Abstract
Background:
The prevalence of genetic arrhythmogenic diseases is unknown. For the long QT syndrome (LQTS), figures ranging from 1:20,000 to 1:5,000 were published but none was based on actual data. Our objective was to define the prevalence of LQTS.
Methods and Results:
In 18 maternity hospitals an ECG was performed in 44,596 infants 15-25 days old (43,080 Caucasians). In infants with a QTc >450 ms the ECG was repeated within 1-2 weeks. Genetic analysis, by screening 7 LQTS genes, was performed in 28/31 (90%) and in 14/28 (50%) of infants with, respectively, a QTc >470 ms or between 461 and 470 ms. A QTc of 451-460, of 461-470, and >470 ms was observed in 184 (0.41%), in 28 (0.06%), and in 31 (0.07%) infants. Among genotyped infants, disease-causing mutations were found in 12/28 (43%) with a QTc >470 ms and in 4/14 (29%) with a QTc of 461-470 ms. One genotype-negative infant (QTc 482 ms) was diagnosed affected by LQTS on clinical grounds. Among family members of genotype-positive infants, 51% were found to carry disease-causing mutations. In total, 17/43,080 Caucasian infants were affected by LQTS demonstrating a prevalence of at least 1:2,534 apparently healthy live-births (95% C.I. 1:1,583- 1:4,350).
Conclusions:
This study provides the first data-based estimate of the prevalence of LQTS among Caucasians. Based on the non-genotyped infants with QTc between 451 and 470 ms we advance the hypothesis that this prevalence might be close to 1:2,000. ECG-guided molecular screening can identify most infants affected by LQTS and unmask affected relatives, thus allowing effective preventive measures.
Keywords: arrhythmia; death, sudden; electrocardiography; genetics; long-QT syndrome
INTRODUCTION
The last 15 years have witnessed growing and widespread interest in arrhythmogenic diseases of genetic origin. These cardiac disorders are regarded as rare but their prevalence remains unknown. The case of the long QT syndrome (LQTS) is paradigmatic. One of the leading contributors to sudden death in the young, LQTS is caused by mutations in genes encoding ion channels involved in the control of ventricular repolarization. Following the identification of the first 3 major LQTS genes1-3, the list now includes 12 disease-causing genes4-8. Fifty years have elapsed since LQTS was described in its two variants with9,10 and without11-13 congenital deafness, but no reliable data exist on its prevalence. The literature offers all sort of rates, ranging from 1:20,00014, to 1:10,00015, to 1:5,00016,17. What these very different rates of prevalence have in common is to be at best educated guesses not supported by any actual data.
A recently completed large prospective electrocardiographic study in 3-4 weeks old infants provides the first opportunity for a data-driven assessment of the prevalence of LQTS. Over a period of 30 years, between 1976 and 2007, after our initial suggestion18 we and others had demonstrated19-23 that approximately 10-15% of cases of Sudden Infant Death Syndrome (SIDS) may actually be caused by LQTS. As death could be prevented in these infants by an early diagnosis and especially as a significant portion of sudden deaths among LQTS patients represents the first manifestation of the disease4,24, the Italian Ministry of Health has considered the opportunity of following our recommendation of introducing a program of neonatal ECG screening as part of the National Health Service25 with the main objective of identifying early on most cases of LQTS. Accordingly, they requested and funded a prospective study to obtain relevant information. This study, recently completed with the enrolment of 44,596 neonates, has indicated that such a program would be highly cost-effective in Europe26. In the infants with a marked QT interval prolongation confirmed in two different ECGs we performed molecular screening with the objective of obtaining a reliable estimate of the prevalence of LQTS.
METHODS
Study Population
The population under study included 44,596 neonates (43,080 Caucasians), 22,967 males (51%) and 21,629 females (49%), consecutively enrolled by 18 maternity hospitals (see Appendix) between January 2001 and June 2006, in whom an electrocardiogram (ECG) was recorded between the 15th and the 25th day of life. At hospital discharge the parents were asked to return with their babies in order to perform an ECG and to fill a questionnaire with personal and clinical data. In no case was the ECG performed because of the presence of LQTS in the family. All neonates were apparently healthy because very premature and sick newborns were usually transferred to intensive care units before they could be enrolled. All parents signed an informed consent. Our records show no refusals of the ECG screening by the parents.
Electrocardiography
Twelve-lead ECGs were recorded at a paper speed of 25 mm/s with a Marquette MAC 5000 recorder. The ECGs were initially analyzed in the participating centers and, as written reports had to be prepared, they were measured manually. All ECGs were then transferred via modem together with personal and clinical data through a dedicated website to the Coordinating Center where they were all reread manually, first those with a QTc > 440 ms (as indicated by the peripheral centers) and then all the others. In other words, all ECG tracings were eventually read centrally.
The guidelines of the European Society of Cardiology for the interpretation of neonatal ECG were followed27. The RR and QT intervals were measured in leads II, V5 and V6 from five non-consecutive beats, and the corrected QT interval (QTc) was calculated according to Bazett's formula. The longest mean QTc found in one of the three leads was considered. Whenever a QTc >450 ms was found, the ECG had to be repeated within 1-2 weeks to confirm the initial finding. If QT prolongation was confirmed or any other ECG abnormality was identified, the infants were managed and treated according to the guidelines27. In the case of a QTc >470 ms, a blood sample was taken from the neonate and from his/her parents for genetic analysis. Toward the end of the study it was decided to extend the genetic analysis to the infants with a QTc between 461 and 470 ms.
Genetic analysis
With informed consent (IRB of our University Hospital), genomic DNA was extracted from peripheral blood lymphocytes obtained from the proband and first degree relatives using standard methods. All coding exons of KCNQ1, KCNH2, SCN5A, KCNE1, KCNE2, CAV3, and SCN4B (the genes currently screened in our laboratory for the routine diagnosis of LQTS) were amplified by polymerase chain reaction using previously published primer pairs or home-designed primers. Amplicons were screened for sequence variants using denaturing high-performance liquid chromatography (DHPLC) analysis performed on two different automated DNA fragment analysis systems (Wave™ models 1100 and 3500HT, Transgenomic, San Jose, CA, USA). Elution profiles were compared with normal control samples. Products exhibiting divergent chromatographic profiles were purified enzymatically (ExoSAP-IT, Amersham Bioscience, Piscataway, NJ, USA) and sequenced using fluorescent dye terminator chemistry (Big-Dye® Terminator system, Applied Biosystems, Inc. Foster City, CA, USA).
All the genetic variants identified were searched in a population of 300 ethnically matched controls (all Caucasians) and in all available online databases. A genetic variant was regarded as a disease-causing mutation if it had been already described in other LQTS families and/or if a functional study was available to prove its functional effect. In case of a novel mutation, we evaluated its absence in control populations, the conservation among different species, the presence of a genotype-phenotype correlation among family members, and whenever possible its functional effect through a cellular electrophysiologic study.
Statistical Analysis
The distribution of values for heart rate, QT interval, and QTc was assessed, and percentile values (2.5th and 97.5th) were calculated. Differences in electrocardiographic measurements between groups were assessed by Student's t-test. Prevalence data are reported as proportions of subjects with confirmed LQTS along with binomial exact 95% confidence intervals (CI). Given the fact that all 300 controls were Caucasians it was conservatively decided to calculate prevalence only in the 43,080 Caucasians, a group which represents 97% of the population under study and the one in which all genetic variants had been identified. Data are presented as means ± SD. A two-sided p value below 0.05 was considered statistically significant.
RESULTS
Electrocardiographic Characteristics
Heart rate, QT interval, and QTc values were normally distributed. Mean heart rate was 153±16 b/min, mean QT interval was 256±18 ms, mean QTc was 406±20 ms and slightly longer in females than in males (407±20 and 405±20 ms, p<0.001). The 97.5th and the 2.5th percentiles, defining the upper and lower normal values, were 443 ms and 364 ms, respectively.
Neonates with QT interval Prolongation
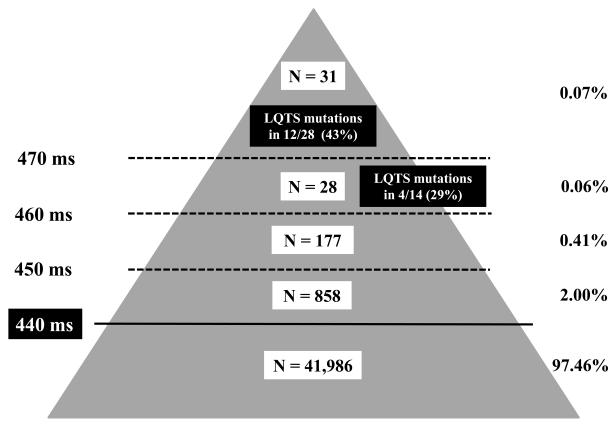
The QT interval was considered prolonged according to the guidelines for the interpretation of neonatal ECG of the European Society of Cardiology27. In 1137 neonates (2.5%) QTc was > 440 ms and in 894 (2.0%) it was between 441 and 450 ms, an area that, for the purpose of this study, we regarded as borderline prolonged. There were 184 infants with a QTc between 451 and 460 ms, 28 between 461 and 470 ms and 31 above 470 ms (Fig. 1). Among these 31 neonates (1:1438, 0.07%, 23 females and 8 males), 4 had a QTc >500 ms.
Figure 1.
Distribution of the 43,080 Caucasian neonates among 5 subgroups (absolute numbers and percentage), according to QTc duration on the screening ECG. Neonates positive at the genetic analysis are also reported.
Two of the 31 neonates with a QTc >470 ms did not return for the second ECG and were lost to follow-up. In all remaining 29 neonates QT interval prolongation was confirmed at the second ECG, which showed a QTc greater than 470 ms in 26 and between 461 and 470 in 3. Mean QTc was 485 ± 17 ms on the first ECG and 484 ± 20 ms on the second. These infants were managed according to the guidelines, also in consideration of their risk for SIDS19-23: they underwent an echocardiogram, which was normal in all cases, and a 24-hour ECG Holter recording which confirmed the QT interval prolongation and showed no arrhythmias. All but one, due to parental refusal, were then treated with propranolol 2 mg/kg per day and none of them experienced side-effects. During follow-up (median 2.8, range 0.7-6.9 years) they all remained free of symptoms.
The neonates with a QTc between 461 and 470 ms were followed with additional ECGs, according to the guidelines27. In all cases the second ECG essentially confirmed the QTc values observed on the first ECG. Two of them were treated with propranolol because of further QTc prolongation on a 24-hour Holter recording.
Genetic Analysis in the Neonates with Markedly Prolonged QT interval (>470 ms)
Blood samples for DNA extraction and molecular analysis were obtained in 28 of 29 (96%) neonates with QTc >470 ms (7 males and 21 females) available during follow-up; the parents of one subject did not consent to genetic analysis.
LQTS mutations were identified in 12/28 neonates (43%): 8 were carrying heterozygous mutations on the KCNQ1 gene (LQT1) and 4 on KCNH2 (LQT2). The distribution among the LQTS subgroups confirmed the higher prevalence of LQT1, similar to what we reported from the Pavia database28, and most of the mutations identified had already been described in other LQTS families. Table 1 provides the information relevant to all the mutations identified and regarded as disease-causing29-39. A novel SCN5A genetic variant (A647V), located in a highly conserved region and never reported in controls, was identified in an infant but was not regarded as disease-causing and not included among the LQTS-related mutations because the same mutation was found in the infant's father and grandmother who were asymptomatic and with a normal QTc. Furthermore, our own cellular electrophysiological study failed to show functional effects. Additionally, to be very conservative we did not include the SCN5A-P2006A which we identified in 1/300 controls. However, we suspect that P2006A might play some contributory role not only because it has a functional effect40 but also because we found it in 2 victims of Sudden Infant Death Syndrome22 and in one stillbirth41. On this basis, we decided not to consider these two variants as related to LQTS.
Table 1.
LQTS-mutations identified in 17 neonates with a prolonged QT interval
| LQTS mutations | Gender | QTc | Inheritance | Mutation carriers among family members# |
Mutation characteristics* | |||
|---|---|---|---|---|---|---|---|---|
| QTc > 470 | ||||||||
| KCNQ1-R190Q | M | 482 | Maternal | 1/3 (33%) | A29 | B29 | C | |
| KCNQ1-R190W | F | 506 | Paternal | 6/10 (60%) | A30 | C | ||
| KCNQ1-R190W | F | 478 | Paternal | 1/3 (33%) | A30 | |||
| KCNQ1-D202H | M | 492 | De novo | A30 | ||||
| KCNQ1-I204M | F | 487 | Maternal | 1/2 (50%) | A30 | C | ||
| KCNQ1-W305X | F | 476 | Paternal | 4/5 (80%) | A31 | C | ||
| KCNQ1-R380G | M | 474 | Maternal | 3/6 (50%) | A32 | C | ||
| KCNQ1-P631+19X | M | 481 | Maternal | 3/12 (25%) | A33 | C | ||
| KCNH2-F29S | M | 555 | Paternal | 1/2 (50%) | B*** | C | ||
| KCNH2-F617L | F | 513 | Maternal | 2/4 (50%) | B*** | C | ||
| KCNH2-delK638 | F | 513 | Paternal | 1/2 (50%) | A34,35 | B35 | C | |
| KCNH2-R744X | F | 474 | Paternal | 9/14 (64%) | A36 | C | ||
| 461 < QTc ≤ 470 | ||||||||
| KCNQ1-T221M | } | A34 | C | |||||
| KCNH2-R922W | F | 461 | Paternal | 4/6 (66%) | B*** | |||
| KCNH2-D102V | F | 465 | Maternal | 2/5 (40%) | B*** | C | ||
| KCNE1-S28L** | M | 465 | Paternal | 2/3 (66%) | A37 | C | ||
| KCNE2-I57T | M | 462 | Maternal | 2/5 (40%) | A38,39 | B38 | C | |
Probands not included
A: Already known LQTS mutation, B: Functional effect, C: Positive Genotype-Phenotype correlation among family-members
Associated with KCNE1-D85N, KCNE1-S38G, KCNH2-K897T, SCN5A-H558R
Besana et al. (unpublished data)
Among the neonates with negative genotyping, the father of one infant with a QTc of 482 ms also had an extremely prolonged QTc (581 ms). These two ECGs, taken together, are diagnostic for LQTS4,42 even in the absence of symptoms; accordingly, we did consider this neonate as definitely affected by LQTS despite negative genotyping. Currently, in approximately 15%-20% of patients with definite LQTS no mutations are identified in the seven genes regularly screened in our laboratory4; QTc was longer in neonates found to have a mutation than in those with negative genotyping (494±24 vs 479±5 ms, p=0.049). If individual values are considered, LQTS mutations were identified in 6/7 neonates with a QTc > 485 ms.
Genetic Analysis in Neonates with Prolonged QT interval (461-470 ms)
Blood samples for DNA extraction and molecular analysis were obtained from 14 of the 28 (50%) neonates with a QTc between 461 and 470 ms and LQTS mutations were identified in 4/14 neonates (29%). When these 4 mutations are added to the 12 identified among infants with a QTc >470 ms, the total number of disease-causing mutations becomes 16. Details are provided in Table 1.
One infant carried two independent mutations: the first was an already described LQTS mutation in the C-terminal region of KCNH2 (R922W)34 and the second was a novel one in the extracellular loop between S3 and S4 of KCNQ1 (T224M). One infant carried a novel mutation in the N-terminal region of KCNH2 (D102V). In both these cases the genotype segregated with the clinical phenotype among family members. In the remaining two cases a mutation in KCNE1 (S28L) and KCNE2 (I57T), respectively, was identified; both had already been described37-39 and one had also been functionally characterized (see Table 1). In the infant with the KCNE2-I57T mutation normalization of the QTc was observed at follow-up suggesting a mild form of LQTS that could manifest itself mainly as a predisposition to drug-induced Torsades-de-Pointes, as previously reported39.
Mutation Status and QTc Normalization
QTc normalization at one year of life occurred in 3/16 (19%) genotype-positive and in 24/25 (96%) genotype-negative infants with an available ECG at follow-up. Importantly, the only genotype-negative child in whom QTc remained prolonged was the one whose father also had marked QT prolongation and who was considered affected by LQTS on clinical criteria. Among the 14 infants whose QT interval remained prolonged at one year of life, a disease-causing mutation was identified in 13 (92%).
Genetic Analysis in the Parents and Family Members
In all 16 cases with LQTS mutations genetic analysis was extended to the parents. Only one case was a de novo mutation, while in the others the mutation was inherited from the father (n=8) or from the mother (n=7) in whom LQTS had not been previously diagnosed. The analysis was then performed in other family members and it allowed the identification of 42/82 (51%) mutation carriers. QTc was prolonged in 32/42 (76%) mutation positive subjects; some family members had striking QT prolongations previously unrecognized (Fig. 2). The family members affected by LQTS had not been previously diagnosed; most of them, following our recommendation based on the European guidelines, are now treated with β-blockers and continued to remain free of symptoms, with one exception. A young man in his early twenties, a member of the family with the KCNH2-R744X mutation, did not take the recommended beta-blockers and actually initiated anti-malaria prophylaxis: he was found dead in his bed. As the autopsy was negative, this sudden death was likely caused by the combination of a QT-prolonging drug with a disease-causing LQTS mutation.
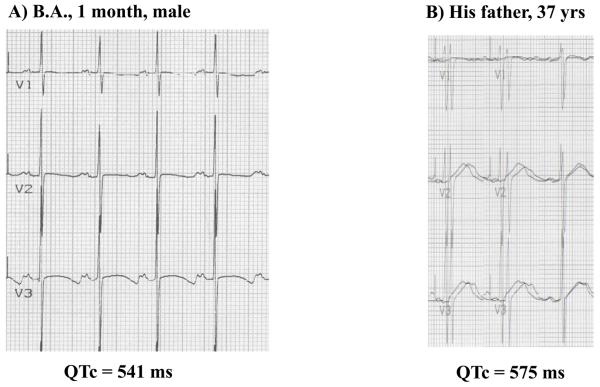
Figure 2.
A) ECG tracing of a neonate with a markedly prolonged QT interval. Genetic analysis identified a mutation on KCNH2 (LQT2). B) ECG tracing of the neonate's father. Genetic analysis identified the same mutation on KCNH2 (LQT2).
Prevalence of LQTS
Our data clearly indicate that at least 17 infants (16 because of disease-causing mutations and one because of clear-cut clinical diagnosis) among this cohort of 44,596 neonates are affected by LQTS. All of them are Caucasians. This indicates a prevalence among Caucasians of 1:2,534 (95% CI: 1:1,583 – 1:4,350). This prevalence is much higher than what has been previously suggested.
DISCUSSION
The present findings provide the first data-based estimate of the prevalence of a clinically important arrhythmogenic disease of genetic origin, the long QT syndrome (LQTS). Until now the prevalence of these diseases, regarded as “rare”, was simply unknown despite the fact that articles and textbooks often mentioned one or another estimate but without support from objective data. Our own data, based on ECG-guided identification of disease-causing mutations, indicate that among Caucasians the prevalence of LQTS is at least of 1:2,534 apparently healthy live births. This finding has direct implications for the early detection of LQTS.
QT Interval Prolongation and Probability of Carrying LQTS Mutations
Besides it being an intuitive concept, our data point to a positive correlation between duration of the QT interval and probability of carrying LQTS disease-causing mutations. This is already evident in the group with marked QT prolongation as among all infants with a QTc >470 ms this probability was 43% (12/28) but it increased to 86% (6/7) in the neonates with a QTc >485 ms. Moreover, the probability of finding disease-causing mutations in the seven genes tested was 92% among the infants whose QTc was >470 ms initially and remained prolonged over 450 ms at one year of life.
Among the infants with a QTc between 461 and 470 ms, 4/14 (29%) had disease-causing mutations but for 14/28 we did not obtain blood for genetic analysis. Considering the possibility that among the non-genotyped 196 infants with a QTc between 450 and 470 ms there might be some LQTS mutation carriers, we advance the hypothesis that the prevalence of LQTS may be closer to 1/2,000.
Our study cannot answer the question of how many neonates carry LQTS mutations in the presence of a normal or borderline prolonged (between 441 and 450 ms) QT interval. In 1975 we had suggested13 that “LQTS is more unrecognized than rare”, and in 200343 had pointed to the until then unsuspected high frequency of patients carrying two independent mutations, respectively of maternal and paternal origin (“compound mutations”), as further evidence of a relatively frequent presence of LQTS mutations in the general population43,44. In 199945, supported also by previous suggestions46,47, we provided the evidence for the existence of low penetrance in LQTS which implied the presence of many “silent” mutation carriers, i.e. subjects with disease-causing mutations but with a QTc within normal limits (<440 ms).
The number of silent mutation carriers cannot be assessed in the general population because it would require mass molecular screening, which is practically unfeasible. We have previously indicated that their percentage varies within the main genotypes, being high (36%) among LQT1 patients and decreasing progressively among LQT2 (17%) and LQT3 (10%) patients28. Unavoidably, the prevalence of LQTS will remain an underestimate because it has to refer to LQTS with QT prolongation and cannot include clinically silent mutation carriers. However, and clinically relevant, the risk of spontaneous major cardiac events among LQTS patients with a normal QT interval is very modest; their main risk is the exposure to drugs with IKr blocking activity with the attendant possibility of developing Torsades-de-Pointes ventricular tachycardia48.
Study Limitations
The present data suffer from one significant limitation which has its origin in the design of the study. We initially decided to follow the recommendations of the European Guidelines27 (of which we share responsibility) and to plan the genetic screening only for infants with a QTc > 470 ms. When we realized that mutation carriers were likely to be found also among infants with a less marked QT prolongation, it was partially too late. Indeed, despite our efforts it proves difficult to trace all the families involved and, when we succeed, to convince the parents of apparently healthy children to return for genetic testing.
At first glance another potential limitation might arise from the fact that the present study was entirely conducted in Italy, thus raising questions about the legitimacy of using the same figures and their relevance to other populations. As a matter of fact, the figures obtained for the Italian population can be expected to be comparable to what would be found in Europe, at least for countries sharing similar historical background. It is important to realize that Italians, with the exception of the inhabitants of the island of Sardinia, do not constitute an “ethnic group”. Historic reasons, beginning with the initial large movements of populations coming through the Middle East and then settling into Europe and especially continuing with the “barbaric” invasions of the first thousand years of the modern era which were characterized by the fact that the “barbarians” instead of returning to Central Europe kept settling in Italy and mixing with the friendly local inhabitants, have resulted in the fact that the genetic characteristics of the Italians are largely similar to most other European countries. For the same reasons, the prevalence estimated in Italy may be a reasonable estimate also for the North American population of European descent49.
Implications
Besides providing the first direct evidence on the prevalence of LQTS, much higher than what previously postulated, these findings carry clinically relevant implications. One is that infants with a QTc >460 ms in the first month of life and whose QT interval remains prolonged at one year have a >90% probability to carry a LQTS-causing mutation. Also, whereas genetic screening should be immediately performed in all infants with a QTc > 485 ms, the normalization within a year for 75% of the infants with an initial QTc between 460 and 485 ms suggests - unless one of the parents shows QT prolongation - to postpone the genetic screening for this group until the end of the first year of life. This simple measure will reduce both costs and unnecessary anxiety.
Another major implication, in a still controversial area25,50, is that a very feasible and relatively inexpensive26 ECG screening would identify most of the neonates affected by LQTS. Moreover, this would guide molecular screening which in turn, as most of these are familial cases, would unmask many affected relatives (approximately half of the family members) thus allowing effective preventive measures. In the United States alone, with over 4 million live-births per year, this would mean at least 2,000 new cases and families per year. Furthermore, this knowledge will allow health authorities in countries with a prevalent Caucasian population to estimate how many new LQTS patients they may expect every year and to approximately assess how many LQTS patients may be living in their countries.
The implications for the prevention of avoidable sudden deaths in the young are evident. The tragic case of the youngster who died suddenly while on anti-malaria treatment is a sad reminder of the life-threatening potential of the LQTS mutations found in apparently healthy newborns and present in their apparently healthy family members. It also fits with the very recent evidence of the frequently devastating effect of QT-prolonging drugs administered to LQTS patients51.
One final practical question concerns the best time for the ECG screening, as the non uncommon QT normalization by one year of age could make this period of life a reasonable choice. At one year of age there would less false positives but the infants with major QT prolongation - possibly at high risk for SIDS between month 2 and 6 - would be missed and avoidable tragedies would not be prevented. With errors unavoidable on both sides (3-4 weeks vs 1 year) we would prefer to err on the safe side and not miss the very high risk infants. Of course, this “safety first” approach comes with a price.
Conclusion
The actual data from this study demonstrate that among Caucasians the prevalence of LQTS is at least 1: 2,534 apparently healthy live-births.
Clinical Impact.
This prospective electrocardiographic study, performed in 44,596 infants 15-25 days old and complemented by molecular screening in those with a markedly prolonged QT interval, indicates that the prevalence of the long QT syndrome (LQTS) among Caucasians is 1:2,534 live-births (95% C.I. 1:1,583- 1:4,350), much higher than previously suspected. Furthermore, reasonable inferences on the infants with a prolonged QT interval who were not genotyped suggest that this prevalence may be close to 1:2,000 live births.
This is the first data-based estimate of the prevalence of an arrhythmogenic disease of genetic origin. As such, it will allow health authorities in countries with a prevalent Caucasian population to estimate how many new LQTS patients they may expect every year and to approximately assess how many LQTS patients may be living in their countries. Of note, 51% of the family members of the affected infants were also mutation-carriers.
The study carries practical implications. Infants with a QTc > 460 ms in the first month of life and whose QTc remains prolonged at one year have > 90% probability to carry a LQTS-causing mutation. Whereas genetic screening should be immediately performed in infants with a QTc > 485 ms, the normalization within one year for 75% of the infants with a QTc between 460 and 485 ms suggests to postpone their genetic screening until the end of their first year of life.
ECG-guided molecular screening can identify most infants affected by LQTS and unmask affected relatives, thus allowing the early institution of effective preventive measures.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to the many physicians and nurses of the 18 participating centers who have contributed with their time and effort to the data acquisition, and to the almost 90,000 parents who have accepted to enroll their infants in the study. We express our gratitude to the Italian Ministry of Health and to Regione Lombardia for encouraging and supporting this initiative.
We thank Dr Alfred L. George Jr. and Dr. Cesare Danesino for constructive criticism, Dr. Milena Perotti for database management, Dr. Gheorgios Michailidis for verification of a large number of ECG tracings, and Ms. Pinuccia De Tomasi for expert editorial support.
FUNDING SOURCES
This study has been made possible by the following grants: NIH grant HL083374 “Neonatal long QT syndrome and sudden infant death”; Italian Ministry of Health grant “Sindrome della morte improvvisa del lattante: meccanismi e prevenzione”; Italian Ministry of Health and Regione Lombardia Ricerca Finalizzata 2001 “Studio sulla prevalenza, il significato clinico e l'evoluzione delle anomalie ECG neonatali associate ad aritmie nell'infanzia”.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
None of the authors has conflicts of interest to disclose.
REFERENCES
- 1.Wang Q, Shen J, Splawski I, Atkinson D, Li Z, Robinson JL, Moss AJ, Towbin JA, Keating MT. SCN5A mutations associated with an inherited cardiac arrhythmia, long QT syndrome. Cell. 1995;80:805–811. doi: 10.1016/0092-8674(95)90359-3. [DOI] [PubMed] [Google Scholar]
- 2.Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80:795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- 3.Wang Q, Curran ME, Splawski I, Burn TC, Millholland JM, VanRaay TJ, Shen J, Timothy KW, Vincent GM, de Jager T, Schwartz PJ, Towbin JA, Moss AJ, Atkinson DL, Landes GM, Connors TD, Keating MT. Positional cloning of a novel potassium channel gene: KvLQT1 mutations cause cardiac arrhythmias. Nature Genetics. 1996;12:17–23. doi: 10.1038/ng0196-17. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz PJ, Crotti L. Long QT and short QT syndromes. In: Zipes DP, Jalife J, editors. Cardiac Electrophysiology: From Cell to Bedside. 5th Edition Elsevier/Saunders; Philadelphia: 2009. pp. 731–744. [Google Scholar]
- 5.Chen L, Marquardt ML, Tester DJ, Sampson KJ, Ackerman MJ, Kass RS. Mutation of an A-kinase-anchoring protein causes long QT syndrome. Proc Natl Acad Sci USA. 2007;104:20990–20995. doi: 10.1073/pnas.0710527105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ueda K, Valdivia C, Medeiros-Domingo A, Tester DJ, Vatta M, Farrugia G, Ackerman MJ, Makielski JC. Syntrophin mutation associated with long QT syndrome through attivation of the nNOS-SCN5A macromulecolar complex. PNAS. 2008;105:9355–9360. doi: 10.1073/pnas.0801294105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vatta M, Ackerman MJ, Ye B, Makielski JC, Ughanze EE, Taylor EW, Tester DJ, Balijepalli RC, Foell JD, Li Z, Kamp TJ, Towbin JA. Mutant Caveolin-3 induces persisten late sodium current and is associated with Long-QT-Syndrome. Circulation. 2006;114:2104–2112. doi: 10.1161/CIRCULATIONAHA.106.635268. [DOI] [PubMed] [Google Scholar]
- 8.Medeiros-Domingo A, Kaku T, Tester DJ, Iturralde-Torres P, Itty A, Ye B, Valdivia C, Ueda K, Canizales-Quinteros S, Tusié-Luna MT, Makielski JC, Ackerman MJ. SCN4B-encoded sodium channel beta4 subunit in congenital long-QT syndrome. Circulation. 2007;116:134–142. doi: 10.1161/CIRCULATIONAHA.106.659086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jervell A, Lange-Nielsen F. Congenital deaf-mutism, functional heart disease with prolongation of the Q-T interval, and sudden death. Am Heart J. 1957;54:59–68. doi: 10.1016/0002-8703(57)90079-0. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz PJ, Spazzolini C, Crotti L, Bathen J, Amlie JP, Timothy K, Shkolnikova M, Berul CI, Bitner-Glindzicz M, Toivonen L, Horie M, Schulze-Bahr E, Denjoy I. The Jervell and Lange-Nielsen Syndrome. Natural history, molecular basis, and clinical outcome. Circulation. 2006;113:783–790. doi: 10.1161/CIRCULATIONAHA.105.592899. [DOI] [PubMed] [Google Scholar]
- 11.Romano C, Gemme G, Pongiglione R. Aritmie cardiache rare dell'età pediatrica. Clin Pediat. 1963;45:656–683. [PubMed] [Google Scholar]
- 12.Ward OC. A new familial cardiac syndrome in children. J Irish Med Ass. 1964;54:103–106. [PubMed] [Google Scholar]
- 13.Schwartz PJ, Periti M, Malliani A. The long Q-T syndrome. Am Heart J. 1975;89:378–390. doi: 10.1016/0002-8703(75)90089-7. [DOI] [PubMed] [Google Scholar]
- 14.Moss AJ, Robinson JL. The long QT syndrome. Circulation. 2002;105:784–786. [Google Scholar]
- 15.Chiang CE, Roden DM. The long QT syndromes: genetic basis and clinical implications. J Am Coll Cardiol. 2000;36:1–12. doi: 10.1016/s0735-1097(00)00716-6. [DOI] [PubMed] [Google Scholar]
- 16.Nemec J, Hejilik JB, Shen WK, Ackerman MJ. Cathecolamine-induced T-wave lability in congenital long QT syndrome: a novel phenomenon associated with syncope and cardiac arrest. Mayo Clin Proc. 2003;78:40–50. doi: 10.4065/78.1.40. [DOI] [PubMed] [Google Scholar]
- 17.Goldenberg I, Moss AJ. Long QT syndrome. J Am Coll Cardiol. 2008;51:2291–2300. doi: 10.1016/j.jacc.2008.02.068. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz PJ. Cardiac sympathetic innervation and the sudden infant death syndrome. A possible pathogenetic link. Am J Med. 1976;60:167–172. doi: 10.1016/0002-9343(76)90425-3. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz PJ, Stramba-Badiale M, Segantini A, Austoni P, Bosi G, Giorgetti R, Grancini F, Marni ED, Perticone F, Rosti D, Salice P. Prolongation of the QT interval and the sudden infant death syndrome. N Engl J Med. 1998;338:1709–1714. doi: 10.1056/NEJM199806113382401. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz PJ, Priori SG, Bloise R, Napolitano C, Ronchetti E, Piccinini A, Goj A, Breithardt G, Schulze-Bahr E, Wedekind H, Nastoli J. A molecular link between the sudden infant death syndrome and the long QT syndrome. N Engl J Med. 2000;343:262–267. doi: 10.1056/NEJM200007273430405. [DOI] [PubMed] [Google Scholar]
- 21.Ackerman MJ, Siu BL, Sturner WQ, Tester DJ, Valdivia CR, Makielski JC, Towbin JA. Postmortem molecular analysis of SCN5A defect in sudden infant death syndrome. JAMA. 2001;286:2264–2269. doi: 10.1001/jama.286.18.2264. [DOI] [PubMed] [Google Scholar]
- 22.Arnestad M, Crotti L, Rognum TO, Insolia R, Pedrazzini M, Ferrandi C, Vege A, Wang DW, Rhodes TE, George AL, Jr, Schwartz PJ. Prevalence of long QT syndrome gene variants in sudden infant death syndrome. Circulation. 2007;115:361–367. doi: 10.1161/CIRCULATIONAHA.106.658021. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz PJ, Priori SG, Bloise R, Napolitano C, Ronchetti E, Piccinini A, Goj C, Breithardt G, Schulze-Bahr E, Wedekind H, Nastoli J. Molecular diagnosis in a child with sudden infant death syndrome. Lancet. 2001;358:1342–1343. doi: 10.1016/S0140-6736(01)06450-9. [DOI] [PubMed] [Google Scholar]
- 24.Tester DJ, Ackerman MJ. Postmortem long QT syndrome genetic testing for sudden unexplained death in the young. J Am Coll Cardiol. 2007;49:240–246. doi: 10.1016/j.jacc.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz PJ. Newborn ECG screening to prevent sudden cardiac death. Heart Rhythm. 2006;3:1353–1355. doi: 10.1016/j.hrthm.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 26.Quaglini S, Rognoni C, Spazzolini C, Priori SG, Mannarino S, Schwartz PJ. Cost-effectiveness of neonatal ECG screening for the Long QT-Syndrome. Eur Heart J. 2006;15:1824–1832. doi: 10.1093/eurheartj/ehl115. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz PJ, Garson A, Jr., Paul T, Stramba-Badiale M, Vetter VL, Villain E, Wren C. Guidelines for the interpretation of the neonatal electrocardiogram. Eur Heart J. 2002;23:1329–1344. doi: 10.1053/euhj.2002.3274. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz PJ, Garson A, Jr., Paul T, Stramba-Badiale M, Vetter VL, Villain E, Wren C. Guidelines for the interpretation of the neonatal electrocardiogram. Eur Heart J. 2002;23:1329–1344. doi: 10.1053/euhj.2002.3274. [DOI] [PubMed] [Google Scholar]
- 29.Chouabe C, Neyroud N, Richard P, Denjoy I, Hainque B, Romey G, Drici MD, Guicheney P, Barhanin J. Novel mutations in KvLQT1 that affect Iks activation through interactions with Isk. Cardiovasc Res. 2000;45:971–980. doi: 10.1016/s0008-6363(99)00411-3. [DOI] [PubMed] [Google Scholar]
- 30.Napolitano C, Priori SG, Schwartz PJ, Bloise R, Ronchetti E, Nastoli J, Bottelli G, Cerrone M, Leonardi S. Genetic testing in the long QT syndrome: A three-tier approach to genotyping in clinical practice. JAMA. 2005;294:2975–2980. doi: 10.1001/jama.294.23.2975. [DOI] [PubMed] [Google Scholar]
- 31.Chen S, Zhang L, Bryant RM, Vincent GM, Flippin M, Lee JC, Brown E, Zimmerman F, Rozich R, Szafranski P, Oberti C, Sterba R, Marangi D, Tchou PJ, Chung MK, Wang Q. KCNQ1 mutations in patients with a family history of lethal cardiac arrhythmias and sudden death. Clin Genet. 2003;63:273–282. doi: 10.1034/j.1399-0004.2003.00048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tester DJ, Will ML, Haglund CM, Ackerman MJ. Compendium of cardiac channel mutations in 541 consecutive unrelated patients referred for long QT syndrome genetic testing. Heart Rhythm. 2005;2:507–517. doi: 10.1016/j.hrthm.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 33.Neyroud N, Richard P, Vignier N, Donger C, Denjoy I, Demay L, Shkolnikova M, Pesce R, Chevalier P, Hainque B, Coumel P, Schwartz K, Guicheney P. Genomic organization of the KCNQ1 K+ channel gene and identification of C-terminal mutations in the long-QT syndrome. Circ Res. 1999;84:290–297. doi: 10.1161/01.res.84.3.290. [DOI] [PubMed] [Google Scholar]
- 34.Splawski I, Shen J, Timothy KW, Lehmann MH, Priori S, Robinson JL, Moss AJ, Schwartz PJ, Towbin JA, Vincent GM, Keating MT. Spectrum of mutations in long-QT syndrome genes. KVLQT1, HERG, SCN5A, KCNE1, and KCNE2. Circulation. 2000;102:1178–1185. doi: 10.1161/01.cir.102.10.1178. [DOI] [PubMed] [Google Scholar]
- 35.De Ferrari GM, Crotti L, Lundquist AL, Pedrazzini M, Insolia R, Vicentini A, Schwartz PJ, George AL., Jr Novel KCNH2 mutation causing both loss and gain of function is associated with long QT syndrome exhibiting transient short QT intervals. Eur Heart J. 2005;26(Abstr Suppl):127. [Google Scholar]
- 36.Moss AJ, Zareba W, Kaufman ES, Gartman E, Peterson DR, Benhorin J, Towbin JA, Keating MT, Priori SG, Schwartz PJ, Vincent GM, Robinson JL, Andrews ML, Feng C, Hall WJ, Medina A, Zhang L, Wang Z. Increased risk of arrhythmic events in long QT syndrome with mutations in the pore region of the human ether-a-go-go-related gene potassium channel. Circulation. 2002;105:794–799. doi: 10.1161/hc0702.105124. [DOI] [PubMed] [Google Scholar]
- 37.Shim SH, Ito M, Maher T, Milunsky A. Gene sequencing in neonates and infants with the long QT syndrome. Genet Test. 2005;9:281–284. doi: 10.1089/gte.2005.9.281. [DOI] [PubMed] [Google Scholar]
- 38.Abbott GW, Sesti F, Splawski I, Buck ME, Lehmann MH, Timothy KW, Keating MT, Goldstein SA. MiRP1 forms IKr potassium channels with HERG and is associated with cardiac arrhythmia. Cell. 1999;97:175–187. doi: 10.1016/s0092-8674(00)80728-x. [DOI] [PubMed] [Google Scholar]
- 39.Sesti F, Abbott GW, Wei J, Murray KT, Saksena S, Schwartz PJ, Priori SG, Roden DM, George AL, Jr, Goldstein SA. A common polymorphism associated with antibiotic-induced cardiac arrhythmia. Proc Natl Acad Sci USA. 2000;97:10613–10618. doi: 10.1073/pnas.180223197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang DW, Desai RR, Crotti L, Arnestad M, Insolia R, Pedrazzini M, Ferrandi C, Vege A, Rognum T, Schwartz PJ, George AL., Jr Cardiac sodium channel dysfunction in sudden infant death syndrome is it time to consider newborn electrocardiographic screening? Circulation. 2007;115:368–376. doi: 10.1161/CIRCULATIONAHA.106.646513. [DOI] [PubMed] [Google Scholar]
- 41.Crotti L, Insolia R, Ghidoni A, Antonazzo P, Facchinetti F, Cetin I, Schwartz PJ. Long QT Syndrome as a cause of stillbirths. Circulation. 2007;116(Abstr Suppl):II_653. [Google Scholar]
- 42.Schwartz PJ, Moss AJ, Vincent GM, Crampton RS. Diagnostic criteria for the long QT syndrome: an update. Circulation. 1993;88:782–784. doi: 10.1161/01.cir.88.2.782. [DOI] [PubMed] [Google Scholar]
- 43.Schwartz PJ, Priori SG, Napolitano C. How really rare are rare diseases? The intriguing case of independent compound mutations in the long QT syndrome. J Cardiovasc Electrophysiol. 2003;14:1120–1121. doi: 10.1046/j.1540-8167.2003.03339.x. [DOI] [PubMed] [Google Scholar]
- 44.Westenskow P, Splawski I, Timothy KW, Keating MT, Sanguinetti MC. Compound mutations: a common cause of severe long-QT syndrome. Circulation. 2004;109:1834–1841. doi: 10.1161/01.CIR.0000125524.34234.13. [DOI] [PubMed] [Google Scholar]
- 45.Priori SG, Napolitano C, Schwartz PJ. Low penetrance in the long QT syndrome. Clinical impact. Circulation. 1999;99:529–533. doi: 10.1161/01.cir.99.4.529. [DOI] [PubMed] [Google Scholar]
- 46.Schwartz PJ. The long QT syndrome. In: Kulbertus HE, Wellens HJJ, editors. SUDDEN DEATH. M Nijhoff; The Hague: 1980. pp. 358–378. [Google Scholar]
- 47.Vincent GM, Timothy KW, Leppert M, Keating M. The spectrum of symptoms and QT intervals in carriers of the gene for the long QT syndrome. N Engl J Med. 1992;327:846–852. doi: 10.1056/NEJM199209173271204. [DOI] [PubMed] [Google Scholar]
- 48.Napolitano C, Schwartz PJ, Brown AM, Ronchetti E, Bianchi L, Pinnavaia A, Acquaro G, Priori SG. Evidence for a cardiac ion channel mutation underlying drug-induced QT prolongation and life-threatening arrhythmias. J Cardiovasc Electrophysiol. 2000;11:691–696. doi: 10.1111/j.1540-8167.2000.tb00033.x. [DOI] [PubMed] [Google Scholar]
- 49.Cavalli-Sforza LL, Menozzi P, Piazza A. THE HISTORY AND GEOGRAPHY OF HUMAN GENES. Princeton University Press; 1994. pp. 255–301. [Google Scholar]
- 50.Van Langen IM, Wilde AAM. Newborn screening to prevent sudden cardiac death? Heart Rhythm. 2006;3:1356–1359. doi: 10.1016/j.hrthm.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 51.Vincent GM, Schwartz PJ, Denjoy I, Swan H, Bithell C, Spazzolini C, Crotti L, Piippo K, Lupoglazoff JM, Villain E, Priori SG, Napolitano C, Zhang L. High efficacy of beta-blockers in long QT syndrome type 1: contribution of non-compliance and QT prolonging drugs to the occurrence of beta-blocker treatment “failures”. Circulation. 2009;119:215–221. doi: 10.1161/CIRCULATIONAHA.108.772533. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.