Abstract
Immune challenge induces behavioral changes including reduced ingestion of palatable food. Multiple pathways likely contribute to this effect, including viscerosensory pathways controlling hypothalamic feeding circuits or by influence on “reward” circuitry previously established to control ingestive behavior. To investigate whether the effects of immune challenge may influence this network, we compared brain activation patterns in animals trained to drink a palatable sweetened milk solution and treated systemically with either the immune stimulant lipopolysaccharide (LPS) or saline. Brain sections were processed for localization of the activation marker c-Fos in neurons of regions implicated in regulation of feeding behavior. Sweetened milk ingestion was associated with increased numbers of c-Fos postive neurons in the caudal core and shell of the nucleus accumbens (NAc), the paraventricular thalamus (PVT), central nucleus of the amygdala (CEA), the basal lateral amygdala (BLA), in orexin-A containing neurons of the lateral hypothalamus (LH), and in cocaine and amphetamine regulated transcript (CART) neurons of the arcuate hypothalamus. In LPS treated animals sweetened milk consumption was significantly reduced, as was c-Fos induction in the hypothalamic orexin-A and CART neurons, and in the BLA. In addition, induction of c-Fos in the rostral regions of the NAc, the PVT, and CEA was increased following LPS treatment, compared to controls. The findings from this study point to a network of brain regions (LH, PVT, NAc and BLA) previously implicated in the modulation of feeding behavior, reward, and arousal that may also contribute to neural substrates involved in the reorganization of behavioral priorities that occurs during sickness.
Immune challenge induces marked behavioral changes, including a reduction in drinking or eating (anorexia), fatigue, reduction in pleasure seeking behavior (anhedonia), or reduction in exploratory behavior (Andreasson et al. 2007; see review Dantzer, 2001, De la Garza, 2005). The neurological substrates by which illness induces behavioral symptoms are not well-established. However, neurovegative symptoms, including inhibition of ingestive behavior, likely involve brain regions that are associated with homeostasis and motivation. Suppressed food intake, in particular, is associated with poorer outcomes of chronic illness (Hauser et al. 2006; Strassburg & Anker 2006). Consequently, increased understanding of the neurobiological substrates impacted by immune challenges and inflammation could lead to clinically important strategies for intervention.
Ingestive behavior is ultimately the result of interplay between peripheral signals related to physiological states, and cognitive and affective drive related to learning, arousal and hedonics (Berthoud, 2004). Circulating signals (e.g. leptin, ghrelin) interacting with brain regions including the arcuate hypothalamus and/or neural pathways originating in the caudal brainstem (e.g. dorsal vagal complex, ventrolateral medulla) contribute to “bottom-up” drive on hypothalamic neural circuits (reviewed in Berthoud, 2004, Elmquist et al. 2005; Jobst et al., 2004) that mediate the induction of eating behavior (orexigenic) or inhibition of eating behavior (anorexigenic). Within the arcuate nucleus, two distinct populations of neurons have been identified that exert opposite effects on feeding behavior. Activity in arcuate neurons that express the neuropeptides pro-opiomelanocortin (POMC) and cocaine and amphetamine regulated transcript (CART) is associated with inhibition of eating, whereas a separate population of neuropeptide Y containing neurons seem to act to enhance feeding. In contrast, “top-down” influence derives from forebrain regions including the medial prefrontal cortex, amygdala, and the nucleus accumbens (Maldonado-Irizarry et al., 1995; Kelley and Swanson, 1997; Petrovich and Gallagher, 2003; Stratford and Kelley, 1997, 1999; Reynolds and Berridge, 2002; Will et al., 2004; Baldo et al., 2005, Zheng et al., 2003), which influence hypothalamic circuits according to ongoing behaviors, learned cues, or “hedonics”. From this view, the effects of immune challenges on ingestive behavior likely occur either via influence on top-down pathways, bottom-up pathways, or both. To date, evidence exists that supports all three possibilities, which, it should be emphasized, are not mutually exclusive.
Although immune challenge influences neural populations believed to be involved in the control or modulation of feeding behavior (Dantzer, 2001; Elmquist et al., 1996; Gaykema et al., 2004; Goehler et al., 2000; Wan et al., 1994), little information exists regarding whether LPS treatment influences these neurons in the context of feeding. Besckei et al. (2007) reported that LPS treatment prevented the activation of arcuate and lateral hypothalamic (LH) orexin neurons following food deprivation, but other neuronal populations were not assessed. Similarly, although Sergeyev et al (2001) reported LPS effects on hypothalamic neuropeptide mRNA, the neural populations in which this effect occurred were not described. To gain a more complete picture of brain responses that mediate the ability of immune challenge to inhibit feeding behavior, further studies are needed that assess both top-down and bottom-up neural influences on neurochemically identified populations of hypothalamic neurons.
As noted earlier, recent work on the neurocircuitry of feeding behavior has identified a network of forebrain nuclei that may represent the top-down pathways that modulate feeding based on learned cues and mood states (e.g. stress, depression/anhedonia) or arousal (Baldo & Kelly 2007). This network seems to involve interconnections between the orexin neurons of the lateral hypothalamus, the paraventricular thalamus (PVT), the nucleus accumbens (NAc), and the central and basolateral amygdala (CEA and BLA) (Baldo and Kelley, 2007; Kelly, 2005a, 2005b; Kirouac & Ganguly, 1995; Parsons et al., 2006; Zheng et al., 2003). To test the idea that immune challenge influences this feeding behavior network, we assessed the effects of LPS on activity of neurons (using nuclear c-Fos protein as a cell activation marker) in these brain regions in animals allowed access to a palatable sweetened milk solution. Rats previously allowed to drink sweetened milk ad libitum for 30 minutes were injected with LPS (i.p), and allowed access to sweetened milk two hours later. Their intake was recorded, and the effects of LPS challenge on activity of the NAc, PVT, hypothalamic areas, and amygdala were determined using immunohistochemistry for c-Fos protein. The pattern of activation in LPS-treated animals given sweetened milk was compared to saline treated animals given sweetened milk, and animals given water (to control for disturbance of the animals during solution presentation, e.g. effects on arousal) and treated with either LPS or saline. This allowed us to discern activation patterns likely related to LPS treatment, related to access to a palatable substance, and to the combined effects of both LPS treatment and sweetened milk.
To evaluate the effects of LPS treatment on the activation of specific hypothalamic neuronal populations that are implicated in feeding behavior, c-Fos immunoreactivity (ir) in the hypothalamus was assessed in neurons stained for orexin-A, MCH, and CART ir. These neurochemically defined populations were chosen based on studies showing that the orexin-A-labeled cells, which are mainly located in the lateral and perifornical hypothalamus, contribute to reward-driven motivated behaviors, and also promote feeding behaviors (Peyron et al., 1998; Dube et al., 1999; Harris and Aston-Jones, 2006), as do the MCH neurons (Tritos and Maratos-Flier, 1999). Because immune challenge typically inhibits feeding behavior, it was expected that orexin- and MCH- positive neurons would show reduced c-Fos expression compared to saline-treated animals, concomitant with reduced intake of sweetened milk. In the arcuate nucleus, CART mRNA expression in neurons that co-express POMC was reported to be enhanced by LPS challenge, and to satiating stimuli (Sergeyev et al. 2001). Thus, if the reduced feeding behavior following LPS administration follows from bottom-up drive of the anorexigenic neurons in the arcuate nucleus, greater numbers of CART-containing neurons should show c-Fos-IR in LPS-treated animals than in saline treated controls.
Because c-Fos expression in animals presented with sweetened milk could be related to either the palatability (hedonics) or the bottom-up viscerosensory signals related to ingesting a large volume of sweetened milk, a second experiment used a “yoked control” approach that limited access of all animals to the average amount of sweetened milk solution consumed by LPS-treated animals in Experiment 1 (about 5 grams). C-Fos induction related to hedonic value should be similar in yoked and ad libitum conditions (because it is the same palatable stimulus), whereas c-Fos induction related to viscerosensory signals derived from e.g. a full stomach, would be expected to be reduced, as the smaller volume of milk provides fewer calories and less gastric distension, and thus constitutes a weaker viscerosensory stimulus.
In this way, we were able to provide evidence that LPS treatment suppresses neuronal activity in hypothalamic feeding-related neurons in the LH, and that this effect may derive from the more “top-down” effects via the NAc, amygdala and PVT, or via bottom-up pathways not investigated in this study. The anorexigenic CART-containing neurons in the arcuate nucleus implicated in “bottom-up” signaling related to satiety do not seem to contribute to this effect.
METHODS
Subjects
Thirty-four male Sprague-Dawley rats (240–250 g) were obtained from Taconic (Germantown, New York) and housed in pairs upon arrival in the vivarium (lights on at 7:00 AM). A week before the experiments, the rats were singly housed with food with water provided ad libitum at all times. Animals were handled on a daily basis at least one week before the experiment started to minimize possible stress. All procedures followed the National Institute of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the University of Virginia Animal Care and Use Committee.
Immune challenge
LPS (serotype 0111:B4; derived from Escherichia coli; Sigma, St Louis, L-2013) was dissolved in sterile pyrogen-free saline (0.9% NaCl) and diluted to a concentration of 0.1mg/ml before use. On the experiment day rats were injected intraperitoneally (i.p.) with either LPS or saline at a volume of 1ml/kg (LPS dose of 0.1 mg/kg) and returned to their home cage two hours before the behavioral test.
Experiment 1
The day following the transfer to single housing, twenty-four rats were divided randomly in two groups and offered sweetened 30 g milk solution [Nestle Carnation Brand sweetened condensed milk (containing milk and sugar, approximately 7.5 % protein, 7.5 % fat, 85% carbohydrate) diluted 1:1 with deionized water] (n = 12) or deionized water (n =12), which were presented in their home cages beginning between 12:00 and 13:00h for 30 minutes. This procedure was repeated during six consecutive days. The rats were also divided across four different experiment days (6 rats per day) to allow procedures to occur within the same time window, and the solution access periods were subsequently staggered. The sweetened milk solution and water were presented in polypropylene tubes with ball-tipped drinking spouts. Each animal received the sweetened milk or water at the same time of day; the presentation was staggered such that the timing of milk presentation would allow for precise timing of transcardial perfusion (see below). Thus, the animals received drink solutions sequentially 10 min apart. To allow avoid confounding effects of food deprivation on c-Fos induction, standard laboratory chow was available at all times. After 30 min, sweetened milk consumption was measured by subtracting the post-exposure weight of the drinking bottles from the pre-exposure weight. Baseline consumption was calculated as the average of the amount of consumed solution for last three days of training period.
On the experiment day between 10:00 and 11:00 h, rats were injected with LPS (i.p.) or with sterile, non-pyrogenic saline. Two hours later, they were presented in their home cage with the same solution (either sweetened milk or water) they were previously offered for a period of 30 min at exactly the same times as during the training period. One hour after removal of the sweetened milk or water, the rats were deeply anesthetized with pentobarbital (i.p) for the analysis of c-Fos protein induction in the brain that reflects the experimental condition.
Experiment 2
This experiments used a “yoked control” approach that limited access of all animals to the average amount of sweetened milk solution consumed by LPS-treated animals in Experiment 1 (about 5 grams). Ten rats were allowed to drink approximately 5–6 grams of sweetened milk solution using the same procedure as Experiment 1. On the experiment day, 5 rats were injected with saline and 5 rats with LPS, as above. They were presented with drink solution 2 hours later for the duration of 30 min, and anesthetized in preparation for transcardial perfusion another hour later. To assess possible differences in motivation to drink sweetened milk, latency (in sec) to drink from the tube was also recorded.
Tissue preparation, immunohistochemistry, and data analysis
The rats were anesthetized with Pentobarbital (60 mg/kg, i.p.) and transcardially perfused briefly with saline followed by 4% paraformaldehyde in 0.1M phosphate buffer (PB) with 15% saturated picric acid. The brains were removed and post-fixed overnight with the same fixative. The brains were sectioned coronally on a Vibratome (Model 1500) at a thickness of 50 µm and sections were collected serially in six-well plates such that each well contained every 6th section equally spaced at 300 µm. The sections were stored in 0.1 M PB-0.1% sodium azide at 4 °C until immunohistochemical staining procedures.
Immunohistochemistry
Prior to incubation in the primary antibody solution, the sections were treated with a mixture of hydrogen peroxide (0.3%) and sodium azide (0.1%) in phosphate buffered saline (PBS, 0.154 M NaCl and 0.01 M PB, pH 7.4) for 30 minutes to block endogenous peroxidase activity. All blocking, primary and secondary antibodies solutions were diluted in PBS containing 0.5% Triton X-100 (PBS-T), to which also 2 % normal goat serum and 0.1% sodium azide were added. The sections were rinsed three times in phosphate buffered saline (PBS, 0.154 M NaCl and 0.01 M phosphate buffer, pH 7.4) between all incubation steps. To reduce nonspecific staining, the sections were immersed in PBS-T containing 4% normal goat serum (Jackson immunoResearch Laboratories, West Grove, PA) and Fab’ fragment of goat anti-rat igG (1:500) overnight at 4°C.The first and fourth sets of sections (when combined containing every third section) were processed for immunoperoxidase labeling of nuclear c-Fos protein. These sections were incubated for 72 h at 4°C in rabbit anti-Fos (Ab5, Oncogene, Cambridge, MA, 1:50,000 for 72 h), followed by biotinylated goat anti-rabbit IgG (Jackson ImmunoResearch; 1:1000 overnight at 4°C), and avidin–biotin peroxidase complex (Vectastain Elite ABC kit, Vector Laboratories, Burlingame, CA,1:500 in PBS-T; overnight at 4°C). After rinses in PBS and Tris-HCl buffer(0.05M, pH 7.6), black immunostaining was obtained with 0.02% 3′3-diaminobenzidine (DAB), 0.15% nickel ammonium sulfate, 0.04% ammonium chloride, and 0.02% glucose oxidase in Tris-HCl buffer. The reaction was initiated by adding β D-glucose (final concentration 0.1%) and terminated by rinses in PBS. Sections were mounted on gelatin coated microscope slides, dehydrated in graded ethanol solutions, cleared in Citrosolve, and coverslipped with DPX (BDH Laboratory Supplies, England).
Dual immunoperoxidase labeling for c-Fos immunoreactivity and orexin-A, melanin-concentrating hormone (MCH), or cocaine amphetamine-related transcript (CART)
These neurochemically defined populations were chosen based on studies showing that the orexin-A labeled cells, which are mainly located in the lateral and perifornical hypothalamus, contribute to reward-driven motivated behaviors, and also promote feeding behaviors (Peyron et al., 1998; Dube et al., 1999; Harris and Aston-Jones, 2005, 2006), as do the MCH neurons (Tritos and Maratos-Flier, 1999). In the arcuate nucleus, CART mRNA expression in neurons that co-express pro-opiomelanocorticotropin is enhanced by LPS challenge, and to satiating stimuli (Sergeyev et al. 2001). Three additional sets of sections through the hypothalamus underwent the c-Fos nuclear immunostaining procedure as described above, after which the sets were subjected to immunoperoxidase cytoplasmic staining of orexin-A, CART, or MCH. For this purpose the sets were incubated in either rabbit anti-orexin-A (AB3704, lot 0506003001, Chemicon Int., Temecula, CA, 1:2000), rabbit anti-MCH (H-070-47, lot 00703, Phoenix Pharmaceuticals, Inc., Belmont CA, 1:2000), or rabbit anti-CART (anti CART (55–102) H-003-62, lot 00807, Phoenix Pharmaceuticals, Inc., Belmont CA, 1:10,000) for 48 h at 4°C. Brown cytoplasmic immunostaining was completed by incubations in biotinylated goat anti-rabbit IgG and ABC (both diluted 1:1000, overnight at 4 °C), followed by reaction with regular DAB (0.04%). All sections were mounted, dehydrated, cleared, and coverslipped as described above.
Microscopy
The sections were examined with an Olympus BX51 microscope and digital images were captured with a Magnafire digital camera (Optronics, Goleta, CA) linked to a Power Mac (OS 9) equipped with NIH Image software for counting the c-Fos-immunoreactive (-ir) profiles. Photomicrographs were cropped, resized and adjusted in brightness and contrast in Adobe Photoshop CS2 (Adobe Systems, Mountain View, CA).
Quantification of c-Fos protein immunoreactivity
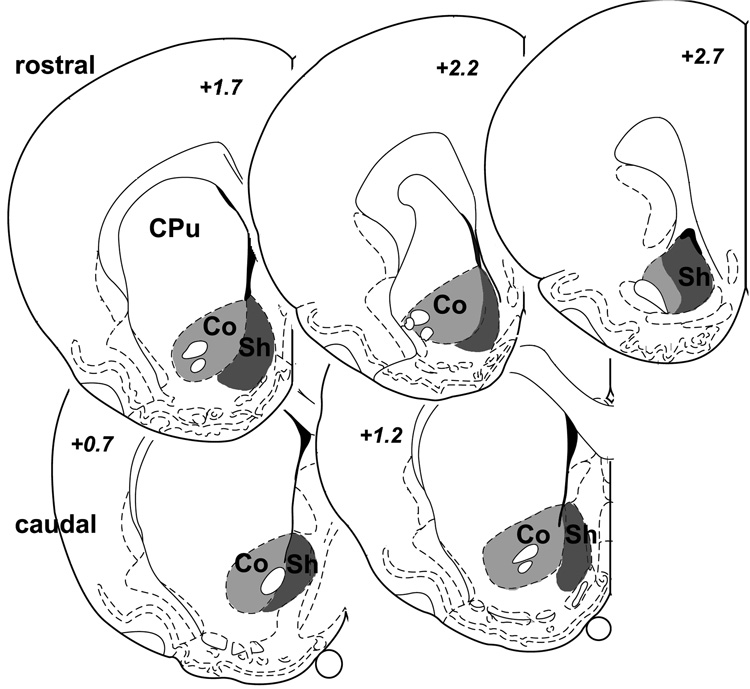
In the series stained only for nuclear c-Fos, numbers of immunopositive neurons in selected brain regions were counted using NIH Image (v. 1.61). The regions analyzed were outlined based on landmark features with the aid of the Rat Brain Atlas (Paxinos and Watson, 1998) as well as adjacent sections stained for immunohistochemical markers that reveal the boundaries of the nuclei of interest. Counts through the amygdala and PVT were performed bilaterally in every sixth section (from one series of sections spaced by 300 µm mid-section distance), and summed to a total for each animal. C-Fos expression in the rostral and caudal NAc were examined separately based on studies suggesting a possible dichotomy of their activity according to emotional valence (Reynolds and Berridge, 2002; 2003). Specifically, activation of rostral NAc shell may promote negative emotional valence, whereas positive or rewarding stimuli seem to be associated with activity in the caudal NAc. Thus, the NAc was subdivided into core and shell regions as well as into rostral (2.7, 2.2, and 1.7 µm anterior to bregma) and caudal parts (1.2 and 0.7 µm anterior to bregma), and five sections were selected that corresponded closest with the diagrams at these 5 brain atlas levels (Fig. 1). For both shell and core divisions, the bilateral counts were summed in both the rostral and caudal portions. The PVT was counted in 6 sections (evenly spaced by 300 µm) through the rostrocaudal extent from −1.6 to −3.3 mm caudal to bregma. C-Fos-positive cells in the BLA and CeA were counted bilaterally through the rostrocaudal extent in 4 sections that were evenly spaced by 300 µm from −2.1 to −3.3 mm caudal to bregma.
Fig. 1.
Brain section diagrams (modified after Paxinos and Watson, 1998) depicting the shell (Sh) and core (Co) divisions of the nucleus accumbens at 5 different rostro-caudal levels, of which the top 3 (+2.7 to +1.7 mm relative to bregma) and the bottom 2 (+1.2 and +0.7 mm) comprised the rostral and caudal portions, respectively.
Analysis of hypothalamic orexin-A, MCH, and CART-containing populations
Using the series with dual staining for c-Fos and one of the hypothalamic peptides, the relative activation of orexin-A-positive perikarya in the lateral/perifornical hypothalamus and CART-labeled cell bodies in the arcuate nucleus was determined. For this purpose, we counted double-stained neurons that harbored both brown cytoplasmic and black nuclear reaction products, as well as the orexin-A- and CART-positive perikarya with a lighter brown or unstained nucleus that clearly lacked grayish-black reaction product (thus labeled as c-Fos-negative). From these counts the total number of orexin-A and CART cells with and the proportion that was c-Fos-positive were calculated. Sections were examined using a 20X objective lens and an 10X eyepiece equipped with reference grid of 10 by 10 square units each 50×50 µm in size. Neurons were counted in both hemispheres. Four sections through the hypothalamus spaced 300 µm apart were included from each animal. The sections covered the part of the hypothalamus between the caudal end of the paraventricular nucleus between −2.3 mm and −3.6 mm caudal to bregma. The CART ir cells present in the arcuate nucleus, but not those present in the supraoptic, lateral, and dorsomedial nuclei, were included in the analysis. At each rostro-caudal level (4 sections), the hypothalamic region containing orexin-A positive cells was divided into 3 regions from medial to lateral, (between −2.3 mm and −3.6 mm). The fornix served as a reference on which the 500 × 500 µm grid was centered. All cells inside, ventral, and dorsal to the grid were included the middle region labeled “perifornical” (PF). Orexin-A-labeled cells lateral to this region were included in the lateral region (LH proper), and those medial from the grid overlay were in the medial group (MH), which partially overlapped with the dorsomedial nucleus of the hypothalamus. No further quantitative analysis was performed on the MCH-positive population as no (or very few) double-labeled cells were encountered in any experimental group.
Data analysis
Consumption data as well as the c-Fos counts were analyzed using analysis of variance (ANOVA) with a two by two design (Experiment 1) or 1-way ANOVA from the sweetened milk-offered rats in Experiment 2). If the ANOVA results were statistically significant (probability of 0.05 or less), Fisher’s protected least significant difference or t-tests were conducted to compare between the individual groups (water/saline, water/LPS, sweetened milk/saline, and sweetened milk/LPS). For double-label studies (c-Fos and peptides) percentages were calculated of activated cells (double-labeled) out of the total number of peptide-IR positive cells in the specified region.
RESULTS
Experiment 1
The rats in this experiment were allowed access to sweetened milk ad libitum during a 30 min exposure period. The animals began to drink immediately and stopped drinking within the 30 min period, without consuming all of the solution. Animals exposed to deionized water approached the drinking tube but in general did not immediately consume water.
LPS effects on sweetened milk intake
While there were no prior differences in solution consumption between groups assigned to the different treatment during the training period (amount consumed averaged over the last three days shown in Fig. 2A), LPS-treated rats showed a statistically significant reduction in solution consumption compared to the saline-treated controls on the experiment day (Fig. 2B; mean = 18.8 and 5.1 g for saline- and LPS-treated, respectively; F1,21 = 44.2, p< .0001). Water consumption, although very limited in the training period (2.3 and 1.1 g, for saline and LPS groups, respectively) was reduced to almost zero by LPS treatment (range between 0.0 and 0.3 g). Rats presented with sweetened milk solution consumed significantly more compared to rats presented with water both during the training period as well as on the experiment day (F1,21 = 191.6 and 88.2, respectively, p < .0001). There was a statistically significant interaction between treatment and solution on the experiment day (F1,21 = 26.2, p < .0001). Comparison of percentage change of sweetened milk and water consumption (calculated from baseline and experiment day values from each rat) showed that LPS treatment strongly reduced the consumption of either solution (shown in Fig. 2C; F1,21 = 25.8, p < .0001).
Fig. 2.
Effect of LPS challenge on the consumption of ad lib sweetened milk solution and deionized water in experiment 1 (left panels A–C) and of restricted sweetened milk intake in experiment 2, where the intake was limited to approx. 5 g (right panels D–F). Bar graphs depict mean and SE; light and dark shading represent saline- and LPS-treated groups, respectively. Baseline intake during the days prior to treatment did not differ between the two treatment groups (A, D). LPS challenge is associated with a large reduction in sweetened milk intake in experiment 1 (B), and a smaller reduction of milk intake in experiment 2 (E). Panels C and F depict the percentage change in consumption that was calculated from the baseline and experimental day values of each individual rat. Both consumption of water and sweetened milk solution when offered ad lib dropped sharply (C), but the drop in intake of the sweetened milk solution was modest when the limited amount was offered (F). * p < 0.05, ** p < 0.005, LPS vs. saline treatment.: ## p < 0.005, sweetened milk vs. water exposure.
Distribution of c-Fos protein in the nucleus accumbens, amygdala, and paraventricular thalamus
Nucleus accumbens
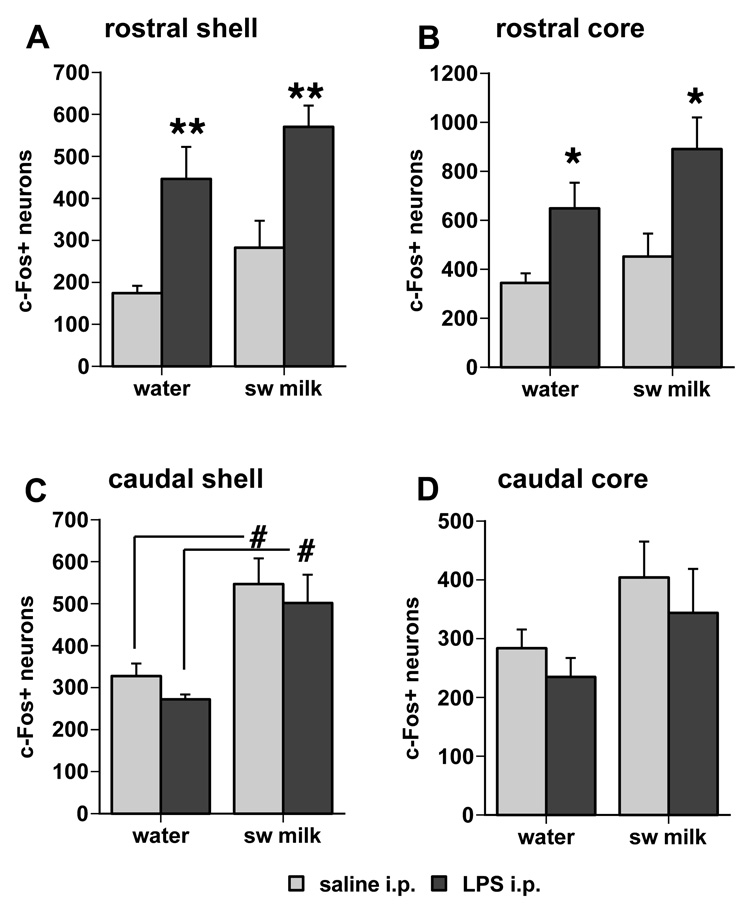
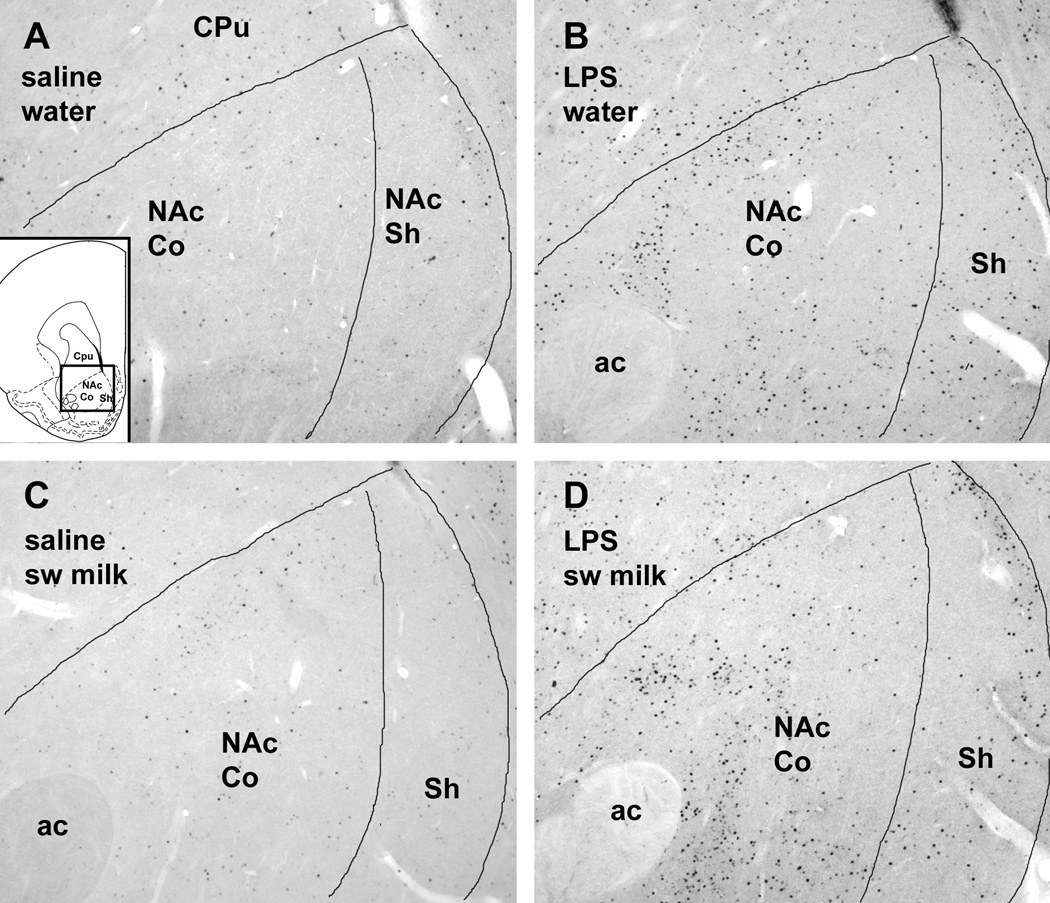
LPS treatment significantly increased c-Fos expression in the rostral part of the NAc (Fig. 3, Fig. 4). The mean number of c-Fos ir cells in the rostral shell and core regions of the NAc of the LPS-treated rats was significantly greater compared to saline-treated rats irrespective of their exposure to water or sweetened milk solution (main effects in the shell: F1,19 = 26.2, p < .0001 [Fig. 3A]; in the core: F1,19 = 14.6, p = .0012 [Fig. 3B]). In addition, groups exposure to sweetened milk solution showed a small, but statistically significant increase in c-Fos expression in the rostral shell (Fig. 3A; main drinking solution effect F1,19 = 4.52, p = .047), whereas in the rostral core of the NAc this effect was marginal (main effect F1,19 = 3.23, p= 0.088). However, post-hoc comparisons between groups revealed that sweetened milk-associated increase in c-Fos was not statistically significant (p = 0.14 and 0.19, for saline- and LPS-treated groups, respectively).
Fig. 3.
Differential effects of LPS treatment and sweetened milk consumption on the number of c-Fos ir profiles in the NAc shell and core subdivisions. The pattern of c-Fos induction differs across regions of the NAc, showing a significant effect of LPS treatment in the rostral shell (A) and core (B) divisions, and a main sweetened milk effect in the caudal shell (C). * p < 0.05, ** p < 0.005, LPS vs. saline treatment; # p < 0.05, sweetened milk vs. water exposure.
Fig. 4.
Photomicrographs of c-Fos immunostaining in the rostral NAc in representative cases offered either water (A,B) or sweetened milk solution (C,D) after i.p. injections of either saline (A,C) or LPS (B,D). Note the strong increase in c-Fos staining following LPS treatment in both core and shell divisions (B,D). Insert in A depicts the area shown in a brain section diagram (modified from Paxinos and Watson, 1998). Scale bar in A = 200 µm.
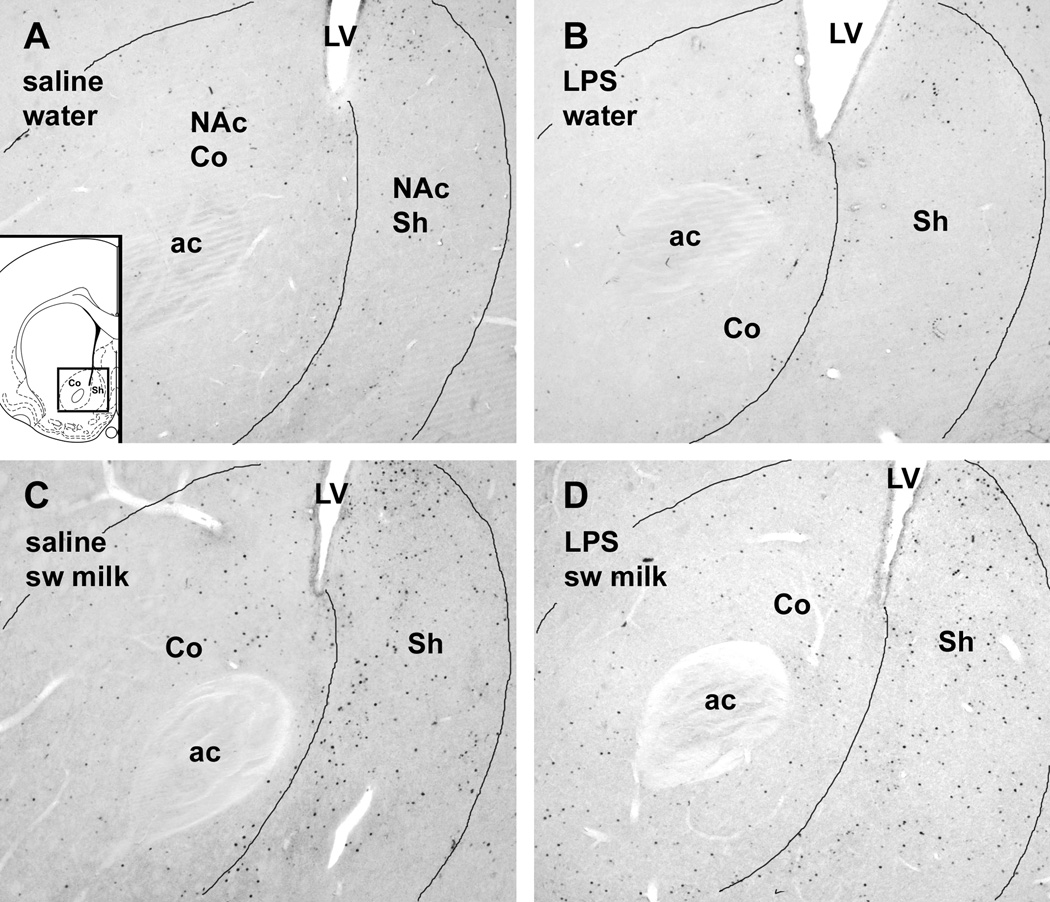
In contrast to the LPS-associated c-Fos increase in the rostral NAc described above, sweetened milk consumption, but not LPS treatment, significantly increased c-Fos expression in the caudal medial shell of the NAc (Fig. 3C, Fig. 5) in both the saline- and LPS-treated animals (main effect, F1,19 =19.6, p = 0.0003). This main effect of sweetened milk was less pronounced in the caudal core of the NAc (Fig. 3D, F1,19= 4.32, p = 0.051). The sweetened milk-related increase in c-Fos expression in the caudal NAc occurred irrespective of the prior saline and LPS treatment (post-hoc comparison of saline groups: p = 0.009, LPS groups: p = 0.014).
Fig. 5.
Photomicrographs of c-Fos immunostaining in the caudal NAc in representative cases offered either water (A,B) or sweetened milk solution (C,D) after i.p. injections of either saline (A,C) or LPS (B,D). Note the strong increase in c-Fos ir in the shell region after sweetened milk was offered (C,D). Insert in A depicts the area shown in a brain section diagram (modified from Paxinos and Watson, 1998). Scale bar in A = 200 µm.
Amygdala
LPS treatment and sweetened milk consumption exerted differential effects on c-Fos expression in the basolateral and central nuclei of the amygdala (BLA & CEA; Fig. 6, Fig. 7). Compared with water, sweetened milk presentation increased c-Fos expression in the BLA of saline-treated rats, an effect that was not present in LPS-injected animals (Fig 6A; interaction injection × milk/water exposure: F1,19 = 9.37, p = 0.007). Post-hoc comparisons showed that LPS treatment marginally increased c-Fos expression in the BLA of the water group (p = 0.07), but slightly decreased the number of c-Fos ir profiles in the BLA of the sweetened milk group (p = 0.04). The c-Fos ir cells were mainly present in the anterior subdivision (as depicted in Fig. 7) and in the medial portion farther caudally.
Fig. 6.
Effects of LPS treatment and sweetened milk consumption on the number of c-Fos ir profiles in the BLA, CEA, and the PVT. In the BLA, sweetened milk is associated with an increase in c-Fos expression only in the saline-treated rats (#), whereas increased c-Fos expression occurs in the water group following LPS treatment, but decreased c-Fos expression was seen in the sweetened milk group. In the CEA, LPS challenge is associated with increased c-Fos expression, in addition to an effect of sweetened milk consumption that was smaller in magnitude. In the PVT, increased c-Fos expression is associated with both sweetened milk consumption and LPS treatment. * p < 0.05, ** p < 0.005, LPS vs. saline treatment.: # p < 0.05, sweetened milk vs. water exposure.
Fig. 7.
Photomicrographs of c-Fos immunostaining in the amygdala in representative cases offered either water (A,B) or sweetened milk solution (C,D) after i.p. injections of either saline (A,C) or LPS (B,D). In the BLA, an increase in the number of c-Fos-labeled cells is apparent following sweetened milk intake (C) but also following LPS treatment (B,D). In the CEA, increases in c-Fos staining can be seen following LPS challenge (B,D), but in addition a modest increase can be seen following sweetened milk exposure (C). Insert in A depicts the area shown in a brain section diagram (modified from Paxinos and Watson, 1998). Scale bar in A = 200 µm.
In the CEA, LPS treatment strongly increased the mean number of c-Fos ir cells (Fig. 6B, Fig. 7; main effect LPS treatment: F1,19 = 36.6, p < 0.0001), and these increases were highly statistically significant in both water (p < 0.0001) and sweetened milk groups(p = 0.007). In addition, sweetened milk consumption increased the number of c-Fos ir cells in the CEA (main effect: F1,19 = 4.47, p = 0.048), and post-hoc comparison revealed a statistically significant effect in the saline-treated groups (means increased from 438 to 840, p = 0.002), but not in the LPS groups. Most c-Fos ir cells were distributed in the lateral division of the CEA (Fig. 7).
Paraventricular thalamus
Both sweetened milk consumption and LPS treatment increased the numbers of c-Fos positive neurons in the PVT (Fig. 6C, Fig. 8, main effects for sweetened milk: F1,19 = 15.19, p = 0.001; main effect for LPS: F1,19 = 10.9, p = 0.004) throughout the entire rostrocaudal extent of the nucleus. Although c-Fos ir cells were present in the water/saline control group, the increase in c-Fos expression by both independent variables was statistically significant (comparison with the sweetened milk-saline group: p = 0.0012; with the water- LPS group: p = 0.012). Although the effects of sweetened milk and LPS seem cumulative, the differences between LPS and saline groups that were offered sweetened milk were not statistically significant (p = 0.13).
Fig. 8.
Photomicrographs of c-Fos immunostaining in the PVT in representative cases offered either water (A,B) or sweetened milk solution (C,D) after i.p. injections of either saline (A,C) or LPS (B,D). An increase in the number of c-Fos-labeled cells is apparent following both LPS challenge (B) and sweetened milk intake (C). Insert in A depicts the area shown in a brain section diagram (modified from Paxinos and Watson, 1998). Scale bars in A = 100 µm.
Hypothalamic peptidergic neuronal responses to sweetened milk and LPS
Orexin-A
Sweetened milk consumption significantly increased, relative to the water controls, the proportion of orexin-A-labeled neurons with nuclear c-Fos staining in the lateral, perifornical and medial subpopulations (Fig. 9; respective main effects: F1,19 = 5.37, p = 0.032; F1,19 = 4.46, p = 0.048; F1,19 = 8.31, p = 0.0095). In saline-treated animals, post-hoc comparisons revealed sweetened milk-associated increases in percentage of double-labeled orexin-c-Fos-positive cells in the LH (p = 0.029), MH (p = 0.025), and less pronounced in the PF portion (p = 0.069). LPS treatment had an effect on c-Fos expression in the LH portion of orexin cells only (main inhibitory LPS effect: F1,19 = 4.45, p = 0.049, trend towards interaction F1,19 = 2.33, p = 0.13). In animals treated with LPS, the sweetened milk-induced increase was absent in the lateral-most (LH) subpopulation of orexin neurons (LPS decreased the percentage double-labeled cells; post hoc comparison between sweetened milk groups: p = 0.035). Total numbers of orexin-positive somata (both c-Fos-positive and -negative) were not different among groups (Fig. 9, right panels). Fisher’s PLSD post hoc test showed that the ratio of orexin neurons expressing c-Fos ir in the LH of rats offered sweetened milk was significantly reduced following LPS treatment (p = 0.035). This effect is illustrated in Fig. 10 showing the LH area double-stained for orexin-A and c-Fos, where double-labeled cells were more abundant in the saline-sweetened milk group (panel B).
Fig. 9.
Effects of LPS treatment and sweetened milk consumption on the proportion of orexin neurons displaying c-Fos ir in lateral (LH, A), perifornical (PF, B) and medial (MH, C) parts of their range of distribution (see diagram of section through the hypothalamus between A’ and B’, modified from Paxinos and Watson, 1998). The sweetened milk-related increase in c-Fos expression, significant in the LH (A) and MH (C) portions of orexin neurons in saline-treated groups, is reduced following LPS challenge, most notably in the lateral-most portion of orexin-containing neurons (LH, in A). In the MH portion of the orexin neurons, however, c-Fos expression is increased following LPS challenge alone (C). There were no differences in the total number of orexin neurons between the groups (right panels A’–C’). * p < 0.05, LPS vs. saline treatment. # p < 0.05, sweetened milk vs. water exposure.
Fig. 10.
Photomicrographs of orexin-A-containing neurons in the lateral hypothalamus (brown cytoplasmic staining), which lack c-Fos ir in rats offered water (A), but show black nuclear staining for c-Fos ir when offered sweetened milk (B, indicated with arrows). When challenged with i.p. LPS prior, however, much less c-Fos staining is apparent in the orexin neurons (C). Insert of brain section diagram depicts the area of the photomicrographs taken. Scale bar in A = 50 µm.
MCH
Neither sweetened milk consumption nor LPS treatment influenced activation of hypothalamic MCH ir cells that were dispersed across the lateral, perifornical and dorsomedial divisions similar to the orexin neurons. Neurons double-labeled for c-Fos and MCH ir were very small in number, despite the numerous MCH-labeled neurons encountered, whereas the distribution of the occasional double-labeled neurons was variable in all groups assessed (data not shown).
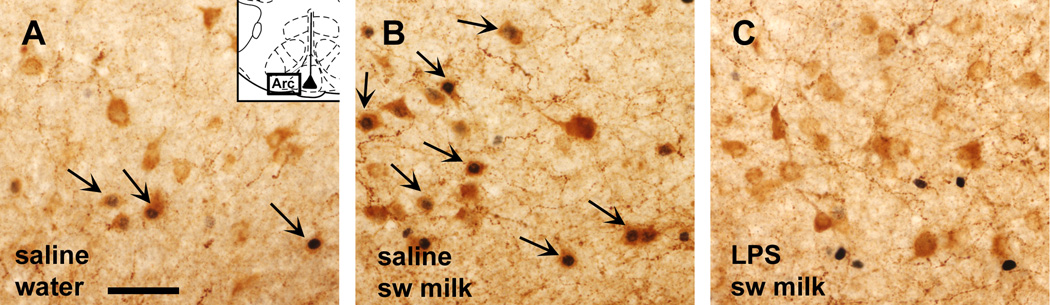
CART
In saline-treated rats only, sweetened milk consumption was associated with a marked increase in the proportion of arcuate CART neurons that expressed c-Fos ir, whereas LPS challenge dramatically reduced c-Fos expression in the CART neurons in rats of both the water and sweetened milk groups (Fig. 11, Fig. 12; interaction F1,19 = 9.83, p = 0.005). Fisher’s PLSD post-hoc test showed that sweetened milk consumption increased the proportion of CART ir neurons with nuclear c-Fos staining (from 43% in the water group to 73%, p = 0.0036; Fig. 11A). Following LPS treatment, c-Fos expression in the arcuate hypothalamus occurred almost totally separate from the CART-labeled neurons (Fig. 12C), whereas arcuate c-Fos expression seen in saline-injected rats predominantly resided within the CART-labeled population (Fig. 12A,B).
Fig. 11.
The percentage of CART-positive neurons in the arcuate nucleus that express c-Fos ir is increased following sweetened milk consumption, but c-Fos expression in CART neurons is almost completely eliminated following LPS treatment, irrespective of drinking solution offered (A). The total number of CART cells is slightly reduced in the LPS-treated groups (B).: * p < 0.05, ** p < 0.005, LPS vs. saline treatment. ## p < 0.005, sweetened milk vs. water exposure.
Fig. 12.
Photomicrographs of CART-containing neurons in the ventral arcuate hypothalamus (brown cytoplasmic staining), several of which express c-Fos ir (black nuclear staining, indicated with arrows) in rats offered water (A). The c-Fos staining appears in the greater majority of CART cells in rats offered sweetened milk (B). When challenged with LPS, however, c-Fos staining is absent from most CART neurons (C). Insert of brain section diagram depicts the area of the photomicrographs taken. Scale bar in A = 50 µm.
Experiment Two
To assess whether the differences in c-Fos expression between LPS- and saline-treated rats that had access to sweetened milk might be due to the changes in hedonic value or to the difference in the amount or volume of sweetened milk solution consumed (differences in viscerosensory signals) this experiment used a “yoked control” approach that limited access of all animals to the average amount of sweetened milk solution consumed by LPS-treated animals in Experiment 1 (about 5 grams). As in experiment 1, the rats began to drink sweetened milk immediately, and during training consumed all of the milk they were able to get out of the tube in approximately 5 min (mean baseline intake 5.3 g for both groups, Fig. 2D). On the day of the experiment, there were no differences in latency to drink between LPS- and saline-treated animals (mean latency 2.6 vs. 2.4 sec, respectively). However, even though the amount of sweetened milk presented to the animals corresponded to the average amount of milk consumed by LPS-treated animals in experiment 1, the LPS-treated animals in experiment 2 still consumed less sweetened milk than the saline-treated controls (3.8 vs. 5.2 g; F1,8= 9.8, p < 0.01; Fig. 2E), although the reduction of sweetened milk compared to controls was much smaller in magnitude; reduced by 23% from baseline (Fig 2F).
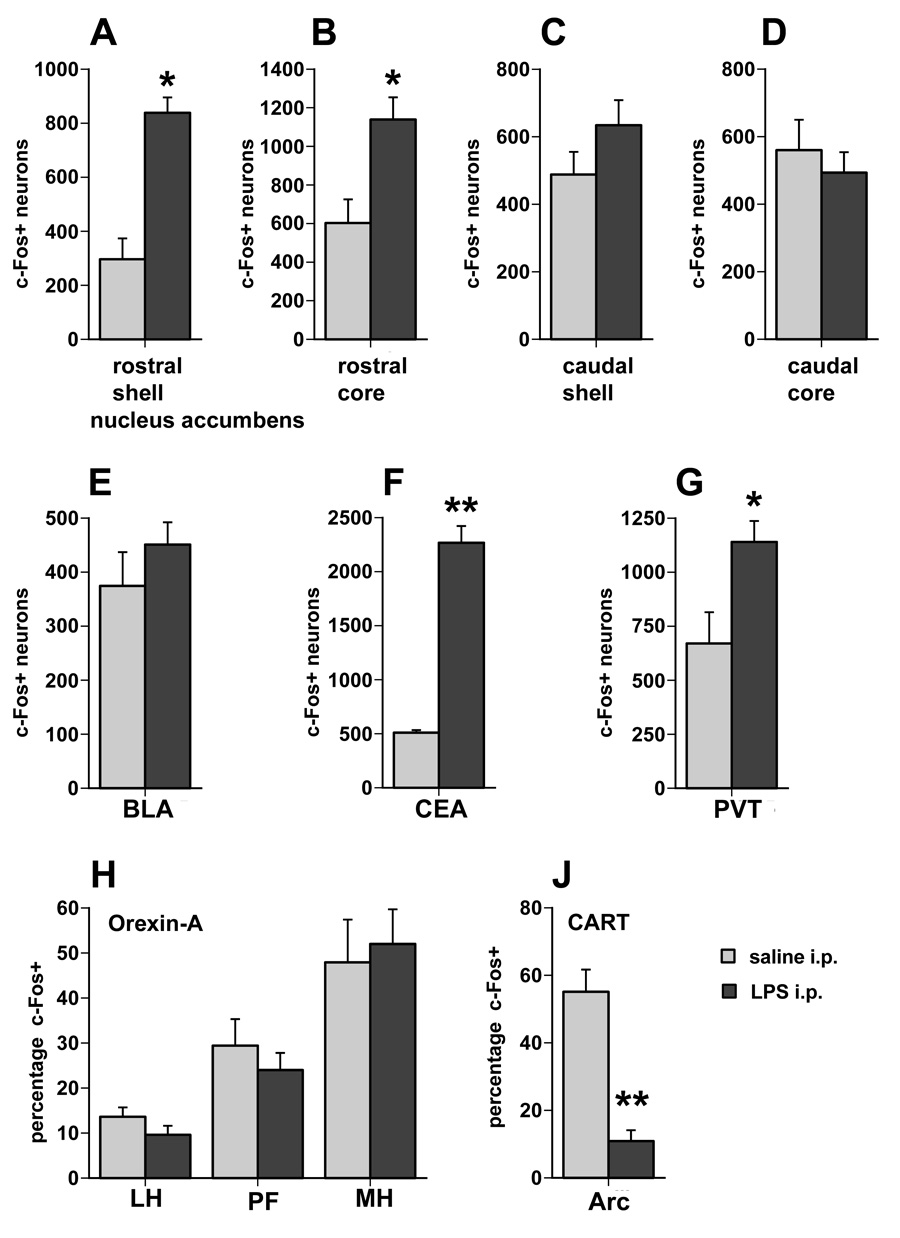
The pattern of c-Fos expression in the NAc in the “yoked” animals was similar to the animals that had ad libitum access to sweetened milk (Expt. 1). The number of c-Fos positive neurons was significantly greater in the rostral shell and core of the NAc of LPS-treated animals compared to saline-treated animals (mean positive neurons in shell: 838 vs. 296, respectively, F1,8=31.69, p=0.0005; core: 1138 vs. 603, respectively, F1,8 = 10.14, p= 0.01). There was no difference in c-Fos positive neurons in either the shell or core of the caudal NAc. There was no statistically significant difference in the BLA between LPS and saline-treated animals (mean= 451 vs. 375, respectively). However, in the PVT and CEA, LPS treatment was associated with increased c-Fos expression compared to saline treatment (PVT: F1,8 =7.29, p=0.027; CEA: F1,8 =125.2, p< 0.0001). In contrast to the animals given ad libitum access to sweetened milk experiment 1 (see Fig. 9A), there were no significant differences in the proportion of orexin-c-Fos double-labeled neurons between LPS and the saline-treated animals in the yoked condition in any of the three regions assessed (Fig. 13H), albeit the proportion double-labeled cells in the LH portion was rather low in the saline-treated group when compared to the ad libitum condition. As in experiment 1, numbers of CART and c-Fos double-labeled neurons in the arcuate nucleus were strongly reduced in the LPS-treated animals compared to the saline-treated controls (F1,8 = 23.99, p < 0.001; Fig. 13J). In saline-treated rats with access to a reduced amount of sweetened milk, the proportion of arcuate CART-positive neurons double-labeled for c-Fos-ir (55%) was intermediate between the saline-treated water (43%) and ad-libitum milk groups (73%) of experiment 1 (compare Fig. 13J with Fig. 11A). The total number of CART-positive neurons did not differ between the two groups.
Fig. 13.
A. c-Fos expression in selected brain regions following LPS or saline treatment and after exposure and consumption of a limited amount of sweetened milk (5 grams). c-Fos expression is increased following LPS challenge in the rostral shell and core of the NAc (A,B), the CEA, and the PVT (G), but c-Fos ir in arcuate CART cells was reduced (J). No significant differences between LPS and saline treated animals are encountered in the caudal NAc shell and core (C,D), the BLA (E) and the orexin neurons in the hypothalamus (H). There is no difference in the total number of orexin and CART cells between the saline and LPS treatment groups. * p < 0.05, ** p < 0.005, LPS vs. saline treatment.
DISCUSSION
As predicted based on previous findings, LPS-treated rats consumed much less sweetened milk than controls when offered sweetened milk ad libitum, although the difference was smaller in the yoked condition. The purpose of this study was to determine whether this effect is concomitant with changes in c-Fos protein expression in forebrain networks that have been previously identified to mediate ingestive behavior. Indeed, LPS treatment was associated with a decrease in c-Fos expression in hypothalamic neurons implicated in feeding (orexin-A and CART containing) and an increase in the PVT, NAc, and BLA (areas implicated in modulating feeding) in animals presented with sweetened milk compared to controls receiving water. When the animals were allowed access to a smaller volume of sweetened milk, which would presumably provide a reduced viscerosensory stimulus (due to milk ingestion per se), the response in saline-treated controls was reduced in the PVT and in orexin neurons of the LH. This suggests that these regions are normally processing “satiety”-related signals related to sweetened milk ingestion. However, in the caudal NAc, which has been implicated in processing of stimuli with positive hedonic value, there was no difference in c-Fos induction between LPS- and saline- treated rats. This might mean that for LPS-treated rats, sweetened milk is still a positive stimulus. The results from this study support the idea that the effects of LPS challenge on palatable food ingestion may involve forebrain neural circuits previously implicated in motivational aspects of the control of feeding behavior, but that these effects are not likely to involve arcuate CART-expressing neurons that are implicated in satiety signaling when tested in this experimental paradigm.
Methodological considerations
The findings reported here are based on immunohistochemical localization of a neuronal activation marker (c-Fos), in the context of an acute immune challenge and a complex gustatory and viscerosensory stimulus, sweetened milk. The use of c-Fos protein as a marker to identify ensembles of activated neurons is by now well-established, but it needs to be borne in mind that the approach may not identify all activated neurons, and that, especially in complex experiments, the specific role of an activated neuron cannot be conclusively established, i.e., the findings are correlational. Nonetheless, c-Fos analysis can provide details regarding activation patterns throughout the neuraxis with a high degree of spatial resolution, and thus provide a template for further study. Sweetened milk was chosen as a stimulus because although it is nutritionally complex and thus could activate more heterogeneous populations of neurons than a simple tastant such as sucrose or saccharine, it is strongly preferred and has been used previously in studies assessing anhedonia (De La Garza, 2005). Finally, although we focused our analysis on selected brain regions previously implicated in behavioral responses to palatable food, other regions likely contribute as well.
Does LPS challenge induce or enhance satiety-related signals?
Cytokines induced by LPS increase satiety signals and may disturb the balance of neuropeptides that maintain homeostasis (Becskei et al., 2008; Gautron et al., 2005; see reviews Plata-Salaman, 2000; Ramos et al., 2004; Langhans, 2007), and thus contribute to anorexia during illness. Further, pro-inflammatory cytokines associated with the development of cancer anorexia-cachexia syndrome have been shown to inhibit orexigenic activity and stimulate anorexigenic neuropeptides in the hypothalamic areas (see reviews Plata-Salaman, 2000; Ramos et al., 2004). Thus, based on previous findings (Sergeyev et al., 2001) that LPS treatment leads to increased expression of CART (a peptide expressed in POMC/β-endorphin-containing neurons of the arcuate nucleus that, when activated, suppress feeding), it was expected that LPS treatment, which leads to suppressed ingestion of sweetened milk, would be associated with activation of CART-expressing neurons. However, when animals were allowed sweetened milk ad-libitum (in expt.1), saline-treated controls showed elevated numbers of activated CART-positive neurons (perhaps associated with satiety induced by the high-volume ingestion). In contrast, the number of activated CART neurons was reduced in LPS-treated animals. This effect was seen in both sweetened milk and water conditions, suggesting that LPS exerts a non-specific inhibition of CART neuron activity, irrespective of feeding status. In experiment 2, the volume of sweetened milk available was restricted to the average amount consumed by LPS-treated animals of experiment 1. C-Fos expression in CART-positive cells of saline-treated animals was reduced in comparison to those receiving sweetened milk ad libitum, but not to the level seen in LPS-treated animals. The mechanisms of the apparent LPS-induced suppression of CART-containing neurons are unknown, but it seems unlikely that the anorectic/hypophagic effects of LPS are mediated by arcuate CART neurons in this experimental paradigm.
Although arcuate CART containing neurons do not seem to play a role in the LPS-induced reduction of sweetened milk ingestion, it is still possible that LPS-induced anorexigenic signals could be conveyed via other pathways, for instance those originating from the caudal viscerosensory nuclei, notably the nucleus of the solitary tract (Rinaman et al., 1998; Fan et al., 2004; Grill et al., 2004). Indeed, in control animals, the orexin-positive LH neurons and the PVT (which both receive ascending viscerosensory inputs) showed reduced c-Fos induction when the volume of milk, and presumably the intensity of satiety signaling, was reduced in experiment 2. These observations imply that these brain regions are normally influenced by peripheral satiety signals or, perhaps, salience of the stimulus (small snack vs. big meal), or both.
Does LPS induce anhedonia?
Evidence suggesting that immune challenge can specifically influence “hedonic” top-down drive on feeding derives from findings that peripheral administration of LPS or cytokines reduces intake of palatable substances, such as sweetened milk or sucrose, in rodents (Borowski et al., 1998; Cross-Mellor et al., 2003; De La Garza, 2005; Dunn and Swiergiel, 2001; Larson, 2006). Findings from previous studies have suggested that anhedonia (the lack of ability to experience pleasure or reward) may contribute to the mechanisms by which immune activation can lead to behavioral deficits (Yirmiya, 1996; Yirmiya et al., 2000). In the experiments reported here, animals consumed considerable amounts of a palatable food, sweetened milk, although they were not food deprived and thus not likely to be motivated by hunger due to prior food restriction. Rather, the rats likely consumed the sweetened milk because it was “pleasurable” or “rewarding”. From this, a plausible explanation for why LPS inhibits ingestion of sweetened milk is that it influences brain regions, notably the NAc, that are implicated in reward, motivation and food intake (Zhang and Kelley, 1997).
The findings from the current study show an increase in c-Fos protein induction in the rostral shell and core of the NAc in LPS-treated rats compared to controls, and a decrease in the activation of orexin neurons in the LH at the lateral side of the fornix. Activation of the rostral regions of the NAc has been associated with negative emotional or hedonic valence (Reynolds and Berridge, 2002, 2003), and via projections to the LH (a notable target being the orexin neurons), the suppression of feeding behavior (Baldo et al., 2004; Zheng et al., 2003). In this way, the rostral NAc can as a “brake”, or “circuit-breaker” on feeding behavior (for more description of this mechanism, see Baldo and Kelly, 2007). Thus, because LPS treatment was associated with a robust induction of c-Fos protein in the rostral NAc, concomitant with a decreased c-Fos induction in the LH orexin neurons, one mechanism by which immune challenge inhibits ingestion of palatable food may well occur via this rostral NAc “brake”.
Based on inputs to the NAc from brain regions that integrate cognitive and homeostatic signals, including the PVT and the amygdala, Kelley (2004) has described the NAc as a “sentinel” that responds to ongoing situations or challenges by shutting down feeding circuits in the LH. This allows behavioral flexibility such that feeding behavior can be interrupted when other behaviors (e.g. escape) are more appropriate. In this study, the amygdala (CEA and BLA) and PVT responded to both sweetened milk and LPS treatment, suggesting that these regions may contribute to an interface between the homeostatic signals derived from LPS challenge and the hedonic drive triggered by the presence of palatable sweetened milk. Thus, processing in these regions could provide the neural mechanisms by which an adaptive inhibition of eating occurs in sickness (i.e. to prevent further ingestion of illness-inducing agents) and contribute to a reorganization of behavioral priorities (Aubert et al., 1997) during sickness.
Although the “top-down” neural network implicated in feeding inhibition by this and other feeding studies (Zheng et al. 2003; reviewed in Kelley, 2004, Baldo & Kelley 2007) may well contribute to the reduction in sweetened milk intake following LPS treatment, it is unlikely that the results from this study reflect solely a reduction in hedonic value of sweetened milk. In the caudal NAc, a region implicated in the processing of stimuli with positive hedonic value (Pecina and Berridge, 2000; Ranaldi and Beninger, 1994; Reynolds and Berridge, 2003), access to sweetened milk was associated with a significant increase in c-Fos induction in both saline- and LPS-treated rats. Similarly, there was no difference in latency to drink the milk between sick and control rats (Expt. 2), indeed sick rats continued to consume milk, in between bouts of immobility. These findings are consistent with those of Aubert and Dantzer (2005) and Cross-Mellor et al. (1999) showing that LPS-treated rats showed no signs of aversive reaction to sucrose in a taste-reactivity test, and of Anisman et al. (1998) in which proinflammatory cytokines (IL-1 and IL-6) did not seem to induce evidence of anhedonia in a brain self-stimulation paradigm. Therefore, in the current study sweetened milk intake may have been reduced for reasons other than (or in addition to) anhedonia, such as psychomotor retardation or fatigue.
Could “fatigue” account for LPS-induced reduction in the sweetened milk intake?
LPS challenge is reliably associated with an inhibition of motor activity (e.g., psychomotor retardation; Engeland et al., 2001; Franklin et al., 2007), which may indicate psychomotor slowing or “fatigue”, and may also contribute to inhibition of sweetened milk intake. According to Cross-Mellor et al. (2003), although LPS treatment reduced consumption of sucrose when the animals had to approach the tube to obtain the solution, it did not reduce intra-orally administered sucrose intake (i.e. that did not require locomotion to acquire the solution) suggesting that inhibition of motor behavior is an important contributor towards the reduction in the ingestion of palatable substances following LPS challenge.
Consonant with the idea that sickness behavior may derive in part from inhibition of motor behavior, we recently reported that immune challenge with LPS is associated with a dramatic inhibition of c-Fos induction in histamine neurons of the posterior hypothalamus normally active during sweetened milk ingestion (Gaykema et al. 2007) and other behavioral situations. Activation of these histamine neurons has been shown (based on pharmacologic and knockout experimental approaches) to be critical for the expression of motor behavior, wakefulness and brain arousal necessary for responses to behavioral challenges (Ko et al. 2003; Lin 2000; Meynard et al. 2005; Takahashi et al. 2006; Valdes et al. 2005). Histamine system knockout animals appear somnolent and display reduced behavior (Inoue et al. 1996, Parmentier et al. 2002), seemingly similar to animals treated with LPS. The LPS-induced inhibition of histamine neurons may therefore contribute to the reduced intake of sweetened milk found in this and other studies.
Finally, any influence of fatigue, or lack of energy, on sweetened milk intake may also be reflected somehow in the increased c-Fos expression in the NAc. Capuron et al. (2007) have recently reported that in human patients with a history of malignant melanoma, four weeks of treatment with interferon-α induced evidence of fatigue and lack of energy, the latter of which was correlated with “hypermetabolism” in the putamen and NAc. The mechanism of this effect is not established, but the similarity with our findings using acute LPS supports the idea that behavioral impairments associated with sickness or immune challenge follow from interference with neural networks mediating motor behavior and motivation.
Conclusions and perspectives
Sweetened milk is a stimulus that activates brain circuits that process both viscerosensory information and hedonics. Although rats avidly consume sweetened milk even when they are not hungry, they consume much less when sick. Because sickness is the manifestation of potentially dangerous physiological states that can occur following consumption of pathogen-contaminated food, inhibition of food intake (of even a preferred food, in which motivational drive may be enhanced) is likely to be adaptive as it prevents further ingestion of pathogens and allows for conservation of energy. Although the full picture of the mechanisms by which sickness depresses consumption of palatable food is incomplete, the findings from this study indicate that one mechanism may operate via interaction with neural networks integrating emotional valence with ingestive behavior. The NAc, in particular, functions as a nodal point for the integration of information relevant for feeding behavior (Kelley, 2004; Kelley et al., 2005), and thus the identification of its potential role in the suppression of feeding behavior due to illness is perhaps unsurprising. Nonetheless, the findings from this study point to a network of brain regions (LH, PVT, NAc, and BLA) that could form the part of the substrate for the reorganization of behavioral priorities that occurs during sickness. The network could be involved, as well, in other manifestations of sickness that may involve anhedonia-like phenomena, including the reduction in social and play behavior, as well as other neurovegetative symptoms (e.g. Capuron et al. 2007, Stone et al. 2006). The precise role that these candidate brain regions play in sickness behavior awaits mechanistic studies.
Acknowledgements
The authors thank Mary Tyler, Meghan Jones, and Gregory Thacker for their expert technical assistance. This work was supported by the NIH grant MH068834.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreasson A, Arborelius L, Erlanson-Albertsson C, Lekander M. A putative role for cytokines in the impaired appetite in depression. Brain Behav Immun. 2007;21:147–152. doi: 10.1016/j.bbi.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Anisman H, Kokkinidis L, Borowski T, Merali Z. Differential effects of interleukin (IL)-1b, IL-2 and IL-6 on responding for rewarding lateral hypothalamic stimulation. Brain Res. 1998;779:177–187. doi: 10.1016/s0006-8993(97)01114-1. [DOI] [PubMed] [Google Scholar]
- Aubert A, Dantzer R. The taste of sickness: Lipopolysaccharide-induced finickiness in rats. Physio.I Behav. 2005;84:437–444. doi: 10.1016/j.physbeh.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Aubert A, Kelley KW, Dantzer R. Differential effect of lipopolysaccharide on food hoarding behavior and food consumption in rats. Brain Behav. Immun. 1997;11:229–238. doi: 10.1006/brbi.1997.0503. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Alsene KM, Negron A, Kelley AE. Hyperphagia induced by GABAA receptor-mediated inhibition of the nucleus accumbens shell: Dependence on intact neural output from the central amygdaloid region. Behav. Neurosci. 2005;119:1195–1206. doi: 10.1037/0735-7044.119.5.1195. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Gual-Bonilla L, Sijapati K, Daniel RA, Landry CF, Kelley A. Activation of a subpopulation of orexin/hypocretin-containing hypothalamic neurons by GABAA receptor-mediated inhibition of the nucleus accumbens shell, but not by exposure to a novel environment. Eur. J. Neurosci. 2004;19:376–386. doi: 10.1111/j.1460-9568.2004.03093.x. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Kelley AE. Discrete neurochemical coding of distinguishable motivational processes: insight from nucleus accumbens control of feeding. Neuropsychopharmacol. 2007;191:439–459. doi: 10.1007/s00213-007-0741-z. [DOI] [PubMed] [Google Scholar]
- Becskei C, Riediger T, Hernadfavley N, Arsenijvic D, Lutz TA, Langhans W. Inhibitory effects of lipopolysaccharide on hypothalamic nuclei implicated in the control of food intake. Brain Behav. Immun. 2008;22:56–64. doi: 10.1016/j.bbi.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Berthoud H-R. Mind versus metabolism in the control of food intake and energy balance. Physiol Behav. 2004;81:781–793. doi: 10.1016/j.physbeh.2004.04.034. [DOI] [PubMed] [Google Scholar]
- Borowski T, Kokkinidis L, Merali Z, Anisman H. Lipopolysaccharide, central in vivo biogenic amine variations, and anhedonia. Neuroreport. 1998;9:3797–3802. doi: 10.1097/00001756-199812010-00006. [DOI] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Demetrashvili MF, Lawson DH, Fornwalt FB, Woolwine B, Berns GS, Nemeroff CB, Miller AH. Basal Ganglia Hypermetabolism and Symptoms of Fatigue during Interferon-a Therapy. Neuropsychopharmacol. 2007;32:2384–2392. doi: 10.1038/sj.npp.1301362. [DOI] [PubMed] [Google Scholar]
- Cross-Mellor SK, Kent WDT, Ossenkopp KP, Kavaliers M. Differential effects of lipopolysaccharide and cholecystokinin on sucrose intake and palatability. Am. J. Physiol. 1999;277:705–715. doi: 10.1152/ajpregu.1999.277.3.R705. [DOI] [PubMed] [Google Scholar]
- Cross-Mellor SK, Roberts S, Kavaliers M, Ossenkopp KP. Activation of the immune system in rats with lipopolysaccharide reduces voluntary sucrose intake but not intraoral intake. Pharmacol. Biochem. Behav. 2003;76:153–159. doi: 10.1016/s0091-3057(03)00210-7. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behavior: Where do we stand? Brain Behav. Immun. 2001;15:7–24. doi: 10.1006/brbi.2000.0613. [DOI] [PubMed] [Google Scholar]
- De La Garza R., 2nd Endotoxin- or pro-inflammatory cytokine-induced sickness behavior as an animal model of depression: Focus on anhedonia. Neurosci. Biobehav. Rev. 2005;29:761–770. doi: 10.1016/j.neubiorev.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Dube MG, Kalra SP, Kalra PS. Food intake elicited by central administration of orexins/hypocretins: Identification of hypothalamic sites of action. Brain Res. 1999;842:473–477. doi: 10.1016/s0006-8993(99)01824-7. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH. The reductions in sweetened milk intake induced by interleukin-1 and endotoxin are not prevented by chronic antidepressant treatment. Neuroimmunomodul. 2001;9:163–169. doi: 10.1159/000049021. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Coppari R, Balthasar N, Ichinosi M, Lowell BB. Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J. Comp. Neurol. 2005;493:63–71. doi: 10.1002/cne.20786. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Scammel ITE, Jacobson CD, Saper CB. Distribution of fos-like immunoreactivity in the rat brain following intravenous lipopolysaccharide administration. J. Comp. Neurol. 1996;371:85–103. doi: 10.1002/(SICI)1096-9861(19960715)371:1<85::AID-CNE5>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Engeland CG, Nielsen DV, Kavaliers M, Ossenkopp KP. Locomotor activity changes following lipopolysaccharide treatment in mice: a multivariate assessment of behavioral tolerance. Physiol. Behav. 2001;72:481–491. doi: 10.1016/s0031-9384(00)00436-4. [DOI] [PubMed] [Google Scholar]
- Fan W, Ellacott KL, Halatchev IG, Takahashi K, Yu P, Cone RD. Cholecystokinin-mediated suppression of feeding involves the brainstem melanocortin system. Nat. Neurosci. 2004;7:335–336. doi: 10.1038/nn1214. [DOI] [PubMed] [Google Scholar]
- Franklin AE, Engeland CG, Kavaliers M, Ossenkopp KP. The rate of behavioral tolerance development to repeated lipopolysaccharide treatments depends upon the time of injection during the light–dark cycle: a multivariable examination of locomotor activity. Behav. Brain Res. 2007;180:161–173. doi: 10.1016/j.bbr.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Gautron L, Mingam R, Moranis A, Combe C, Laye S. Influence of feeding status on neuronal activity in the hypothalamus during lipopolysaccharide-induced anorexia in rats. Neurosci. 2005;134:933–946. doi: 10.1016/j.neuroscience.2005.03.063. [DOI] [PubMed] [Google Scholar]
- Gaykema RPA, Goehler LE, Lyte M. Brain response to cecal infection with Camplyobacter jejuni: analysis with Fos immunohistochemistry. Brain, Behav Immun. 2004;18:238–245. doi: 10.1016/j.bbi.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Gaykema RPA, Park S-M, McKibbin CR, Goehler LE. Lipopolysaccharide suppresses activation of the tuberomammillary histaminergic system concomitant with behavior: a novel target of immune-sensory pathways. Neurosci. 2007 doi: 10.1016/j.neuroscience.2007.10.042. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goehler LE, Gaykema RP, Hansen MK, Anderson K, Maier SF, Watkins LR. Vagal immune-to-brain communication: A visceral chemosensory pathway. Auton. Neurosci. 2000;85:49–59. doi: 10.1016/S1566-0702(00)00219-8. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Carmody JS, Sadacca LA, Williams DL, Kaplan JM. Attenuation of lipopolysaccharide anorexia by antagonism of caudal brainstem but not forebrain GLP-1-R. Am J. Physiol. Regul. Integr. Comp. Physiol. 2004;287:R1190–R1193. doi: 10.1152/ajpregu.00163.2004. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Arousal and reward: A dichotomy in orexin function. Trends Neurosci. 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Hauser CA, Stockler MR, Tattersall MHN. Prognostic factors in patients with recently diagnosed incurable cancer: a systematic review. Support. Care Cancer. 2006;14:999–1011. doi: 10.1007/s00520-006-0079-9. [DOI] [PubMed] [Google Scholar]
- Inoue I, Yanai K, Kitamura D, Taiuchi I, Kobavachi T, Niimura KK, Watanabe T, Watanabe T. Impaired locomotor activity and exploratory behavior in mice lacking H1 receptors. Proc. Natl. Acad. Sci. USA. 1996;93:13316–13320. doi: 10.1073/pnas.93.23.13316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobst EE, Enriori P, Cowley MA. The electrophysiology of feeding circuits. TRENDS Endocrinol. Metab. 2004;15:488–499. doi: 10.1016/j.tem.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Ventral striatal control of appetitive motivation: Role in ingestive behavior and reward-related learning. Neurosci Biobehav. Rev. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Swanson CJ. Feeding induced by blockade of AMPA and kainate receptors within the ventral striatum: A microinfusion mapping study. Behav. Brain. Res. 1997;89:107–113. doi: 10.1016/s0166-4328(97)00054-5. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE. A proposed hypothalamic-thalamic-striatal axis for the integration of energy balance, arousal, and food reward. J. Comp. Neurol. 2005a;493:72–85. doi: 10.1002/cne.20769. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol. Behav. 2005b;86:773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- Kirouac GJ, Ganguly PK. Topographical organization in the nucleus accumbens of afferents from the basolateral amygdala and efferents to the lateral hypothalamus. Neurosci. 1995;67:625–630. doi: 10.1016/0306-4522(95)00013-9. [DOI] [PubMed] [Google Scholar]
- Ko EM, Estabrooke IV, McCarthy M, Scammell TE. Wake-related activity of tuberomammillary neurons in rats. Brain Res. 2003;992:220–226. doi: 10.1016/j.brainres.2003.08.044. [DOI] [PubMed] [Google Scholar]
- Langhans W. Signals generating anorexia during acute illness. Proc. Nutrition Soc. 2007;66:321–330. doi: 10.1017/S0029665107005587. [DOI] [PubMed] [Google Scholar]
- Larson SJ. Lipopolysaccharide and interleukin-1beta decrease sucrose intake but do not affect expression of place preference in rats. Pharmacol. Biochem. Behav. 2006;84:429–435. doi: 10.1016/j.pbb.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Lin JS. Brain structures and mechanisms involved in the control of cortical activation and wakefulness, with emphasis on the posterior hypothalamus and histaminergic neurons. Sleep Med. Rev. 2000;4:471–503. doi: 10.1053/smrv.2000.0116. [DOI] [PubMed] [Google Scholar]
- Maldonado-Irizarry CS, Swanson CJ, Kelley AE. Glutamate receptors in the nucleus accumbens shell control feeding behavior via the lateral hypothalamus. J. Neurosci. 1995;15:6779–6788. doi: 10.1523/JNEUROSCI.15-10-06779.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meynard MM, Valdes JL, Recabarren M, Seron-Ferre M, Torrealba F. Specific activation of histaminergic neurons during daily feeding anticipatory behavior in rats. Behav. Brain Res. 2005;158:311–319. doi: 10.1016/j.bbr.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Parmentier R, Ohtsu H, Djebbara-Hannas Z, Valatx J-L, Watanabe T, Lin J-S. Anatomical, physiological, and pharmacological characteristics of histidine decarboxylase knock-out mice: evidence for the role of brain histamine in behavioral and sleep-wake control. J. Neurosci. 2002;22:7695–7711. doi: 10.1523/JNEUROSCI.22-17-07695.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons MP, Li S, Kirouac GJ. The paraventricular nucleus of the thalamus as an interface between the orexin and CART peptides and the shell of the nucleus accumbens. Synapse. 2006;59:480–490. doi: 10.1002/syn.20264. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Fourth Ed. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Pecina S, Berridge KC. Opioid site in nucleus accumbens shell mediates eating and hedonic 'liking' for food: Map based on microinjection fos plumes. Brain Res. 2000;863:71–86. doi: 10.1016/s0006-8993(00)02102-8. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Gallagher M. Amygdala subsystems and control of feeding behavior by learned cues. Ann. N.Y. Acad. Sci. 2003;985:251–262. doi: 10.1111/j.1749-6632.2003.tb07086.x. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J. Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plata-Salaman CR. Central nervous system mechanisms contributing the cachexia-anorexia syndrome. Nutrition. 2000;16:1009–1012. doi: 10.1016/s0899-9007(00)00413-5. [DOI] [PubMed] [Google Scholar]
- Ramos EJ, Suzuki S, Marks D, Inui A, Asakawa A, Meguid MM. Cancer anorexia-cachexia syndrome: Cytokines and neuropeptides. Curr. Opinion Clin. Nutrition Metabolic Care. 2004;7:427–434. doi: 10.1097/01.mco.0000134363.53782.cb. [DOI] [PubMed] [Google Scholar]
- Ranaldi R, Beninger RJ. Rostro-caudal differences in effects of nucleus accumbens amphetamine and VTA ICSS. Brain Res. 1994;642:251–258. doi: 10.1016/0006-8993(94)90929-6. [DOI] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Positive and negative motivation in nucleus accumbens shell: Bivalent rostrocaudal gradients for GABA-elicited eating, taste "liking"/"disliking" reactions, place preference/avoidance, and fear. J. Neurosci. 2002;22:7308–7320. doi: 10.1523/JNEUROSCI.22-16-07308.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Glutamate motivational ensembles in nucleus accumbens: Rostrocaudal shell gradients of fear and feeding. Eur. J. Neurosci. 2003;17:2187–2200. doi: 10.1046/j.1460-9568.2003.02642.x. [DOI] [PubMed] [Google Scholar]
- Rinaman L, Baker EA, Hoffman GE, Stricker EM, Verbalis JG. Medullary c-Fos activation in rats after ingestion of a satiating meal. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1998;275:R262–R268. doi: 10.1152/ajpregu.1998.275.1.R262. [DOI] [PubMed] [Google Scholar]
- Sergeyev V, Broberger C, Hokfelt T. Effect of LPS administration on the expression of POMC, NPY, galanin, CART and MCH mRNAs in the rat hypothalamus. Mol. Brain Res. 2001;90:93–100. doi: 10.1016/s0169-328x(01)00088-2. [DOI] [PubMed] [Google Scholar]
- Stone EA, Lehmann ML, Li Y, Quartermain D. Depressive behavior in mice due to immune stimulation is accompanied by reduced neural activity in brain regions in positively motivated behavior. Biol. Psychiatry. 2006;60:803–811. doi: 10.1016/j.biopsych.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Strassburg S, Anker SD. Metabolic and immunologic derangements in cardiac cachexia: where to from here? Heart Fail. Rev. 2006;11:57–64. doi: 10.1007/s10741-006-9193-5. [DOI] [PubMed] [Google Scholar]
- Stratford TR, Kelley AE. GABA in the nucleus accumbens shell participates in the central regulation of feeding behavior. J. Neurosci. 1997;17:4434–4440. doi: 10.1523/JNEUROSCI.17-11-04434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford TR, Kelley AE. Evidence of a functional relationship between the nucleus accumbens shell and lateral hypothalamus subserving the control of feeding behavior. J. Neurosci. 1999;19:11040–11048. doi: 10.1523/JNEUROSCI.19-24-11040.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Lin J-S, Sakai K. Neuronal activity of histaminergic tuberomammillary neurons during wake-sleep states in the mouse. J. Neurosci. 2006;26:10292–10298. doi: 10.1523/JNEUROSCI.2341-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe AJ, Cleary JP, Levine AS, Kotz CM. Centrally administered orexin A increases motivation for sweet pellets in rats. Psychopharmacol. 2005;182:75–83. doi: 10.1007/s00213-005-0040-5. [DOI] [PubMed] [Google Scholar]
- Tritos NA, Maratos-Flier E. Two important systems in energy homeostasis: melanocortins and melanin-concentrating hormone. Neuropept. 1999;33:339–349. doi: 10.1054/npep.1999.0055. [DOI] [PubMed] [Google Scholar]
- Valdes JL, Farias P, Ocampo-Garces A, Cortes N, Seron-Ferre M, Torrealba F. Arousal and differential Fos expression in histaminergic neurons of the ascending arousal system during a feeding-related motivated behaviour. Eur. J. Neurosci. 2005;21:1931–1942. doi: 10.1111/j.1460-9568.2005.04013.x. [DOI] [PubMed] [Google Scholar]
- Wan W, Wetmore L, Sorensen CM, Greenberg AM, Nance DM. Neural and biochemical mediators of endotoxin and stress-induced c-fos expression in the rat brain. Brain Res. Bull. 1994;34:7–14. doi: 10.1016/0361-9230(94)90179-1. [DOI] [PubMed] [Google Scholar]
- Will MJ, Franzblau EB, Kelley AE. The amygdala is critical for opioid-mediated binge eating of fat. Neuroreport. 2004;15:1857–1860. doi: 10.1097/00001756-200408260-00004. [DOI] [PubMed] [Google Scholar]
- Yirmiya R. Endotoxin produces a depressive-like episode in rats. Brain Res. 1996;711:163–174. doi: 10.1016/0006-8993(95)01415-2. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Pollak Y, Morag M, Reichenberg A, Barak O, Avitsur R, Shavit Y, Ovadia H, Weidenfeld J, Morag A, Newman ME, Pollmacher T. Illness, cytokines, and depression. Ann. N. Y. Acad. Sci. 2000;917:478–487. doi: 10.1111/j.1749-6632.2000.tb05412.x. [DOI] [PubMed] [Google Scholar]
- Zhang M, Kelley AE. Opiate agonists microinjected into the nucleus accumbens enhance sucrose drinking in rats. Psychopharmacology (Berl) 1997;132:350–360. doi: 10.1007/s002130050355. [DOI] [PubMed] [Google Scholar]
- Zheng H, Corkern M, Stoyanova I, Patterson LM, Tian R, Berthoud H-R. Peptides that regulate food intake: Appetite-inducing accumbens manipulation activates hypothalamic orexin neurons and inhibits POMC neurons. Am. J. Physiol. Reg. Integr. Comp. Physiol. 2003;284:R1436–R1444. doi: 10.1152/ajpregu.00781.2002. [DOI] [PubMed] [Google Scholar]