallin can act as a molecular chaperone. Proc Natl Acad Sci USA. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jakob U, Gaestel M, Engel K, Buchner J. Small heat shock proteins are molecular chaperones. J Biol Chem. 1993;268:1517–20. [PubMed] [Google Scholar]
- 4.Andley UP. The lens epithelium: Focus on the expression and function of the α-crystallin chaperones. Int J Biochem Cell Biol. 2008;40:317–323. doi: 10.1016/j.biocel.2007.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wistow GJ, Piatigorsky J. Recruitment of enzymes as lens structural proteins. Science. 1987;236:1554–1556. doi: 10.1126/science.3589669. [DOI] [PubMed] [Google Scholar]
- 6.Brady JP, Garland D, Duglas-Tabor Y, Robison WG, Jr, Groome A, Wawrousek EF. Targeted disruption of the mouse alpha A-crystallin gene induces cataract and cytoplasmic inclusion bodies containing the small heat shock protein alpha B-crystallin. Proc Natl Acad Sci USA. 1997;94:884–9. doi: 10.1073/pnas.94.3.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Litt M, Kramer P, LaMorticella DM, Murphey DM, Lovrien EW, Weleber RG. Autosomal dominant congenital cataract associated with a missence mutation in human α-crystallin gene CRYAA. Hum Mol Genet. 1999;7:471–474. doi: 10.1093/hmg/7.3.471. [DOI] [PubMed] [Google Scholar]
- 8.Bova MP, Yaron O, Haung Q, Ding L, Haley DAA, Stewart PL, Horwitz J. Mutation R120G in αB-crystallin, which is linked to a desmin-related myopathy, results in an irregular structure and defective chaperone-like function. Proc Natl Acad Sci USA. 1999;96:6137–6142. doi: 10.1073/pnas.96.11.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vogt W. Oxidation of methionyl residues in proteins: tools, targets, and reversal. Free Radic Biol and Med. 1995;18:93–105. doi: 10.1016/0891-5849(94)00158-g. [DOI] [PubMed] [Google Scholar]
- 10.Cherian M, Abraham EC. Decreased molecular chaperone property of α-crystallins due to post-translational modifications. Biochim Biophys Res Comm. 1995;208:675–679. doi: 10.1006/bbrc.1995.1391. [DOI] [PubMed] [Google Scholar]
- 11.van Boekel MA, Hoogakker SE, Harding JJ, de Jong WW. The influence of some post-translational modifications on the chaperone-like activity of α-crystallin. Opthalmic Res. 1996;28:32–38. doi: 10.1159/000267940. [DOI] [PubMed] [Google Scholar]
- 12.Kim HY, Gladyshev VN. Methionine sulfoxide reductases: selenoprotein forms and roles in antioxidant protein repair in mammals. Biochem J. 2007;407:321–329. doi: 10.1042/BJ20070929. [DOI] [PubMed] [Google Scholar]
- 13.Stadtman ER, Van Remmem H, Richardson A, Wehr NB, Levine RL. Methionine oxidation and aging. Biochim Biophys Acta. 2005;1703:135–140. doi: 10.1016/j.bbapap.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 14.Kantorow M, Hawse JR, Cowell TL, et al. Methionine sulfoxide reductase A is important for lens cell viability and resistance to oxidative stress. Proc Natl Acad Sci USA. 2004;101:9654–9659. doi: 10.1073/pnas.0403532101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marchetti MA, Lee W, Cowell TL, Wells TM, Weissbach H, Kantorow M. Silencing of the methionine sulfoxide reductase A gene results in loss of mitochondrial membrane potential and increased ROS production in human lens cells. Exp Eye Res. 2006;83:1281–1286. doi: 10.1016/j.exer.2006.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brennan LA, Lee W, Cowell T, Giblin F, Kantorow M. Deletion of mouse MsrA results in HBO-induced lens opacity and MsrA repairable cytochrome c oxidation. Mol Vis. 2009;15:985–999. [PMC free article] [PubMed] [Google Scholar]
- 17.Truscott RJ, Augusteyn RC. Oxidative changes in human lens proteins during senile nuclear cataract formation. Biochim Biophys Acta. 1977;492:43–52. doi: 10.1016/0005-2795(77)90212-4. [DOI] [PubMed] [Google Scholar]
- 18.Garner MH, Spector A. Selective oxidation of cysteine and methionine in normal and senile cataractous lenses. Proc Natl Acad Sci USA. 1980;77:1274–1277. doi: 10.1073/pnas.77.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korlimbinis A, Hains PG, Truscott RJW, Aquilina JA. 3-Hydroxykynurenine oxidizes α-crystallin: Potential role in cataractogenesis. Biochemistry. 2006;45:1852–1860. doi: 10.1021/bi051737+. [DOI] [PubMed] [Google Scholar]
- 20.Hanson SRA, Hasan ADL, Smith, Smith JB. The major in vivo modifications of the human water-insoluble lens crystallins are disulphide bonds, deamidation, methionine oxidation and backbone cleavage. Exp Eye Res. 2000;71:195–207. doi: 10.1006/exer.2000.0868. [DOI] [PubMed] [Google Scholar]
- 21.Lund AL, Smith JB, Smith DL. Modifications of water-insoluble human lens α-crystallins. Exp Eye Res. 1996;63:661–672. doi: 10.1006/exer.1996.0160. [DOI] [PubMed] [Google Scholar]
- 22.Fujii N, Takeuchi N, Fujii N, Tezuka T, Kuge K, Takata T. Comparison of port-translational modifications of alphaA-crystallin from normal and hereditary cataract rats. Amino Acids. 2007;26:147–152. doi: 10.1007/s00726-003-0050-8. [DOI] [PubMed] [Google Scholar]
- 23.Schroff NP, Bera S, Cherian-Shaw M, Abraham EC. Substituted hydrophobic and hydrophilic residues at methionine-68 influence the chaperone-like function of αB-crystallin. Mol Cell Biochem. 2001;220:127–133. doi: 10.1023/a:1010834107809. [DOI] [PubMed] [Google Scholar]
- 24.Nakata K, Crabb JW, Hollyfield JG. Crystallin distribution in Bruch’s membrane-choroid complex from AMD and age-matched donor eyes. Exp Eye Res. 2005;80:821–6. doi: 10.1016/j.exer.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 25.Lowe J, Landon M, Pike I, Spendlove I, McDermott H, Mayer RT. Dementia with beta-amyloid deposition: involvement of alpha B crystallin supports two main diseases. 1990;336:515–516. doi: 10.1016/0140-6736(90)92075-s. [DOI] [PubMed] [Google Scholar]
- 26.Rekas A, Adda CG, Aquilina JA, et al. Interaction of the Molecular Chaperone aB-Crystallin with a-Synuclein: Effects on Amyloid Fibril Formation and Chaperone Activity. J Mol Biol. 2004;340:1167–1183. doi: 10.1016/j.jmb.2004.05.054. [DOI] [PubMed] [Google Scholar]
- 27.Renkawek K, Stege GJ, Bosman GJ. Dementia, gliosis and expression of small heat shock proteins hsp27 and alphaB crystallin in Parkinson’s disease. Neuroreport. 1999;10:2273–2276. doi: 10.1097/00001756-199908020-00009. [DOI] [PubMed] [Google Scholar]
- 28.Renkawek K, Voorter CE, Bosman GJ, van Workum FP, de Jong WW. Expression of alpha B-crystallin in Alzheimer’s disease. Acta Neuropathol. 1994;87:155–60. doi: 10.1007/BF00296185. [DOI] [PubMed] [Google Scholar]
- 29.Simpson RJ. Peptide Mapping and Sequence Analysis of Gel-Resolved Proteins. In: Simpson RJ, editor. Proteins and Proteomics. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2003. pp. 385–387. [Google Scholar]
- 30.Agbar S, Yevlampieva N, Aerts T, Vanhoudt J, Clauwaert J. Chaperone-like activity of bovine lens α-crystallin in the presence of dithiothreitol-destabilized proteins: Characterization of the formed complexes. Biochemical and Biophysical Communications. 2000;276:619–625. doi: 10.1006/bbrc.2000.3518. [DOI] [PubMed] [Google Scholar]
- 31.Ueda Y, Duncan MK, David LL. Lens proteomics: The accumulation of crystallin modifications in the mouse lens with age. Invest Ophthalmol Vis Sci. 2002;45:205–215. [PubMed] [Google Scholar]
- 32.Hawkins CL, Pattison DI, Davis MJ. Hypochlorite-induced oxidation of amino acids, peptides and proteins. Amino Acids. 2003;25:259–274. doi: 10.1007/s00726-003-0016-x. [DOI] [PubMed] [Google Scholar]
- 33.Chen YR, Deterding LJ, Sturgeon BE, Tomer KB, Mason RP. Protein oxidation of cytochrome c by reactive halogen species enhances its peroxidase activity. J Biol Chem. 2002;277:29781–29791. doi: 10.1074/jbc.M200709200. [DOI] [PubMed] [Google Scholar]
- 34.Dalle-Donne I, Rossi R, Giustarini D, et al. Methionine oxidation as a major cause of the functional impairment of oxidized actin. Free Radic Biol Med. 2002;32:927–937. doi: 10.1016/s0891-5849(02)00799-2. [DOI] [PubMed] [Google Scholar]
- 35.Szuchman-Sapir AJ, Pattison DI, Ellis NA, Hawkins CL, Davies MJ, Witting PK. Hypochlorous acid oxidizes methionine and tryptophan residues in myoglobin. Free Radic Biol Med. 2008;46:789–798. doi: 10.1016/j.freeradbiomed.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 36.Shao B, Belaaouaj A, Verlinde CL, Fu X, Heinecke JW. Methionine sulfoxide and proteolytic cleavage contribute to the inactivation of cathepsin G by hypochlorous acid: an oxidative mechanism for regulation of serine proteinases by myeloperoxidase. J Biol Chem. 2005;280:29311–29321. doi: 10.1074/jbc.M504040200. [DOI] [PubMed] [Google Scholar]
- 37.Marchetti MA, Pizarro GO, Sagher D, et al. Methionine sulfoxide reductases B1, B2 and B3 are present in the human lens and confer oxidative stress resistance to lens cells. Invest Ophthalmol Vis Sci. 2005;46:2107–2112. doi: 10.1167/iovs.05-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicholl ID, Quinlan RA. Chaperone activity of alpha-crystallins modulates intermediate filament assembly. Embo J. 1994;13:945–953. doi: 10.1002/j.1460-2075.1994.tb06339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rajan S, Horn C, Abraham EC. Effect of αA- and αB-crystallins on their structure, oligomerization and chaperone function. Mol Cell Biol. 2006;288:125–134. doi: 10.1007/s11010-006-9128-4. [DOI] [PubMed] [Google Scholar]
- 40.Smith JB, Jiang X, Abraham EC. Identification of hydrogen peroxide oxidation sites of αA- and αB-crystallins. Free Rad Res. 1997;26:103–111. doi: 10.3109/10715769709097789. [DOI] [PubMed] [Google Scholar]
- 41.Sreelakshmi Y, Sharma KK. Recognition sequence 2 (residues 60–71) plays a role in oligomerization and exchange dynamics of αB-crystallin. Biochemistry. 2005;14:12245–12252. doi: 10.1021/bi051005h. [DOI] [PubMed] [Google Scholar]
- 42.Schroff NP, Cherian-Shaw M, Bera S, Abraham EC. Mutation of R116C results in highly oligomerized αA-crystallin with modified structure and defective chaperone function. Biochemistry. 2000;39:1420–1426. doi: 10.1021/bi991656b. [DOI] [PubMed] [Google Scholar]
- 43.Kumar LVS, Ramakrishna T, Rao CH. Structural and functional consequences of the mutation of a conserved arginine residue in αA- and αB-crystallins. J Biol Chem. 1999;274:24137–24141. doi: 10.1074/jbc.274.34.24137. [DOI] [PubMed] [Google Scholar]
- 44.Singh D, Raman B, Ramakrishna T, Rao CH. The cataract-causing mutation G98R in human αA-crystallin leads to folding defects and loss of chaperone activity. Mol Vis. 2006;12:1372–1379. [PubMed] [Google Scholar]
- 45.Petropoulos I, Mary J, Perichon M, Friguet B. Rat peptide methionine sulphoxide reductase: cloning of the cDNA, and down-regulation of gene expression and enzyme activity during aging. Biochem J. 2001;355:819–825. doi: 10.1042/bj3550819. [DOI] [PMC free article] [PubMed] [Google Scholar]