Summary
Recent experiments demonstrate that aggressive competition for potential mates involves different neural mechanisms than does territorial, resident-intruder aggression. However, despite the obvious importance of mate competition aggression, we know little about its regulation. Immediate early gene experiments show that in contrast to territorial aggression, mate competition in finches is accompanied by the activation of neural populations associated with affiliation and motivation, including vasotocin (VT) neurons in the medial bed nucleus of the stria terminalis (BSTm) and midbrain dopamine (DA) neurons that project to the BSTm. Although VT is known to facilitate mate competition aggression, the role of DA has not previously been examined. We now show that in male zebra finches (Taeniopygia guttata), mate competition aggression is inhibited by the D2 agonist quinpirole, though not the D1 agonist SKF-38393 or the D4 agonist PD168077. The D3 agonist 7-OH-DPAT also inhibited aggression, but only following high dose treatment that may affect aggression via non-specific binding to D2 receptors. Central VT infusion failed to restore D2 agonist-inhibited aggression in a subsequent experiment, demonstrating that D2 does not suppress aggression by inhibiting VT release from BSTm neurons. In a final experiment, we detected D2 agonist-induced increases in immunofluorescent colocalization of the product of the immediate early gene c-fos and the steroid-converting enzyme aromatase (ARO) within VT neurons of the BSTm. Thus, although VT and DA appear to influence mate competition aggression independently, BSTm VT neurons are clearly influenced by the activation of D2 receptors, which may modify future behaviors.
Keywords: aggression, bird, courtship, dopamine, vasotocin, vasopressin
Introduction
Across vertebrates, the neurotransmitter dopamine (DA) modulates motivated, goal-directed behaviors and thereby a wide diversity of social behaviors (e.g., Balthazart et al., 1997; Rodriguiz et al., 2004; Triemstra et al., 2005; Watt et al., 2007). For instance, DA release within limbic regions of the brain occurs during both anticipation of and involvement in behaviors associated with aggression, feeding, and reproduction (Dominguez and Hull, 2005; Miczek et al., 2002; Schultz, 2007). Dopaminergic regulation of aggression seems to primarily involve mesocorticolimbic circuits and the D2 receptor subtype (Miczek et al., 2002). However, research suggesting the involvement of this circuitry has often examined the role of DA in the regulation of territorial or defensive aggression, or has involved animals in artificial situations such as isolation or reinforcement withdrawal (Miczek et al., 2002). Recent immediate early gene and pharmacology experiments in songbirds demonstrate that aggressive competition over mates likely involves different neural circuits than do other forms of aggression such as territorial aggression (Goodson and Kabelik, in press). Mate competition aggression may therefore also involve different dopaminergic circuitry. Because little is known about the involvement of DA in mate competition aggression, we here examine whether, and by what mechanisms, DA regulates this behavior. These studies are conducted in male zebra finches (Taeniopygia guttata), a gregarious bird species in which such aggression has been extensively investigated (Goodson and Kabelik, in press).
Previous studies of dopaminergic regulation of social behaviors have often demonstrated opposing effects of excitatory and inhibitory DA receptor subtypes on social behaviors. For instance, Balthazart et al. (1997), found that activation of D1 and D2 receptor subtypes exerts opposing effects on male copulatory behavior in Japanese quail. In fact, a variety of DA receptor subtypes have been differentially implicated in the regulation of social behaviors such as aggression (Fresan et al., 2007; Miczek et al., 2002). Therefore, we here examine the effects of DA agonists targeting the excitatory D1 receptor and the inhibitory D2, D3, and D4 receptors on both courtship singing and aggressive mate competition among males.
Of particular interest among dopaminergic target regions are those containing the neuropeptide vasotocin (VT; nonmammalian homologue of vasopressin, VP). VT regulation of aggression has been shown to differ between mate competition and territorial aggression, with blockage of VT receptors in songbirds inhibiting aggression within the mate competition context, while increasing aggression during the defense of a territory or a nest (Goodson and Kabelik, in press). The population of VT neurons thought to promote mate competition aggression lies within the medial bed nucleus of the stria terminalis (BSTm), as these neurons show increased expression of the immediate early gene product Fos (an indirect marker of neural activation) in the context of mate competition, but not during resident-intruder aggression (Goodson and Kabelik, in press; Goodson and Wang, 2006). Because dopaminergic projections from the ventral tegmental area and the central gray target the BSTm and other VT-containing brain regions (Bailhache and Balthazart, 1993; Balthazart and Absil, 1997), the second experiment in the present study tests the hypothesis that D2 agonist treatment inhibits mate competition aggression by inhibiting VT release from the BSTm. We test this hypothesis by accompanying D2 agonist treatment with central infusions of VT, to determine whether VT can rescue D2 agonist-induced inhibition of aggression.
While VT neurons in the BSTm express increased Fos colocalization during mate competition aggression, VT neurons within the hypothalamic paraventricular nucleus (PVN) show a negative correlation between Fos expression and levels of territorial aggression (Goodson and Kabelik, in press). Hence, in a third experiment, we examined the effects of D2 agonist treatment on immunofluorescent colocalization of VT and the immediate early gene product Fos in the BSTm and PVN. Because VT neurons in BSTm and PVN appear to exert opposing effects on aggressive behaviors, this study tests the hypothesis that activation of the D2 receptor inhibits aggression by increasing activation of VT neurons in the PVN but not BSTm. Furthermore, we also examined colocalization of these neurons with the steroid-converting enzyme aromatase (ARO), which is of interest as steroid hormones regulate both aggression and VT production in various taxa (e.g., Kabelik et al., 2008; Plumari et al., 2004). Hence, we examined the effects of D2 agonist treatment on both Fos induction in VT neurons specifically expressing ARO, and on the expression of the ARO enzyme itself.
Methods
Subjects
A total of 82 adult male zebra finch subjects were used in these studies. Additional adult male and female zebra finches from our housing colony were used as stimulus animals during behavioral tests. To minimize animal use, some subjects were used in multiple behavioral experiments, with at least two weeks allowed between experiments. Birds were group housed in same-sex cages with ad libitum access to food and vitamin-enriched water (except during experimental trials), and were maintained on a 14:10 light:dark cycle. All procedures were conducted humanely and in a way as to minimize animal suffering, and in compliance with federal and institutional guidelines.
Experiment 1: Dopamine Agonists and Social Behaviors
Drug Treatments
In order to test the effects of DA on mate competition aggression and directed song (a courtship behavior) in male zebra finches, we injected subjects with the following drugs in a repeated measures design, with 48 hours between treatments: the D1 agonist SKF-38393 hydrochloride (Sigma-Aldrich, St. Louis, MO, USA; catalog item D047), the D2 agonist quinpirole dihydrochloride (Sigma Q111), the D3 agonist 7-OH-DPAT (Sigma H8653), and the D4 agonist PD168077 maleate (Sigma P233). Table 1 lists representative binding affinities of these agonists for the various DA receptor subtypes, of rat or human origin. The initial dose for the D1, D2, and D4 agonists was 1 mg/kg (as in Balthazart et al., 1997); a lower regular dose of 100 ug/kg was used for the D3 agonist, because this agonist can non-specifically bind to D2 receptors at high doses (Pritchard et al., 2003). Low doses were also run in mate competition trials for the drugs that had significant effects on behavior: 100 ug/kg for the D2 agonist and 10 ug/kg for the D3 agonist. All agonists were administered in 50 ul of 0.9% NaCl, injected subcutaneously into the inguinal fold of the left leg. Control injections consisted of 50 ul of 0.9% NaCl.
Table 1.
A representative list of affinities (Kd or Ki) for the dopamine (DA) agonists employed in this study at rat or human DA receptor subtypes. Lower values represent higher affinities. Much variation in affinity values is present in the literature, largely depending on experimental parameters, whether both high- and low-affinity receptors are considered, and cell line within which cloned receptors are expressed.
| Drug | target receptor |
source species |
Kd or Ki (nM) |
reference |
|---|---|---|---|---|
|
SKF38393 SKF38393 |
D1 D1 |
rat human |
26.6 45.6 |
Neumeyer et al, 2003 Yanagawa et al., 1997 |
| SKF38393 | D2 | rat | 9560 | Sokoloff et al., 1990 |
| SKF38393 | D3 | rat | 5000 | Sokoloff et al., 1990 |
| Quinpirole | D1 | rat | >1000 | Mottola et al., 2002 |
| Quinpirole Quinpirole |
D2 D2A |
rat human |
4 3.7 |
Malmberg and Mohell, 1995 Malmberg and Mohell, 1995 |
| Quinpirole | D3 | rat | 47 | Boundy et al., 1993 |
| Quinpirole | D3 | human | 0.6 | Malmberg and Mohell, 1995 |
| Quinpirole | D4.2 | human | 140-250 | Tallman et al., 1997 |
| 7-OH-DPAT 7-OH-DPAT |
D2 D2 |
rat human |
3.6 125 |
Gonzalez and Sibley, 1995 Damsma et al., 1993 |
| 7-OH-DPAT | D3 | rat | 0.5 | Gonzalez and Sibley, 1995 |
| 7-OH-DPAT | D3 | human | 0.1 | Damsma et al., 1993 |
| 7-OH-DPAT | D4.2 | human | 520-1469 | Tallman et al., 1997 |
| PD168077 | D1 | human | 4600 | Moreland et al., 2004 |
| PD168077 | D2 | human | 1280 | Moreland et al., 2004 |
| PD168077 | D3 | human | 2300 | Moreland et al., 2004 |
| PD168077 | D4 | human | 11.9 | Moreland et al., 2004 |
Mate Competition Paradigm
Unpaired zebra finches will court robustly when given the opportunity, and exposure of two males to a single female elicits aggressive behavior as males compete for access to the female. We here used this mate competition paradigm to investigate dopaminergic effects on aggression. Each subject was injected with a drug and placed into one half of a 31 cm W × 20 cm H × 36 cm D wire cage, split into left and right halves by a wire partition. Fifteen min later, a stimulus male was placed into the same half of the cage as the focal male, and a female was placed in the opposite half of the cage. Aggressive displacements of the stimulus male by the focal male were recorded for 7 min. Focal and stimulus male pairings had been prescreened, and only pairs in which the focal male was dominant were used. Male pairings were maintained across drug treatments, while females novel to both males were used during each trial. These experiments were conducted in the subjects' housing room and all animals were moved among cages with the lights off.
Courtship Paradigm
We employed a directed song paradigm to assess male courtship of females. In this test, a focal male subject was injected with a drug and placed into one half of a test cage (as in Experiment 1). Fifteen min later, a novel female was placed into the other half of the cage. The number of directed songs performed was recorded for 2 min. The female was then replaced by a novel female for another 2-min period, and then by a third female for a third 2-min period. Multiple females were presented to each male in order to minimize variation resulting from male preferences, female attractiveness, and female behavior. The average number of directed songs performed by each male was recorded as a measure of courtship. So that songs could be heard over the sounds of other birds, the first courtship experiment was conducted in an empty room adjacent to the subjects' main housing room. Walk-in sound isolation chambers were later acquired, so the second experiment was conducted within these chambers. Prior to experimental runs, subjects were habituated to either the empty room or the sound isolation chambers for several hours each time, over the course of three days.
Experimental Design and Statistical Analyses
In order to avoid excessive testing over short periods of time, not all drug treatments were administered in the course of a single experiment. We first compared a high dose of the D1 agonist, the D2 agonist, and saline vehicle on aggression. Because the D2 agonist was found to affect aggression, we also tested a low dose of the D2 agonist relative to vehicle. Next, we examined the effects of a high dose of the D3 agonist, the D4 agonist, and vehicle on aggression. Because the D3 agonist was also found to affect aggression, we tested a low dose of this drug relative to vehicle. We further compared the high doses of the D1 agonist, the D2 agonist, and vehicle, and later the D3 agonist, D4 agonist, and vehicle on courtship.
All tests were analyzed using one-way repeated-measures analysis of variance (ANOVA) or paired t-test. Post hoc analyses for ANOVA results were conducted using paired t-test pairwise comparisons at α=0.05. Data that did not conform to assumptions of these tests were log-transformed, although figures present untransformed data for ease of interpretation. Only subjects that displayed at least five displacements during each experiment (across all treatments combined) were included for analysis.
Experiment 2: Dopamine-Vasotocin Interaction and Mate Competition Aggression
As shown in the Results for Experiment 1, D2 agonist treatments inhibited mate competition aggression without a corresponding effect on courtship behavior. This pattern of results mimics what we have previously observed using VP V1 antagonists to block VT receptors (Goodson et al., 2004; Kabelik et al., 2009). Hence, we hypothesized that D2 agonist treatment may act to inhibit aggression by limiting release from VT neurons in the BSTm, since these neurons show increased Fos expression in response to mate competition (Goodson and Wang, 2006). We here tested this hypothesis by examining whether exogenous VT infusion would restore the aggression that is inhibited by D2 agonist treatment.
Surgeries
Stereotaxic cannulation surgeries were conducted on 26 males, using isoflurane vapor anesthesia at 2-5% of a compressed air flow. Coordinates were referenced to the vascular convergence at the rostral tip of the cerebellum. A 26-gauge guide cannula for small animals (Plastics One, Roanoke, VA) with a 4.6 mm extension beyond the pedestal was inserted 3.1 mm rostral, 1.7 mm right lateral, and 2.6 mm deep, at a 21° angle toward medial. These coordinat es target the caudal portion of the lateral ventricle. The guide cannula was adhered to the skull using a combination of Nexaband S/C cyanoacrylate glue (Abbott Laboratories, North Chicago, IL) and Stoelting dental cement (Stoelting, Wood Dale, IL). A sterile dummy cannula with attached dust cap (Plastics One) was inserted into the guide cannula at all times other than during infusion procedures. Injection cannulae, but not dummy cannulae, projected 1 mm beyond the length of the guide cannula. Subjects were allowed at least five days of recovery before subsequent testing. Following experimental procedures, birds were infused with ink to verify cannula placement.
Drug Treatments and Experimental Design
In this experiment, subjects received an injection of either 1 mg/kg D2 agonist or vehicle, plus an infusion of either 50 ng VT (Sigma V0130) in 0.9% NaCl or vehicle. The infusion dose of 50 ng of VT into the lateral ventricle has been previously demonstrated to alter mate competition aggression in zebra finches (Goodson et al., 2004), and doses of VT ranging from 1 ng to 100 ng have been found to alter reproductive behaviors following intracerebroventricular infusion in newts (Moore and Miller, 1983). These were delivered in a repeated-measures design with 48 hr between treatments as follows: 1. vehicle injection + vehicle infusion; 2. vehicle injection + VT infusion; 3. D2 agonist injection + VT infusion. The subjects were tested in a mate competition aggression paradigm as in Experiment 1. Only subjects that displayed at least five displacements during each experiment (across all treatments combined) were included for analysis.
Experiment 3: D2 Agonist Effects on Vasotocin-Aromatase-Fos Colocalization
To further assess the effects of the D2 agonist on VT neurons, we examined the colocalization of VT, ARO, and Fos in brain sections of animals that received an injection of either 1 mg/kg D2 agonist or 0.9% NaCl vehicle 90 min prior to sacrifice. To limit animal use, we conducted this experiment on cannulated animals that were to be euthanized for verification of cannula placement. Because the right hemisphere was cannulated, we restricted our analyses of the BSTm to the intact left hemisphere.
Experimental Design
Subjects were acclimated to individual housing within a quiet room (containing five or fewer birds) for several hours over two days. The birds were left in this room overnight on the second day. On the morning of the third day, prior to lights-on, the subjects were injected with D2 agonist (n=10) or vehicle (n=10) as in the previous experiments. Ninety min later, allowing time for the drug to take effect and for Fos induction to peak, subjects were euthanized by isoflurane overdose.
Tissue Processing
Immediately after euthanasia, subjects were transcardially perfused with 0.1 M phosphate buffered saline (PBS; pH 7.4) followed by 4% paraformaldehyde. The brains were dissected out and postfixed overnight in 4% paraformaldehyde in PBS, followed by two days of cryoprotection in 30% sucrose in 0.1 M phosphate buffer (pH 7.4). The brains were then frozen and sectioned on a cryostat at 40 mm. Three series were collected and stored in cyroprotectant at −80° C until use. Tissue was rinsed 5 x 10 min in PBS and then incubated for 1 hr in block (PBS + 5% normal donkey serum + 0.3% Triton-X-100). Next, the sections were incubated for 40 h at 4° C in PBS + 2.5% normal donkey serum + 0.3% Triton-X-100 containing guinea pig anti-VP (Bachem, Torrance, CA) and rabbit anti-Fos (Santa Cruz Biotechnology, Santa Cruz, CA), both at 1 μ/ml (as in Goodson et al., 2009b), as well as a custom-made sheep anti-aromatase antibody (Bethyl Laboratories, Inc., Montgomery, TX) at 4 μ/ml. The aromatase antibody was generated against the 3′ end of the zebra finch aromatase coding region according to Saldanha et al. (2000). Aromatase immunoreactivity was greatly reduced by preadsorption with 0.1 mg/ml peptide and completely eliminated with 1 mg/ml peptide. The primary incubation was followed by 2 × 30 min rinses in PBS; 1 hr in a donkey anti-guinea pig secondary conjugated to biotin (8 μl/ml; Vector Laboratories, Burlingame, CA); 2 × 15 min rinses in PBS; 2 hr at room temperature in streptavidin conjugated to Alexa Fluor 488 at 3 μl/ml, donkey anti-rabbit secondary conjugated to Alexa Fluor 594 at 5 μl/ml, and donkey anti-sheep secondary conjugated to Alexa Fluor 680 at 7 μl/ml (all from Molecular Probes, Eugene, OR) in PBS + 2.5% normal donkey serum + 0.3% Triton-X-100. Following extensive rinsing in PBS, the sections were mounted on slides subbed with gelatin and chrome alum and coverslipped with ProLong Gold mounting medium containing DAPI nuclear stain (Molecular Probes).
Image analyses
The BSTm was photographed unilaterally in the left hemisphere at two levels: 1. directly above the anterior commisure and 2. at the level of the diagonal band of the BSTm, just caudal to the anterior commisure. Limited evidence suggests that these levels are not functionally equivalent (Goodson and Evans, 2004; Goodson et al., 2005; Meddle et al., 1999), thus we considered them separately in the analyses. The PVN was photographed bilaterally and at the same rostrocaudal levels as the BSTm. Monochrome images were captured using a Zeiss AxioImager microscope (Carl Zeiss Inc., Göttingen, Germany) outfitted with a Z-drive and a Zeiss Apotome optical dissector (which yields focal plane resolution at near-confocal levels), a Zeiss Axiocam HRm camera, and a high intensity X-Cite fluorescence illuminator (EXFO Photonic Solutions Inc., Mississauga, Canada). Images were captured in Zeiss AxioVision 4.6.3.0 and exported into Photoshop CS3 (Adobe Systems Inc., San Jose, CA). Data for all subjects were collected using standardized acquisition parameters. All cell counts were conducted blind to experimental treatment and by a single observer per brain region. Statistical comparisons were conducted by t-test.
Results
Experiment 1: Dopamine Agonists and Social Behaviors
Mate Competition Aggression
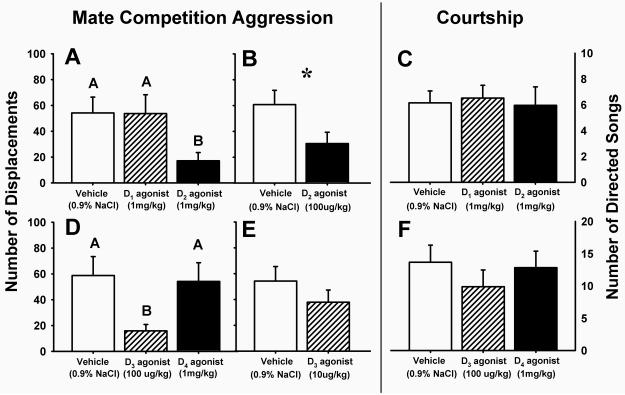
Aggression was significantly reduced by 1 mg/kg treatment with the D2 agonist, relative to either vehicle control or 1 mg/kg of the D1 agonist (F(2,18)=5.72, p=0.012; Fig. 1A). To establish drug specificity, we also conducted a second experiment using a lower dose and found that treatment with 100 μg/kg of the D2 agonist was likewise effective in reducing aggression relative to vehicle control (t=2.74, df=16, p=0.015; Fig. 1B). Next, we examined the effects of the D3 and D4 agonists on mate competition aggression. We found that aggression was reduced by 100 μg/kg treatment with the D3 agonist, relative to either vehicle control or 1 mg/kg of the D4 agonist (F(2,13)=6.19, p=0.013; Fig. 1D). However, D3 agonist treatment at the lower dose of 10 μg/kg did not alter aggression relative to vehicle control (t=0.27, df=16, p=0.79; Fig. 1E).
Figure 1.
The effects of subcutaneous injections of dopamine D1-D4 receptor agonists on social behaviors in male zebra finches. (A) Treatment with 1 mg/kg of the D2 agonist quinpirole, but not 1 mg/kg of the D1 agonist SKF-38393, inhibited mate competition aggression (per 7 min session) relative to treatment with 0.9% NaCl vehicle (n=20, p=0.012). Posthoc analyses show that the D2 agonist and vehicle treatment groups differ at p=0.003. (B) A lower dose, 100 μg/kg, of the D2 agonist also inhibited aggression (n=17, p=0.015). (C) Neither treatment with 1 mg/kg of the D1 agonist, nor with 1 mg/kg of the D2 agonist resulted in altered numbers of directed songs (per 2 min session) to females relative to treatment with vehicle (n=19, p=0.16). (D) Treatment with 100 μg/kg of the D3 agonist 7-OH-DPAT, but not 1 mg/kg of the D4 agonist PD168077, inhibited aggression relative to treatment with vehicle (n=15, p=0.013). Posthoc analyses show that the D3 agonist and vehicle treatment groups differ at p=0.042. (E) However, a lower dose, 10 μg/kg, of the D3 agonist failed to inhibit aggression (n=17, p=0.79), thus leaving it unclear whether the higher dose was acting via D3 receptors, or by binding to D2 receptors. (F) Likewise, neither treatment with 100 μg/kg of the D3 agonist or with 1 mg/kg of the D4 agonist resulted in altered numbers of directed songs to females relative to vehicle (n=16, p=0.37). Bars represent means + 1 S.E.M. Different letters above bars and * indicate treatment groups differing at α<0.05.
Courtship
Directed song was not affected by 1 mg/kg treatment with either the D1 agonist or the D2 agonist, relative to vehicle controls (F(2,17)=2.08, p=0.16; Fig. 1C). Similarly, we found no effect of 100 ug/kg treatment with the D3 agonist, or of 1 mg/kg treatment with the D4 agonist, relative to vehicle controls (F(2,14)=1.07, p=0.37; Fig. 1E). The difference in absolute mean song numbers between the two experiments likely stems from the first experiment being run in a large room, whereas small sound isolation booths were used for behavioral testing in the second experiment.
Experiment 2: Dopamine-Vasotocin Interaction and Mate Competition Aggression
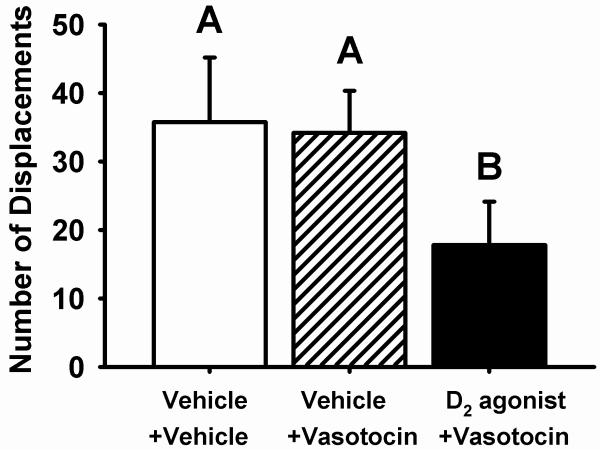
Contrary to our prediction that VT infusions would eliminate the behavioral effects of 1 mg/kg D2 agonist treatment, we found that aggression was still inhibited in subjects that received a D2 agonist injection + VT infusion, relative to either vehicle injection + VT infusion, or vehicle injection + vehicle infusion (F(2,19)=3.99, p=0.036; Fig. 2).
Figure 2.
The effects of subcutaneous injection of dopamine D2 agonist quinpirole, in conjunction with central vasotocin (VT) infusion, on mate competition aggression (per 7 min session) in male zebra finches. Infusions of VT did not reverse the inhibitory effects of D2 agonist treatment on mate competition aggression: subcutaneous injection of 1 mg/kg of the D2 agonist + infusion of 50 ng VT into the lateral ventricle inhibited aggression in comparison to injection of 0.9% NaCl vehicle (VEH) + infusion of 50 ng VT or injection of VEH + infusion of VEH (n=21, p=0.036). Different letters above bars indicate treatment groups differing at α<0.05 in posthoc comparisons. Bars represent means + 1 S.E.M.
Experiment 3: D2 Activation Effects on Vasotocin-Aromatase-Fos Colocalization
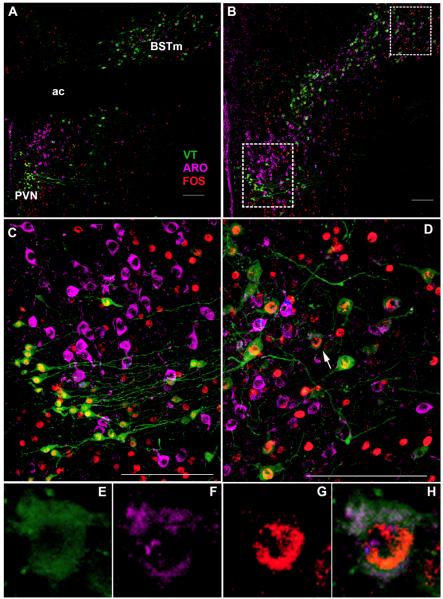
We next examined the effects of D2 agonist treatment on the expression of Fos within VT-immunoreactive (-ir) and ARO-ir cells in the BSTm and PVN. VT-ir and ARO-ir neurons are spatially segregated in the PVN, but show extensively intermingling and colocalization within the BSTm (Fig. 3). The average colocalization frequencies among VT, ARO, and Fos in control and D2 agonist-treated animals are listed in Table 2.
Figure 3.
Photomicrographs depicting vasotocin (VT), aromatase (ARO), and Fos immunofluorescence within the (A) rostral, and (B) caudal subdivisions of the paraventricular nucleus (PVN) and medial bed nucleus of the stria terminalis (BSTm) of a male zebra finch from the control group in Experiment 3. (C) A magnified view of the PVN (from B, lower box) shows the spatial segregation of VT and ARO neurons in this region. (D) A magnified view of the BSTm (from B, upper box) that shows the higher levels of intermingling and colocalization between VT and ARO in this region. The white arrow indicates a triple-labeled neuron, which is shown separately below for VT (E), ARO (F), and Fos (G) immunofluorescence, as well as in a composite (H). Scale bars = 100 μm; ac = anterior commisure.
Table 2.
Neurons within the medial bed nucleus of the stria terminalis (BSTm) and paraventricular nucleus (PVN) of male zebra finches treated either with D2 agonist (D2ag) or vehicle (Veh). Mean and standard error of the mean (S.E.M.) values are displayed for vasotocin (VT) and aromatase (ARO) neurons colocalized with one another or the immediate early gene Fos. Values are of combined counts for rostral and caudal subdivisions of each nucleus.
| %VT colocalizing Fos | %VT colocalizing ARO | %ARO colocalizing Fos | %ARO colocalizing VT | |||||
|---|---|---|---|---|---|---|---|---|
| mean | S.E.M. | mean | S.E.M. | mean | S.E.M. | mean | S.E.M. | |
|
BSTm Veh D2ag |
57.97 65.59 |
4.05 2.84 |
27.26 32.97 |
2.29 4.02 |
23.31 24.57 |
2.70 2.93 |
15.03 13.78 |
1.10 1.99 |
|
PVN Veh D2ag |
71.00 74.60 |
2.08 3.90 |
6.33 5.65 |
1.23 0.84 |
13.28 14.20 |
2.06 2.34 |
4.79 4.20 |
0.60 0.72 |
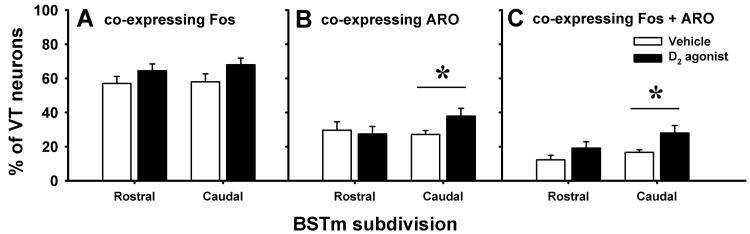
Despite trends in the data, treatment with 1 mg/kg of the D2 agonist, relative to vehicle, failed to significantly affect Fos colocalization with VT neurons at both the rostral (t=1.29, df=18, p=0.21) and caudal (t=1.65, df=18, p=0.12) levels of the BSTm (Fig. 4A). However D2 agonist treatment did increase ARO colocalization with VT in the caudal (t=2.11, df=18, p=0.049), though not rostral (t=0.33, df=18, p=0.11), BSTm (Fig. 4B). The D2 agonist produced even more prominent effects on the colocalization of all three substances, such that D2 agonist-treated subjects exhibited a significantly higher percentage of VT-ir neurons that colocalized both ARO and Fos than did saline controls. However, these effects were again only apparent with the caudal (t=2.46, df=18, p=0.024) and not rostral (t=1.48, df=18, p=0.16) BSTm (Fig. 4C).
Figure 4.
The effects of subcutaneous injections of the D2 agonist quinpirole on Fos and aromatase (ARO) colocalization within vasotocin (VT) neurons in the medial bed nucleus of the stria terminalis (BSTm) of male zebra finches. (A) Relative to treatment with 0.9% NaCl vehicle alone, subcutaneous injection of 1 mg/kg of the D2 agonist failed to affect Fos colocalization with VT at the rostral (n=20, p=0.21) or caudal (n=20, p=0.12) BSTm. (B) Treatment with the D2 agonist did increase ARO colocalization with VT in the caudal (n=20, p=0.049), though not rostral (n=20, p=0.74), BSTm. (C) Overall, treatment with the D2 agonist increased Fos+ARO colocalization with VT (triple labeling) in the caudal (n=20, p=0.024), though not rostral (n=20, p=0.16), BSTm. Bars represent means + 1 S.E.M. * indicates treatment groups differing at p<0.05.
D2 agonist treatment did not affect absolute VT or ARO cell numbers, or Fos colocalization within ARO neurons, at either the rostral or caudal levels of the BSTm (p>0.05 for all; data not shown). Likewise, no effects of D2 agonist treatment were observed on the number of ARO or VT neurons, or on double- and triple-labeling within ARO cells at either level of the PVN (p>0.05 for all; data not shown).
Discussion
This study demonstrates that activation of the D2 DA receptor subtype inhibits aggressive competition over mates, and that none of the tested DA agonists affect courtship behavior per se. Furthermore, VT infusion does not restore D2 agonist-inhibited aggression levels, thereby demonstrating at least partial independence of the neural circuitry by which these substances regulate mate competition aggression. Nevertheless, D2 agonist treatment did induce ARO and Fos colocalization in VT neurons of the caudal BSTm, suggesting some input or feedback of the DA system onto these VT neurons. Therefore, although the mechanisms by which D2 agonist treatment inhibits mate competition aggression do not seem to involve the regulation of VT release, activation of D2 receptors induces changes in BSTm VT neurons that may alter the future probability of aggression or other social behaviors.
Dopaminergic Regulation of Mate Competition Aggression
DA is known to regulate many social behaviors, including resident-intruder aggression (e.g., Balthazart et al., 1997; Rodriguiz et al., 2004; Watt et al., 2007). However, the mechanisms involved in this behavioral regulation are unclear. For instance, both agonists and antagonists of the D1 and D2 receptors have been shown to decrease aggression in rodent studies, although some of these findings seem due to high treatment doses that achieve effects via inhibition of motor activity and nonspecific binding to other neural systems and receptor subtypes (Miczek et al., 2002).
In this study, we demonstrate that treatment with a D2 agonist leads to a robust inhibition of mate competition aggression, while the D1 and D4 agonists are without effect. Although our results also indicate an inhibitory effect of the D3 agonist, the specificity of this agonist is not entirely clear. Indeed, it is important to note that the affinities of the employed agonists for particular receptor subtypes have not been ascertained in the zebra finch or related bird species, and while such affinities are usually similar across species, there is always uncertainty of action and affinity, even within well-studied species (e.g., Seeman and Schaus, 1991). We interpret our D3 agonist findings conservatively because studies in D3 receptor knockout mice demonstrate that doses above 10 ug/kg of 7-OH-DPAT non-specifically activate the D2 receptor, causing equal behavioral changes in knockouts and wild-type individuals (Pritchard et al., 2003). Only doses of 10 ug/kg or below exert specific D3 effects, at least in mice. However, songbirds have higher metabolic rates than do rodents and often require higher doses of drugs to achieve effects comparable to those in rodents (Schroeder and Riters, 2006). Hence, it is unclear whether the inhibition of aggression observed here with 100 ug/kg of this D3 agonist is due to binding at D2 or D3 receptors, precluding definitive conclusions regarding the involvement of D3 receptors in mate competition aggression. Clearly, however, dopaminergic signaling via D2-like receptors, and likely primarily at the D2 receptor, inhibits mate competition aggression.
Dopaminergic Regulation of Courtship
It is surprising that none of the DA agonist treatments in the present study altered directed singing to females because DA has been strongly implicated in the regulation of courtship and reproduction in birds (Bharati and Goodson, 2006; Charlier et al., 2005), and DA projections target song control nuclei of the telencephalon (Appeltants et al., 2000; Appeltants et al., 2001, 2002). In Japanese quail, D1 receptors mediate positive DA effects on consummatory sexual behavior, whereas D2 receptors mediate negative effects on both appetitive and consummatory components of behavior (Balthazart et al., 1997). Furthermore, DA is released within Area X of the song control circuit during directed singing in zebra finches, and more DA is released in association with directed than undirected song (Sasaki et al., 2006). Directed song is also correlated with changes in Fos induction in several DA populations, with a positive correlation present between song number and percent of DA neurons colocalized with Fos in the central gray and ventral tegmental area of zebra finches (Bharati and Goodson, 2006; Goodson et al., 2009a).
However, the relationship between DA and song is not simple. For instance, a recent study has reported negative correlations between different types of song and D1 receptor densities in several brain regions of European starlings within a breeding context (Heimovics et al., 2009), but a positive correlation between song and D1 receptor densities in nonbreeding birds. Our subjects were housed under reproductively non-stimulatory conditions (same-sex housing), but were highly motivated to court. It is thus possible that we encountered a ceiling effect, and that further examination may reveal D1 effects on directed song. Alternately, because zebra finches are opportunistic breeders that do not exhibit typical seasonal changes in reproductive physiology and behavior (Zann, 1996), the relationship between DA and song behavior may be different in this species than in seasonally breeding species. However, it is important to note that a lack of effect does not conclusively demonstrate the noninvolvement of any particular receptor subtype, as access of the agonists to the brain regions regulating song may be more limited than to regions that regulate aggression. Another possibility is that no relationship between DA agonists and directed song was observed in this study because this behavior may be regulated via different receptors. For instance, Cornil et al. (2008) demonstrated that in zebra finches, DA released in the striatal song nucleus Area X (and other brain regions) binds at α2-adrenoceptors, thereby raising the possibility that activation of α2-adrenoceptors and not DA receptors may be primarily responsible for the regulation of directed song. Additionally, in Japanese quail, DA has been shown to activate both α1- and α2-adrenoceptors in the POA (Cornil et al., 2002), suggesting that the binding of DA at adrenoceptors may be somewhat common. One previous finding does suggest a direct action of DA on DA receptors. Rauceo et al. (2008) found effects of the mixed D1/D2 DA antagonist on directed song in zebra finches. However, these findings were obtained only following 5 days of high dose antagonist treatment in testosterone-treated males, and the effect disappeared by the subsequent testing period, at which time the treatment was found to generally decrease behavioral activity levels. These results leave unclear whether the effects on directed song were due to specific antagonism of DA receptors, or whether they are the result of other compensatory changes induced by this treatment.
Dopamine-Vasotocin Interactions
Vasotocin release from the BSTm is thought to promote aggressive competition over mates for a number of reasons. First, VT neurons within the BSTm of zebra finches show increased Fos colocalization in the mate competition paradigm (Goodson and Wang, 2006). Second, VT infusions into the lateral septum or adjoining lateral ventricle (mimicking release from the BSTm) facilitate mate competition aggression, though they do not alter directed song (Goodson and Adkins-Regan, 1999; Goodson et al., 2004). Third, central treatment of zebra finches with antagonists to block VT receptors inhibits mate competition aggression but not directed song (Goodson et al., 2004; Kabelik et al., 2009). Fourth, endogenous VT likewise promotes mate competition aggression in territorial waxbills, supporting the hypothesis that this is a general mechanism across species (Goodson et al., in press).
We here tested the hypothesis that D2 agonist treatment inhibits aggression by inhibiting VT release from the BSTm because the behavioral results of D2 agonist treatment in Experiment 1 of this study were highly similar to those described above, following treatment with an antagonist of VT receptors. Furthermore, the BSTm receives direct TH-ir innervation (Appeltants et al., 2001; Balthazart et al., 1998; Bottjer, 1993; Reiner et al., 1994) strongly suggesting that there are direct dopaminergic inputs into the VT system. However, we here found that VT infusions into the lateral ventricle failed to recover D2 agonist-induced inhibition of aggression, suggesting that DA and VT influence mate competition aggression independently. The failure of VT alone to facilitate aggression beyond that of our other (vehicle infused) control group is not highly surprising as previous effects in this direction have been weak, likely due to a ceiling effect in these highly motivates individuals, following same-sex housing (Goodson and Adkins-Regan, 1999; Goodson et al., 2004). However, four separate studies using VP antagonists have clearly demonstrated that endogenous VT release in this region causally facilitates mate competition aggression (Goodson and Adkins-Regan, 1999; Goodson et al., in press; Goodson et al., 2004; Kabelik et al., 2009), and the VT dose infused in this study has been shown to be effective in previous studies of this and other species (Goodson et al., 2004; Moore and Miller, 1983). Importantly, the lack of significant facilitation by VT (whether due to a ceiling effect or not) does not impact our ability to test the hypothesis that the inhibition of aggression by the D2 agonist is mediated by a reduction in the release of VT. Indeed, we can clearly reject that hypothesis, since the D2 agonist strongly inhibited aggressive behavior even after central administrations of this fairly high dose of VT.
In an additional test of our hypothesis, we used fluorescent immunolabeling to examine the effect of a D2 agonist on VT-Fos colocalization in the BSTm, as well as the PVN. Although no significant effects of D2 agonist treatment on VT-Fos colocalization were present, trends in the BSTm hinted at a possible induction of Fos. Such upregulation would be unexpected, as Fos induction in VT neurons of the BSTm is associated with the exhibition of mate competition aggression, not its suppression. In contrast, such results would have been expected within the PVN, where VT-Fos colocalization is negatively correlated with aggressive response to a simulated territorial intrusion in male song sparrows (Goodson and Kabelik, in press). However, no such results were obtained for the PVN.
D2 agonist treatment did, however, significantly increase the percentage of VT neurons in the caudal BSTm that co-expressed aromatase. Evidence of neural activation in these neurons is even more pronounced in relation to triple-labeling; i.e., D2 agonist treatment increased the colocalization of both ARO and Fos within VT neurons. Thus, it is clear that D2 agonist treatment did have a causal effect on gene transcription within VT neurons of the BSTm, although our behavioral experiments suggest that these changes do not underlie the immediate effects of D2 agonist treatment on aggression.
Dopamine-Aromatase Interactions
The conversion of testosterone to 17β-estradiol via the enzyme ARO is known to be essential for the regulation of numerous reproductive social behaviors (Balthazart and Ball, 2006; Balthazart et al., 1996b), and we therefore hypothesized that aromatization may also regulate mate competition aggression. Given that 1) VT and ARO are expressed in many of the same brain areas (Aste et al., 1998; Balthazart et al., 1996a); 2) VT/VP neurons of the BSTm are strongly regulated by estradiol in many vertebrate species (De Vries and Panzica, 2006); 3) ARO activity is at least partly regulated by DA (Absil et al., 2001); and 4) dopaminergic fibers are known to converge onto most ARO-containing brain regions, including the BSTm (Balthazart et al., 1998), we predicted that D2 agonist treatment would influence ARO expression within VT neurons, and within the BSTm and hypothalamus more generally.
Because in vitro studies have show that cAMP has a direct stimulatory effect on aromatase activity within the brain and other tissues (Callard, 1981; Verhoeven et al., 1979), and because the D2 receptor works via Gi inactivation of adenylate cyclase production of cAMP (Absil et al., 2001), we expected to observe either changes in Fos immunoreactivity within ARO cells, or a decrease in ARO expression. Instead, D2 agonist treatment increased ARO immunoreactivity in VT neurons of the caudal BSTm (likely due to genomic ARO upregulation). These results suggest an indirect route of action by which the activation of D2 receptors on unknown cells leads to signaling that targets the caudal BSTm, thereby causing an increase in ARO within these VT neurons. Further experiments are necessary to more precisely describe these neural connections and their functional significance.
Conclusions
The activation of D2 receptors inhibits aggressive competition over mates in male zebra finches, just as D2 activation inhibits other forms of aggression in other species (Miczek et al., 2002). However, this inhibition of aggression appears to be independent of the vasotocinergic mechanisms by which mate competition is regulated in this and other songbird species. Nevertheless, some cross-talk does exist between dopaminergic and vasotocinergic systems, in that D2 agonist treatment induces Fos+ARO immunoreactivity within VT neurons of the caudal BSTm. The induction of ARO in these neurons by the D2 agonist likely does not reflect a direct action on VT neurons, because activation of D2 receptors should inhibit ARO transcription via Gi inactivation of adenylate cyclase production of cAMP. These findings therefore suggest an inhibition of mate competition aggression by the D2 agonist with subsequent indirect feedback to VT neurons in the caudal BSTm, possibly to regulate future behaviors.
Acknowledgements
We would like to thank J.A. Morrison, L.C. Ayres, C.R. Theisen, and M.E. Wade for help with behavioral trials, S.E. Schrock and J.H. Murray for help with tissue processing and image quantification, and two anonymous reviewers for comments on the manuscript. This study was funded by NIMH grant RO1 MH-0-62656 to J.L.G.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Absil P, Baillien M, Ball GF, Panzica GC, Balthazart J. The control of preoptic aromatase activity by afferent inputs in Japanese quail. Brain Res Brain Res Rev. 2001;37:38–58. doi: 10.1016/s0165-0173(01)00122-9. [DOI] [PubMed] [Google Scholar]
- Appeltants D, Ball GF, Balthazart J. The distribution of tyrosine hydroxylase in the canary brain: demonstration of a specific and sexually dimorphic catecholaminergic innervation of the telencephalic song control nuclei. Cell Tissue Res. 2001;304:237–259. doi: 10.1007/s004410100360. [DOI] [PubMed] [Google Scholar]
- Aste N, Balthazart J, Absil P, Grossmann R, Mulhbauer E, Viglietti-Panzica C, Panzica GC. Anatomical and neurochemical definition of the nucleus of the stria terminalis in Japanese quail (Coturnix japonica) J Comp Neurol. 1998;396:141–157. [PubMed] [Google Scholar]
- Bailhache T, Balthazart J. The catecholaminergic system of the quail brain: immunocytochemical studies of dopamine beta-hydroxylase and tyrosine hydroxylase. J Comp Neurol. 1993;329:230–256. doi: 10.1002/cne.903290206. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Absil P. Identification of catecholaminergic inputs to and outputs from aromatase-containing brain areas of the Japanese quail by tract tracing combined with tyrosine hydroxylase immunocytochemistry. J Comp Neurol. 1997;382:401–428. [PubMed] [Google Scholar]
- Balthazart J, Absil P, Foidart A, Houbart M, Harada N, Ball GF. Distribution of aromatase-immunoreactive cells in the forebrain of zebra finches (Taeniopygia guttata): implications for the neural action of steroids and nuclear definition in the avian hypothalamus. J Neurobiol. 1996a;31:129–148. doi: 10.1002/(SICI)1097-4695(199610)31:2<129::AID-NEU1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 2006;29:241–249. doi: 10.1016/j.tins.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Castagna C, Ball GF. Differential effects of D1 and D2 dopamine-receptor agonists and antagonists on appetitive and consummatory aspects of male sexual behavior in Japanese quail. Physiol Behav. 1997;62:571–580. doi: 10.1016/s0031-9384(97)00163-7. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Foidart A, Absil P, Harada N. Effects of testosterone and its metabolites on aromatase-immunoreactive cells in the quail brain: Relationship with the activation of male reproductive behavior. J Steroid Biochem Mol Biol. 1996b;56:185–200. doi: 10.1016/0960-0760(95)00236-7. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Foidart A, Baillien M, Harada N, Ball GF. Anatomical relationships between aromatase and tyrosine hydroxylase in the quail brain: double-label immunocytochemical studies. J Comp Neurol. 1998;391:214–226. [PubMed] [Google Scholar]
- Bharati IS, Goodson JL. Fos responses of dopamine neurons to sociosexual stimuli in male zebra finches. Neuroscience. 2006;143:661–670. doi: 10.1016/j.neuroscience.2006.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottjer SW. The distribution of tyrosine hydroxylase immunoreactivity in the brains of male and female zebra finches. J Neurobiol. 1993;24:51–69. doi: 10.1002/neu.480240105. [DOI] [PubMed] [Google Scholar]
- Boundy VA, Luedtke RR, Gallitano AL, Smith JE, Filtz TM, Kallen RG, Molinoff PB. Expression and characterization of the rat D3 dopamine receptor: pharmacologic properties and development of antibodies. J Pharmacol Exp Ther. 1993;264:1002–1011. [PubMed] [Google Scholar]
- Damsma G, Bottema T, Westerink BHC, Tepper PG, Dijkstra D, Pugsley TA, MacKenzie RG, Heffner TG, Wikström H. Pharmacological aspects of R-(+)-7-OH-DPAT, a putative dopamine D3 receptor ligand. Eur J Pharmacol. 1993;249:R9–R10. doi: 10.1016/0014-2999(93)90533-n. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Panzica GC. Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: different mechanisms, similar endpoints. Neuroscience. 2006;138:947–955. doi: 10.1016/j.neuroscience.2005.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez AM, Sibley DR. [3H]7-OH-DPAT is capable of labeling dopamine D2 as well as D3 receptors. Eur J Pharmacol. 1995;272:R1–3. doi: 10.1016/0014-2999(94)00738-s. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Adkins-Regan E. Effect of intraseptal vasotocin and vasoactive intestinal polypeptide infusions on courtship song and aggression in the male zebra finch (Taeniopygia guttata) J Neuroendocrinol. 1999;11:19–25. doi: 10.1046/j.1365-2826.1999.00284.x. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Kabelik D. Dynamic limbic networks and social diversity in vertebrates: From neural context to neuromodulatory patterning. Front Neuroendocrinol. doi: 10.1016/j.yfrne.2009.05.007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Kabelik D, Kelly AM, Rinaldi J, Klatt JD. Midbrain dopamine neurons reflect affiliation phenotypes in finches and are tightly coupled to courtship. Proc Natl Acad Sci U S A. 2009a;106:8737–8742. doi: 10.1073/pnas.0811821106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Kabelik D, Schrock SE. Dynamic neuromodulation of aggression by vasotocin: Influence of social context and social phenotype in territorial songbirds. Biol Lett. doi: 10.1098/rsbl.2009.0316. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Lindberg L, Johnson P. Effects of central vasotocin and mesotocin manipulations on social behavior in male and female zebra finches. Horm Behav. 2004;45:136–143. doi: 10.1016/j.yhbeh.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Rinaldi J, Kelly AM. Vasotocin neurons in the bed nucleus of the stria terminalis preferentially process social information and exhibit properties that dichotomize courting and non-courting phenotypes. Horm Behav. 2009b;55:197–202. doi: 10.1016/j.yhbeh.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Wang Y. Valence-sensitive neurons exhibit divergent functional profiles in gregarious and asocial species. Proc Natl Acad Sci USA. 2006;103:17013–17017. doi: 10.1073/pnas.0606278103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimovics SA, Cornil CA, Ball GF, Riters LV. D1-like dopamine receptor density in nuclei involved in social behavior correlates with song in a context-dependent fashion in male European starlings. Neuroscience. 2009;159:962–973. doi: 10.1016/j.neuroscience.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabelik D, Klatt JD, Kingsbury MA, Goodson JL. Endogenous vasotocin exerts context-dependent behavioral effects in a semi-naturalistic colony environment. Horm Behav. 2009;56:101–107. doi: 10.1016/j.yhbeh.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmberg A, Mohell N. Characterization of [3H]quinpirole binding to human dopamine D2A and D3 receptors: effects of ions and guanine nucleotides. J Pharmacol Exp Ther. 1995;274:790–797. [PubMed] [Google Scholar]
- Miczek KA, Fish EW, De Bold JF, De Almeida RM. Social and neural determinants of aggressive behavior: pharmacotherapeutic targets at serotonin, dopamine and gamma-aminobutyric acid systems. Psychopharmacology (Berl) 2002;163:434–458. doi: 10.1007/s00213-002-1139-6. [DOI] [PubMed] [Google Scholar]
- Moore FL, Miller LJ. Arginine vasotocin induces sexual behavior of newts by acting on cells in the brain. Peptides. 1983;4:97–102. doi: 10.1016/0196-9781(83)90173-0. [DOI] [PubMed] [Google Scholar]
- Moreland RB, Terranova MA, Chang R, Uchic ME, Matulenko MA, Surber BW, Stewart AO, Brioni JD. [3H] A-369508 ([2-[4-(2-cyanophenyl)-1-piperazinyl]-N-(3-methylphenyl) acetamide): an agonist radioligand selective for the dopamine D4 receptor. Eur J Pharmacol. 2004;497:147–154. doi: 10.1016/j.ejphar.2004.06.049. [DOI] [PubMed] [Google Scholar]
- Mottola DM, Kilts JD, Lewis MM, Connery HS, Walker QD, Jones SR, Booth RG, Hyslop DK, Piercey M, Wightman RM, Lawler CP, Nichols DE, Mailman RB. Functional selectivity of dopamine receptor agonists. I. Selective activation of postsynaptic dopamine D2 receptors linked to adenylate cyclase. J Pharmacol Exp Ther. 2002;301:1166–1178. doi: 10.1124/jpet.301.3.1166. [DOI] [PubMed] [Google Scholar]
- Neumeyer JL, Kula NS, Bergman J, Baldessarini RJ. Receptor affinities of dopamine D1 receptor-selective novel phenylbenzazepines. Eur J Pharmacol. 2003;474:137–140. doi: 10.1016/s0014-2999(03)02008-9. [DOI] [PubMed] [Google Scholar]
- Pritchard LM, Logue AD, Hayes S, Welge JA, Xu M, Zhang J, Berger SP, Richtand NM. 7-OH-DPAT and PD 128907 selectively activate the D3 dopamine receptor in a novel environment. Neuropsychopharmacology. 2003;28:100–107. doi: 10.1038/sj.npp.1300018. [DOI] [PubMed] [Google Scholar]
- Rauceo S, Harding CF, Maldonado A, Gaysinkaya L, Tulloch I, Rodriguez E. Dopaminergic modulation of reproductive behavior and activity in male zebra finches. Behav Brain Res. 2008;187:133–139. doi: 10.1016/j.bbr.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A, Karle EJ, Anderson KD, Medina L. Catecholaminergic perikarya and fibers in the avian nervous system. In: Smeets WJ, Reiner A, editors. Phylogeny and development of catecholamine systems in the CNS of vertebrates. Cambridge University Press; Cambridge: 1994. pp. 135–181. [Google Scholar]
- Sasaki A, Sotnikova TD, Gainetdinov RR, Jarvis ED. Social context-dependent singing-regulated dopamine. J Neurosci. 2006;26:9010–9014. doi: 10.1523/JNEUROSCI.1335-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P, Schaus JM. Dopamine receptors labelled by [3H] quinpirole. Eur J Pharmacol. 1991;203:105–109. doi: 10.1016/0014-2999(91)90796-s. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347:146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- Tallman JF, Primus RJ, Brodbeck R, Cornfield L, Meade R, Woodruff K, Ross P, Thurkauf A, Gallager DW. I. NGD 94-1: Identification of a Novel, High-Affinity Antagonist at the Human Dopamine D4 Receptor. J Pharmacol Exp Ther. 1997;282:1011–1019. [PubMed] [Google Scholar]
- Yanagawa T, Kishimoto Y, Tada K, Arai F, Kondo Y, Kudo T. Presence of dopamine DA-1 receptors in human decidua. Placenta. 1997;18:169–172. doi: 10.1016/s0143-4004(97)90089-8. [DOI] [PubMed] [Google Scholar]
- Zann RA. The zebra finch: a synthesis of field and laboratory studies. Oxford; Oxford University Press: 1996. [Google Scholar]