Abstract
This work explores several quantitative aspects of radiation-induced bystander mutagenesis in WTK1 human lymphoblast cells. Gamma-irradiation of cells was used to generate conditioned medium containing bystander signals, and that medium was transferred onto naïve recipient cells. Kinetic studies revealed that it required up to one hour to generate sufficient signal to induce the maximal level of mutations at the thymidine kinase locus in the bystander cells receiving the conditioned medium. Furthermore, it required at least one hour of exposure to the signal in the bystander cells to induce mutations. Bystander signal was fairly stable in the medium, requiring 12–24 hours to diminish. Medium that contained bystander signal was rendered ineffective by a 4-fold dilution; in contrast a greater than 20-fold decrease in the cell number irradiated to generate a bystander signal was needed to eliminate bystander-induced mutagenesis. This suggested some sort of feedback inhibition by bystander signal that prevented the signaling cells from releasing more signal. Finally, an ionizing radiation-induced adaptive response was shown to be effective in reducing bystander mutagenesis; in addition, low levels of exposure to bystander signal in the transferred medium induced adaptation that was effective in reducing mutations induced by subsequent γ-ray exposures.
Keywords: Bystander effect, Adaptive response, Ionizing radiation mutagenesis
1. INTRODUCTION
Non-targeted effects, where unexposed cells are affected by nearby cells exposed to ionizing radiation, have a long history (reviewed in [1, 2]). Bystander effects have been reported to result from exposures to both low and high LET radiations (reviewed in [1, 3]), although most studies have used the latter. In some experimental in vitro systems, bystander signals can be transmitted through the growth medium, while in others, gap junctions seem to be required (reviewed in [4, 5]). These modes of transfer are of course not mutually exclusive; gap junctions are likely to accentuate the effects of media transfer. Increasing evidence suggests that reactive oxygen species (ROS) may be mediating damage in unexposed cells [6–12], and the involvement of mitochondria in generating ROS has been explored [13–17]
Bystander effects are generally considered to be deleterious, and in cells exposed to bystander signals, effects include, among others, changes in gene expression [18], DNA damage [10, 15, 19], gene mutations [12, 20, 21], sister chromatid exchanges [22], chromosome aberrations [23], cell killing [24], and cell transformation [25]. Defective repair of ionizing radiation-induced DNA damage is associated with increased bystander responses [12, 20, 26–30]. Nevertheless, despite considerable effort over the past 17 years, much of the bystander literature is descriptive or qualitative, and there are numerous gaps in our understanding of the quantitative and temporal aspects which need to be addressed.
The adaptive response to radiation was first described in 1984 by Olivieri et al. [31] who reported that peripheral blood lymphocytes cultured in 3H-thymidine showed a reduced frequency of chromosome aberrations following a challenge with an acute moderate dose of X-rays. The phenomenon was subsequently studied by many different laboratories in a variety of test systems [32–39]. Adaptation is most efficiently induced by doses of 0.005–0.2 Gy. The usual protocol is to prime cells with a low dose of ionizing radiation and then follow 4 or more hours later with a challenge to a much higher dose of 0.5 – 2 Gy. The mechanism behind the adaptive response is unclear. Some have suggested that it involves the induction of signaling pathways, including DNA repair pathways or down regulation of heat-shock-related proteins [39–43]. More recently, Coleman et al. [44] reported a number of different transcription elements associated with the adaptive response.
There are several published studies which link bystander and adaptive responses. Ionizing radiation-induced adaptation can render cells resistant to bystander signals (e.g., [45, 46]), and bystander signals themselves can induce the adaptive response (e.g., [32, 33, 47–49]). This may be mediated in part by reactive oxygen species (ROS) such as H2O2 and reactive nitrogen species, which have also been reported to induce adaptation directly [50–52]. A bystander signal-induced adaptive response would tend to make cells more resistant to a subsequent high dose challenge, and such an adaptive effect might also reduce DNA damage induced from any long-term exposure to bystander factors. Thus there is the potential for bystander effects to be advantageous.
The work reported here defines some key kinetic and temporal aspects of bystander-induced mutagenesis in human lymphoblastoid cells, including induction of the adaptive response.
2. MATERIALS AND METHODS
WTK1 human lymphoblastoid cells [53] were maintained as exponentially growing cultures at densities of 1–10 × 105 cells/ml in RPMI 1640 medium supplemented with 10% heat inactivated horse serum, 100 U/ml penicillin, and 100 µg/ml streptomycin. Incubator conditions were 37oC in 5% CO2 and 100% humidity.
γ-irradiations were performed in a calibrated Mark I 137Cs γ-irradiator (J. L. Shepherd and Associates, Glendale, CA). Exponentially growing cultures were removed from the incubator, and immediately irradiated at a dose rate of 2.5 Gy/min for high dose (2 Gy), or 0.25 Gy/min for low dose (0.05 Gy). Cells then were returned to the incubator. Cultures were insulated in Styrofoam containers except for during the actual irradiation, and therefore temperatures of the cultures were maintained between 34–35°C during the treatment. Temperatures were restored to 37°C within 5 minutes after re-incubation.
Bystander signal-containing medium was prepared using a protocol modified from Mothersill and Seymour [54]. Briefly, a total of 2.5 × 106 WTK1 cells in 5 ml, were irradiated with 2 Gy γ-rays and returned to the incubator. At the appropriate time, medium containing bystander signal was obtained by centrifuging for 10 minutes at 1000 rpm to pellet the cells, at 37oC. To avoid removing any directly irradiated cells, only the upper 4 ml of medium was removed; thus the standard protocol utilized bystander signal from the equivalent of 2 × 106 cells in a total of 4 ml. That directly irradiated cells were not contained in the bystander medium was confirmed by demonstrating that the plating efficiency of this medium was < 10−6 (data not shown).
Mutant fractions (MF) were analyzed at the heterozygous autosomal thymidine kinase locus, using standard protocols [53]. In order to reduce the background MF prior to the experiment, cells were treated for 2 days with CHAT (complete RPMI 1640 medium with 10−5 M deoxycytidine, 2×10−4 M hypoxanthine, 2×10−7 M aminopterin, and 1.75×10−5 M thymidine), followed by 1 day in CHT (CHAT without aminopterin); cells were then used within 5 days. After the end of any particular potentially mutagenic treatment, cells were maintained in normal RPMI medium for 3 days to allow for the expression of induced mutants. Cells then were seeded into 96-well dishes to determine the MF by limiting dilution. Cells were seeded at 2000 cells/well in the presence of 2 µg/ml trifluorothymidine to select tk− mutants, and also at 1 cell/well in normal medium to determine plating efficiency. Mutation plates were fed with fresh trifluorothymidine after 11 days and colonies were scored after 21 days incubation. The MF was calculated using the Poisson distribution [55].
Background MFs shown in various figures are for completely untreated cultures. These were determined separately for each experiment.
Statistical comparisons were made with the Student’s t-test, using SigmaStat 3.5.
3. RESULTS
This manuscript presents studies testing key kinetic aspects of the ionizing radiation-induced bystander effect, and its effects on the adaptive response, specifically on the endpoint of mutagenesis at the thymidine kinase locus in WTK1 human lymphoblasts. In these experiments, medium transfer was employed; typically, cells were irradiated with 2 Gy of γ-rays, and the medium was harvested by centrifugation at various times; this medium then was used to culture untreated, naïve cells.
Kinetic and temporal aspects of bystander mutagenesis
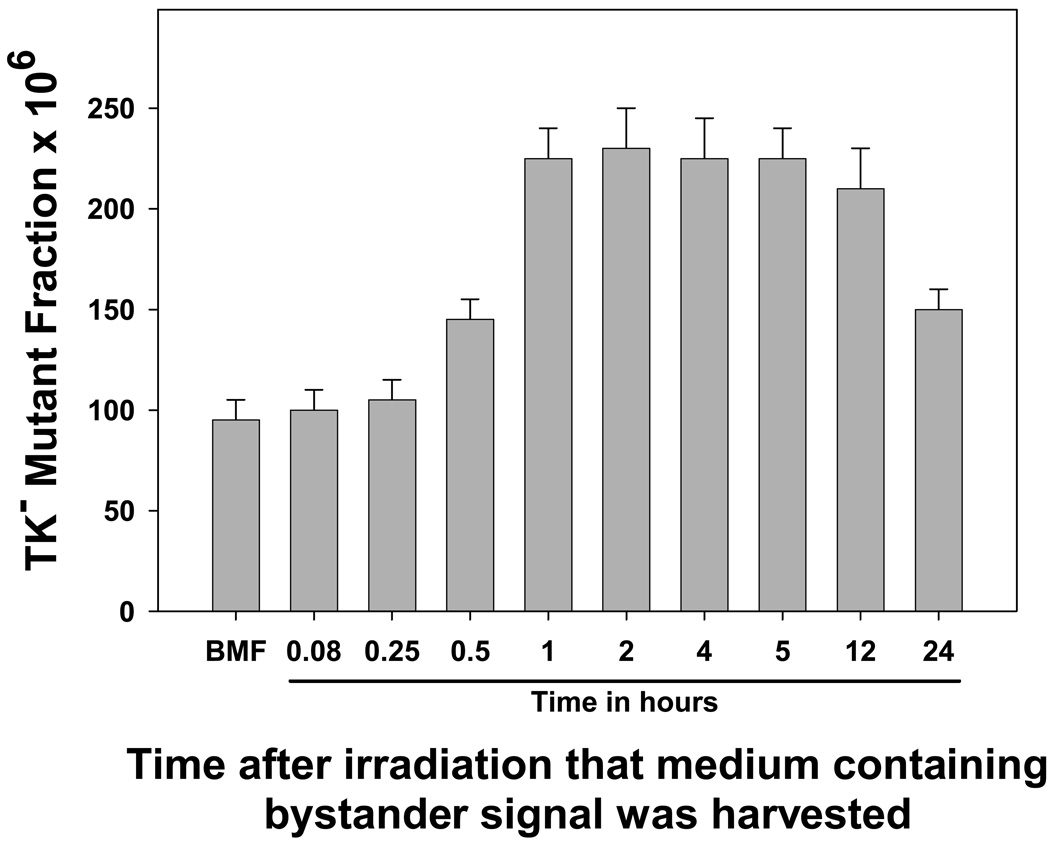
In the first experiment, the medium was harvested at various times after irradiation, and utilized to resuspend untreated, naïve cells. As can be seen in Figure 1, shorter post-irradiation culture times of 5 or 15 minutes did not allow sufficient bystander signal to accumulate such that no increase in mutagenesis was observed when the medium was transferred to bystander cells. An accumulation time of 30 minutes resulted in an intermediate level of induced mutation (30 minutes compared to background, p=0.004; 30 minutes compared to 1 hr, p=0.002), showing that the bystander effect is not an all or nothing response. One hour was required to generate sufficient signal in the medium to produce a full bystander effect. Post-irradiation culture times of 1–12 hours produced approximately equal levels of bystander mutagenesis, approximately a 2.5-fold increase over background (no statistical differences among these data points, p≥0.35; all are significantly different from the background, p < 0.01). However, when the medium transfer was done 24 hours after irradiation, bystander mutagenesis was still present but significantly reduced (24 hr compared to background, p=0.003; 24 hr compared to 12 hr, p=0.01), suggesting that the signal has a finite lifetime somewhat greater than 24 hours.
Figure 1. Kinetics of bystander signal production after ionizing radiation treatment: Time required for cells to generate sufficient bystander signal to induce significant levels of gene mutation.
Aliquots of WTK1 cells were irradiated with 2 Gy of γ-rays, and the medium was harvested by centrifugation at the indicated times. It was applied to naïve cells for 24 hours, and the mutant fractions were subsequently determined. BMF is background mutation frequency. Data are mean of three experiments and error bars are SD.
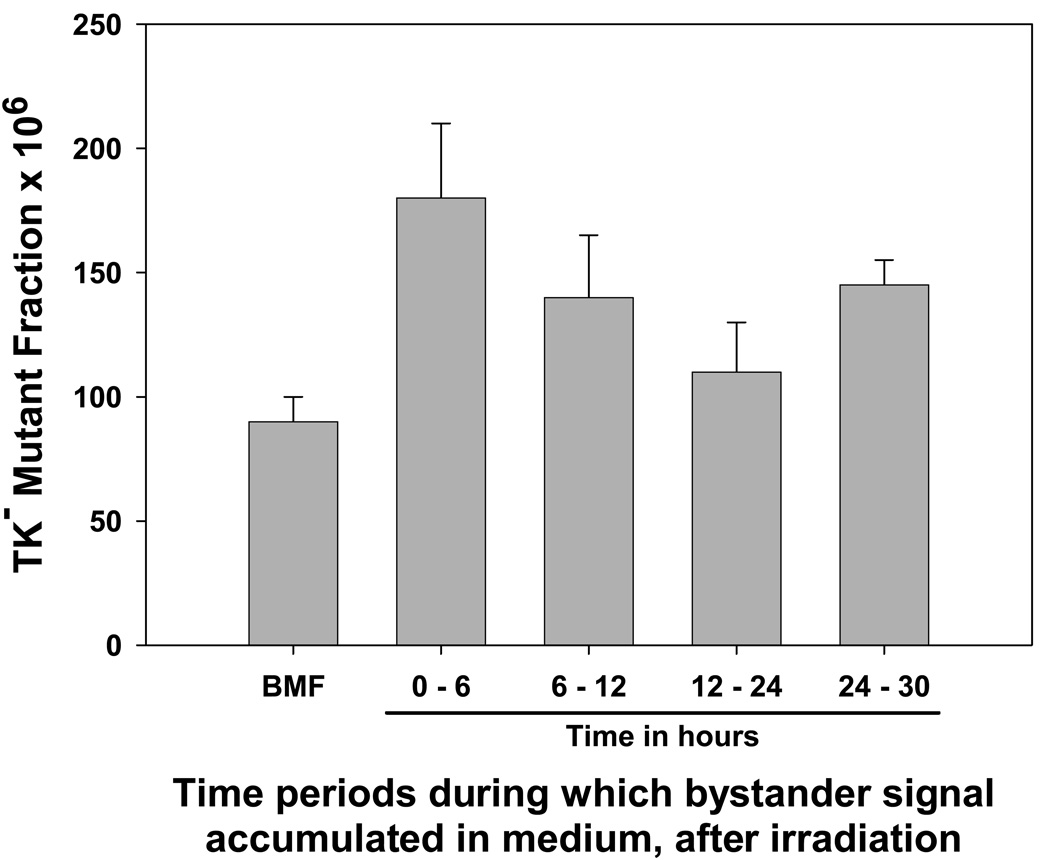
The time intervals during which bystander signal was secreted into medium by irradiated cells were determined. For this experiment, cells were treated with 2 Gy, and the medium from those cells was harvested in various time intervals (Figure 2). As can be seen, the strongest level of bystander signal was present in the medium obtained from 0 – 6 hr after irradiation compared to background, p=0.008). It was still present in the 6–12 hour interval (compared to background, p=0.032); although it appeared to be diminished the difference was not significant (p=0.15). There was no significant increase in mutagenesis in the 12–24 hour interval (p=0.196), suggesting that no signal was produced in this time interval. Interestingly, there appeared to be a second wave of bystander signal produced between 24–30 hours (compared to background, p=0.003).
Figure 2. Kinetics of appearance of bystander signal in medium, after irradiation.
Aliquots of WTK1 cells were irradiated with 2 Gy of γ-rays. The 0–6 hr sample was the medium collected by centrifugation after 6 hr. For the 6–12 hour sample, cells were centrifuged after 6 hr, and fresh medium was added; at t=12 hr, this medium was removed and applied to naïve cells. An identical approach was used to collect the 12–24 and 24–30 hour samples. Mutant fractions were subsequently determined. Data are mean of three experiments and error bars are SD.
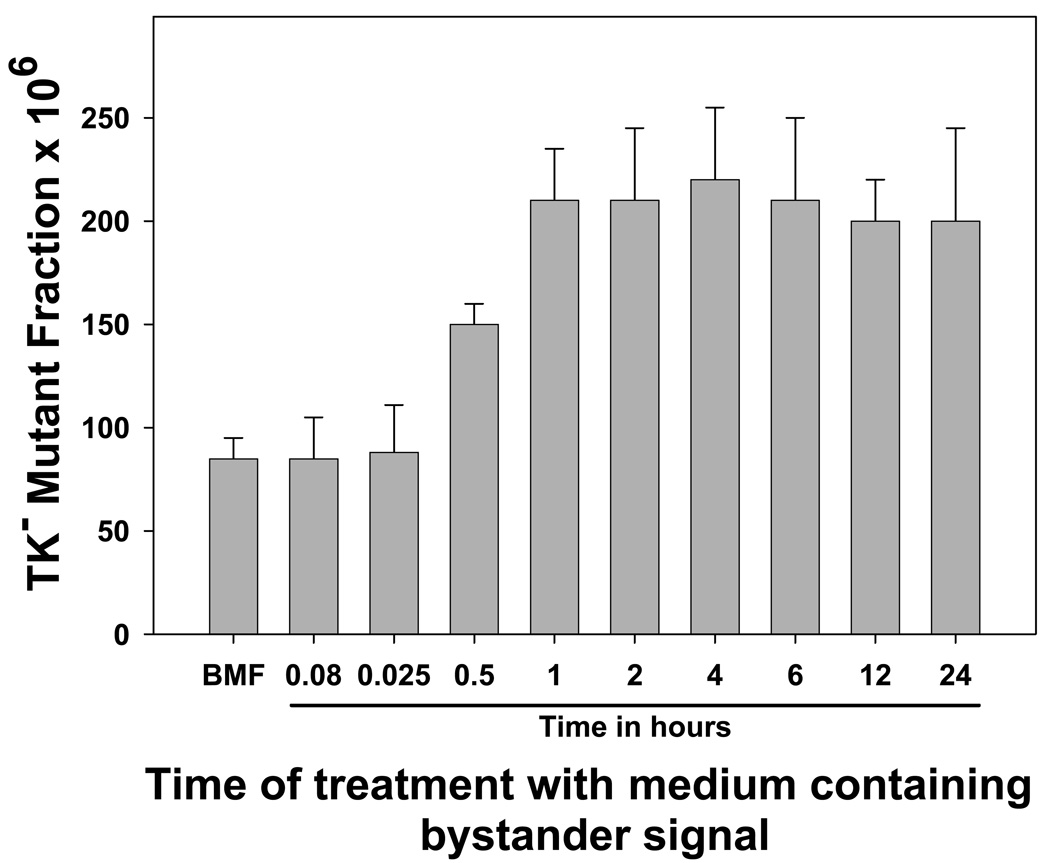
Next, batches of medium containing bystander signal were prepared by irradiating aliquots of cells with 2 Gy and harvesting the medium 2 hours later. Naïve cells were incubated with this bystander-signal-containing medium for various times, and it was found that at least 1 hour of exposure to the bystander signal was required to induce maximal levels of mutagenesis; exposure times of 5 or 15 minutes were ineffective (Figure 3). Similar to Figure 1, an intermediate response was observed with the 30 minute exposure (30-min compared to background, p=0.001; 30-min compared to 1-hr, p=0.018), again showing that the bystander effect is not all-or-nothing, but can exhibit a graded response.
Figure 3. Length of exposure to bystander signal required to induce significant levels of gene mutation.
Aliquots of WTK1 cells were irradiated with 2 Gy of γ-rays, and the medium was harvested 2 hr later. Aliquots were used to treat naïve WTK1 cells for the indicated times, and the mutant fractions were subsequently determined. Data are mean of three experiments and error bars are SD.
Dilution of bystander signals
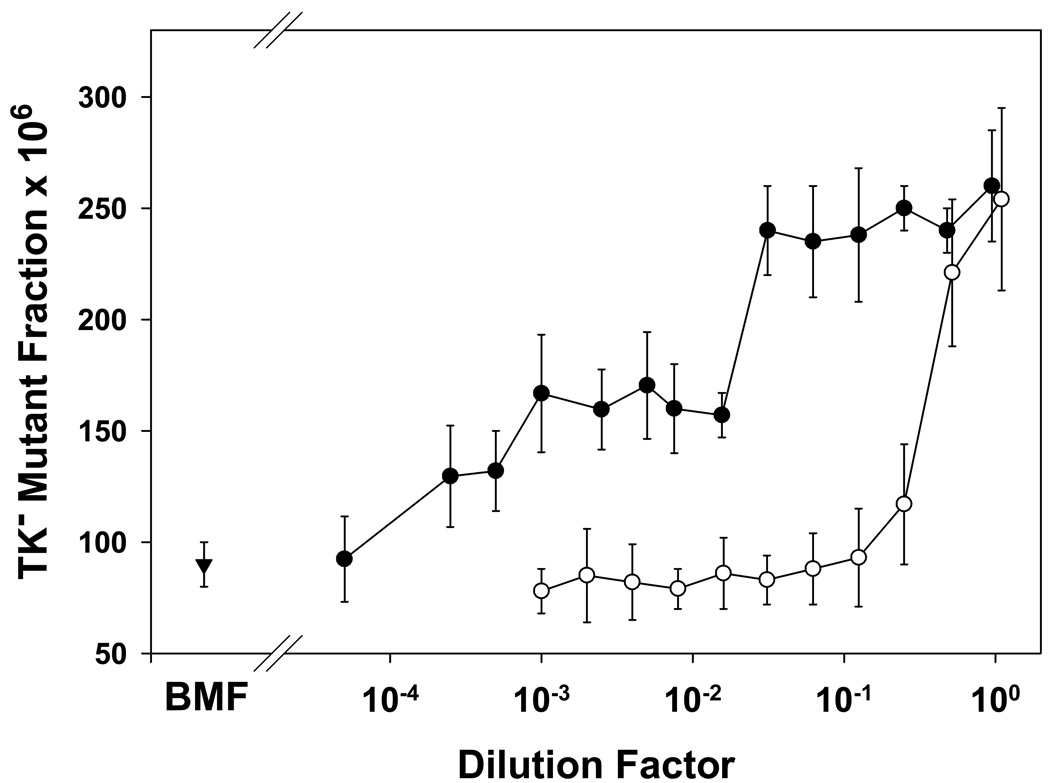
Two experiments were conducted to investigate how the levels of bystander signal could be modulated to affect the induction of gene mutations. Figure 4 shows that the bystander signal produced by 2 Gy of γ-rays to 2 million cells, harvested at 2 hours after irradiation, easily could be diluted away to ineffective levels. In fact a 4-fold dilution was sufficient to eliminate bystander mutagenesis. However, Figure 4 also shows that when progressively fewer cells were irradiated to produce bystander signal, it was required to reduce the number of cells in the irradiated sample by >20-fold (i.e., from 2 × 106 to ≤ 5 × 104) to observe a reduced level of bystander mutagenesis. This leads us to speculate that under the defined experimental conditions, the amount of bystander signals reach a plateau. In other words, treatment of 105 to 2 × 106 cells with 2 Gy resulted in the same levels of bystander signal in the medium.
Figure 4. Dilution of bystander signal.
Dilution of medium containing bystander signal. (○) Aliquots of WTK1 cells were irradiated with 2 Gy of γ-rays, and the medium was collected by centrifugation 2 hr later. These medium samples were diluted as indicated, with fresh complete medium at 37°C, pH 7.2, and applied to naïve cells for 24 hr. Mutant fractions were subsequently determined. Data are mean of three experiments and error bars are SD.
Variation in bystander effects induced by treatment of varying cell number. (•) Aliquots of WTK1 cells at various cell numbers were irradiated with 2 Gy of γ-rays, and the medium was collected by centrifugation 2 hr later. These medium samples were applied to naïve cells for 24 hr. Mutant fractions were subsequently determined. Data are mean of three experiments and error bars are SD.
BMF (▾) is the background mutant fraction.
Bystander effects and the adaptive response
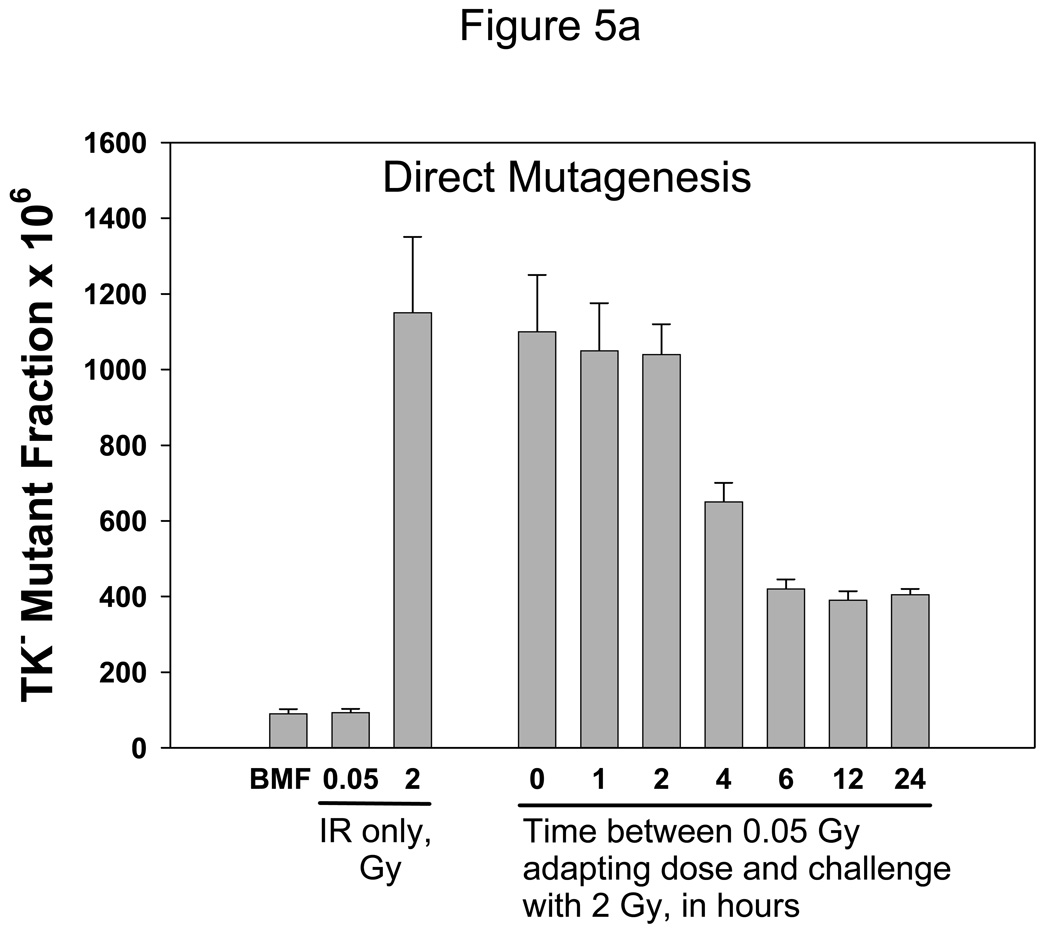
Experiments then were done to determine how bystander signals affect the adaptive response. Figure 5A shows “classical” adaptation. WTK1 cells were pretreated with 0.05 Gy γ-rays as the priming dose, and these cells were challenged with 2 Gy at various times afterwards. The priming dose of 0.05 Gy of γ-rays did not induce a measurable change in the MF, but as can be seen, a dose of 2 Gy was significantly less mutagenic when administered 4–24 hr after the adapting dose (p<0.01); at earlier time points there was no effect. The 4-hr time point yielded an intermediate response, as it was significantly different from both the 2-hr and the 6-hr points (p=0.002 for both comparisons).
Figure 5. Ionizing radiation-induced adaptive response in WTK1 cells.
Fig. 5A shows the “classical” ionizing radiation-induced adaptive response, for direct radiation mutagenesis. The leftmost bar represents untreated cells and is the background mutant fraction; next is cells treated with 0.05 Gy of γ-rays only, and the third bar is cells treated with 2 Gy of γ-rays only. The rightmost seven bars represent cells that first received a 0.05 Gy priming dose, followed by a 2 Gy challenge dose at the indicated time. Mutant fractions were subsequently determined.
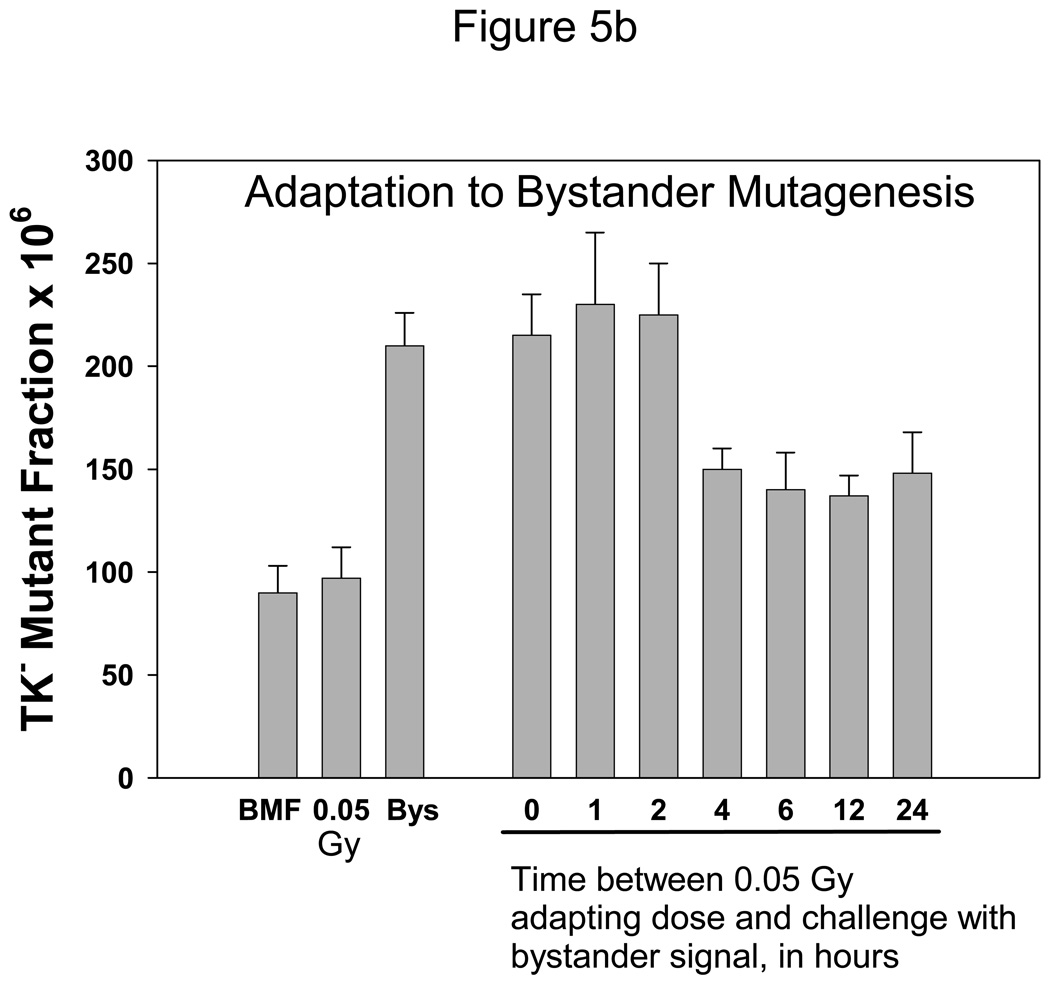
Fig 5B also shows an ionizing radiation-induced adaptive response; however, this time the challenge dose was bystander medium harvested from WTK1 cells, 2 hr after treatment with 2 Gy of γ-rays. As in 5A, the leftmost bar represents untreated cells, the background mutant fraction; next is cells treated with 0.05 Gy of γ-rays only, and the third bar is cells treated with bystander medium only (2 hr). The rightmost seven bars represent cells that first received the 0.05 Gy priming dose, followed by the bystander medium challenge dose (delivered for 2 hours) at the indicated time. Mutant fractions were subsequently determined. Data are means of three experiments and error bars are SD.
Figure 5B shows a similar experiment, in which it was demonstrated that the 0.05 Gy priming dose also renders cells resistant to a subsequent challenge with bystander medium (p < 0.01 for 0, 1 or 2 compared to 4, 6, 12 or 24 hr). The kinetics were different in that the 2-hr point did not exhibit an intermediate response. This experiment suggests that cells can adapt after ionizing radiation exposure to be protected against deleterious bystander effects.
A series of control experiments also were done to insure that cell handling did not induce adaptation. Here, sham irradiations simulating the 0.05 Gy priming dose were done at various times prior to irradiations with 0 or 2 Gy of γ-rays. To accomplish this, cells were removed from the incubator for 10 minutes (a time equivalent to that needed to do the transport to and from the irradiator and perform the irradiations), during which time they cooled down to approximately 34°C and likely became more oxygenated due to the unavoidable shaking of flasks. Cells were challenged with 0 or 2 Gy at 0, 1, 2, 4, 8 or 24 hr later. These treatments had absolutely no effect on the background or on radiation-induced mutant fractions (data not shown).
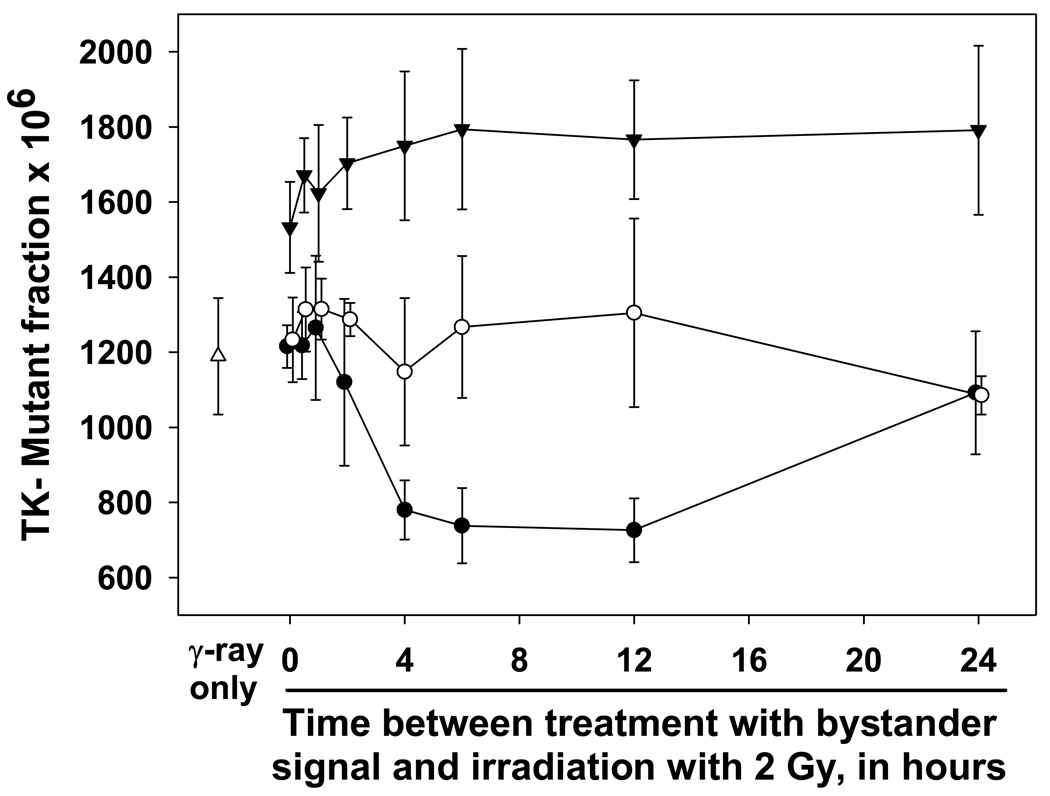
In the final set of experiments, the ability of bystander signal to induce adaptation was examined. Medium containing bystander signal was prepared by irradiating aliquots of cells with 2 Gy of γ-rays, and the media were harvested 2 hours later. The priming treatment consisted of exposing cells to that conditioned medium for 5, 30 or 120 minutes. The challenge dose was 2 Gy of γ-rays, 0–24 hours after the end of the priming dose. These results are shown in Figure 6. Clearly the 5-min priming treatment was sufficient to produce adaptation, when at least 4 hr was allowed for development (compared to γ-rays alone, for 4 to 12 hrs all p < 0.02). However, bystander-induced adaptation was not as persistent as that for ionizing radiation, since protection was not evident at the 24 hr point. Interestingly, all of the mutation frequencies for the 30-min priming were approximately equal (compared to γ-rays alone, all p>0.10), and for the 2-hr priming time, mutation frequencies were uniformly higher (compared to γ-rays alone, all p < 0.05), but none were different from one another (comparisons among primed cells, all p > 0.15). Here, bystander mutagenesis and direct γ-ray-induced mutagenesis appeared to be additive, without adaptation. This is reminiscent of what has been seen for ionizing radiation, where higher doses (> 0.5 Gy) generally do not induce the adaptive response.
Figure 6. Bystander signal-induced adaptive response in WTK1 cells.
In this experiment, WTK1 cells were primed with bystander signal, which had been generated by exposing WTK1 cells to 2 Gy of γ-rays and harvesting 2 hr later. These media with bystander signals were used to prime cells: they were applied to naïve cells for 5 minutes (•), 30 minutes (○), or 2 hours (▾). The primed cells were treated with 2 Gy of γ-rays at the indicated times after priming. Mutant fractions were subsequently determined. Data are mean of three experiments and error bars are SD.
4. DISCUSSION
The experiments presented in this paper investigate key kinetic aspects of radiation-induced bystander mutagenesis, and leads to the overall conclusion that both directly irradiated cells and unirradiated cells can adapt over time to bystander signals.
Bystander mutagenesis plateaus
A simple model for bystander-induced mutagenesis would be that the bystander effectors are DNA damaging agents such as ROS. If this were true, then one would expect that increasing the time of exposure of unirradiated cells to bystander signal ought to increase the mutational yields, at least for as long as the signal was still present. From Figure 2, it can be seen that the signal remained active for more than 6 hours, and therefore one might predict that the mutation frequencies ought to have increased with exposure time in the range of 1 to ≥6 hours. However, as seen in Figure 3, the kinetics of induction of bystander mutagenesis seems to follow a sigmoid-shaped curve, and in fact the bystander effect plateaus with time of treatment with medium containing bystander signal.
One possible explanation would be that the processes of bystander-induced mutagenesis and bystander-induced adaptation were competing and essentially canceling each other out. In other words, at longer exposure times, even though mutagenic bystander signals were present, additional mutagenic damage was not induced because the adaptive response had also been activated and was protective. However, this idea is inconsistent with the data in Figure 6, where there was no evidence of adaptation in the cells exposed to higher levels of bystander signal for longer times. An alternative explanation would be that the sigmoid-shaped curve actually reflects the induction of a mutagenic process in bystander cells rather than the direct production of DNA damage. To fit our data, that mutagenic process would need to be a damped effect; i.e., cells would have to become resistant to continued receipt of bystander signal. We do not have any evidence of what that mutagenic process might be. One possibility would be a burst of reactive oxygen species from the mitochondria. Recent data have in fact predicted a role for mitochondria in bystander responses [13–17], but mainly this has regarded the generation of signal from the irradiated cell. One of these reports did show that cells depleted of mitochondria could still respond to bystander signals, as measured by DNA damage/repair-related focus formation [15], and this would argue against the hypothesis of a burst of reactive oxygen species in the bystander cells. But perhaps even a very small remaining mitochondrial compartment could be sufficient to generate enough ROS to induce mutations, without overtly increasing the frequency of foci. Clearly this will be an area for future research.
Bystander-feedback inhibition
We performed two ‘dilution’ experiments, one of bystander-medium and one of the number of cells available to produce bystander signal (both in Figure 4). It is quite interesting that although dilution of medium containing bystander signal rapidly eliminated the ability of that medium to induce mutations, a much greater dilution of the cell number available to produce bystander signal was required to reduce the ability of the transferred medium to induce mutation. One possible explanation for this is that there may be some kind of feedback mechanism operating. In other words, once the bystander signal reached a certain concentration, it prevented the production and/or release of further signal.
Bystander mutagenesis probably contributes to the overall mutation frequencies observed in directly irradiated cell populations
In these experiments, the fact that it took 4 hours for cells irradiated with 0.05 Gy to become resistant to bystander signals suggests the following. An acute mutagenic response to a single high dose of ionizing radiation, where all cells are directly damaged, is likely to include two components. First would be the effects of the direct radiation damage, and second would be the bystander signals secreted from and returning to the irradiated cells. This idea is consistent with our previous observation that extracellular catalase appeared to slightly reduce the mutagenicity of γ-irradiation [12].
Adaptation develops from bystander signaling
We have shown that bystander signals induce the adaptive response, which protects these human lymphoblast cells from radiation mutagenesis. Bystander-induced adaptation also has been reported by others [47–49]. This could provide a new importance for the adaptive response. In theory, exposure of a cell to a very low dose of ionizing radiation (the ultimate low dose being a single photon) could generate extra-cellular bystander signals which in turn could affect up to hundreds of cells. In one study, a low γ-ray dose of 5 mGy, which was probably fewer than 10 photons per cell, induced a bystander effect for cell killing [48]. If passage of a single photon can induce bystander signals, then a fairly large proportion of cells in the body could be adapted at any given time, from background radiation (which can be expected to hit each cell in the body 1 – 3 times per year). Then it would become significant that there is inter-individual variability in the human population as to whether the adaptive response functions or not. In fact there are reports that the proportion of people who exhibit adaptation varies; in limited studies, 50–80% of individuals have shown a reduction after the challenge dose, while the remainder have not [39, 56–58]. Variation is likely to derive from genetic factors [56–59]. Thus it will be important to determine whether individuals without an adaptive response are actually more sensitive to the deleterious effects of ionizing radiation.
In the adaptation experiments (Figure 6), minimal exposure to bystander signal appeared to be protective while longer exposures were ineffective or detrimental, at least for the endpoint of mutagenesis. Thus the bystander effect can be expected to vary dramatically, depending on the specific conditions present. This could account for some of the disparate results in the literature.
Acknowledgements
This work was supported by NIH Grant P01CA095227 (K.D. Held).
J. Baldwin was supported by a summer fellowship from the National Science Foundation (DBI-0552425). K. Prise was partially supported by grants from Cancer Research UK [CUK] (C1513/A7047) and European Union NOTE project (FI6R 036465).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that there are no conflicts of interest.
Reference List
- 1.Little JB. Genomic instability and bystander effects: a historical perspective. Oncogene. 2003;22:6978–6987. doi: 10.1038/sj.onc.1206988. [DOI] [PubMed] [Google Scholar]
- 2.Mothersill C, Seymour C. Radiation-induced bystander effects: past history and future directions. Radiat. Res. 2001;155:759–767. doi: 10.1667/0033-7587(2001)155[0759:ribeph]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 3.Mothersill C, Seymour C. Radiation-induced bystander effects: evidence for an adaptive response to low dose exposures? Dose. Response. 2006;4:283–290. doi: 10.2203/dose-response.06-111.Mothersill. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lorimore SA, Coates PJ, Wright EG. Radiation-induced genomic instability and bystander effects: inter-related nontargeted effects of exposure to ionizing radiation. Oncogene. 2003;22:7058–7069. doi: 10.1038/sj.onc.1207044. [DOI] [PubMed] [Google Scholar]
- 5.Morgan WF. Is there a common mechanism underlying genomic instability, bystander effects and other nontargeted effects of exposure to ionizing radiation? Oncogene. 2003;22:7094–7099. doi: 10.1038/sj.onc.1206992. [DOI] [PubMed] [Google Scholar]
- 6.Azzam EI, de Toledo SM, Spitz DR, Little JB. Oxidative metabolism modulates signal transduction and micronucleus formation in bystander cells from alpha-particle-irradiated normal human fibroblast cultures. Cancer Res. 2002;62:5436–5442. [PubMed] [Google Scholar]
- 7.Azzam EI, de Toledo SM, Little JB. Oxidative metabolism, gap junctions and the ionizing radiation-induced bystander effect. Oncogene. 2003;22:7050–7057. doi: 10.1038/sj.onc.1206961. [DOI] [PubMed] [Google Scholar]
- 8.Bishayee A, Hill HZ, Stein D, Rao DV, Howell RW. Free radical-initiated and gap junction-mediated bystander effect due to nonuniform distribution of incorporated radioactivity in a three-dimensional tissue culture model. Radiat. Res. 2001;155:335–344. doi: 10.1667/0033-7587(2001)155[0335:friagj]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hei TK, Zhou H, Ivanov VN, Hong M, Lieberman HB, Brenner DJ, Amundson SA, Geard CR. Mechanism of radiation-induced bystander effects: a unifying model. J. Pharm. Pharmacol. 2008;60:943–950. doi: 10.1211/jpp.60.8.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang H, Asaad N, Held KD. Medium-mediated intercellular communication is involved in bystander responses of X-ray-irradiated normal human fibroblasts. Oncogene. 2005;24:2096–2103. doi: 10.1038/sj.onc.1208439. [DOI] [PubMed] [Google Scholar]
- 11.Yang H, Anzenberg V, Held KD. The time dependence of bystander responses induced by iron-ion radiation in normal human skin fibroblasts. Radiat. Res. 2007;168:292–298. doi: 10.1667/RR0864.1. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Zhou J, Held KD, Redmond RW, Prise KM, Liber HL. Deficiencies of double-strand break repair factors and effects on mutagenesis in directly gamma-irradiated and medium-mediated bystander human lymphoblastoid cells. Radiat. Res. 2008;169:197–206. doi: 10.1667/RR1189.1. [DOI] [PubMed] [Google Scholar]
- 13.Chen S, Zhao Y, Han W, Zhao G, Zhu L, Wang J, Bao L, Jiang E, Xu A, Hei TK, Yu Z, L Wu. Mitochondria-dependent signalling pathway are involved in the early process of radiation-induced bystander effects. Br.J. Cancer. 2008;98:1839–1844. doi: 10.1038/sj.bjc.6604358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen S, Zhao Y, Zhao G, Han W, Bao L, Yu KN, Wu L. Up-regulation of ROS by mitochondria-dependent bystander signaling contributes to genotoxicity of bystander effects. Mutat. Res. 2009;666:68–73. doi: 10.1016/j.mrfmmm.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Tartier L, Gilchrist S, Burdak-Rothkamm S, Folkard M, Prise KM. Cytoplasmic irradiation induces mitochondrial-dependent 53BP1 protein relocalization in irradiated and bystander cells. Cancer Res. 2007;67:5872–5879. doi: 10.1158/0008-5472.CAN-07-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang G, Wu L, Chen S, Zhu L, Huang P, Tong L, Zhao Y, Zhao G, Wang J, Mei T, Xu A, Y Wang. Mitochondrial dysfunction resulting from loss of cytochrome c impairs radiation-induced bystander effect. Br. J. Cancer. 2009;100:1912–1916. doi: 10.1038/sj.bjc.6605087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou H, Ivanov VN, Lien YC, Davidson M, Hei TK. Mitochondrial function and nuclear factor-kappaB-mediated signaling in radiation-induced bystander effects. Cancer Res. 2008;68:2233–2240. doi: 10.1158/0008-5472.CAN-07-5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azzam EI, de Toledo SM, Gooding T, Little JB. Intercellular communication is involved in the bystander regulation of gene expression in human cells exposed to very low fluences of alpha particles. Radiat. Res. 1998;150:497–504. [PubMed] [Google Scholar]
- 19.Sokolov MV, Dickey JS, Bonner WM, Sedelnikova OA. gamma-H2AX in bystander cells: not just a radiation-triggered event, a cellular response to stress mediated by intercellular communication. Cell Cycle. 2007;6:2210–2212. doi: 10.4161/cc.6.18.4682. [DOI] [PubMed] [Google Scholar]
- 20.Nagasawa H, Huo L, Little JB. Increased bystander mutagenic effect in DNA double-strand break repair-deficient mammalian cells. Int. J. Radiat. Biol. 2003;79:35–41. [PubMed] [Google Scholar]
- 21.Zhou H, Randers-Pehrson G, Waldren CA, Vannais D, Hall EJ, Hei TK. Induction of a bystander mutagenic effect of alpha particles in mammalian cells. Proc. Natl. Acad. Sci. U. S. A. 2000;97:2099–2104. doi: 10.1073/pnas.030420797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagasawa H, Little JB. Induction of sister chromatid exchanges by extremely low doses of alpha-particles. Cancer Res. 1992;52:6394–6396. [PubMed] [Google Scholar]
- 23.Nagasawa H, Little JB. Bystander effect for chromosomal aberrations induced in wild-type and repair deficient CHO cells by low fluences of alpha particles. Mutat. Res. 2002;508:121–129. doi: 10.1016/s0027-5107(02)00193-8. [DOI] [PubMed] [Google Scholar]
- 24.Mothersill C, Seymour C. Medium from irradiated human epithelial cells but not human fibroblasts reduces the clonogenic survival of unirradiated cells. Int. J. Radiat. Biol. 1997;71:421–427. doi: 10.1080/095530097144030. [DOI] [PubMed] [Google Scholar]
- 25.Sawant SG, Randers-Pehrson G, Geard CR, Brenner DJ, Hall EJ. The bystander effect in radiation oncogenesis: I. Transformation in C3H 10T1/2 cells in vitro can be initiated in the unirradiated neighbors of irradiated cells. Radiat. Res. 2001;155:397–401. doi: 10.1667/0033-7587(2001)155[0397:tbeiro]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 26.Burdak-Rothkamm S, Short SC, Folkard M, Rothkamm K, Prise KM. ATR-dependent radiation-induced gamma H2AX foci in bystander primary human astrocytes and glioma cells. Oncogene. 2007;26:993–1002. doi: 10.1038/sj.onc.1209863. [DOI] [PubMed] [Google Scholar]
- 27.Burdak-Rothkamm S, Rothkamm K, Prise KM. ATM acts downstream of ATR in the DNA damage response signaling of bystander cells. Cancer Res. 2008;68:7059–7065. doi: 10.1158/0008-5472.CAN-08-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mothersill C, Seymour RJ, Seymour CB. Increased radiosensitivity in cells of two human cell lines treated with bystander medium from irradiated repair-deficient cells. Radiat. Res. 2006;165:26–34. doi: 10.1667/rr3488.1. [DOI] [PubMed] [Google Scholar]
- 29.Nagasawa H, Peng Y, Wilson PF, Lio YC, Chen DJ, Bedford JS, Little JB. Role of homologous recombination in the alpha-particle-induced bystander effect for sister chromatid exchanges and chromosomal aberrations. Radiat. Res. 2005;164:141–147. doi: 10.1667/rr3420. [DOI] [PubMed] [Google Scholar]
- 30.Kanasugi Y, Hamada N, Wada S, Funayama T, Sakashita T, Kakizaki T, Kobayashi Y, Takakura K. Role of DNA-PKcs in the bystander effect after low- or high-LET irradiation. Int. J. Radiat. Biol. 2007;83:73–80. doi: 10.1080/09553000601121116. [DOI] [PubMed] [Google Scholar]
- 31.Olivieri G, Bodycote J, Wolff S. Adaptive response of human lymphocytes to low concentrations of radioactive thymidine. Science. 1984;223:594–597. doi: 10.1126/science.6695170. [DOI] [PubMed] [Google Scholar]
- 32.Kadhim MA, Moore SR, Goodwin EH. Interrelationships amongst radiation-induced genomic instability, bystander effects, and the adaptive response. Mutat. Res. 2004;568:21–32. doi: 10.1016/j.mrfmmm.2004.06.043. [DOI] [PubMed] [Google Scholar]
- 33.Mothersill C, Seymour C. Radiation-induced bystander effects and adaptive responses--the Yin and Yang of low dose radiobiology? Mutat. Res. 2004;568:121–128. doi: 10.1016/j.mrfmmm.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 34.Olivieri G. Adaptive response and its relationship to hormesis and low dose cancer risk estimation. Hum. Exp. Toxicol. 1999;18:440–442. doi: 10.1191/096032799678840336. [DOI] [PubMed] [Google Scholar]
- 35.Preston RJ. Bystander effects, genomic instability, adaptive response, and cancer risk assessment for radiation and chemical exposures. Toxicol. Appl. Pharmacol. 2005;207:550–556. doi: 10.1016/j.taap.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 36.Rigaud O. The adaptive response to ionizing radiation: low dose effects unpredictable from high dose experiments. Hum. Exp. Toxicol. 1999;18:443–446. doi: 10.1191/096032799678840345. [DOI] [PubMed] [Google Scholar]
- 37.Skov KA. Perspectives on the adaptive response from studies on the response to low radiation doses (or to cisplatin) in mammalian cells. Hum. Exp. Toxicol. 1999;18:447–451. doi: 10.1191/096032799678840354. [DOI] [PubMed] [Google Scholar]
- 38.Streffer C. Bystander effects, adaptive response and genomic instability induced by prenatal irradiation. Mutat. Res. 2004;568:79–87. doi: 10.1016/j.mrfmmm.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 39.Wolff S. The adaptive response in radiobiology: evolving insights and implications. Environ. Health Perspect. 1998;106 Suppl 1:277–283. doi: 10.1289/ehp.98106s1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crawford DR, Davies KJ. Adaptive response and oxidative stress. Environ. Health Perspect. 1994;102 Suppl 10:25–28. doi: 10.1289/ehp.94102s1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sasaki MS, Ejima Y, Tachibana A, Yamada T, Ishizaki K, Shimizu T, Nomura T. DNA damage response pathway in radioadaptive response. Mutat. Res. 2002;504:101–118. doi: 10.1016/s0027-5107(02)00084-2. [DOI] [PubMed] [Google Scholar]
- 42.Wojewodzka M, Kruszewski M, Szumiel I. Effect of signal transduction inhibition in adapted lymphocytes: micronuclei frequency and DNA repair. Int. J. Radiat. Biol. 1997;71:245–252. doi: 10.1080/095530097144111. [DOI] [PubMed] [Google Scholar]
- 43.Wolff S, Afzal V, Jostes RF, Wiencke JK. Indications of repair of radon-induced chromosome damage in human lymphocytes: an adaptive response induced by low doses of X-rays. Environ. Health Perspect. 1993;101 Suppl 3:73–77. doi: 10.1289/ehp.93101s373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coleman MA, Yin E, Peterson LE, Nelson D, Sorensen K, Tucker JD, Wyrobek AJ. Low-dose irradiation alters the transcript profiles of human lymphoblastoid cells including genes associated with cytogenetic radioadaptive response. Radiat. Res. 2005;164:369–382. doi: 10.1667/rr3356.1. [DOI] [PubMed] [Google Scholar]
- 45.Sawant SG, Randers-Pehrson G, Metting NF, Hall EJ. Adaptive response and the bystander effect induced by radiation in C3H 10T(1/2) cells in culture. Radiat. Res. 2001;156:177–180. doi: 10.1667/0033-7587(2001)156[0177:aratbe]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 46.Zhou H, Randers-Pehrson G, Geard CR, Brenner DJ, Hall EJ, Hei TK. Interaction between radiation-induced adaptive response and bystander mutagenesis in mammalian cells. Radiat. Res. 2003;160:512–516. doi: 10.1667/rr3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iyer R, Lehnert BE. Low dose, low-LET ionizing radiation-induced radioadaptation and associated early responses in unirradiated cells. Mutat. Res. 2002;503:1–9. doi: 10.1016/s0027-5107(02)00068-4. [DOI] [PubMed] [Google Scholar]
- 48.Maguire P, Mothersill C, McClean B, Seymour C, Lyng FM. Modulation of radiation responses by pre-exposure to irradiated cell conditioned medium. Radiat. Res. 2007;167:485–492. doi: 10.1667/RR0159.1. [DOI] [PubMed] [Google Scholar]
- 49.Mitchell SA, Marino SA, Brenner DJ, Hall EJ. Bystander effect and adaptive response in C3H 10T(1/2) cells. Int. J. Radiat. Biol. 2004;80:465–472. doi: 10.1080/09553000410001725116. [DOI] [PubMed] [Google Scholar]
- 50.Dominguez I, Panneerselvam N, Escalza P, Natarajan AT, Cortes F. Adaptive response to radiation damage in human lymphocytes conditioned with hydrogen peroxide as measured by the cytokinesis-block micronucleus technique. Mutat. Res. 1993;301:135–141. doi: 10.1016/0165-7992(93)90036-u. [DOI] [PubMed] [Google Scholar]
- 51.Shankar B, Pandey R, Sainis K. Radiation-induced bystander effects and adaptive response in murine lymphocytes. Int. J. Radiat. Biol. 2006;82:537–548. doi: 10.1080/09553000600877114. [DOI] [PubMed] [Google Scholar]
- 52.Tapio S, Jacob V. Radioadaptive response revisited. Radiat. Environ. Biophys. 2007;46:1–12. doi: 10.1007/s00411-006-0078-8. [DOI] [PubMed] [Google Scholar]
- 53.Amundson SA, Xia F, Wolfson K, Liber HL. Different cytotoxic and mutagenic responses by X-rays in two human lymphoblastoid cell lines derived from a single donor. Mutation Res. 1993;286:233–241. doi: 10.1016/0027-5107(93)90188-l. [DOI] [PubMed] [Google Scholar]
- 54.Mothersill C, Seymour CB. Bystander and delayed effects after fractionated radiation exposure. Radiat. Res. 2002;158:626–633. doi: 10.1667/0033-7587(2002)158[0626:badeaf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 55.Furth EE, Thilly WG, Penman BW, Liber HL, Rand WM. Quantitative assay for mutation in diploid human lymphoblasts using microtiter plates. Anal. Biochem. 1981;110:1–8. doi: 10.1016/0003-2697(81)90103-2. [DOI] [PubMed] [Google Scholar]
- 56.Kalina I, Nemethova G. Variability of the adaptive response to low dose radiation in peripheral blood lymphocytes of twins and unrelated donors. Folia Biol. (Praha) 1997;43:91–95. [PubMed] [Google Scholar]
- 57.Sorensen KJ, Attix CM, Christian AT, Wyrobek AJ, Tucker JD. Adaptive response induction and variation in human lymphoblastoid cell lines. Mutat. Res. 2002;519:15–24. doi: 10.1016/s1383-5718(02)00110-9. [DOI] [PubMed] [Google Scholar]
- 58.Vijayalaxmi, Leal BZ, Deahl TS, Meltz ML. Variability in adaptive response to low dose radiation in human blood lymphocytes: consistent results from chromosome aberrations and micronuclei. Mutat. Res. 1995;348:45–50. doi: 10.1016/0165-7992(95)90020-9. [DOI] [PubMed] [Google Scholar]
- 59.Schwartz JL, Jordan R, Slovic J, Moruzzi AM, Kimmel RR, Liber HL. Induction and loss of a TP53-dependent radioadaptive response in the human lymphoblastoid cell model TK6 and its abrogation by BCL2 over-expression. Int. J. Radiat. Biol. 2007;83:153–159. doi: 10.1080/09553000601146949. [DOI] [PubMed] [Google Scholar]