Abstract
PTEN is a tumor suppressor gene that is either mutated or deleted in a number of human cancers. PTEN acts as a negative regulator of the PI3K/Akt survival pathway and thus plays an important role in cell fate, proliferation, growth, and migration. Recent evidence suggests that PTEN may also be involved in the pathophysiology of neurodegenerative disorders such as spinal cord injury (SCI). Overexpression of PTEN appears to cause inactivation/dephosphorylation of Akt in neurons, resulting in increased cell death. Given this newly discovered role for PTEN, it has been identified as a potential molecular target for the development of novel therapeutic strategies against neurodegeneration. Motoneuron degeneration following SCI may occur due to upregulation of pro-inflammatory and cytotoxic cytokines including IFN-γ. Exposure of VSC4.1 motoneurons to IFN-γ (10 ng/ml) for 24 h resulted in significant overexpression of PTEN and decreased levels of activated Akt. Upregulation of PTEN following IFN-γ exposure was associated with decreased overall cell viability due to increased apoptosis, as assessed by Wright staining and analysis of cell death markers including Bax, Bcl-2, calpain activity, and caspase-3 activity, indicating a prominent role for PTEN in suppression of the PI3K/Akt survival pathway to promote motoneuron death. Addition of estrogen (100 nM) to VSC4.1 cells for 1 h prior to IFN-γ exposure partially decreased PTEN expression, allowing adequate activation or phosphorylation of Akt (p-Akt) to prevent apoptotic cell death. Thus, it appears that estrogen may mediate neuroprotection through decrease in PTEN expression. In conclusion, our studies suggest that PTEN inactivation may be used as an important parameter for evaluation of the efficacy of estrogen in prevention of neuronal loss in neurodegenerative disorders.
Keywords: Akt, caspase, estrogen, IFN-γ, motoneurons, PTEN
1. Introduction
PTEN (phosphatase and tensin homologue deleted from chromosome 10) was first identified as a tumor suppressor gene. Mutations and deletions of PTEN have been implicated in a number of malignancies including glioblastomas, endometrial carcinomas, melanomas, and prostate tumors (Li et al., 1997; Steck et al., 1997). In addition, germ-line mutations in PTEN may result in Cowden syndrome, in which patients have a high-risk of developing brain, endometrial, and thyroid cancers (Marsh et al., 1998). Studies suggest that PTEN plays an essential role in cell proliferation, cell differentiation, cell growth, and cell migration through negative regulation of the phosphatidylinositol-3′-kinase (PI3K)/Akt signaling pathway. PTEN acts as a lipid phosphatase to cause dephosphorylation of phosphatidylinositol (3,4,5)-triphosphate into phosphatidylinositol (4,5)-biphosphate, thereby opposing the activity of PI3K (Di Cristofano and Pandolfi, 2000; Myers et al., 1998). Increased PIP3 levels following growth factor stimulation of PI3K play a critical role in the normal activation and activity of Akt (Bellacosa et al., 2005). Numerous studies indicate that overexpression of PTEN results in decreased Akt activity leading to increase in apoptotic cell death (Cantley and Neel, 1999; Datta et al., 1999; Di Cristofano and Pandolfi, 2000). Akt is known to phosphorylate a number of downstream effectors including Bad, nuclear factor-κB (NF-κB), caspase-9, glycogen synthase kinase 3 (GSK-3), forkhead transcription factor (FKHR), GLUT-4, and p70S6K (Chang et al., 2007). Thus, PTEN-mediated inactivation of Akt may modulate a number of important cellular processes.
PTEN is expressed widely in the mouse brain and in both neurons and glial cells (Sano et al., 1999; Lachyankar et al., 2000; Li et al., 2002; Li et al., 2003). Given the expression of PTEN throughout the central nervous system (CNS), recent studies have focused attention on the role of PTEN in CNS development and pathology. Research shows that PTEN is involved in neuronal migration, neuronal cell growth, and in the pathophysiology characteristic of a variety of neurological and mental disorders including transient cerebral ischemia and drug addiction (Backman et al., 2002; Ji et al., 2006; Leslie et al., 2005; Omori et al., 2002). Overexpression of PTEN in cultured neurons has been shown to decrease levels of activated or phosphorylated Akt (p-Akt) and promote neuronal death following glutamate exposure. However, inactivation of PTEN in vitro has been shown to have a neuroprotective effect against both glutamate excitotoxicity and oxidative stress (Gary and Mattson, 2002; Li et al., 2002). In vivo results suggest that levels of phosphorylated PTEN are altered in damaged brain areas following transient cerebral ischemia (Choi et al., 2004; Omori et al., 2002). Further studies suggest that chronic gestational exposure to ethanol in rats leads to increased PTEN expression and is associated with increased neuronal death and cerebellar hypoplasia (Xu et al., 2003; Yeon et al., 2003). Because PTEN overexpression appears to play a role in neurodegeneration, PTEN is a potential molecular target for the development of a therapeutic strategy against neurodegenerative disorders including amyotrophic lateral sclerosis (ALS), cerebral ischemia, spinal cord injury (SCI), multiple sclerosis (MS), and Parkinson’s disease (PD).
Estrogen (17β-estradiol) has been proposed as a potential therapeutic agent in the treatment of neurodegenerative disorders. Findings suggest that estrogen provides neuroprotection in animal models of ALS, cerebral ischemia, SCI, MS, and PD (Veldink et al., 2003; Sribnick et al., 2006; Choi et al., 2008; Garay et al., 2008; Morissette et al., 2008; Jia et al., 2009). Estrogen-mediated neuroprotection may be attributed to its multiple actions in attenuating inflammation, apoptosis, and oxidative stress (Moosmann and Behl, 1999; Dimayuga et al., 2005). Findings suggest that estrogen prevents apoptosis of injured cortical neurons via increased p-Akt in vitro (Wilson et al., 2002). Recent evidence further indicates that estrogen treatment prevents ischemic damage following middle cerebral artery occlusion in rats via decreased phosphorylation of PTEN and subsequent activation of both Akt and cyclic AMP response element binding protein (CREB) (Choi et al., 2004). However, little is known about the mechanisms by which estrogen regulates interactions between PTEN and Akt signaling.
The current study was designed to further elucidate the role of estrogen in modulation of neurodegenerative processes that are mediated by alterations in both PTEN and the PI3K/Akt signaling pathway. We hypothesized that estrogen treatment of ventral spinal cord 4.1 (VSC4.1) motoneurons following exposure to interferon-gamma (IFN-γ) would result in decreased levels of PTEN, enhanced activation of Akt, and increased cell survival markers. Exposure of VSC4.1 motoneurons, formed by the fusion of embryonic rat ventral spinal cord neuron with mouse N18TG2 neuroblastoma cell, to 10 ng/ml IFN-γ for 24 h resulted in significant overexpression of PTEN and decreased p-Akt (Crawford et al., 1992; Smith et al., 1994). These findings indicate a prominent role for PTEN in suppression of the PI3K/Akt survival pathway to promote neuronal cell death. In our studies, addition of 100 nM estrogen to VSC4.1 motoneurons 1 h prior to IFN-γ exposure partially down regulated PTEN so as to allow activation of Akt, suggesting that inactivation of PTEN could be partially responsible for the neuroprotective effects of estrogen. Estrogen treatment also caused decreases in Bax:Bcl-2 ratio to prevent apoptosis in VSC4.1 motoneurons following exposure to IFN-γ. Therefore, our studies provide evidence that down regulation of PTEN may be used as an important index for the evaluation of the efficacy of estrogen in the prevention of neurodegenerative processes.
2. Results
2.1. Estrogen partially restored viability to VSC4.1 cells exposed to IFN-γ
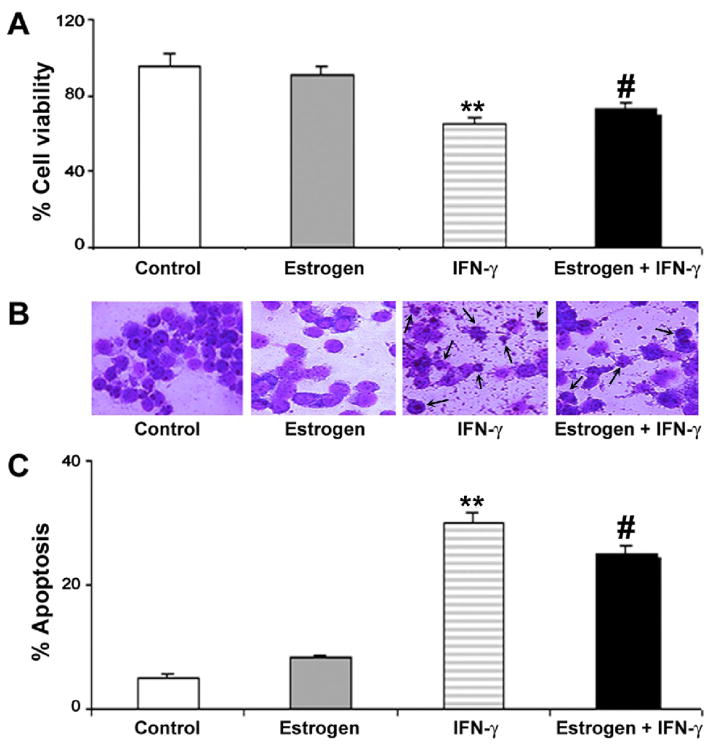
We examined the efficacy of estrogen in preventing cell death in VSC4.1 cells following exposure to IFN-γ (Fig. 1). Motoneuron viability was assessed by the trypan blue dye exclusion test (Fig. 1A). Cells with compromised membranes took up the trypan blue dye and were counted as dead. Exposure of VSC4.1 motoneurons to IFN-γ for 24 h significantly reduced viability (~30%). Treatment of VSC4.1 motoneurons with estrogen prior to exposure to IFN-γ provided partial restoration of cell viability. These results suggest that estrogen provides neuroprotection against inflammatory conditions in an in vitro setting, as we reported in other studies (Sribnick et al., 2009). Treatment of control VSC4.1 cells with estrogen had no appreciable effect on cell viability.
Fig. 1.
Cell viability and apoptosis in VSC4.1 motoneurons following estrogen treatment and IFN-γ exposure. Treatments (24 h): control; 100 nM estrogen; IFN-γ (10 ng/ml); and 100 nM estrogen (1-h pretreatment) + IFN-γ (10 ng/ml). (A) Determination of residual cell viability using trypan blue dye exclusion test. (B) Wright staining to determine morphological features of apoptosis. (C) Determination of percentage of apoptosis based on Wright staining.
2.2. Estrogen partially attenuated morphological features of apoptosis in VSC4.1 cells exposed to IFN-γ
The apoptotic cell death based on morphological features was examined using Wright staining and optical microscopy (Fig. 1B). Apoptotic cells were characterized by specific morphological features including chromatin condensation, cell membrane blebbing, and a reduction in cell volume. VSC4.1 cells exposed to IFN-γ exhibited a significantly greater percentage of apoptosis (~25%) than control cells (Fig. 1C). Estrogen treatment of cells exposed to IFN-γ partially attenuated apoptotic cell death. These findings provide further evidence that estrogen is a neuroprotective agent. Treatment of control VSC4.1 cells with estrogen showed no significant effect of apoptotic cell death.
2.3. Estrogen reversed increases in PTEN expression in VSC4.1 cells following IFN-γ exposure
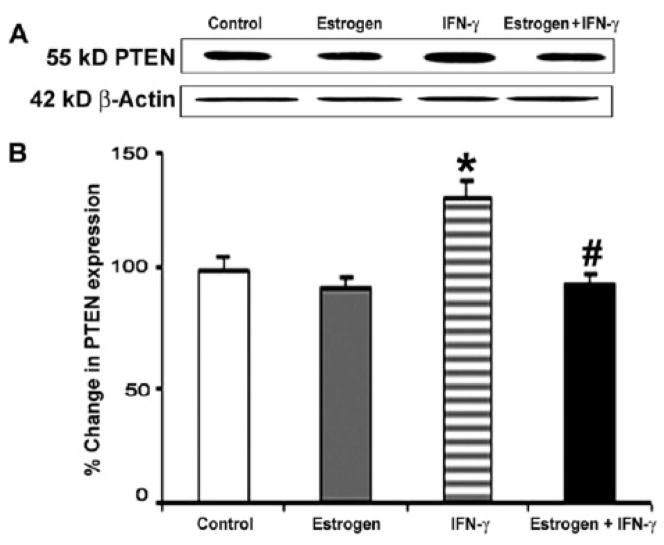
PTEN expression was measured using Western blotting for specific proteins (Fig. 2). Treatment of cells caused alterations in PTEN expression (Fig. 2A). Exposure of VSC4.1 motoneurons to IFN-γ resulted in significantly increased PTEN expression (Fig. 2B). However, treatment of cells with estrogen prior to IFN-γ exposure restored PTEN expression to the levels observed in the control cells. These results indicate that estrogen may partially mediate neuroprotection through down regulation of PTEN expression following insult.
Fig. 2.
Western blot analysis of PTEN expression following treatment of VSC4.1 cells. Treatments (24 h): control; 100 nM estrogen; IFN-γ (10 ng/ml); and 100 nM estrogen (1-h pretreatment) + IFN-γ (10 ng/ml). (A) Representative Western blots showing levels of expression of PTEN and β-actin. (B) Densitometric analysis of level of PTEN in VSC4.1 cells.
2.4. Estrogen restored levels of activated Akt (p-Akt) in IFN-γ exposed VSC4.1 motoneurons
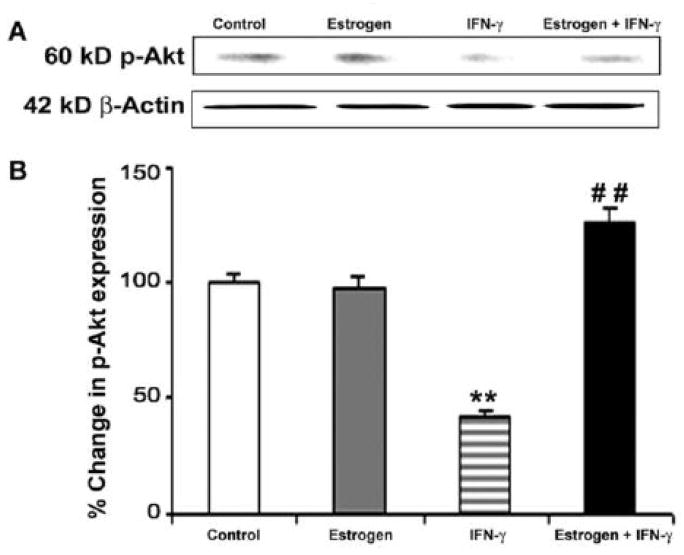
Levels of p-Akt were assessed using Western blotting (Fig. 3). Treatment of VSC4.1 motoneurons with estrogen alone did not affect p-Akt expression (Fig. 3A). But exposure of VSC4.1 motoneurons to IFN-γ resulted in significantly decreased levels of p-Akt (Fig. 3B). Estrogen treatment of cells exposed to IFN-γ restored activation of Akt to levels greater than untreated control cells (Fig. 3B). These findings suggest that attenuation of PTEN expression mediated by estrogen is associated with an increase in phosphorylation/activation of the Akt to provide survival signaling.
Fig. 3.
Western blotting to examine levels of activated/phosphorylated Akt (p-Akt) in VSC4.1 cells. Treatments (24 h): control; 100 nM estrogen; IFN-γ (10 ng/ml); and 100 nM estrogen (1-h pretreatment) + IFN-γ (10 ng/ml). (A) Representative Western blots demonstrating levels of p-Akt and β-actin following treatments. (B) Densitometric analysis of levels of p-Akt after treatments.
2.5. Increases in the Bax:Bcl-2 ratio due to IFN-γ exposure were reversed by estrogen treatment
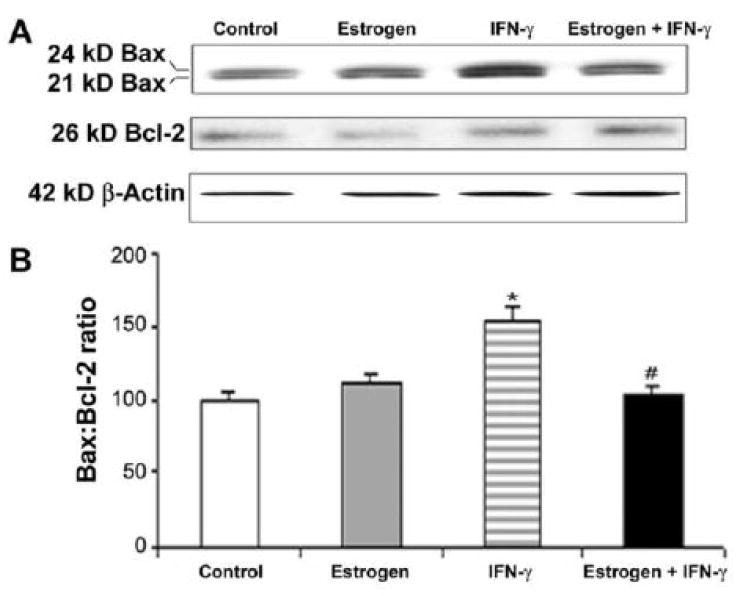
Activation of apoptotic process was also assessed by Western blot analysis of increases in the ratio of Bax (pro-apoptotic) to Bcl-2 (anti-apoptotic) (Fig. 4). We used an antibody that could detect 21 and 24 kD Bax isoforms (Fig. 4A). We took both Bax isoforms into account in our estimation of total Bax (Fig. 4B). Exposure of VSC4.1 motoneurons to IFN-γ caused significant elevation of the Bax:Bcl-2 ratio, indicating a commitment of cells to apoptosis (Fig. 4B). However, estrogen treatment of VSC4.1 motoneurons insulted with IFN-γ caused a significant decrease in the Bax:Bcl-2 ratio (Fig. 4B). These findings further indicate that decrease in PTEN expression due to estrogen treatment results in decrease in Bax:Bcl-2 ratio to increase neuronal survival.
Fig. 4.
Western blot analysis to show alterations in the levels of Bax and Bcl-2 in VSC4.1 motoneurons. Treatments (24 h): control; 100 nM estrogen; IFN-γ (10 ng/ml); and 100 nM estrogen (1-h pretreatment) + IFN-γ (10 ng/ml). (A) Representative Western blots showing levels of Bax, Bcl-2, and β-actin following treatments. (B) Determination of Bax:Bcl-2 ratio in VSC4.1 cells following treatments.
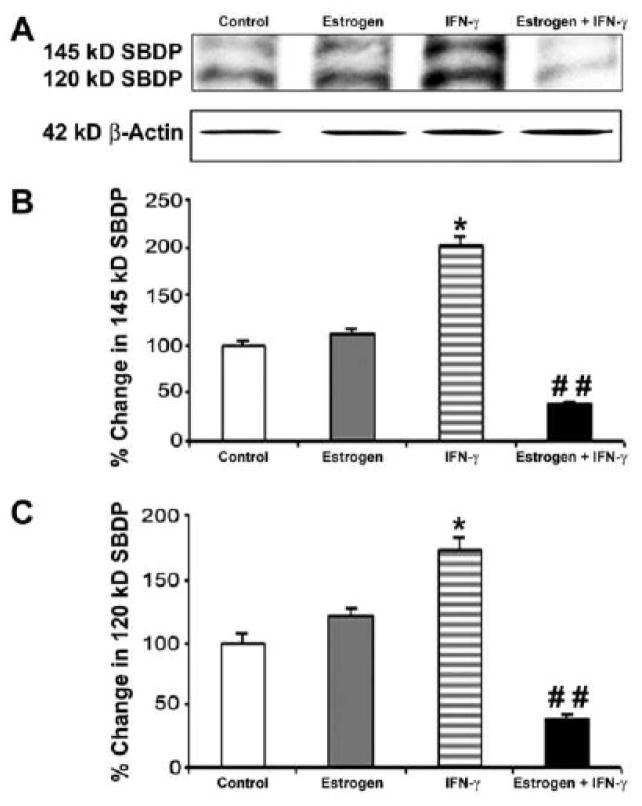
2.6. Estrogen attenuated calpain and caspase-3 proteolytic activities in VSC4.1 cells following IFN-γ exposure
Degradation of 270 kD α-spectrin to the 145 kD specific spectrin breakdown product (SBDP) and the 120 kD SBDP has been attributed to the activities of calpain and caspase-3, respectively (Nath et al., 1996; Wang et al., 1998). Calpain and caspase-3 activities were assessed by Western blot analysis of both 145 kB SBDP and 120 kD SBDP, respectively (Fig. 5). We used an antibody that could detect both 145 kB SBDP and 120 kD SBDP on the same Western blot (Fig. 5A). Uniform levels of β-actin in all lanes indicated loading of equal amounts of protein. Level of the calpain-specific 145 kD SBDP were significantly increased following exposure of VSC4.1 motoneurons to IFN-γ (Fig. 5B). However, treatment of cells with estrogen prior to IFN-γ exposure significantly attenuated elevation of the 145 kD SBDP, indicating a decrease in calpain activity. Similarly, estrogen treatment caused a significant decrease in 120 kD SBDP due to decrease in caspase-3 activity (Fig. 5B). These findings further indicated that estrogen decreased PTEN expression and proteolytic activities so as to prevent apoptosis and provide protection to VSC4.1 motoneurons.
Fig. 5.
Western blotting to examine proteolytic activities of calpain and caspase-3 in VSC4.1 motoneurons. Treatments (24 h): control; 100 nM estrogen; IFN-γ (10 ng/ml); and 100 nM estrogen (1-h pretreatment) + IFN-γ (10 ng/ml). (A) Representative Western blots demonstrating levels of 145 kD SBDP, 120 kD SBDP, and β-actin. (B) Determination of levels of the calpain-specific 145 kD SBDP following treatments. (C) Determination of levels of the caspase-3-specific 120 kD SBDP following treatments.
3. Discussion
PTEN has been identified as an important mediator of neuronal death in animal models of neurodegeneration, likely because of its role as a negative regulator of the PI3K/Akt survival pathway (Omori et al., 2002; Xu et al., 2003; Yeon et al., 2003). Since PTEN expression appears to be one dominant determinant of neuronal death, PTEN may be an important target of novel therapeutic agents to prevent the progression of neurodegenerative disorders like SCI, MS, and PD. Estrogen has been proposed as a potential therapeutic agent against neurodegeneration due to the potent anti-oxidant, anti-inflammatory, and anti-apoptotic properties of this steroid hormone (Sribnick et al., 2004). The current study was designed to investigate the effects of estrogen on PTEN expression following exposure of VSC4.1 motoneurons to IFN-γ. Previous studies suggest that estrogen may mediate neuroprotection through inactivation of PTEN following transient cerebral ischemia in rats (Choi et al., 2004). However, to our knowledge, no studies have explored the effects of estrogen on PTEN expression and cell viability in vitro, particularly in motoneurons. Our findings expand upon current understanding of estrogen-mediated neuroprotection. In particular, in vitro studies allowed us to further elucidate the mechanisms by which estrogen acts as a neuroprotective agent.
Numerous studies indicate that neuronal death following insult may be attributed in part to changes in PTEN expression (Gary and Mattson, 2002; Li et al., 2002). Cell death may result because elevated PTEN expression can be correlated with decreased activation of the cell survival kinase Akt (Myers et al., 1998). Overall, our studies are consistent with others indicating that exposure of VSC4.1 motoneurons to IFN-γ resulted in elevated levels of PTEN expression, increased apoptosis, and decreased overall cell viability. Furthermore, we found that elevated PTEN expression following IFN-γ exposure was correlated with decreased phosphorylation/activation of Akt. These results suggest that upregulation of PTEN following neuronal insult may be responsible for cell death by alterations in the PI3K/Akt signaling pathway.
We have also shown that changes in PTEN and p-Akt following IFN-γ exposure were associated with alterations in expression of numerous apoptotic markers. In particular, IFN-γ-mediated upregulation of PTEN resulted in a significant increase in Bax:Bcl-2 ratio. These findings suggest that upregulation of PTEN following neuronal insult may cause changes in the balance of pro-apoptotic and anti-apoptotic proteins, resulting in apoptotic cell death. Our findings also suggest that upregulation of PTEN following IFN-γ exposure may result in increased activities of both calpain and caspase-3, as indicated by the degradation of α-spectrin into 145 kD SBPD and the 120 kD SBPD, respectively. Both of these cysteine proteases are known to be involved in the cell death mechanism in in vitro and in vivo mdoels of SCI and EAE. Together, these studies suggest overexpression of PTEN following neuronal insult may be closely associated with activation of the proteolytic cascade for apoptosis. Because apoptotic cell death is characteristic of neurodegeneration, down regulation of PTEN may be desirable to prevent the progression of neurodegenerative disorders.
Our studies indicate that treatment of VSC4.1 motoneurons with estrogen prior to IFN-γ exposure partially attenuates increases in PTEN expression. PTEN expression was significantly decreased in cells treated with estrogen prior to IFN-γ exposure. Estrogen-mediated down regulation of PTEN expression was associated with restored phosphorylation/activation of the cell survival kinase Akt, suggesting that estrogen may confer neuroprotection via the PI3K/Akt pathway. Furthermore, decreased PTEN expression following estrogen treatment of VSC4.1 cells resulted in down regulation of cell death markers. In particular, estrogen restored Bax:Bcl-2 ratio to control levels following IFN-γ exposure. Calpain and caspase-3 activities in VSC4.1 motoneurons following IFN-γ exposure was also attenuated following treatment with estrogen. These results indicate that estrogen may act as a neuroprotectant through decrease in PTEN expression and Akt activation.
In conclusion, the current study elucidated a role for PTEN in degeneration of motoneurons. Exposure of VSC4.1 motoneurons to the pro-inflammatory cytokine IFN-γ resulted in increased PTEN expression, inactivated the PI3K/Akt survival pathway, and increased apoptosis. Upregulation of PTEN following IFN-γ exposure was correlated with increases in Bax:Bcl-2 ratio and calpain and caspase-3 activities, indicating a shifting of cell fate toward apoptotic cell death. However, we have shown that treatment of cells with estrogen before exposure to IFN-γ attenuated both PTEN expression and apoptotic cell death. Further, estrogen-mediated changes in PTEN expression were associated with increase in Akt activation and decreases in Bax:Bcl-2 ratio and calpain and caspase-3 activities. Because PTEN appears to play an important role in neurodegeneration, estrogen may be an effective new treatment approach to prevent neuronal loss. Nevertheless, more studies are needed to determine the cellular mechanisms by which estrogen mediates changes in PTEN expression. Our studies provide evidence that down regulation of PTEN may be used as an important index for the evaluation of the efficacy of estrogen in the prevention of neurodegenerative disorders.
4. Experimental procedures
4.1. Cell culture
Ventral spinal cord 4.1 (VSC4.1) motoneurons were grown in monolayer to 80% confluency in 75-cm2 flasks containing 10 ml DMEM/F-12 medium with 15 mM HEPES, pyridoxine, and NaHCO3 (Sigma, St. Louis, MO) supplemented with 2% Sato’s components, 1% penicillin and 1% streptomycin (GIBCO-Invitrogen, Grand Island, NY), and 2% heat-inactivated fetal bovine serum (FBS) (HyClone, Logan, UT). Once the motoneurons were 80% confluent, cells were starved in DMEM/F-12 medium supplemented with 0.5% heat-inactivated FBS for 24 h. Cells were maintained in this medium for treatment with 10 ng/ml IFN-γ, 100 nM estrogen, or a combination of both for 24 h. Cells receiving both IFN-γ (R&D Systems, Minneapolis, MN) and estrogen (Sigma, St. Louis, MO) were treated with estrogen for 1 h prior to IFN-γ exposure. Cells were grown in an incubator at 37°C with 5% CO2 and full humidity. Cells were harvested at 24 h after all treatments.
4.2. Trypan blue dye exclusion test for cell viability
Cell viability was measured using the trypan blue dye exclusion test, which we previously described (Das et al., 2004). Briefly, viable cells maintained membrane integrity and did not take up trypan blue. Compromised cells took up trypan blue and were counted as dead. At least 600 cells were counted in four different fields, and the number of viable cells was calculated as a percentage of the total cell population.
4.3. Wright staining for morphological features of apoptosis
Cells from each treatment group were detached with a cell scraper to harvest both attached and detached cells together. Cells were then washed twice in phosphate-buffered saline (PBS) and sedimented onto microscopic slides using an Eppendorf 5804R centrifuge (Brinkmann Instruments, Westbury, NY) at 106×g for 5 min. Cells were fixed and stained with Wright stain, as previously outlined (Ray et al., 1999a; Ray et al., 1999b). Cellular morphology was assessed using optical microscopy to detect apoptotic cells. Cells were counted as apoptotic if they demonstrated reduction in cell volume, chromatin condensation, and/or cellular membrane blebbing. At least 600 cells were counted in each treatment, and the percentage of apoptotic cells was calculated.
4.4. Western blotting for specific proteins
Cells were washed with Hank’s balanced salt solution without Ca2+ (GIBCO-Invitrogen) in culture flasks, then washed twice in PBS (pH 7.4), and centrifuged in an Eppendorf 5804R centrifuge at 106×g for 10 min. Cells were resuspended in a homogenizing buffer consisting of 50 mM Tris-HCl (pH 7.4), 1 mM phenylmethylsulfonyl fluoride (BRL, Gaithersburg, MD), and 5 mM EGTA (Sigma). Total protein was extracted and concentration was determined using Coomassie-Plus Protein Assay Reagent (Pierce, Rockford, IL). Samples were then diluted 1:1 in sample buffer composed of 62.5 mM Tris-HCl (pH 6.8), 2% sodium dodecyl sulfate (SDS), 5 mM β-mercaptoethanol, and 10% glycerol and boiled for 5 min. Samples were diluted in a solution of equal volumes of sample buffer and homogenizing buffer to a final protein concentration of 1 mg/ml. Diluted samples were then loaded onto a 4–20% gradient gels (Bio-Rad, Hercules, CA) and electrophoresed at 200 V for 30 min. Following electrophoresis, resolved total protein from the gel was transferred to a nylon membrane (Millipore, Bedford, MA) in a Genie transfer apparatus (IDEA Scientific, Minneapolis, MN). The membrane was then blocked in 5% powdered nonfat milk in a Tris/Tween solution (20 mM Tris-HCl (pH 7.6), 0.1% Tween-20 in saline) for 2 h. Primary IgG antibodies were diluted (1:200 for Bax, Bcl-2, p-Akt, PTEN, and SBDP; 1:20000 for β-actin) in blocking solution and then added to the membrane for 1 h. The membrane was then covered with the appropriate secondary IgG antibody (goat anti-rabbit or goat anti-mouse) conjugated with alkaline horseradish peroxidase at a 1:2000 dilution for 1 h. Between steps membranes were washed three times with Tris/Tween solution. Blots were incubated with enhanced chemiluminescence (ECL) plus reagents (Amersham Pharmacia, Buckinghamshire, United Kingdom) and exposed to X-Omat AR films (Eastman Kodak, Rochester, NY). The ECL autoradiograms were scanned on a Umax PowerLook Scanner (Umax Technologies, Fremont, CA) using Adobe Photoshop, and optical density (OD) of each band was measured using Quantity One Software (Bio-Rad).
4.5. Statistical analysis
Results were analyzed using StatView software (Abacus Concepts, Berkeley, CA) and compared using one-way analysis of variance (ANOVA) with Fisher’s protected least significant difference (PLSD) post hoc test at a 95% confidence interval. Data are presented as mean ± standard deviation (SD) of separate experiments (n ≥ 3). Difference between two treatments was considered significant at P ≤ 0.05. Significant difference between control and IFN-γ was indicated by *, P ≤ 0.05 or **, P ≤ 0.001. Significant difference between IFN-γ and estrogen + IFN-γ was indicated by #, P ≤ 0.05 or ##, P ≤ 0.001.
Acknowledgments
This work was supported in part by the R01 grants (NS-31662, NS-45967, NS-57811, and CA-91460) from the National Institutes of Health (Bethesda, MD), a Medical Scientist Training Program (GM008716-11), and the spinal cord injury research fund grants (SCIRF-0803 and SCIRF-1205) from the State of South Carolina.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Backman S, Stambolic V, Mak T. PTEN function in mammalian cell size regulation. Curr Opin Neurobiol. 2002;12:516–522. doi: 10.1016/s0959-4388(02)00354-9. [DOI] [PubMed] [Google Scholar]
- Bellacosa A, Kumar CC, Di Cristofano A, Testa JR. Activation of AKT kinases in cancer: implications for therapeutic targeting. Adv Cancer Res. 2005;94:29–86. doi: 10.1016/S0065-230X(05)94002-5. [DOI] [PubMed] [Google Scholar]
- Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA. 1999;96:4240–4245. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang N, El-Hayek YH, Gomez E, Wan Q. Phosphatase PTEN in neuronal injury and brain disorders. Trends Neurosci. 2007;30:581–586. doi: 10.1016/j.tins.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Choi CI, Lee YD, Gwag BJ, Cho SI, Kim SS, Suh-Kim H. Effects of estrogen on lifespan and motor functions in female hSOD1 G93A transgenic mice. J Neurol Sci. 2008;268:40–47. doi: 10.1016/j.jns.2007.10.024. [DOI] [PubMed] [Google Scholar]
- Choi YC, Lee JH, Hong KW, Lee KS. 17 Beta-estradiol prevents focal cerebral ischemic damages via activation of Akt and CREB in association with reduced PTEN phosphorylation in rats. Fundam Clin Pharmacol. 2004;18:547–557. doi: 10.1111/j.1472-8206.2004.00284.x. [DOI] [PubMed] [Google Scholar]
- Crawford GD, Jr, Le WD, Smith RG, Xie WJ, Stefani E, Appel SH. A novel N18TG2 x mesencephalon cell hybrid expresses properties that suggest a dopaminergic cell line of substantia nigra origin. J Neurosci. 1992;12:3392–3398. doi: 10.1523/JNEUROSCI.12-09-03392.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Banik NL, Patel SJ, Ray SK. Dexamethasone protected human glioblastoma U87MG cells from temozolomide induced apoptosis by maintaining Bax:Bcl-2 ratio and preventing proteolytic activities. Mol Cancer. 2004;3:36. doi: 10.1186/1476-4598-3-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- Di Cristofano A, Pandolfi PP. The multiple roles of PTEN in tumor suppression. Cell. 2000;100:387–390. doi: 10.1016/s0092-8674(00)80674-1. [DOI] [PubMed] [Google Scholar]
- Dimayuga FO, Reed JL, Carnero GA, Wang C, Dimayuga ER, Dimayuga VM, Perger A, Wilson ME, Keller JN, Bruce-Keller AJ. Estrogen and brain inflammation: effects on microglial expression of MHC, costimulatory molecules and cytokines. J Neuroimmunol. 2005;161:123–136. doi: 10.1016/j.jneuroim.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Garay L, Gonzalez Deniselle MC, Gierman L, Meyer M, Lima A, Roig P, De Nicola AF. Steroid protection in the experimental autoimmune encephalomyelitis model of multiple sclerosis. Neuroimmunomodulation. 2008;15:76–83. doi: 10.1159/000135627. [DOI] [PubMed] [Google Scholar]
- Gary DS, Mattson MP. PTEN regulates Akt kinase activity in hippocampal neurons and increases their sensitivity to glutamate and apoptosis. Neuromolecular Med. 2002;2:261–269. doi: 10.1385/NMM:2:3:261. [DOI] [PubMed] [Google Scholar]
- Ji SP, Zhang Y, Van Cleemput J, Jiang W, Liao M, Li L, Wan Q, Backstrom JR, Zhang X. Disruption of PTEN coupling with 5-HT2C receptors suppresses behavioral responses induced by drugs of abuse. Nat Med. 2006;12:324–329. doi: 10.1038/nm1349. [DOI] [PubMed] [Google Scholar]
- Jia J, Guan D, Zhu W, Alkayed NJ, Wang MM, Hua Z, Xu Y. Estrogen inhibits Fas-mediated apoptosis in experimental stroke. Exp Neurol. 2009;215:48–52. doi: 10.1016/j.expneurol.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachyankar MB, Sultana N, Schonhoff CM, Mitra P, Poluha W, Lambert S, Quesenberry PJ, Litofsky NS, Recht LD, Nabi R, Miller SJ, Ohta S, Neel BG, Ross AH. A role for nuclear PTEN in neuronal differentiation. J Neurosci. 2000;20:1404–1413. doi: 10.1523/JNEUROSCI.20-04-01404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie NR, Yang X, Downes CP, Weijer CJ. The regulation of cell migration by PTEN. Biochem Soc Trans. 2005;33:1507–1508. doi: 10.1042/BST0331507. [DOI] [PubMed] [Google Scholar]
- Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner SH, Giovanella BC, Ittmann M, Tycko B, Hibshoosh H, Wigler MH, Parsons R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- Li L, Liu F, Salmonsen RA, Turner TK, Litofsky NS, Di Cristofano A, Pandolfi PP, Jones SN, Recht LD, Ross AH. PTEN in neural precursor cells: regulation of migration, apoptosis, and proliferation. Mol Cell Neurosci. 2002;20:2129. doi: 10.1006/mcne.2002.1115. [DOI] [PubMed] [Google Scholar]
- Li L, Liu F, Ross AH. PTEN regulation of neural development and CNS stem cells. J Cell Biochem. 2003;88:24–28. doi: 10.1002/jcb.10312. [DOI] [PubMed] [Google Scholar]
- Marsh DJ, Coulon V, Lunetta KL, Rocca-Serra P, Dahia PL, Zheng Z, Liaw D, Caron S, Duboue B, Lin AY, Richardson AL, Bonnetblanc JM, Bressieux JM, Cabarrot-Moreau A, Chompret A, Demange L, Eeles RA, Yahanda AM, Fearon ER, Fricker JP, Gorlin RJ, Hodgson SV, Huson S, Lacombe D, Eng C, et al. Mutation spectrum and genotype-phenotype analyses in Cowden disease and Bannayan-Zonana syndrome, two hamartoma syndromes with germline PTEN mutation. Hum Mol Genet. 1998;7:507–515. doi: 10.1093/hmg/7.3.507. [DOI] [PubMed] [Google Scholar]
- Moosmann B, Behl C. The antioxidant neuroprotective effects of estrogens and phenolic compounds are independent from their estrogenic properties. Proc Natl Acad Sci USA. 1999;96:8867–8872. doi: 10.1073/pnas.96.16.8867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morissette M, Al Sweidi S, Callier S, Di Paolo T. Estrogen and SERM neuroprotection in animal models of Parkinson’s disease. Mol Cell Endocrinol. 2008;290:60–69. doi: 10.1016/j.mce.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Myers MP, Pass I, Batty IH, Van der Kaay J, Stolarov JP, Hemmings BA, Wigler MH, Downes CP, Tonks NK. The lipid phosphatase activity of PTEN is critical for its tumor supressor function. Proc Natl Acad Sci USA. 1998;95:13513–13518. doi: 10.1073/pnas.95.23.13513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath R, Raser KJ, Stafford D, Hajimohammadreza I, Posner A, Allen H, Talanian RV, Yuen P, Gilbertsen RB, Wang KK. Non-erythroid α-spectrin breakdown by calpain and interleukin-1β-converting-enzyme-like protease(s) in apoptotic cells: contributory roles of both protease families in neuronal apoptosis. Biochem J. 1996;319 (Pt 3):683–690. doi: 10.1042/bj3190683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori N, Jin G, Li F, Zhang WR, Wang SJ, Hamakawa Y, Nagano I, Manabe Y, Shoji M, Abe K. Enhanced phosphorylation of PTEN in rat brain after transient middle cerebral artery occlusion. Brain Res. 2002;954:317–322. doi: 10.1016/s0006-8993(02)03366-8. [DOI] [PubMed] [Google Scholar]
- Ray SK, Wilford GG, Crosby CV, Hogan EL, Banik NL. Diverse stimuli induce calpain overexpression and apoptosis in C6 glioma cells. Brain Res. 1999a;829:18–27. doi: 10.1016/s0006-8993(99)01290-1. [DOI] [PubMed] [Google Scholar]
- Ray SK, Wilford GG, Matzelle DC, Hogan EL, Banik NL. Calpeptin and methylprednisolone inhibit apoptosis in rat spinal cord injury. Ann N Y Acad Sci. 1999b;890:261–269. doi: 10.1111/j.1749-6632.1999.tb08001.x. [DOI] [PubMed] [Google Scholar]
- Sano T, Lin H, Chen X, Langford LA, Koul D, Bondy ML, Hess KR, Myers JN, Hong YK, Yung WK, Steck PA. Differential expression of MMAC/PTEN in glioblastoma multiforme: relationship to localization and prognosis. Cancer Res. 1999;59:1820–1824. [PubMed] [Google Scholar]
- Smith RG, Alexianu ME, Crawford G, Nyormoi O, Stefani E, Appel SH. Cytotoxicity of immunoglobulins from amyotrophic lateral sclerosis patients on a hybrid motoneuron cell line. Proc Natl Acad Sci USA. 1994;91:3393–3397. doi: 10.1073/pnas.91.8.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sribnick EA, Ray SK, Banik NL. Estrogen as a multi-active neuroprotective agent in traumatic injuries. Neurochem Res. 2004;29:2007–2014. doi: 10.1007/s11064-004-6874-0. [DOI] [PubMed] [Google Scholar]
- Sribnick EA, Matzelle DD, Ray SK, Banik NL. Estrogen treatment of spinal cord injury attenuates calpain activation and apoptosis. J Neurosci Res. 2006;84:1064–1075. doi: 10.1002/jnr.21016. [DOI] [PubMed] [Google Scholar]
- Sribnick EA, Del Re AM, Ray SK, Woodward JJ, Banik NL. Estrogen attenuates glutamate-induced cell death by inhibiting Ca2+ influx through L-type voltage-gated Ca2+ channels. Brain Res. 2009;1276:159–170. doi: 10.1016/j.brainres.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T, Frye C, Hu R, Swedlund B, Teng DH, Tavtigian SV. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15:356–362. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- Veldink JH, Bär PR, Joosten EA, Otten M, Wokke JH, van den Berg LH. Sexual differences in onset of disease and response to exercise in a transgenic model of ALS. Neuromuscul Disord. 2003;13:737–743. doi: 10.1016/s0960-8966(03)00104-4. [DOI] [PubMed] [Google Scholar]
- Wang KK, Posmantur R, Nath R, McGinnis K, Whitton M, Talanian RV, Glantz SB, Morrow JS. Simultaneous degradation of alphaII- and betaII-spectrin by caspase 3 (CPP32) in apoptotic cells. J Biol Chem. 1998;273:22490–22497. doi: 10.1074/jbc.273.35.22490. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Liu Y, Wise PM. Estradiol enhances Akt activation in cortical explant cultures following neuronal injury. Mol Brain Res. 2002;102:48–54. doi: 10.1016/s0169-328x(02)00181-x. [DOI] [PubMed] [Google Scholar]
- Xu J, Yeon JE, Chang H, Tison G, Chen GJ, Wands J, de la Monte S. Ethanol impairs insulin-stimulated neuronal survival in the developing brain: role of PTEN phosphatase. J Biol Chem. 2003;278:26929–26937. doi: 10.1074/jbc.M300401200. [DOI] [PubMed] [Google Scholar]
- Yeon JE, Califano S, Xu J, Wands JR, De La Monte SM. Potential role of PTEN phosphatase in ethanol-impaired survival signaling in the liver. Hepatology. 2003;38:703–714. doi: 10.1053/jhep.2003.50368. [DOI] [PubMed] [Google Scholar]