Abstract
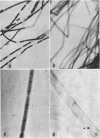
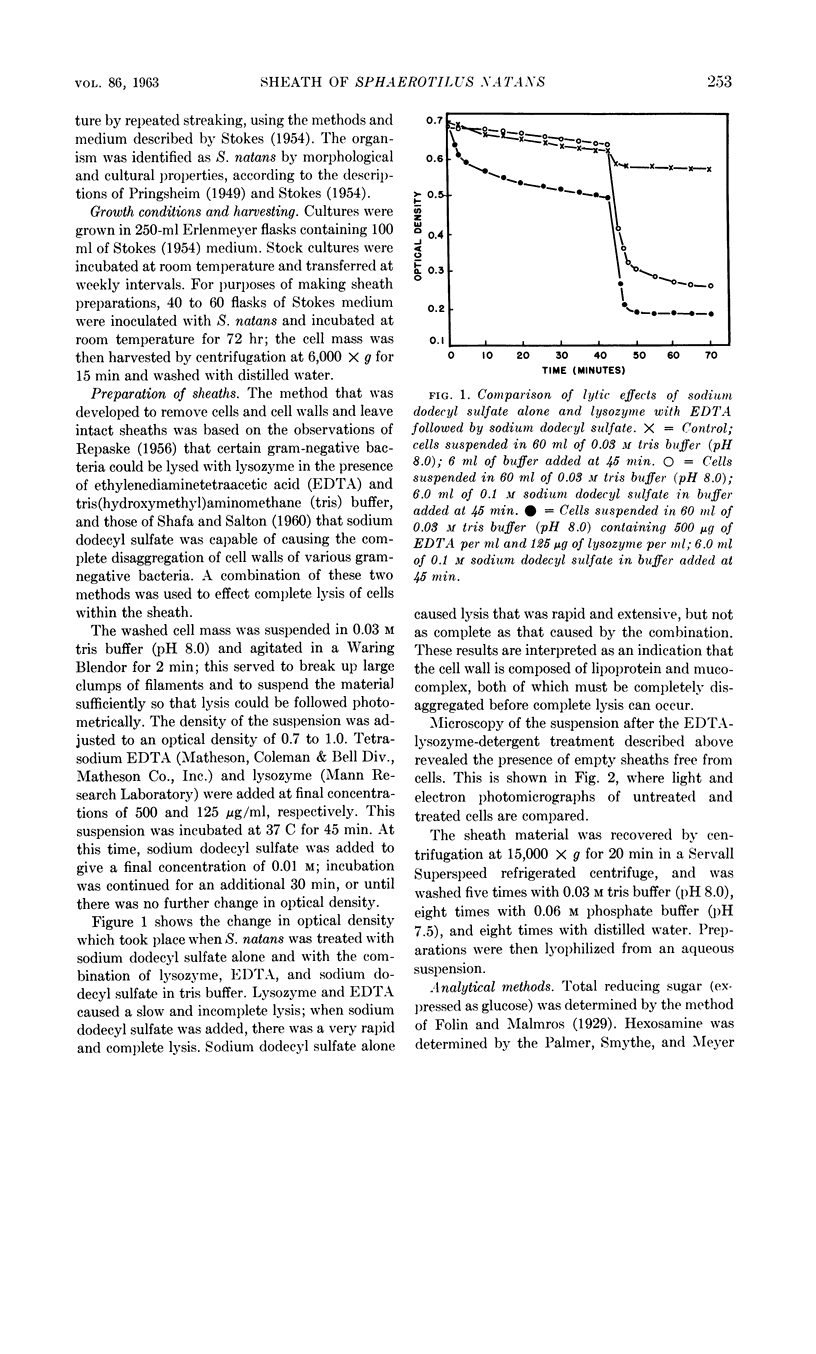
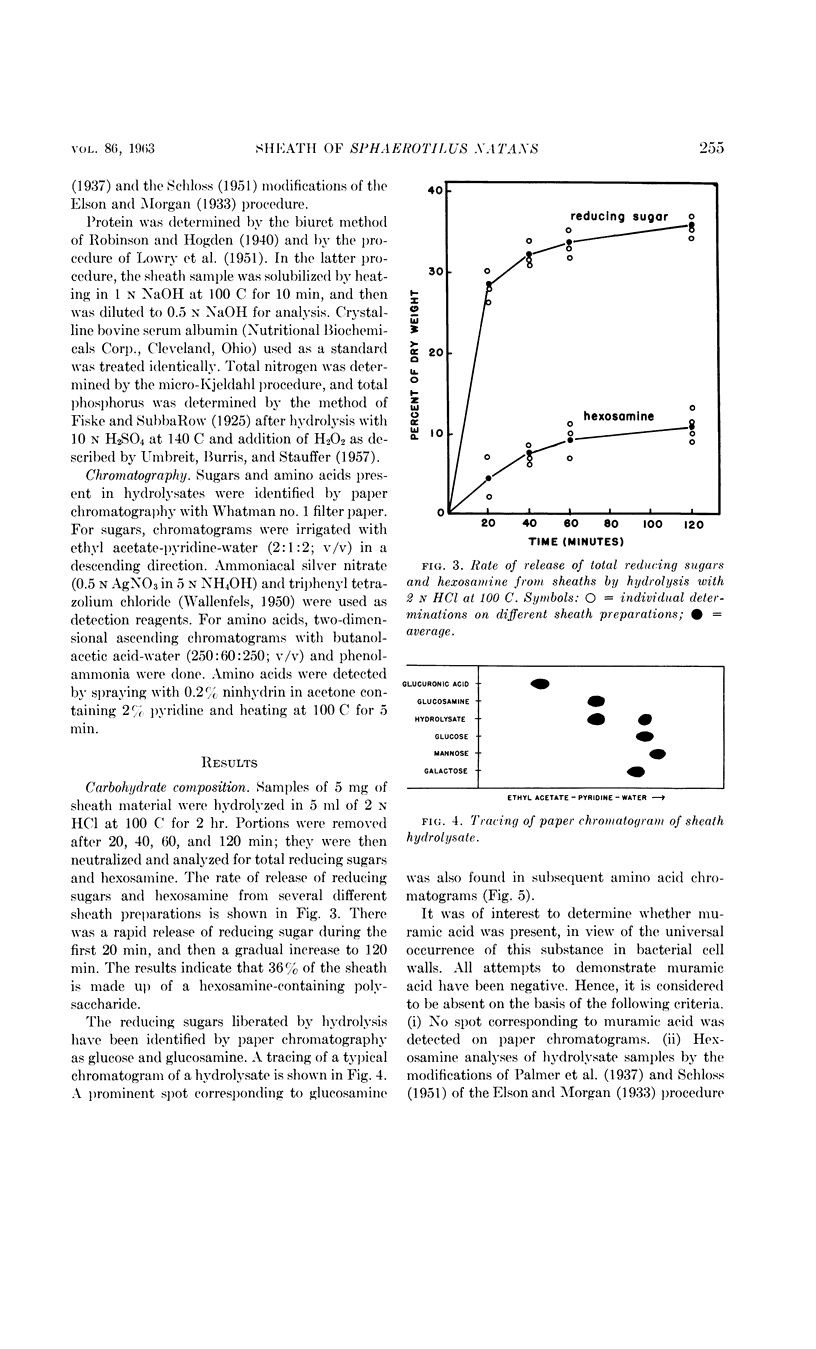
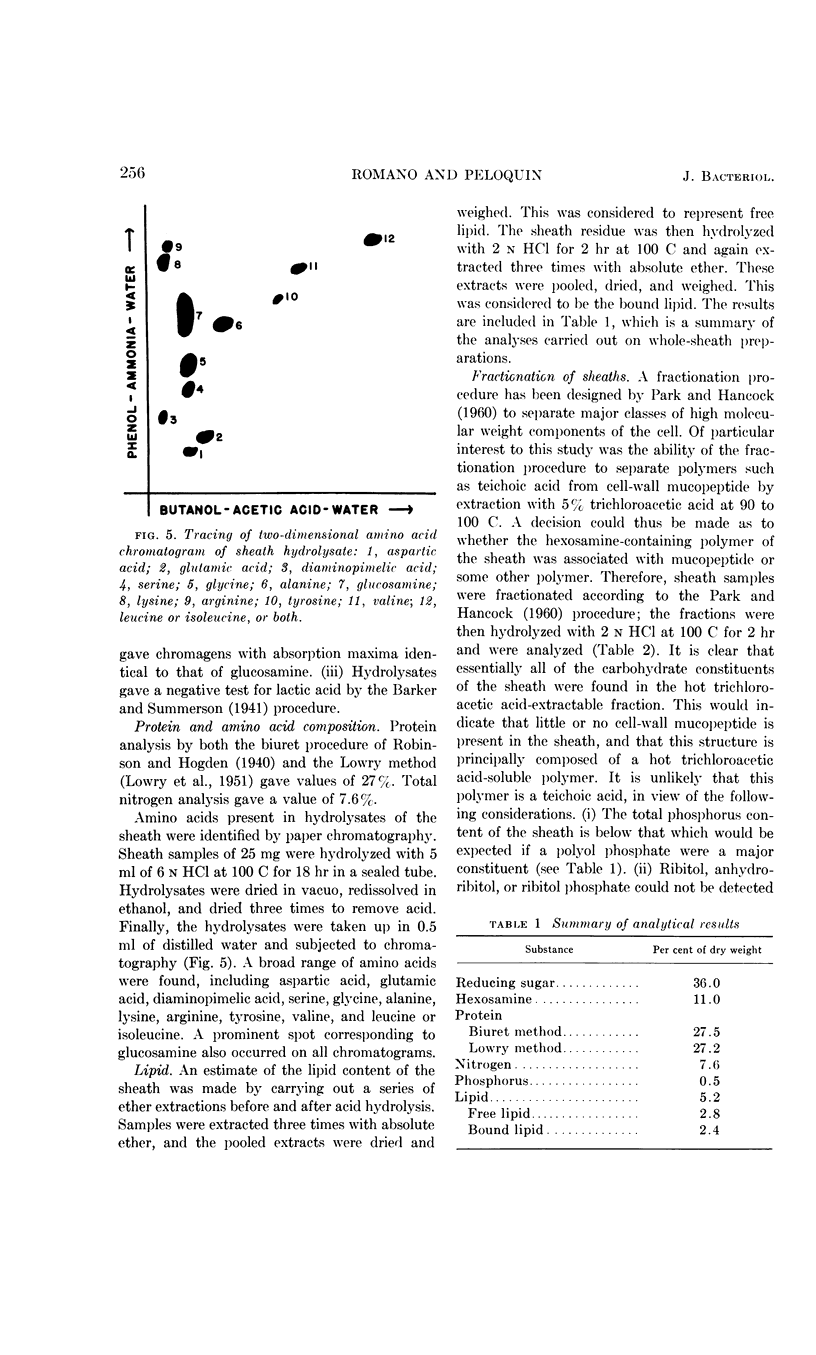
Romano, Antonio H. (University of Cincinnati, Cincinnati, Ohio) and Joyce P. Peloquin. Composition of the sheath of Sphaerotilus natans. J. Bacteriol. 86:252–258. 1963.—The sheath of Sphaerotilus natans was isolated and subjected to chemical analysis. Isolation of the sheaths was accomplished by incubating cells in the presence of lysozyme and ethylenediaminetetraacetic acid in tris(hydroxymethyl)aminomethane buffer and adding sodium dodecyl sulfate subsequently. Under these conditions, there was complete dissolution of cells. The sheaths, which were left intact by this treatment, were recovered by centrifugation, washed exhaustively, lyophilized, and subjected to analysis. Hydrolysis of the sheath material with 2 n HCl at 100 C resulted in the liberation of reducing sugars amounting to 36% of the dry weight. Amino sugar accounted for 11% of the dry weight. Paper chromatography of hydrolysates showed the presence of glucose and hexosamine. Tests for muramic acid were negative. In addition to carbohydrate, 27% protein and 5.2% lipid were found to be present. Fractionation studies indicated that essentially all of the polysaccharide was associated with a trichloroacetic acid-soluble fraction. The sheath is therefore considered to be a protein-polysaccharide-lipid complex, which is chemically and anatomically distinct from the cell wall and the slime layer. It is hypothesized that this unique structure may be related to the microcapsule found in many gram-negative bacteria, and may represent a structural specialization of this more common structure.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Elson L. A., Morgan W. T. A colorimetric method for the determination of glucosamine and chondrosamine. Biochem J. 1933;27(6):1824–1828. doi: 10.1042/bj0271824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAUDY E., WOLFE R. S. Composition of an extracellular poly-saccharide produced by Sphaerotilus natans. Appl Microbiol. 1962 May;10:200–205. doi: 10.1128/am.10.3.200-205.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- PARK J. T., HANCOCK R. A fractionation procedure for studies of the synthesis of cell-wall mucopeptide and of other polymers in cells of Staphylococcus aureus. J Gen Microbiol. 1960 Feb;22:249–258. doi: 10.1099/00221287-22-1-249. [DOI] [PubMed] [Google Scholar]
- REPASKE R. Lysis of gram-negative bacteria by lysozyme. Biochim Biophys Acta. 1956 Oct;22(1):189–191. doi: 10.1016/0006-3002(56)90240-2. [DOI] [PubMed] [Google Scholar]
- SHAFA F., SALTON M. R. Disaggregation of bacterial cell walls by anionic detergents. J Gen Microbiol. 1960 Aug;23:137–141. doi: 10.1099/00221287-23-1-137. [DOI] [PubMed] [Google Scholar]
- STOKES J. L. Studies on the filamentous sheathed iron bacterium Sphaerotilus natans. J Bacteriol. 1954 Mar;67(3):278–291. doi: 10.1128/jb.67.3.278-291.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILKINSON J. F. The extracellualr polysaccharides of bacteria. Bacteriol Rev. 1958 Mar;22(1):46–73. doi: 10.1128/br.22.1.46-73.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WUHRMANN K., MECHSNER K. [On the structure of the sheath of the bacterium Sphaerotilus natans Kuetz]. Z Wiss Mikrosk. 1960 Nov;64:474–481. [PubMed] [Google Scholar]