Abstract
Objectives
To determine whether preoperative optimisation of oxygen delivery improves outcome after major elective surgery, and to determine whether the inotropes, adrenaline and dopexamine, used to enhance oxygen delivery influence outcome.
Design
Randomised controlled trial with double blinding between inotrope groups.
Setting
York District Hospital, England.
Subjects
138 patients undergoing major elective surgery who were at risk of developing postoperative complications either because of the surgery or the presence of coexistent medical conditions.
Interventions
Patients were randomised into three groups. Two groups received invasive haemodynamic monitoring, fluid, and either adrenaline or dopexamine to increase oxygen delivery. Inotropic support was continued during surgery and for at least 12 hours afterwards. The third group (control) received routine perioperative care.
Main outcome measures
Hospital mortality and morbidity.
Results
Overall, 3/92 (3%) preoptimised patients died compared with 8/46 controls (17%) (P=0.007). There were no differences in mortality between the treatment groups, but 14/46 (30%) patients in the dopexamine group developed complications compared with 24/46 (52%) patients in the adrenaline group (difference 22%, 95% confidence interval 2% to 41%) and 28 patients (61%) in the control group (31%, 11% to 50%). The use of dopexamine was associated with a decreased length of stay in hospital.
Conclusion
Routine preoperative optimisation of patients undergoing major elective surgery would be a significant and cost effective improvement in perioperative care.
Key messages
Major elective surgery in UK general hospitals still carries significant mortality and morbidity
Preoperative administration of fluid and inotropes, guided by invasive monitoring, can significantly reduce mortality, morbidity, and length of hospital stay
The choice of inotrope may influence the extent of improvements in outcome
Routine preoperative optimisation would require initial investment in high dependency care facilities but is likely to be cost effective by reducing complications and length of hospital stay
Introduction
Major elective surgery contributes to intensive care occupancy, with a significant mortality rate.1,2 In the United Kingdom most patients are taken from the general ward directly to the operating theatre before major elective surgery. The extent of perioperative monitoring is dependent on the anaesthetist, and the site of postoperative care will depend on the anticipated development of complications and the availability of intensive care beds or high dependency beds.
The enhancement of oxygen delivery to the tissues, guided by data obtained with pulmonary artery catheters, has been shown to improve outcome of patients deemed to be at high risk from major surgery.3,4
Oxygen delivery is dependent on the amount of oxygen in the blood and the cardiac index. Optimisation of cardiac index requires fluid and inotrope therapy to increase cardiac contractility. Inotropic agents, however, have different effects on circulation to the gut, which may possibly affect postoperative morbidity.5 Dopexamine (Dopacard, Ipsen, Maidenhead), is a peripheral vasodilator, which is associated with improved splanchnic oxygenation,6,7 whereas adrenaline (epinephrine), commonly used in intensive care, may reduce splanchnic flow.8
Our study compared the outcome in a population of elective surgical patients receiving either preoperative optimisation of oxygen delivery (treatment groups) or undergoing current hospital practice (control group). We also tested whether the inotrope dopexamine, given in a double blind fashion, affects outcome.
Subjects and methods
Approval for our study was obtained from the ethics committee of York District Hospital, and written consent was obtained from all the patients. We considered all patients undergoing major elective surgical procedures in general surgery, vascular surgery, and urology. Patients were identified as being at high risk of developing perioperative complications on the basis of either surgical criteria or the presence of coexisting medical conditions (table 1).
Table 1.
Age and admission criteria for patients undergoing major elective surgery. Values are number (percentage) unless stated otherwise
| Criteria | Adrenaline group (n=46) | Dopexamine group (n=46) | Control group (n=46) |
|---|---|---|---|
| Median age (years; interquartile range) | 71.5 (64-77) | 70.0 (65-74) | 71.5 (65-76) |
| Surgical admission criteria: | |||
| Repair of aortic or common iliac aneurysm | 14 (30) | 15 (33) | 13 (28) |
| Planned resection of upper gastrointestinal malignancy | 9 (20) | 10 (22) | 8 (17) |
| Anterior resection | 12 (26) | 4 (9) | 12 (26) |
| Cystectomy | 4 (9) | 5 (11) | 1 (2) |
| Medical criteria: | |||
| Ischaemic heart disease | 13 (28) | 13 (28) | 18 (39) |
| Myocardial infarction in past 5 years | 7 (15) | 4 (9) | 4 (9) |
| Congestive cardiac failure | 4 (9) | 1 (2) | 3 (7) |
| Cerebrovascular disease | 6 (13) | 2 (4) | 5 (11) |
| Hypertension | 18 (39) | 11 (24) | 15 (33) |
| Peripheral vascular disease | 4 (9) | 3 (7) | 2 (4) |
| Obstructive airways disease | 3 (7) | 3 (7) | 7 (15) |
| Pulmonary embolus | 0 (0) | 1 (2) | 0 (0) |
| Chronic renal insufficiency | 1 (2) | 0 (0) | 0 (0) |
| Diabetes mellitus with end organ damage | 1 (2) | 0 (0) | 6 (13) |
| Long term systemic steroid therapy | 2 (4) | 4 (9) | 2 (4) |
| Total No of patients with one or more medical conditions* | 34 (74) | 30 (65) | 34 (74) |
P=0.57.
The care of control patients was determined by the individual surgeon and anaesthetist according to their routine practices for the operation. Generally, the patients remained on the general surgical ward until surgery and were then returned to either intensive care, high dependency care, or the ward postoperatively. No routine preoperative fluid protocol was followed for these patients.
Patients in the adrenaline group and dopexamine group were admitted to either intensive care or high dependency care a minimum of 4 hours before surgery. A large intravenous cannula was inserted in the patient’s forearm and an intra-arterial cannula was placed in the patient’s radial artery for measurement of blood pressure and for blood sampling. A pulmonary artery catheter enabling continuous measurement of cardiac index (Baxter Swan Ganz IntelliCath, Baxter Healthcare, Irvine, CA) was inserted via a central vein. All line insertions were carried out under local anaesthesia, with sedation where required.
Oxygen delivery was measured using the standard formula: oxygen delivery (ml/min/m2) = cardiac index (l/min/m2) × oxygen content of blood (haemoglobin (g/l) × oxygen saturation × 1.34).
Optimisation of oxygen delivery consisted of two phases: fluid optimisation and inotrope optimisation.
Fluid optimisation—
All patients received 1 litre of Hartmann’s solution during line insertion. Human albumin solution 4.5% was then infused until a pulmonary artery occlusion pressure of 12 mm Hg was achieved. If haemoglobin concentration was <110 g/l, red blood cells were transfused instead of the albumin solution. If oxygen saturation was <94%, supplemental oxygen was provided.
Inotrope optimisation—
Inotrope was commenced at a rate (ml/hour) calculated from a chart according to the patient’s weight and equated to 0.025 μg/kg/min for adrenaline and 0.125 μg/kg/min for dopexamine. Blinding was achieved by administering the inotrope in a syringe that had been preprepared in the pharmacy. The infusion was increased by single multiples of the initial rate until the target oxygen delivery of >600 ml/min/m2 was achieved or the onset of side effects was noted (increase in heart rate >30% above baseline or development of chest pain or a new dysrhythmia). If side effects were noted, the infusion was decreased. All patients were started on the study inotrope even if the target oxygen delivery had been achieved after the fluid phase. The infusion was maintained at the preoperative rate throughout the remainder of the perioperative period.
Intraoperative care was the responsibility of the anaesthetist, including provision of additional inotropes if thought necessary.
After surgery, patients were returned to intensive care or high dependency care. The study inotrope was continued at the preoperative rate for 12 to 24 hours postoperatively. The time of discontinuation of the inotrope was at the discretion of the intensive care team. On a routine clinical basis the intensive care and surgical teams determined all other aspects of care including removal of the pulmonary artery catheter and timing of discharge from intensive care or high dependency care.
Statistical analysis
We required 46 patients in each group, calculated by matching reductions in mortality from 25% to 5% from the most similar previous study, to give a study power of 80%.4 The randomisaton sequence was generated from a Unix computer program. Allocation was concealed until trial entry by sealed opaque envelope. Randomisation was stratified into three subgroups: vascular surgery, surgery for upper gastrointestinal malignancy, and others. This was to ensure even distribution of these surgical subgroups across the three groups.
Outcome measures
Primary outcome measures were hospital mortality and morbidity (number of patients developing one or more of a predefined range of complications). Secondary measures were length of stay in hospital, use of intensive care or high dependency care, and haemodynamic measurements (for adrenaline and dopexamine groups). We analysed hospital mortality by Kaplan-Meier survival estimates, using the log-rank test for comparison, and by Fisher’s exact test. The differences in proportions of patients with morbidity were calculated. Standardised ratios were constructed for morbidity and mortality, comparing actual incidences to those predicted by the POSSUM score (physiological and operative severity score for the enumeration of mortality and morbidity).9 Where appropriate, we calculated 95% confidence intervals.10 Kaplan-Meier estimates were constructed for hospital length of stay, treating non-survivors as censored values, and analysed using the log-rank test.
Analysis was on an intention to treat basis.
Results
Overall, 203 patients were identified and approached over a 16 month period; 65 did not enter the study either because of refusal to consent (40), lack of intensive care or high dependency care beds (16), or other reasons (9) (see website).
Table 1 shows the entry criteria for each group.
Table 2 outlines the variables for haemodynamics and oxygen transport obtained at the key stages of preoperative optimisation. One patient in the adrenaline group and three patients in the dopexamine group failed to reach target oxygen delivery owing to the development of side effects.
Table 2.
Haemodynamic and oxygen transport in inotrope groups only. Values are median (interquartile range) unless stated otherwise
| Variable | Adrenaline group (n=46) | Dopexamine group (n=46) |
|---|---|---|
| Baseline: | ||
| Pulmonary artery occlusion pressure (mm Hg) | 12.0 (9.0-15.0) | 10.0 (8.0-12.0) |
| Cardiac index (ml/min/m2) | 3.2 (2.6-3.6) | 3.6 (2.9-4.1) |
| Oxygen delivery (ml/min/m2) | 530 (435-590) | 564 (486-636) |
| End fluid optimisation phase: | ||
| Fluid given (ml) | 1250 (1000-1500) | 1100 (875-1300) |
| Pulmonary artery occlusion pressure (mm Hg) | 14.0 (13.0-15.0) | 13.5 (12.0-15.0) |
| Cardiac index | 3.5 (2.8-4.0) | 3.8 (3.3-4.5) |
| Oxygen delivery | 541 (438-590) | 604 (521-686) |
| End inotrope optimisation phase: | ||
| No of increments | 1.0 | 1.0 |
| Pulmonary artery occlusion pressure (mm Hg) | 13.5 (11.0-15.0) | 13.0 (10.2-14.0) |
| Cardiac index | 4.7 (4.0-5.3) | 4.3 (3.8-4.8) |
| Oxygen delivery | 721 (638-827) | 665 (632-769) |
| Total fluid given (ml) | 1500 (1000-2000) | 1525 (1000-2000) |
| Duration of preoptimisation (minutes) | 276 (200-345) | 275 (214-331) |
| No of patients who failed to reach target oxgen delivery | 1 | 3 |
| No of patients with new dysrhythmias: | 4 | 1 |
| Ventricular ectopics | 2 | 0 |
| Ventricular tachycardia | 0 | 1 |
| Sinus tachycardia | 2 | 0 |
| Additional therapy: | ||
| Blood transfusion | 2 | 2 |
| Oxygen therapy | 8 | 5 |
According to the POSSUM scores, there were no differences in operative risk. One patient in the adrenaline group returned from theatre to the ward because of an inoperable lesion. One patient in the dopexamine group pulled out his pulmonary artery catheter immediately before surgery because he was confused. All other patients completed the minimum requirement of 12 hours’ infusion of study inotrope postoperatively.
Overall, preoperative optimisation of oxygen delivery significantly reduced hospital mortality; 3/92 (3%) patients who were preoptimised died compared with 8/46 controls (17%, P=0.007; table 3). Compared with both the control and the adrenaline group, there was a significant reduction in morbidity in the dopexamine group (table 4). Optimisation with adrenaline alone did not significantly reduce morbidity compared with control. Compared with the value predicted from the POSSUM score, dopexamine is associated with a significantly reduced incidence of morbidity.
Table 3.
Mortality data for patients receiving inotrope or standard hospital practice
| Adrenaline group (n=46) | Dopexamine group (n=46) | Control group (n=46) | |
|---|---|---|---|
| No of patients surviving | 45 | 44 | 38 |
| Hospital survival (%; 95% CI) | 98 (94 to 100) | 96 (90 to 100) | 83 (72 to 94) |
| Combined treatment groups: 97 (93 to 100)* | |||
| Actual mortality (%) | 2 | 4 | 17 |
| Predicted mortality (%) | 12 | 15 | 13 |
| Standardised mortality ratio (95% CI) | 0.19 (0.00 to 1.05) | 0.28 (0.04 to 1.08) | 1.36 (0.6 to 2.75) |
Fisher’s test for combined treatment groups v control, P=0.007.
Table 4.
Morbidity data for patients receiving inotrope or standard hospital practice
| Variable | Adrenaline group (n=46) | Dopexamine group (n=46) | Control group (n=46) |
|---|---|---|---|
| Respiratory: | |||
| Prolonged weaning | 1 | 3 | 1 |
| Adult respiratory distress syndrome | 1 | 1 | 4 |
| Pleural effusion | 3 | 3 | 2 |
| Secondary ventilation | 6 | 4 | 8 |
| Sputum retention | 4 | 1 | 5 |
| Cardiovascular: | |||
| Myocardial infarction | 4 | 2 | 3 |
| New arrhythmia | 8 | 7 | 11 |
| Cardiac arrest | 1 | 1 | 3 |
| Pulmonary embolus | 1 | 1 | 2 |
| Cerebrovascular accident | 0 | 0 | 1 |
| Transient ischaemic attack | 1 | 0 | 0 |
| Cardiac failure | 14 | 4 | 12 |
| Gastrointestinal: | |||
| Infarction of gastrointestinal tract | 1 | 0 | 1 |
| Gastrointestinal haemorrhage | 0 | 0 | 4 |
| Renal or metabolic: Acute renal failure | 2 | 0 | 3 |
| Haematological or immune: Coagulopathy | 2 | 1 | 4 |
| Infection: | |||
| Bacteraemia | 2 | 0 | 2 |
| Sepsis syndrome | 2 | 0 | 1 |
| Septic shock | 0 | 2 | 0 |
| Respiratory sepsis | 8 | 2 | 7 |
| Urinary sepsis | 0 | 1 | 5 |
| Abdominal sepsis | 2 | 0 | 2 |
| Wound sepsis | 3 | 0 | 3 |
| Line sepsis | 0 | 1 | 0 |
| Other sepsis | 5 | 0 | 2 |
| Surgical: | |||
| Anastomotic breakdown | 0 | 0 | 3 |
| Deep haemorrhage | 2 | 2 | 4 |
| Wound haemorrhage | 3 | 0 | 2 |
| Total | 87 | 44 | 109 |
| No of patients with complications | 24 | 14 | 28 |
| Proportion of patients | 0.52 | 0.30 | 0.61 |
| Differences in proportions of patients with complications (95% CI): | |||
| Dopexamine v control | 0.30 (0.11 to 0.50) | ||
| Dopexamine v adrenaline | 0.21 (0.02 to 0.41) | ||
| Adrenaline v control | 0.09 (−0.11 to 0.28) | ||
| Actual morbidity (%) | 52 | 30 | 61 |
| Predicted morbidity (%) | 54 | 61 | 57 |
| Standardised morbidity ratio (95% CI) | 0.96 (0.62 to 1.44) | 0.50 (0.27 to 0.84) | 1.07 (0.71 to 1.54) |
The length of hospital stay for the dopexamine group was significantly reduced when individually compared with both the adrenaline group (P=0.02) and the control group (P=0.009). There was no overall increase in intensive care resources or high dependency care resources in the treated groups compared with control, although only 32/46 (70%) of control patients were admitted to these areas at any time during their hospital stay.
Discussion
Our study is a pragmatic one of the effect of a package of preoperative interventions on the outcome from major elective surgery in a typical UK hospital. The package comprises several components, each of which may have contributed to improvements in outcome.
Surgical risk
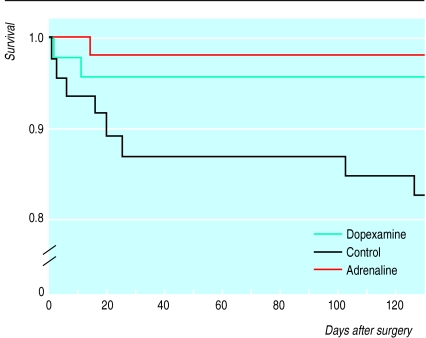
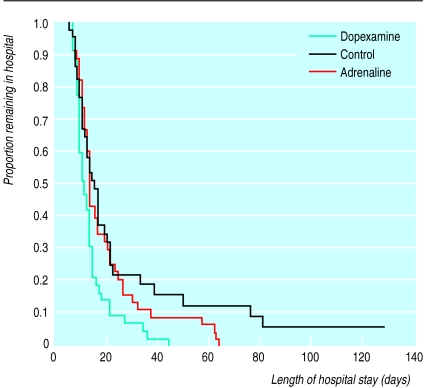
The criteria for patient selection were as a result of reviewing the elective surgical population for intensive care or high dependency care in our own hospital; outcome of control patients would seem to justify their use. We were unable to effect true blinding between patients in the control and treatment groups, but we have no evidence to suggest that this may have biased our results. The POSSUM scoring system was specifically developed for, and validated in, a UK general surgical population and can be used to explain differences in surgical outcome on the basis of different risk.9,12,13 The range of surgical procedures and POSSUM scores for surgical risk would suggest that the three groups were evenly balanced. The hospital mortality of 17% in the control group seems high, but two late deaths contributed to this value (fig 1). POSSUM was derived from data recorded for 6 weeks postoperatively; at that time our control group mortality was 13%—exactly that predicted by POSSUM. After major surgery a proportion of patients will still be in hospital at 6 weeks (fig 2) and, because of the serious morbidity delaying discharge, are likely to have significant mortality.
Figure 1.
Survival after surgery (proportion of original study population)
Figure 2.
Length of stay in hospital after surgery (proportion of original study population)
Use of intensive care beds or high dependency beds
—Sixteen control patients went back to the general ward after surgery (table 5). The location of postoperative care was the decision of the clinical team responsible for the patient, and no patient was denied access to intensive care or high dependency care owing to lack of beds. The improvements we have shown, however, can also be observed in study populations consisting exclusively of patients in intensive care.3,4
Table 5.
Perioperative data for patients receiving inotrope or standard hospital practice. Values are median (interquartile range) unless stated otherwise
| Variable | Adrenaline group (n=46) | Dopexamine group (n=46) | Control group (n=46) |
|---|---|---|---|
| Surgery type (No of patients): | |||
| Aortic surgery | 14 | 15 | 13 |
| Upper gastrointestingal malignancy | 9 | 10 | 8 |
| Other | 23 | 21 | 25 |
| Anaesthetic data: | |||
| Operation length (minutes) | 135 (75-190) | 130 (78-315) | 116 (70-145) |
| General anaesthesia only (No of patients) | 8 | 14 | 11 |
| General anaesthesia and epidural block (No of patients) | 38 | 32 | 35 |
| Total fluid given (ml) | 3025 (2000-4000) | 3375 (2200-4500) | 3500 (2500-4000) |
| Additional inotropes in theatre (No of patients) | 15 | 5 | 18 |
| POSSUM score: | |||
| Physiological component | 20 (17-24) | 20 (16-24) | 20 (16-24) |
| Operative component | 15 (11-20) | 17 (13-20) | 16 (12-17) |
| Total | 35 (32-39) | 37 (31-41) | 36 (32-39) |
| Postoperative location (No of patients): | |||
| Intensive care unit | 19 | 16 | 18 |
| High dependency unit | 26 | 30 | 12 |
| General ward | 1 | 0 | 16 |
| No intensive care or high dependency care | 0 | 0 | 14 |
| Bed usage (days): | |||
| Total hospital bed use for group | 875 | 596 | 1008 |
| Intensive care unit or high dependency unit (No of patients) | 192 (46) | 152 (46) | 176 (32) |
| Intensive care unit or high dependency unit per patient | 4.2 | 3.3 | 5.5 |
| Per patient | 19 | 13 | 22 |
Oxygen delivery as a goal—
High risk surgery is one of the few areas in which reasonable evidence now exists of the benefits of optimising oxygen delivery. When oxygen delivery falls below 390 l/min/m2, tissue oxygenation becomes physiologically inadequate in surgical patients at high risk.14 This is a reduction of just 30% from the median baseline measurements for oxygen delivery in our study groups. Because of blood loss during surgery, reductions in haemoglobin concentration and cardiac output are common occurrences in patients who are not monitored, with consequent falls in tissue oxygenation and the increased likelihood of complications.
Fluid optimisation
Intraoperative fluid requirements were the same in all groups. The treatment groups, however, received an average of 1500 ml of additional fluid preoperatively, on the basis of measurements from the pulmonary artery catheter. Considerable evidence exists that provision of optimal fluid improves outcome after surgery.3,4,11,15,16 All of these studies, however, used additional “non-routine” monitoring to estimate fluid requirements; it is therefore implicit that routine, less invasive monitoring may leave patients relatively depleted of fluid and at higher risk of adverse outcomes. Because of the low doses of inotropes used in our study, we suggest that fluid optimisation is the major contributor to improved oxygen delivery in our patients.
Choice of inotrope
Both inotropes produced the desired preoperative increase in oxygen delivery and a similar decrease in mortality. Only dopexamine, however, reduced morbidity and hospital stay. Although POSSUM scores were equal, there is a suggestion that the patients who received dopexamine may have had less pre-existing cardiovascular disease and a higher baseline oxygen delivery. In the dopexamine group, however, there was a reduction in infective complications. Dopexamine has significant anti-inflammatory properties, reducing the release of toxic mediators in response to an infective challenge.17 This contrasts with the effects of inotropes with α1 receptor activity such as adrenaline.18 Thus dopexamine may confer an additional advantage to fluid optimisation by reducing the effect of infective complications.
Conclusion
The incidence of morbidity in our control group suggests that there is a substantial population of surgical patients in the United Kingdom who are likely to benefit from the interventions described. Only 5% of all planned elective surgical admissions to intensive care are currently admitted preoperatively.2 Formal cost benefit analysis was not performed in this study, but values for usage of intensive carebeds or high dependency care beds (table 5) and length of stay in hospital suggest there may be overall savings in hospital costs when preoptimising patients for major elective surgery. An initial investment in resources may lead to economic gains for hospitals as well as a better outcome for surgical patients.
Supplementary Material
Acknowledgments
We thank the patients, their surgeons, and the medical and nursing staff of the intensive care, anaesthetic, and operating theatre departments for their patience and cooperation. Andy Vale, senior medical statistician at the University of Leeds, gave advice on statistical analysis.
Editorial by Treasure and Bennett
Footnotes
Funding: Grant of £45 000 from the National Hospital Lotteries Fund.
Competing interests: York District Hospital’s intensive care unit research fund was reimbursed by Ipsen, the manufacturer of dopexamine, after RJTW and IW spoke at meetings arranged by Ipsen.
website extra: Details of the flow of patients through the trial appear on the BMJ’s website www.bmj.com
References
- 1.Campling EA, Devlin HB, Hoile RW, Lunn JN. London: Royal College of Surgeons; 1992. National Confidential Enquiry into Perioperative Deaths. [DOI] [PubMed] [Google Scholar]
- 2.Intensive Care National Audit and Research Centre. Annual report from the national case mix programme database. London: Intensive Care National Audit and Research Centre; 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shoemaker WC, Appel PL, Kram HB, Waxman K, Lee T. Prospective trial of supranormal values of survivors as therapeutic goals in high-risk surgical patients. Chest. 1987;94:1176–1186. doi: 10.1378/chest.94.6.1176. [DOI] [PubMed] [Google Scholar]
- 4.Boyd O, Grounds RM, Bennett ED. A randomised clinical trial of the effect of deliberate perioperative increase of oxygen delivery on mortality in high-risk surgical patients. JAMA. 1993;270:2699–2707. [PubMed] [Google Scholar]
- 5.Mythen MG, Webb AR. The role of gut mucosal hypoperfusion in the pathogenesis of post-operative organ dysfunction. Intensive Care Med. 1994;20:203–209. doi: 10.1007/BF01704701. [DOI] [PubMed] [Google Scholar]
- 6.Brown RA, Dixon J, Farmer JB, Hall JC, Humphries RG, Ince F, et al. Dopexamine: a novel agonist at peripheral dopamine receptors and β-receptors. Br J Pharm. 1985;85:599–608. doi: 10.1111/j.1476-5381.1985.tb10554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smithies M, Yee TH, Jackson L, Beale R, Bihari D. Protecting the gut and the liver in the critically ill: effects of dopexamine. Crit Care Med. 1994;22:789–795. doi: 10.1097/00003246-199405000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Meier-Hellmann A, Reinhart K, Bredle DL, Specht M, Spies CD, Hannemann L. Adrenaline impairs splanchnic perfusion in septic shock. Crit Care Med. 1997;25:399–404. doi: 10.1097/00003246-199703000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Copeland GP, Jones MW. POSSUM: a scoring system for surgical audit. Br J Surg. 1991;78:355–360. doi: 10.1002/bjs.1800780327. [DOI] [PubMed] [Google Scholar]
- 10.Gardner MJ, Altman DG. Statistics with confidence. London: BMJ Books; 1989. [Google Scholar]
- 11.Berlauk JF, Abrams JH, Gilmore IJ, O’Conner SR, Knighton DR, Cerra FB. Preoperative optimisation of cardiovascular hemodynamics improves outcome in peripheral vascular surgery. Ann Surg. 1991;214:289–297. doi: 10.1097/00000658-199109000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Copeland GP, Jones D, Wilcox A, Harris PL. Comparative vascular audit using the POSSUM scoring system. Ann R Coll Surg Engl. 1993;75:175–177. [PMC free article] [PubMed] [Google Scholar]
- 13.Sagar PM, Hartley MN, Mancey-Jones B, Sedman PC, May J, Macfie J. Comparative audit of colorectal resection with the POSSUM scoring system. Br J Surg. 1994;81:1492–1494. doi: 10.1002/bjs.1800811031. [DOI] [PubMed] [Google Scholar]
- 14.Lugo G, Arizpe D, Dominguez G, Ramirez M, Tamariz O. Relationship between oxygen consumption and oxygen delivery during anesthesia in high-risk surgical patients. Crit Care Med. 1993;21:64–69. doi: 10.1097/00003246-199301000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Sinclair S, James S, Singer M. Intraoperative intravascular volume optimisation and length of stay after repair of proximal femoral fracture: randomised controlled trial. BMJ. 1997;315:909–912. doi: 10.1136/bmj.315.7113.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mythen MG, Webb AR. Peroperative plasma volume expansion reduces the incidence of gut mucosal hypoperfusion in cardiac surgery. Arch Surg. 1995;130:423–429. doi: 10.1001/archsurg.1995.01430040085019. [DOI] [PubMed] [Google Scholar]
- 17.Erkens U, Scholz SE, Cordes T, Petzinger E, Hemplemann G. Dopexamine impairs hepatic cytokine secretion after stimulation by endotoxin via β-receptor. Intensive Care Med. 1998;24:428S. [Google Scholar]
- 18.Tighe D, Moss R, Bennett ED. Adrenergic receptor α1 agonist and β2 antagonist increase hepatic injury during sepsis. Intensive Care Med. 1998;24:397S. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.