Abstract
Nanoparticles have been explored recently as an efficient means of delivering photosensitizers for cancer diagnosis and photodynamic therapy (PDT). Silicon phthalocyanine 4 (Pc4) is currently being clinically tested as a photosensitizer for PDT. Unfortunately, Pc4 aggregates in aqueous solutions, which dramatically reduces its PDT efficacy and therefore limits its clinical application. We have encapsulated Pc4 using silica nanoparticles (Pc4SNP), which not only improved the aqueous solubility, stability, and delivery of the photodynamic drug but also increased its photodynamic efficacy compared to free Pc4 molecules. Pc4SNP generated photo-induced singlet oxygen more efficiently than free Pc4 as measured by chemical probe and EPR trapping techniques. Transmission electron microscopy and dynamic light scattering measurements showed that the size of the particles is in the range of 25-30 nm. Cell viability measurements demonstrated that Pc4SNP was more phototoxic to A375 or B16-F10 melanoma cells than free Pc4. Pc4SNP photodamaged melanoma cells primarily through apoptosis. Irradiation of A375 cells in the presence of Pc4SNP resulted in a significant increase in intracellular protein-derived peroxides, suggesting a Type II (singlet oxygen) mechanism for phototoxicity. More Pc4SNP than free Pc4 was localized in the mitochondria and lysosomes. Our results show that these stable, monodispersed silica nanoparticles may be an effective new formulation for Pc4 in its preclinical and clinical studies. We expect that modifying the surface of silicon nanoparticles encapsulating the photosensitizers with antibodies specific to melanoma cells will lead to even better early diagnosis and targeted treatment of melanoma in the future.
Keywords: Photodynamic therapy, Nanoparticles, Melanoma, Phthalocyanine, Singlet oxygen, Apoptosis
Introduction
Melanoma is the most aggressive form of skin cancer. Although melanoma accounts for only 4 percent of all dermatologic cancers, it is responsible for 80 percent of the deaths. The existing chemotherapeutic strategies have shown little effect against metastatic melanoma. In addition, they all cause serious side effects because of the loss of normal cell function due to non-specific targeting of the treatments. A potential new approach for treatment of dermal melanoma is photodynamic therapy, in which a photosensitizing compound preferentially accumulates in tumors, and when the tumor is irradiated with visible light, the photosensitizer produces singlet oxygen that destroys the surrounding tumor cells (Dolmans et al., 2003; Brown et al., 2004).
Photodynamic Therapy using a porphyrin derivative (Photofrin®) and visible light below 640 nm was approved by the FDA for treatment against human tumors in 1998 (Dougherty et al., 1998). Over the last decade, a substantial effort has been directed towards improving this treatment. We have previously developed a method of selective protection of normal tissues for the eye, bladder, and skin (Bedwell et al., 1991; Reme et al., 1991; Roberts et al., 1991; Roberts and Dillon, 1993; Roberts et al., 1994) while allowing PDT therapy to destroy tumors. There has also been continuous development of several new classes of photosensitizers that can be excited at wavelengths longer than 640 nm, which allows deeper penetration, greater tumor specificity, and less cutaneous photosensitivity than Photofrin (Ali and van Lier, 1999; Detty et al., 2004; Nyman and Hynninen, 2004; Wainwright, 2008).
A recent class of dyes that shows great potential as second-generation photosensitizers is the phthalocyanines, which absorb strongly in the red visible region, allowing a deeper light penetration, high efficiency of singlet oxygen generation, and ease of chemical modification to facilitate the tailoring of properties such as aggregation and cellular uptake (Ali and van Lier, 1999; Lukyanets, 1999; Allen et al., 2001; Detty et al., 2004; Nyman and Hynninen, 2004; Lo et al., 2008). One of the most efficient phthalocyanine-based photosensitizers, silicon phthalocyanine 4 (Pc4, Figure 1a), exhibits high photodynamic activities, both in vitro and in vivo, against a range of model cell lines and tumors (Ahmad et al., 1998; Kalka et al., 2000; Whitacre et al., 2000; Chiu et al., 2001; Srivastava et al., 2001; Morris et al., 2002; Usuda et al., 2003). Pc4 has already entered Phase I clinical trials. However, like most of the phthalocyanines, Pc4 is a lipophilic compound and under physiological conditions is essentially insoluble, unstable, and easily aggregated in water, which not only complicates the normal administration and biodistribution of this drug in vivo, but also dramatically reduces its photodynamic activity. This may be a major limiting factor for its potential clinical application.
Figure 1.

(a) Chemical structure of Pc4 and (b) schematic diagram of Pc4 containing silica nanoparticles (Pc4SNP). (c) TEM images of Pc4SNP. (d) DLS determination of particle size and distribution of Pc4SNP. The number (%) in the Y axis represents the percent of mean number of nanoparticles while the X axis represents the size of the particles.
Some of these problems were addressed by incorporating photodynamic photosensitizers into various nano-carriers including liposomes (Nishiwaki et al., 2002), gold nanoparticles (Wieder et al., 2006), polymeric nanoparticles (Konan et al., 2003), and silica nanoparticles (Roy et al., 2003; Tu et al., 2009). Incorporating them into nanoparticle carriers means they can be engineered to modulate their release and stability and to prolong the circulation time, protecting them from elimination by phagocytic cells or premature degradation. The extremely high surface-to-volume ratios of nanocarriers can also dramatically improve the reactivity of the drug. Moreover, nanoscale carriers can be tailored to accumulate in tumor cells and tissues, due to both enhanced permeability and retention in tumor cells and active targeting using ligands designed to recognize tumor-associated molecular markers (Brigger et al., 2002; Konan et al., 2002; Haag, 2004; Wang et al., 2004; Chatterjee et al., 2008). Recently silica-based nanoparticles have been widely developed as an efficient means for drug and gene delivery platforms due to their unique advantages such as small and uniform pore size, large surface area and pore volume, as well as nontoxicity and biocompatibility (Roy et al., 2003; Brevet et al., 2009; Cheng et al., 2009; Tu et al., 2009). The porous structure of silica nanoparticles can not only act as a suitable carrier for hydrophobic photosensitizers, but also allow the oxygen and generated singlet oxygen permeability that is essential for PDT therapy (Roy et al., 2003; Bechet et al., 2008).
In the current work, we have encapsulated Pc4 into a silica nanoparticle that not only preserves the photodynamic activity of Pc4 but also provides a basis for future conjugation with targeted molecules. Our results demonstrate that the encapsulation in nanoparticles greatly improves the photo-stability and photodynamic efficacy of Pc4. In addition, we have measured and compared the generation of singlet oxygen, and carried out in vitro experiments to elucidate the mechanisms of phototoxicity. This formulation may represent an innovative delivery system for photosensitizers used for PDT therapy of tumors.
Materials and methods
Drugs and reagents
Pc4 was obtained from the Developmental Therapeutics Program, NCI/NIH (Bethesda, MD) (NSC 676418/5). Tween-80, 1-butanol, triethoxyvinylsilane (TEVS), aqueous ammonia (28-30%), 9,10-anthracenediyl-bis(methylene)dimalonic acid (ABMDMA) and 2,2,6,6-tetramethyl-4-piperidone (TEMP) were all purchased from Sigma-Aldrich, Inc. (St. Louis, MO). Dulbecco's Modified Eagle's Medium (DMEM, Cat. No. 30-2002) was from ATCC (Manassas, VA). Fetal bovine serum (FBS) was from Biofluids (Rockville, MD, USA). Trypsin-EDTA was from GIBCO Invitrogen Corporation (Carlsbad, CA, USA).
Preparation of Pc4-containing silica nanoparticles (Pc4SNP) and free Pc4 solution
Pc4SNP was prepared by the method of Ohulchanskyy, et al. (Ohulchanskyy et al., 2007) with some modification. Briefly, to 10 mL of 2% aqueous Tween-80 solution, 300 μL of the co-surfactant 1-butanol was added. Then, 50 μL of a solution of Pc4 in DMF (10 mM) was added by magnetic stirring. Next, 100 μL of TEVS was added and the system was stirred until it became clear (about one hour). After that, 10 μL of aqueous ammonia was added and the system was stirred for about 24 h in the dark. After the formation of the nanoparticles, surfactant Tween-80 and 1-butanol were removed by dialyzing the solution against D.I. water in a 15- kDa cutoff cellulose ester membrane for 48 h. The dialyzed solution then was filtered through a 0.22-μm cutoff membrane (surfactant free cellulose acetate) filter and was stored at 4 °C for further experimentation. The concentration of the Pc4 in the silica nanoparticles was determined by spectrophotometric methods. A 50 μL sample was dissolved in 1.5 mL of DMF and the concentration was calculated via the Lambert-Beer law by the use of absorbance at 668 nm (log ε = 5.13). Free Pc4 solution was prepared by dilution of the 1 mM Pc4/DMF stock solution with water or medium.
Characterization
Transmission electron microscopy (TEM) images were taken on a Tecnai-12 Bio-Twin transmission electron microscope (FEI, The Netherlands) operating at 80 kV. TEM grids were prepared by evaporating approximately 20 μL of Pc4SNP solution onto a 300 mesh carbon-coated copper grid. Dynamic light scattering (DLS) measurements of particle size were carried out using a light scattering Zetasizer Nano-S90 light scattering instrument (Malvern Instruments, Enigma Business Park, UK). All absorption spectra were recorded on an Agilent 8453 UV-visible spectrophotometer (Agilent Technologies, Inc., Santa Clara, CA). Fluorescence spectra and lifetime decays were recorded on an FL3-22 spectrofluorometer (HORIBA Jobin Yvon Inc., NJ).
Photobleaching measurements
Water solutions containing 10 μM Pc4SNP and Pc4 were prepared respectively. The solution was irradiated in an open quartz cuvette with continuous stirring. The absorbance measurements followed by irradiation were carried out every 2 min. A 450 W xenon-mercury lamp was used as the light source. A long-pass glass filter (Barloworld Scientific) was used to cut off wavelengths below 550 nm.
Singlet oxygen detection by photobleaching of ABMDMA
Photo-induced singlet oxygen generation was determined by photobleaching of the chemical probe 9,10-anthracenediyl-bis(methylene)dimalonic acid (ABMDMA). ABMDMA is a water soluble derivative of anthracene that can be photobleached by singlet oxygen to its corresponding endoperoxide. The reaction was monitored spectrophotometrically by recording the decrease in optical density at 400 nm (λmax of ABMDMA). In a typical experiment, 0.15 mM of the sodium salt of ABMDMA in water was mixed with Pc4SNP or Pc4 to give a final concentration of 2 μM. The control experiment used 0.15 mM of the sodium salt of ABMDMA and void silica nanoparticles in water. The procedure and light source was the same as listed above (Photobleaching Measurements).
Detection of singlet oxygen by EPR
Generation of singlet oxygen was also detected by the EPR trapping technique using 2,2,6,6-tetramethyl-4-piperidone (TEMP). It has been previously reported that TEMPO, a nitroxide radical detectable by EPR spectra, is generated from TEMP and singlet oxygen (Eq. 1) (Lion et al., 1976).
 |
(1) |
All EPR spectra were recorded at room temperature in a quartz flat cell on a Bruker EMX EPR spectrometer equipped with a super high-Q cavity (Bruker, Billerica, MA) and a Varian E-109 Century line magnet (Varian Associates, Palo Alto, CA) operating at 9.78 GHz with 100-kHz modulation. Spectra were recorded using an IBM-compatible computer interfaced with the spectrometer with the following instrument settings and conditions: 10 mW microwave power, 100 kHz modulation frequency, 1 G modulation amplitude, 20.5 ms time constant, 21 s scan time, and multiple scans of 80 G. Where indicated, samples were placed in a quartz flat cell and irradiated directly inside the microwave cavity of the spectrometer using a 1 kW Xe arc lamp. Radiation from the lamp was passed through a long-pass glass filter to remove wavelengths below 550 nm.
Cell culture and in vitro PDT
The non-pigmented human melanoma A375 and pigmented mouse melanoma (B16F10) cell lines were used in these studies. Cells were grown in Dulbecco's Modified Eagle's Medium (DMEM) containing 10% FBS in an atmosphere of 5% CO2 /95% air at 37°C. Cells were fed 3 times a week and after attaining confluence were passaged using 0.25% trypsin–EDTA. For in vitro PDT tests, cells were exposed in the dark for 2 h at 37°C to different concentrations of Pc4SNP or Pc4 in DMEM (FBS free). Control cells were treated with DMEM alone. After incubation the medium was removed and replaced by PBS. Cells were then irradiated with a 300 W halogen lamp, a water tank for cooling, and a long-pass glass filter (Barloworld Scientific) with a cut-off of 550 nm. The fluence rate (λ > 550 nm) was 25 mW cm-2 as measured with a SPR-4001 Spectroradiometer (Luzchem Research Inc., Ottawa Ontario, Canada). An irradiation time of 15 min led to a total fluence of 22.5 J cm-2 (6.6 J cm-2 for light between 600 to 700 nm). After exposure, the PBS solution was removed and replaced with DMEM containing 2% FBS and the cells were kept in the incubator overnight. The medium was removed, and the cells washed with PBS and then treated with 120 μL/well of PBS/glucose containing the MTS reagent (CellTiter 96 Aqueous Proliferation Assay; Promega Corp.). After incubation for 2 h at 37°C, the absorbance at 492 nm was recorded using a microplate reader (Spectrafluor Plus, Tecan US, RTP, NC).
Measurement of apoptotic and necrotic cells
Apoptotic and necrotic cells were quantitatively evaluated by flow cytometry (Martin et al., 1995; Reno et al., 1998). After treatment, the cells were harvested by trypsinization and collected by centrifugation at 300 g for 5 min at room temperature. Cells were washed with cold phosphate buffered saline (PBS) and stained with Annexin V-FITC and propidium iodide (PI) using a TACS™ Apoptosis Detection Kit according to the manufacturer's instructions (Trevigen, Gaithersburg, MD). Cells positive for PI, for Annexin V-FITC, or for both were quantified by flow cytometry using a Becton Dickinson FACSort (Becton Dickinson, Mountain View, CA). In the fluorescence dot plot histogram of Annexin V/PI stained cells, the lower left quadrant shows normal viable cells which are negative for both Annexin V and PI; the lower right quadrant shows early-apoptotic cells which are positive for Annexin V; the upper left quadrant shows necrotic cells which are positive for PI; while the upper right quadrant shows late-apoptotic cells which are positive for both Annexin V and PI (Martin et al., 1995; Reno et al., 1998).
Measurement of protein peroxides
To measure protein peroxides, A375 cells were suspended in PBS, and 2 mL aliquots containing 8 × 106 cells were placed in individual wells of a 6-well plate (Becton Dickinson, Franklin Lakes, NJ). After the addition of 2 μM Pc4SNP or Pc4, the cells were incubated at 37°C for 2 h and then exposed to the light as described above for 15 min. Control cells either contained no Pc4 or were kept in the dark. Where indicated, sodium azide (20 mM) was present during treatment. Intracellular protein peroxides were assayed using a modified FOX assay as described by Wright et al. (Wright et al., 2003). The absorbance was measured at 560 nm and compared to a standard curve prepared using H2O2.
Fluorescence confocal imaging
We employed fluorescence microscopy to study cellular uptake and subcellular localization. A375 and B16F10 cells were seeded into 35 mm dishes containing a glass coverslip-covered 14 mm cutout (MatTek, Ashland, MA) for live cell microscopy measurement. Cells were incubated with 2 μM Pc4 or Pc4SNP for 2 hours at 37°C. For measurement of subcellular localization, cells were further stained with 100 nM MitoTracker Green FM (Invitrogen/Molecular Probes, Eugene, OR), which is a specific fluorescence dye for mitochondria, or 60 nM LysoTraker Green DND-26 (Invitrogen/Molecular Probes, Eugene, OR), which is a specific fluorescence dye for lysosomes. Confocal fluorescence imaging was performed with a Zeiss LSM-510 META confocal microscope. For Pc4 fluorescence, excitation was carried out at 633 nm and emission was collected for wavelengths greater than 650 nm. For MitoTracker Green FM or LysoTracker Green DND-26, fluorescence, excitation was carried out at 488 nm and emission was collected for wavelengths between 505 and 550 nm.
Statistical analyses
Data were expressed as the mean of three independent experiments and were analyzed by Student's t test or ANOVA. A two-sided P value of <0.05 was considered significant in all cases.
Results
Particle size and distribution of Pc4SNP
Hydrophobic Pc4 photosensitizer was successfully encapsulated by silica nanoparticles (Pc4SNP, Figure 1a, b) in a Tween-80/1-butanol/water microemulsion. The TEM (Figure 1c) images clearly showed that the Pc4SNPs were spherical, uniform, and monodispersed with sizes of about 25-30 nm. DLS measurements (Figure 1d) confirmed that the particle size was about 28 nm with a narrow distribution.
Spectroscopic characterization
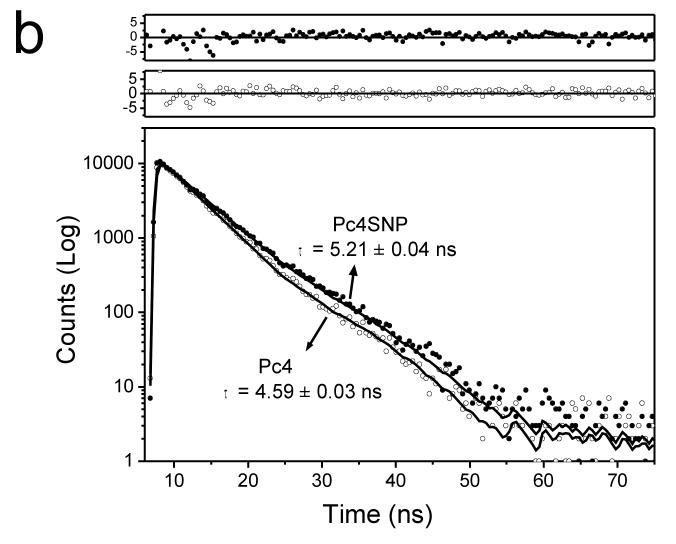
The UV-visible absorption spectra and fluorescence spectra of 10 μM Pc4 encapsulated in silica nanoparticles (Pc4SNP) were similar to those of free Pc4 molecules in aqueous solution (Figure 2a). The absorption spectra of both show a clear Q-band at 674 nm, which suggests the non-aggregation of the phthalocyanines (Choi et al., 2000). Both absorption and fluorescence spectra of Pc4SNP were blue-shifted compared to Pc4. At the excitation wavelength, the fluorescence intensity of Pc4SNP was higher than that of Pc4 at the same absorbance. The fluorescence lifetime of Pc4SNP was estimated to be 5.21 ns (single exponential), which was longer than that of Pc4 (4.59 ns, single exponential, Figure 2b).
Figure 2.
(a) Differences in UV-Vis absorption and fluorescence emission spectra (inset, Ex: 636 nm) of 10 μM Pc4SNP and Pc4 in aqueous solution. (b) Fluorescence decays of 5 μM Pc4SNP and Pc4 in aqueous solution (Ex: 636 nm, Em: 680 nm). Both decays are fit to a single exponential function with a time constant of 5.21 ± 0.04 ns for Pc4SNP and 4.59 ± 0.03 ns for Pc4. The top set of residuals is for Pc4SNP; the bottom set, for Pc4.
Photobleaching of Pc4SNP and Pc4
It is well known that photosensitizers used in PDT can be photobleached, followed by the loss of absorbance, fluorescence and photoactivity (Ma et al., 1994). Encapsulation of Pc4 in the silica nanoparticles reduced and delayed the photobleaching and thus protected its photodynamic effectiveness. Figure 3 shows the absorbance changes of Pc4SNP (673 nm) and Pc4 (676 nm) in aqueous solution as a function of irradiation time. It clearly shows that the encapsulated Pc4 molecules were more photo-stable than free Pc4 during irradiation. We also found that there are three phases for photobleaching of Pc4SNP. In contrast to the approximately 54% decrease of absorbance in free Pc4 solution, the Pc4-containing silica nanoparticles exhibited a minor absorbance change (12%) in the first 12 min (Phase I). From 12 to 22 min (Phase II) Pc4SNP exhibited acceleration in photobleaching and then a slowdown after 22 min of irradiation (Phase III).
Figure 3.
Photobleaching of 10 μM Pc4SNP and Pc4 in aqueous solution during irradiation (>550 nm) measured by UV-Vis absorption at 673 nm for Pc4SNP and 676 nm for Pc4 as a function of time. Values are the means ± SEM (n = 3).
Detection of singlet oxygen generation
Singlet oxygen (1O2) is a highly reactive oxygen species and believed to play a key role in the efficacy of PDT. To verify the generation of singlet oxygen we used both the chemical probe 9,10-anthracenediyl-bis(methylene)dimalonic acid (ABMDMA) and the EPR trapping technique using 2,2,6,6-tetramethyl-4-piperidone (TEMP).
As shown in Figure 4, the chemical measurements using ABMDMA showed a significant decrease in absorbance at 400 nm (line 3) for Pc4SNP with ABMDMA after irradiation, suggesting a high efficiency in generation of reactive 1O2. For the Pc4 sample (line 2), the photobleaching rate caused by 1O2 was much lower than that of Pc4SNP. There was no obvious decrease in absorbance for a solution containing ABMDMA and void silica nanoparticles (line 1).
Figure 4.
Photobleaching of 0.15 mM 9,10-anthracenediyl-bis(methylene)dimalonic acid (ABMDMA) by singlet oxygen generated by (i) void silica nanoparticles as control; (ii, iii) 2 μM Pc4SNP and Pc4 water solution. The change in ABMDMA absorption at 400 nm was measured as a function of irradiation time. Inset: absorption spectra of 0.15 mM ABMDMA water solution containing 2 μM Pc4SNP as a function of irradiation times (0 to 360 sec). Values are the means ± SEM (n = 3).
The generation of singlet oxygen was also determined by the EPR trapping technique using TEMP. Irradiation of a Pc4SNP or Pc4 sample (10 μM) containing 20 mM TEMP resulted in EPR spectra of three lines with equal intensities (aN = 16.0 G), typical of nitroxide radicals (inset, Figure 5). The hyperfine splitting constant and g factor of the photosensitized oxidation product of TEMP were identical to those of commercial TEMPO. Figure 5 shows the EPR intensity of the TEMPO signal as a function of time during irradiation of different solutions. The Pc4SNP (line 1) solution showed the fastest increasing rate while Pc4 (line 2) was much slower. A control solution with TEMP only (line 4) indicated no EPR signal increase. For Pc4SNP, addition of a singlet oxygen quencher, NaN3, caused a dramatic decrease in rate of increase of the EPR signal (line 3).
Figure 5.
Plot of the intensity of TEMPO signal as a function of time during irradiation. The TEMPO signal was measured by the EPR technique for solutions containing: 20 mM TEMP and (i, ii) 10 μM Pc4SNP and Pc4 water solution; (iii) 10 μM Pc4SNP in addition to 10 mM NaN3 in water (iv) H2O only as control. Measurements were made for 21-second periods, with 9-second intervals between each measurement. During the measurements, the samples were exposed to irradiation (λ>550 nm). Values are the means ± SEM (n = 3). Inset: EPR signal of TEMPO (aN = 16.0 G). Instrumental settings: microwave power, 10 mW; modulation frequency, 100 kHz; modulation amplitude, 1 G; time constant, 20.5 ms; 21.0 s scan time; scan range, 80 G.
In vitro PDT studies on melanoma cells
The photodynamic activity of Pc4SNP and Pc4 towards A375 and B16F10 melanoma cells was measured using the MTS assay (which mainly measures the activity of mitochondrial dehydrogenases) (Figure 6). While no essential effect on viability was observed when cells were exposed to either Pc4 or Pc4SNP in the dark or to light (>550 nm) alone, they caused a concentration-dependent loss of mitochondrial activity in the presence of light irradiation. As shown in Figure 6, they both exhibited high phototoxicity, with IC50 values of ∼5 and ∼30 nM for A375 cells, respectively. Apparently, the phototoxicity of Pc4SNP was much higher than that of Pc4. Also the photodynamic activity of Pc4 photosensitization was reduced in pigmented B16F10 melanoma cells (Figure 6b) compared to non-pigmented A375 cells (Figure 6a).
Figure 6.
Photodynamic effect of different concentrations of Pc4 and Pc4SNP exposure on the viability of (a) A375 and (b) B16F10 melanoma cells in the absence and presence of light (22.5 J/cm2) as measured by the MTS assay (see Materials and methods). Values are the means ± SEM (n = 4).
Analysis of Pc4 and Pc4SNP photo-induced apoptosis and necrosis
We used flow cytometry to quantify necrotic and apoptotic A375 cells induced by Pc4SNP and light (> 550 nm) irradiation. Early apoptosis is characterized by plasma membrane reorganization (Martin et al., 1995; Reno et al., 1998; Singh, 2000) and is detected by positive staining for Annexin V-FITC while later stage apoptosis indicating DNA damage shows positive staining for both Annexin V and PI. We measured necrosis by determining the percentage of cells which were positive for only PI. In the absence of light, Pc4 or Pc4SNP concentrations up to 40 nM had no effect on apoptosis or necrosis (Figure 7g). However, cells exposed to 5, 10, 20 and 40 nM Pc4 or Pc4SNP plus light exhibited a concentration-dependent increase in apoptosis (Figure 7b–e, g). The photo-induced apoptosis by Pc4SNP was much higher than that of Pc4 (p < 0.05). Pretreatment of cells with the singlet oxygen quencher NaN3 showed significant protection against the Pc4/Pc4SNP-induced apoptosis on light exposure (Figure 7f, g, p < 0.05). Exposure of A375 cells to light alone had no obvious effect on apoptosis and necrosis (Figure 7a).
Figure 7.

Pc4 and Pc4SNP induced apoptotic and necrotic death in A375 cells after irradiation. (a–e) Cells were seeded in individual wells of a 6-well plate, pretreated with Pc4SNP of different concentrations in DMEM (FBS free) for 2 hours, and then exposed to the light (15 J/cm2). After irradiation, the cells were incubated overnight in cell culture medium (2% FBS) and then stained with Annexin V-FITC and propidium iodide. Apoptotic and necrotic cell death were determined with flow cytometry. For NaN3 protection assay (f), cells were incubated with 20 mM NaN3 during the incubation period as well as the irradiation period. (g) The graphs illustrate photo-induced Pc4 and Pc4SNP dose-dependent apoptosis and necrosis and the protection effect by NaN3.
Intracellular formation of protein peroxides
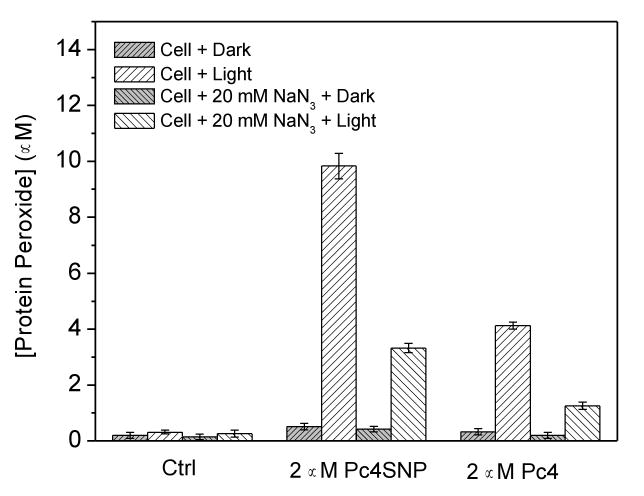
To further investigate the mechanism of Pc4SNP phototoxicity, we used a modified FOX (Wright et al., 2003; Ohulchanskyy et al., 2007) assay to measure the formation of protein peroxides in A375 cells loaded with Pc4SNP or Pc4. It is well known that singlet oxygen can oxidize many biomolecules, including membrane lipids, amino acids, cholesterol, and thiols. Furthermore, proteins are known to be the major intracellular targets for singlet oxygen due to their abundance and fast rates of reaction (Kochevar et al., 1994; Wilkinson et al., 1995; Wright et al., 2003). As shown in Figure 8, negligible levels of protein peroxides were detected in control cells or in cells which had been pre-incubated with Pc4SNP or Pc4 and then kept in the dark. Exposure of the control cells to light (>550 nm) irradiation generated 0.31 μM protein peroxides, which increased to 9.8 μM and 4.1 μM in the presence of 2 μM Pc4SNP and Pc4, respectively. When the singlet oxygen quencher NaN3 (20 mM) was present during irradiation, the protein peroxide level decreased to 0.26 μM, 3.3 μM, and 1.3 μM, respectively. These data indicate the presence of high levels of singlet oxygen-mediated protein peroxides in A375 cells exposed to Pc4SNP or Pc4 and light irradiation. Also, the level of protein peroxides caused by Pc4SNP was much higher than that of Pc4, which is consistent with the observed singlet oxygen generation and cell viability results.
Figure 8.
Effect of Pc4SNP and Pc4 exposure on the formation of protein peroxides during illumination of A375 cells in the absence or presence of 20 mM sodium azide. Cells (4 × 106 cells ml-1) were incubated with 2 μM Pc4SNP or Pc4 for 2 hours before illumination (22.5 J/cm2).
In vitro imaging and subcellular localization
After incubation with Pc4 or Pc4SNP for 2 h and upon excitation at 633 nm, the A375 (Figure 9a1, a2) and B16F10 (Figure 9b1, b2) cells showed a strong intracellular fluorescence, indicating substantial uptake of the Pc4 dyes. After treatment with Pc4 or Pc4SNP, we further stained the cells with either MitoTracker Green FM or LysoTracker Green DND-26, which is a specific fluorescence dye for mitochondria and lysosomes, respectively. Figures 9c and 9e show the images for Pc4 and Pc4SNP in A375 cells, which are similar to those for both Pc4 and Pc4SNP in B16F10 cells (data not shown). It can be seen that the fluorescence caused by the Pc4 (Ex/Em, 633 nm/LP 650 nm) is diffused throughout the cytoplasm and is partially taken up by the mitochondria as indicated by the fluorescence caused by MitoTracker (Ex/Em, 488 nm/BP 505-550 nm). More Pc4SNP than free Pc4 was localized in mitochondria. In addition, Pc4 and Pc4SNP were partially localized in lysosomes as visualized by co-staining cells with LysoTracker (Figure 9d, 9f). It appears that more Pc4SNP than free Pc4 is co-localized with LysoTracker staining, suggesting that silica nanoparticle encapsulation enhances lysosomal accumulation of Pc4.
Figure 9.

Confocal fluorescence images of (a1, a2) A375 and (b1, b2) B16F10 melanoma cells after incubation with 2 μM Pc4 or Pc4SNP (a1 and b1: Pc4; a2 and b2: Pc4SNP) for 2 hours (Ex/Em, 633 nm/LP 650 nm). (c), (d), (e), (f) Visualization of intracellular fluorescence of A375 cells using filter sets specific for Pc4 or Pc4SNP (in red; Ex/Em, 633 nm/LP650 nm), the MitoTracker or LysoTracker (in green; Ex/Em, 488 nm/BP 505-550 nm), and the corresponding superimposed image.
Discussion
Melanoma is a deadly disease that is often difficult to detect in its early stages and difficult to cure in the late stages. Early detection can be accomplished non-invasively with fluorescence imaging (Ntziachristos, 2006). Photosensitizers used for PDT therapy, including Pc4, are taken up by tumors and fluoresce, allowing for detection of suspicious lesions in the very early stages.
Current clinical trials are investigating the potential of Pc4 to treat melanoma using PDT Therapy. It has been found that ultra small size particles (less than 50 nm) are more easily taken up and retained in tumor cells and achieve better targeting by avoiding the reticuloendothelial system (RES) (Brannon-Peppas and Blanchette, 2004). Consequently, we formulated silica nanoparticles containing the hydrophobic phthalocyanine photosensitizer Pc4 with a typical diameter of 25-30 nm (Figure 1). We have found that encapsulation of the hydrophobic photosensitizer Pc4 into silica nanoparticles has the potential to reveal previously undetected tumors because of its enhanced fluorescence. Encapsulation of Pc4 into silicon nanoparticles also improved its tumor targeting properties and singlet oxygen production while decreasing photobleaching and aggregation.
We examined the absorption and fluorescence properties of Pc4 versus the encapsulated Pc4. There was little difference (Figure 2) between Pc4 and Pc4 in silica nanoparticles: both have several Q bands between 600 and 700 nm with a sharp Q-band at 674 nm. We did find enhanced fluorescence intensity of the encapsulated molecules compared to free Pc4 in aqueous solution (Figure 2a), and the fluorescence lifetime of Pc4SNP was slightly longer than that of free Pc4 (Figure 2b), possibly due to reduced nonradiative transitions of the confined photosensitizer molecules inside the nanoparticles. Encapsulated Pc4SNP (Figure 9a2, b2) also fluoresced more strongly in cells than free Pc4 (Figure 9a1, b1) and remained fluorescent longer, thus improving its precision in diagnosis. We have also used fluorescence to determine the exact location of the sensitizer in the cell. Confocal fluorescent microscopy confirmed that both Pc4 photosensitizer and Pc4 encapsulated inside silica nanoparticles enter the melanoma cells and have a relatively selective affinity to specific subcellular components. Both Pc4 photosensitizer and Pc4SNP are taken up into the cytoplasm and at least partially by the mitochondria and lysosomes. More Pc4SNP than free Pc4 is localized in mitochondria. There is substantial fluorescence in the cytoplasm (Figure 9) that is enhanced in the encapsulated Pc4. Although the uptake by the mitochondria is not exclusive, it is important because mitochondria are believed to be the targets for the initiation of apoptosis by PDT (Oleinick et al., 2002). In addition, more Pc4SNP than its free form localizes in lysosome and thus may lead to increased apoptosis of melanoma cells upon photosensitization. Rupture of lysosomes, leading to the release of their cathepsin content, has long been recognized to be potentially harmful to tumor cells, including melanoma cells (Turk et al., 2001). Tumor cells may be preferentially sensitive to agents that trigger the lysosomal apoptosis pathway. Thus increased accumulation of Pc4SNP in lysosomes should increase killing of the melanoma cells upon exposure to light.
Encapsulation of Pc4 in silica nanoparticles not only enhances its properties in melanoma detection by enhancing its fluorescence and stability, but also addresses some of the problems associated with PDT treatment. One of the problems of using hydrophobic photosensitizers for PDT therapy is that they are administered dispersed in Cremophor EL/ethanol/normal saline and then self-aggregate. Hydrophilic photosensitizers are solubilized in aqueous solutions by hydrogen bonding with water, which does not occur with hydrophobic photosensitizers, where the failure to solubilize leads to aggregation. This aggregation significantly reduces the fluorescence of the photosensitizer and the generation of singlet oxygen, making it less effective for both diagnosis and PDT. Our approach of encapsulation of the hydrophobic photosensitizer Pc4 into silica nanoparticles reduced aggregation, which enhanced aqueous solubility, ease of delivery, and drug stability.
Another problem with PDT therapy is the lack of stability of the sensitizer. With treatment of light the sensitizer bleaches and loses its effectiveness, requiring increased light exposure and/or photosensitizer concentration for cell inactivation. Our results showed that encapsulation of Pc4 in the silica nanoparticles substantially decreased photobleaching, especially during the first 12 minutes (Figure 3, Phase I). The acceleration in photobleaching during Phase II could be due to the damage to nanoparticles induced by photo-generated singlet oxygen. It has been previously reported that photobleaching may play a role in protecting normal tissue from damage by PDT and enhancing the therapeutic ratio of PDT (Moan, 1986; Ma et al., 1994). Thus with proper control of both the drug/light dose and exposure time, this pattern of photobleaching could be potentially useful for improving PDT therapeutic efficacy and suppressing side effects.
It is generally accepted that the singlet oxygen mechanism (photochemical reaction of type II) predominates during PDT and that singlet oxygen is the most important cytotoxic species produced by phthalocyanine photosensitizers (Lukyanets, 1999; Allen et al., 2001). Pc4 in silica nanoparticles generated photo-induced singlet oxygen more efficiently than Pc4 alone, as measured by two independent assays, the chemical probe ABMDMA (Figure 4) and the EPR trapping technique using TEMP (Figure 5). Using two techniques allowed us to measure singlet oxygen at different concentrations of Pc4 and encapsulated Pc4 (2 μM for the chemical probe method and 10 μM for the ESR technique). At the higher concentration in aqueous solutions, Pc4 aggregates, whereas encapsulated Pc4 is less likely to aggregate. Aggregation results in reduced singlet oxygen production. We found that the free Pc 4 molecule aggregated in an aqueous system, resulting in decreased singlet oxygen production, whereas Pc4 encapsulation by silica nanoparticles protected the Pc4 from aggregation and thus had a higher singlet oxygen production than the free Pc4. Therefore singlet oxygen was generated more efficiently in encapsulated Pc4 over Pc4 alone.
The increased singlet oxygen generation by the encapsulated Pc4 versus free Pc4 is reflected in greater photo-induced toxicity of Pc4SNP than free Pc4 towards A375 and B16F10 melanoma cells as measured by the MTS assay (cell viability, Figure 6) and flow cytometery (apoptosis, Figure 7). More than half of the A375 and B16F10 cells were destroyed with encapsulated Pc4 as low as 5 nM and 10nm after irradiation, respectively. The phototoxicity of free Pc4 towards both A375 and B16F10 cells at the same concentrations was much lower than the encapsulated Pc4, as measured by MTS. Due to the porous nature of silica nanoparticles used in this study (Roy et al., 2003), the above in vitro studies also indicate that the encapsulated Pc4 permits not only efficient photo-induced generation of singlet oxygen, but also free diffusion out of the nano-platform to cause damage to tumor cells.
PDT is known to elicit both necrosis and apoptosis, depending on the light/drug dose, cell type, its genetic and metabolic potential, the nature of the photosensitizer used, and its subcellular localization (Granville et al., 1998; Mooney et al., 2002; Oleinick et al., 2002). Under the optimized experimental protocol conditions used to elicit cell death, our results show that apoptosis was greater with Pc4SNP than the free Pc4. As seen in Figure 7, apoptosis is involved in the photo-induced A375 cell killing (Figure 7), similar to the early findings in Pc4-PDT treatment of other cells (Miller et al., 2007). Furthermore, the decrease of phototoxic damage in the presence of the singlet oxygen quencher NaN3 (Figure 7f) confirms that the phototoxic mechanism of Pc4 with irradiation very likely involves singlet oxygen as one of the main reactive oxygen species (Type II mechanism).
Singlet oxygen is known to directly react with proteins and unsaturated lipids located in cell membranes to give the corresponding peroxides. That singlet oxygen is indeed generated inside the cells is strongly suggested by the detection of protein peroxides (Figure 8) as the major product during PDT treatment of A375 cells containing Pc4SNP or Pc4 and the quenching effect by azide. Once again, Pc4SNP demonstrates a remarkably greater increase in protein peroxides via 1O2-mediated reactions than that of Pc4, suggesting that photodynamic efficacy is enhanced by encapsulation of Pc4 in silica nanoparticles. Because the diffusion distance of singlet oxygen is very short (<70 nm (Moan, 1990)), this result also provides indirect evidence that photoreactive Pc4 is taken up into cells.
Considering the ease with which its surface can be chemically modified, silica nanoparticle formulation with Pc4 may be a novel platform for formulating photodynamic drugs for melanoma diagnosis and therapy with the advantage of adding targeting molecules at the surface. The surface modification of nanoparticles containing photosensitizers using melanoma targeting molecules (antibody and peptide) is under investigation.
In summary, melanoma cells are photo-damaged with Pc4 and encapsulated Pc4 primarily through apoptosis by singlet oxygen (Type II). The photo-stability, generation of singlet oxygen, and therapeutic efficiency of photosensitizer Pc4 were significantly improved by encapsulation into porous silica nanoparticles. This nano-platform not only imparts solubility to the hydrophobic Pc4 in aqueous solution with less aggregation, but also transports Pc4 into cells efficiently. Encapsulated Pc4 fluoresces more strongly in cells than free Pc4 and remains fluorescent and photoactive longer, thus improving its potential for use in both early diagnosis and PDT treatment of melanoma. Furthermore, we believe that the surface modification of photosensitizers encapsulated in silicon nanoparticles with antibodies specific to melanoma cells will lead to better early diagnosis and targeted treatment of melanoma.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences. The authors are indebted to Dr. Ann Motten, NIEHS, for critical reading of the manuscript and Dr. Carl Bortner and Maria Sifre for their assistance with flow cytometry.
Abbreviations
- ABMDMA
9,10-anthracenediyl-bis(methylene)dimalonic acid
- EPR
electron paramagnetic resonance
- FBS
fetal bovine serum
- DLS
Dynamic light scattering
- DMEM
Dulbecco's Modified Eagle's Medium
- MTS
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
- PBS
phosphate buffered saline
- PDT
photodynamic therapy
- ROS
reactive oxygen species
- RT
room temperature
- TEM
Transmission electron microscopy
- TEMP
2,2,6,6-tetramethyl-4-piperidone
- TEMPO
2,2,6,6-tetramethyl-4-piperidone-N-oxyl radical
- TEVS
triethoxyvinylsilane
Footnotes
Conflict of interest statement: The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmad N, Feyes DK, Agarwal R, Mukhtar H. Photodynamic therapy results in induction of WAF1/CIP1/P21 leading to cell cycle arrest and apoptosis. Proc Nat Acad Sci USA. 1998;95:6977–6982. doi: 10.1073/pnas.95.12.6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali H, van Lier JE. Metal complexes as photo- and radiosensitizers. Chem Rev. 1999;99:2379–2450. doi: 10.1021/cr980439y. [DOI] [PubMed] [Google Scholar]
- Allen CM, Sharman WM, Van Lier JE. Current status of phthalocyanines in the photodynamic therapy of cancer. J Porphyr Phthalocyanines. 2001;5:161–169. [Google Scholar]
- Bechet D, Couleaud P, Frochot C, Viriot ML, Guillemin F, Barberi-Heyob M. Nanoparticles as vehicles for delivery of photodynamic therapy agents. Trends Biotechnol. 2008;26:612–621. doi: 10.1016/j.tibtech.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Bedwell J, Chatlani PT, Macrobert AJ, Roberts JE, Barr H, Dillon J, Bown SG. Enhanced Tumor Selectivity of Photodynamic Therapy in the Rat Colon Using a Radioprotective Agent. Photochem Photobiol. 1991;53:753–756. doi: 10.1111/j.1751-1097.1991.tb09888.x. [DOI] [PubMed] [Google Scholar]
- Brannon-Peppas L, Blanchette JO. Nanoparticle and targeted systems for cancer therapy. Adv Drug Deliv Rev. 2004;56:1649–1659. doi: 10.1016/j.addr.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Brevet D, Gary-Bobo M, Raehm L, Richeter S, Hocine O, Amro K, Loock B, Couleaud P, Frochot C, Morere A, Maillard P, Garcia M, Durand JO. Mannose-targeted mesoporous silica nanoparticles for photodynamic therapy. Chem Commun. 2009:1475–1477. doi: 10.1039/b900427k. [DOI] [PubMed] [Google Scholar]
- Brigger I, Dubernet C, Couvreur P. Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev. 2002;54:631–651. doi: 10.1016/s0169-409x(02)00044-3. [DOI] [PubMed] [Google Scholar]
- Brown SB, Brown EA, Walker I. The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol. 2004;5:497–508. doi: 10.1016/S1470-2045(04)01529-3. [DOI] [PubMed] [Google Scholar]
- Chatterjee DK, Fong LS, Zhang Y. Nanoparticles in photodynamic therapy: an emerging paradigm. Adv Drug Deliv Rev. 2008;60:1627–1637. doi: 10.1016/j.addr.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Cheng SH, Lee CH, Yang CS, Tseng FG, Mou CY, Lo LW. Mesoporous silica nanoparticles functionalized with an oxygen-sensing probe for cell photodynamic therapy: potential cancer theranostics. J Mater Chem. 2009;19:1252–1257. [Google Scholar]
- Chiu SM, Evans HH, Lam M, Nieminen AL, Oleinick NL. Phthalocyanine 4 photodynamic therapy-induced apoptosis of mouse L5178Y-R cells results from a delayed but extensive release of cytochrome c from mitochondria. Cancer Lett. 2001;165:51–58. doi: 10.1016/s0304-3835(01)00422-0. [DOI] [PubMed] [Google Scholar]
- Choi MTM, Li PPS, Ng DKP. A direct comparison of the aggregation behavior of phthalocyanines and 2,3-naphthalocyanines. Tetrahedron. 2000;56:3881–3887. [Google Scholar]
- Detty MR, Gibson SL, Wagner SJ. Current clinical and preclinical photosensitizers for use in photodynamic therapy. J Med Chem. 2004;47:3897–3915. doi: 10.1021/jm040074b. [DOI] [PubMed] [Google Scholar]
- Dolmans DEJGJ, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3:380–387. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. Photodynamic therapy. J Natl Cancer Inst. 1998;90:889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granville DJ, Carthy CM, Jiang HJ, Shore GC, McManus BM, Hunt DWC. Rapid cytochrome c release, activation of caspases 3, 6, 7 and 8 followed by Bap31 cleavage in HeLa cells treated with photodynamic therapy. FEBS Lett. 1998;437:5–10. doi: 10.1016/s0014-5793(98)01193-4. [DOI] [PubMed] [Google Scholar]
- Haag R. Supramolecular drug-delivery systems based on polymeric core-shell architectures. Angew Chem Int Ed Engl. 2004;43:278–282. doi: 10.1002/anie.200301694. [DOI] [PubMed] [Google Scholar]
- Kalka K, Ahmad N, Criswell T, Boothman D, Mukhtar H. Up-regulation of clusterin during phthalocyanine 4 photodynamic therapy-mediated apoptosis of tumor cells and ablation of mouse skin tumors. Cancer Res. 2000;60:5984–5987. [PubMed] [Google Scholar]
- Kochevar IE, Bouvier J, Lynch M, Lin CW. Influence of Dye and Protein Location on Photosensitization of the Plasma-Membrane. Biochim Biophys Acta-Biomembr. 1994;1196:172–180. doi: 10.1016/0005-2736(94)00236-3. [DOI] [PubMed] [Google Scholar]
- Konan YN, Berton M, Gurny R, Allemann E. Enhanced photodynamic activity of meso-tetra(4-hydroxyphenyl)porphyrin by incorporation into sub-200 nm nanoparticles. Eur J Pharm Sci. 2003;18:241–249. doi: 10.1016/s0928-0987(03)00017-4. [DOI] [PubMed] [Google Scholar]
- Konan YN, Gurny R, Allemann E. State of the art in the delivery of photosensitizers for photodynamic therapy. J Photochem Photobiol B-Biol. 2002;66:89–106. doi: 10.1016/s1011-1344(01)00267-6. [DOI] [PubMed] [Google Scholar]
- Lion Y, Delmelle M, van de Vorst A. New method of detecting singlet oxygen production. Nature. 1976;263:442–443. doi: 10.1038/263442a0. [DOI] [PubMed] [Google Scholar]
- Lo PC, Fong WP, Ng DK. Effects of peripheral chloro substitution on the photophysical properties and in vitro photodynamic activities of galactose-conjugated silicon(IV) phthalocyanines. ChemMedChem. 2008;3:1110–1117. doi: 10.1002/cmdc.200800042. [DOI] [PubMed] [Google Scholar]
- Lukyanets EA. Phthalocyanines as photosensitizers in the photodynamic therapy of cancer. J Porphyr Phthalocyanines. 1999;3:424–432. [Google Scholar]
- Ma LW, Moan J, Berg K. Comparison of the Photobleaching Effect of 3 Photosensitizing Agents - Meso-Tetra(M-Hydroxyphenyl)Chlorin, Meso-Tetra(M-Hydroxyphenyl)Porphyrin and Photofrin during Photodynamic Therapy. Lasers Med Sci. 1994;9:127–132. [Google Scholar]
- Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, LaFace DM, Green DR. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JD, Baron ED, Scull H, Hsia A, Berlin JC, McConnick T, Colussi V, Kenney ME, Cooper KD, Oleinick NL. Photodynarnic therapy with the phthalocyanine photosensitizer Pc 4: The case experience with preclinical mechanistic and early clinical-translational studies. Toxicol Appl Pharmacol. 2007;224:290–299. doi: 10.1016/j.taap.2007.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moan J. Effect of Bleaching of Porphyrin Sensitizers during Photodynamic Therapy. Cancer Lett. 1986;33:45–53. doi: 10.1016/0304-3835(86)90100-x. [DOI] [PubMed] [Google Scholar]
- Moan J. On the diffusion length of singlet oxygen in cells and tissues. J Photochem Photobiol B-Biol. 1990;6:343–347. [Google Scholar]
- Mooney LM, Al-Sakkaf KA, Brown BL, Dobson PR. Apoptotic mechanisms in T47D and MCF-7 human breast cancer cells. Br J Cancer. 2002;87:909–917. doi: 10.1038/sj.bjc.6600541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RL, Varnes ME, Kenney ME, Li YS, Azizuddin K, McEnery MW, Oleinick NL. The peripheral benzodiazepine receptor in photodynamic therapy with the phthalocyanine photosensitizer Pc 4. Photochem Photobiol. 2002;75:652–661. doi: 10.1562/0031-8655(2002)075<0652:tpbrip>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Nishiwaki H, Zeimer R, Goldberg MF, D'Anna SA, Vinores SA, Grebe R. Laser targeted photo-occlusion of rat choroidal neovascularization without collateral damage. Photochem Photobiol. 2002;75:149–158. doi: 10.1562/0031-8655(2002)075<0149:ltpoor>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Ntziachristos V. Fluorescence molecular imaging. Annu Rev Biomed Eng. 2006;8:1–33. doi: 10.1146/annurev.bioeng.8.061505.095831. [DOI] [PubMed] [Google Scholar]
- Nyman ES, Hynninen PH. Research advances in the use of tetrapyrrolic photosensitizers for photodynamic therapy. J Photochem Photobiol B-Biol. 2004;73:1–28. doi: 10.1016/j.jphotobiol.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Ohulchanskyy TY, Roy I, Goswami LN, Chen Y, Bergey EJ, Pandey RK, Oseroff AR, Prasad PN. Organically modified silica nanoparticles with covalently incorporated photosensitizer for photodynamic therapy of cancer. Nano Lett. 2007;7:2835–2842. doi: 10.1021/nl0714637. [DOI] [PubMed] [Google Scholar]
- Oleinick NL, Morris RL, Belichenko T. The role of apoptosis in response to photodynamic therapy: what, where, why, and how. Photochem Photobiol Sci. 2002;1:1–21. doi: 10.1039/b108586g. [DOI] [PubMed] [Google Scholar]
- Reme CE, Braschler UF, Roberts J, Dillon J. Light Damage in the Rat Retina - Effect of a Radioprotective Agent (Wr-77913) on Acute Rod Outer Segment Disk Disruptions. Photochem Photobiol. 1991;54:137–142. doi: 10.1111/j.1751-1097.1991.tb01997.x. [DOI] [PubMed] [Google Scholar]
- Reno F, Burattini S, Rossi S, Luchetti F, Columbaro M, Santi S, Papa S, Falcieri E. Phospholipid rearrangement of apoptotic membrane does not depend on nuclear activity. Histochem Cell Biol. 1998;110:467–476. doi: 10.1007/s004180050308. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Dillon J. Improvement of photodynamic therapy by selective protection of normal tissues. In: Jung EG, Holick MF, editors. Biological effects of light. Gruyter & Co.; Berlin, New York: 1993. pp. 393–399. [Google Scholar]
- Roberts JE, Kinley JS, Young AR, Jenkins G, Atherton SJ, Dillon J. Invivo and Photophysical Studies on Photooxidative Damage to Lens Proteins and Their Protection by Radioprotectors. Photochem Photobiol. 1991;53:33–38. doi: 10.1111/j.1751-1097.1991.tb08464.x. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Selman S, Morgan A, Dillon J. Selective Protection Against the Side Effects of Photodynamic Therapy by Radioprotectors. In: Jung EG, Holick MF, editors. Biological effects of light. Gruyter & Co.; Berlin, New York: 1994. pp. 400–406. [Google Scholar]
- Roy I, Ohulchanskyy TY, Pudavar HE, Bergey EJ, Oseroff AR, Morgan J, Dougherty TJ, Prasad PN. Ceramic-based nanoparticles entrapping water-insoluble photosensitizing anticancer drugs: A novel drug-carrier system for photodynamic therapy. J Am Chem Soc. 2003;125:7860–7865. doi: 10.1021/ja0343095. [DOI] [PubMed] [Google Scholar]
- Singh N. A simple method for accurate estimation of apoptotic cells. Exp Cell Res. 2000;256:328–337. doi: 10.1006/excr.2000.4810. [DOI] [PubMed] [Google Scholar]
- Srivastava M, Ahmad N, Gupta S, Mukhtar H. Involvement of Bcl-2 and Bax in photodynamic therapy-mediated apoptosis - Antisense Bcl-2 oligonucleotide sensitizes RIF 1 cells to photodynamic therapy apoptosis. J Biol Chem. 2001;276:15481–15488. doi: 10.1074/jbc.M006920200. [DOI] [PubMed] [Google Scholar]
- Tu HL, Lin YS, Lin HY, Hung Y, Lo LW, Chen YF, Mou CY. In vitro Studies of Functionalized Mesoporous Silica Nanoparticles for Photodynamic Therapy. Adv Mater. 2009;21:172–+. [Google Scholar]
- Turk V, Turk B, Turk D. Lysosomal cysteine proteases: facts and opportunities. EMBO J. 2001;20:4629–4633. doi: 10.1093/emboj/20.17.4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usuda J, Chiu SM, Murphy ES, Lam M, Nieminen AL, Oleinick NL. Domain-dependent photodamage to Bcl-2 - A membrane anchorage region is needed to form the target of phthalocyanine photosensitization. J Biol Chem. 2003;278:2021–2029. doi: 10.1074/jbc.M205219200. [DOI] [PubMed] [Google Scholar]
- Wainwright M. Photodynamic therapy: the development of new photosensitisers. Anticancer Agents Med Chem. 2008;8:280–291. doi: 10.2174/187152008783961888. [DOI] [PubMed] [Google Scholar]
- Wang SZ, Gao RM, Zhou FM, Selke M. Nanomaterials and singlet oxygen photosensitizers: potential applications in photodynamic therapy. J Mater Chem. 2004;14:487–493. [Google Scholar]
- Whitacre CM, Feyes DK, Satoh T, Grossmann J, Mulvihill JW, Mukhtar H, Oleinick NL. Photodynamic therapy with the phthalocyanine photosensitizer Pc 4 of SW480 human colon cancer xenografts in athymic mice. Clin Cancer Res. 2000;6:2021–2027. [PubMed] [Google Scholar]
- Wieder ME, Hone DC, Cook MJ, Handsley MM, Gavrilovic J, Russell DA. Intracellular photodynamic therapy with photosensitizer-nanoparticle conjugates: cancer therapy using a ‘Trojan horse’. Photochem Photobiol Sci. 2006;5:727–734. doi: 10.1039/b602830f. [DOI] [PubMed] [Google Scholar]
- Wilkinson F, Helman WP, Ross AB. Rate Constants for the Decay and Reactions of the Lowest Electronically Excited Singlet-State of Molecular-Oxygen in Solution - an Expanded and Revised Compilation. J Phys Chem Ref Data. 1995;24:663–1021. [Google Scholar]
- Wright A, Hawkins CL, Davies MJ. Photo-oxidation of cells generates long-lived intracellular protein peroxides. Free Radic Biol Med. 2003;34:637–647. doi: 10.1016/s0891-5849(02)01361-8. [DOI] [PubMed] [Google Scholar]