Abstract
Targeting viral vectors encoding tumor-associated antigens to dendritic cells (DCs) in vivo is likely to enhance the effectiveness of immunotherapeutic cancer vaccines. We have previously shown that genetic modification of adenovirus (Ad) 5 to incorporate CD40 ligand (CD40L) rather than native fiber allows selective transduction and activation of DCs in vitro. Here, we examine the capacity of this targeted vector to induce immune responses to the tumor antigen CEA in a stringent in vivo canine model. CD40-targeted Ad5 transduced canine DCs via the CD40-CD40L pathway in vitro, and following vaccination of healthy dogs, CD40-targeted Ad5 induced strong anti-CEA cellular and humoral responses. These data validate the canine model for future translational studies and suggest targeting of Ad5 vectors to CD40 for in vivo delivery of tumor antigens to DCs is a feasible approach for successful cancer therapy.
Keywords: cancer immunotherapy, adenovirus, dendritic cell, dog, vaccine
1. Introduction
Many immunological features of cancers that allow evasion of immune surveillance and destruction have been revealed, enabling the development of new and more effective immunotherapeutic strategies. Immune evasion is now recognized to be due primarily to a breakdown in the normal route of tumor antigen presentation to T cells [1]. In order to mount an immune response against a tumor, antigen presenting cells (APCs) monitoring peripheral tissues must present tumor-associated antigens (TAA) to T cells. In a fully functional immune system, APCs capture and present TAA to CD8+ T cells (lymphocytes) (CTL) and CD4+ helper T (Th) cells in lymph nodes (LN), leading to the expansion and activation of antigen-specific effector T cells. Once activated, CTLs recognize tumor cells expressing the presented TAA. The identification of a variety of cancer-specific TAA has provided more options for immunotherapy; however, TAA are often self-antigens to which a considerable immunological tolerance is maintained, especially depending on the route of T cell presentation [2]. Therefore, strategies for overcoming tolerance and generating effective immune responses against TAA are being developed based on harnessing the immunostimulatory activity of APCs.
Delivering TAA specifically to DCs with an antigen-delivery system offers tremendous potential for the development of new cancer vaccines [3]. Supporting this, pre-clinical and clinical trials have focused on adoptive transfer of TAA-exposed DCs. These approaches involve isolating DCs from the blood of patients, exposing the DCs to TAA and other maturation stimuli in culture, and, finally, re-injecting them into the patient. Although encouraging results have been reported, there are substantial medical, economic, and logistic complexities to this approach. In addition, while pre-clinical trials in mice demonstrated highly promising immune responses, including tumor regression and remission, clinical trials in humans resulted in far fewer cases of tumor stability or regression, suggesting these vaccines are not yet optimal [4-8]. Possibly underlying these results, recent evidence indicates that DCs matured ex vivo do not accurately mimic DCs matured in vivo, precluding optimal immune system stimulation [9]. Thus, a strategy to facilitate DC transduction to mediate TAA delivery in vivo that is universally efficacious, regardless of haplotype, is required.
In regard to DC transduction efficiency, gene transfer and immune stimulation, viral transduction methods have been found to be superior to non-viral methods [10-12]. In addition to high gene transfer efficiency, transduction of DCs with adenovirus serotype 5 (Ad5)-based vectors offers many benefits over other viral vectors. Ad5-mediated transduction does not require cell proliferation and poses a low risk for insertional mutagenesis [13-16]. Furthermore, non-replicating Ads provide the additional benefit of delivering TAA for only a finite amount of time, thus minimizing the chances of inducing hyporesponsiveness to chronic antigen presentation. Despite these advantages, DCs are relatively refractory to Ad5 transduction due to limited cell surface expression of the primary Ad5 receptor, coxsackie virus and adenovirus receptor (CAR) [17]. Thus, efficient Ad5-mediated gene transfer to DCs requires high multiplicities of infection (MOI). To overcome this, our lab has retargeted Ad5 to CD40 expressed on cell surfaces, achieving efficient and specific DC transduction and antigen-specific immune responses using low doses of Ad5 administered subcutaneously, a site enriched for DCs [17-25]. In addition to providing a means for efficient DC transduction, CD40 ligation and activation induces migration of mature DCs to the T cell populated areas of the draining lymph node, thus CD40-targeted vectors are likely to provide additional immunotherapeutic benefits.
In the experiments presented here, the transduction potential of CD40-targeted Ad5 was determined in canine DCs, and the immunostimulatory capacity of this targeting strategy was evaluated in dogs, the most relevant translational animal model available for evaluating many therapeutic modalities designed to combat human diseases. Of particular relevance to our studies, dogs are naturally susceptible to several cancers which mimic the onset, progression and symptoms of the corresponding human cancers, allowing therapeutic evaluation with increased clinical relevance [26-29]. Indeed, many advances in human cancer therapies have been made or improved through studies in canine patients, including the first evaluations of cancer vaccines, and the analysis of cytokine and chemotherapeutic regimens for pulmonary metastases [26]. In addition, canine models provide dosing and vector production challenges similar to those encountered in human clinical trials [26, 30, 31].
The data presented from this pilot study in healthy dogs validate the utility of the canine model for our translational studies, and suggest that specific targeting of Ad5 vectors to DCs for in vivo delivery of genes encoding TAA may provide an enhanced immune response to disease-related antigens.
2. Materials and Methods
2.1. Cell lines
The human embryonic kidney cell line 293 was purchased from Microbix (Toronto, Ontario, Canada). The 293F28 and 293/hCD40 cell lines are derivatives of 293 cells which express either Ad5 wild-type fiber (for mosaic virus propagation as described below) or human CD40 as previously described [21]. The 293/cCD40 cell line is a derivative of the 293 cell line which expresses canine CD40, and was generated by transfection of 293 cells with the plasmid pcDNA3.1canineCD40, and subsequent selection with 1000 μg/ml of Geneticin (G418). A cell clone derived from this population that expressed high levels of canine CD40 was identified by RT-PCR. All 293 and 293-derived cell lines were propagated in a 50:50 mixture of Dulbecco's modified Eagle's medium and Ham's F-12 medium (DMEM/F-12) supplemented with 10% (v/v) fetal calf serum (FCS), L-glutamine (2 mM), penicillin (100 units/ml) and streptomycin (100 μg/ml). FCS was purchased from Gibco-BRL (Grand Island, NY) and media and supplements were from Mediatech (Herndon, VA). RT-PCR analysis of cell lines revealed 4.47 × 107 copies/μg of human CD40 mRNA in 293/hCD40 compared to 5.6 × 10-2 copies/μg in control 293 cells, and 6.44 × 103 copies/μg of canine CD40 mRNA in 293/cCD40 cells. 293F28 cells were maintained with 100 μg/ml Zeocin (Invitrogen), and 293/hCD40 and 293/cCD40 cells were maintained with 100 μg/ml G418. All cell lines were cultured at 37°C in 5% CO2.
2.2. Gene Therapy Vectors
Ad5.FFhCD40L vectors expressing artificial fiber proteins containing FF-CD40L and encoding either luciferase, CEA or GFP/CEA were constructed as previously described [20, 21]. Ad5 vectors expressing the native fiber protein and encoding either luciferase, GFP/luciferase or CEA were employed as controls [20, 32, 33]. Briefly, Ad5 vectors encoding the native fiber protein were generated by transfection of 293 cells with Pac I-digested Ad rescue vectors. Vectors with FF-CD40L were generated by transfection of 293F28 cells with Pac I-digested Ad rescue vectors. 293F28 cells stably express the native Ad5 fiber, thus viruses rescued at this point were mosaic in the sense that the Ad5 virions randomly incorporated a mixture of native fibers and FF-CD40L chimeras. After additional rounds of amplification on 293F28 cells, the viruses were amplified in 293 cells, which do not express native Ad5 fiber, to obtain virus particles containing only FF-CD40L [21].
All Ad5 vectors were isolated from infected cells and purified by equilibrium centrifugation in CsCl gradients according to a standard protocol [34]. The protein concentrations in the viral preparations were determined using the DC protein assay (Bio-Rad, Hercules, CA) with purified bovine serum albumin (BSA) as a standard. The virus titers were calculated using the formula: 1 μg of protein = 4 × 109 viral particles (vp).
2.3. Preparation of canine peripheral blood mononuclear cell populations
Whole blood (40-60 ml) was collected from normal outbred dogs in EDTA tubes (Becton, Dickinson), gently and thoroughly mixed and centrifuged 30 min at 1000 × g at RT. The buffy coat containing the peripheral blood mononuclear cell (PBMC) population was extracted with a pipette in 1-2 ml and diluted with 8 ml HBS (HEPES-buffered saline, no Mg/Ca, Gibco/BRL). The cell suspension was layered over 5 ml Histopaque 1077 (Sigma-Aldrich) and centrifuged at 1000 × g at RT for 30 min. The band containing the PBMC population was extracted in 1-2 ml into a new sterile tube and diluted with 2 ml HBS, mixed, and centrifuged once more. The supernatant was removed and the cells gently resuspend in 5 ml HBS. The cells were centrifuged once more and resuspended in 4 ml flow wash buffer (FWB - HBS containing 10% fetal bovine serum) and incubated at RT at least 40 min to allow blocking of nonspecific sites.
2.4. Flow cytometry and fluorescent activated cell sorting for canine dendritic cells
Antibodies were obtained that recognize canine CD11c, (monoclonal mouse anti-dog CD11c, Serotec) and human CD40 (monoclonal mouse clone B-B20, prelabeled with Zenon reagent PE/Alexa Fluor 610, Invitrogen). The PBMC suspension was centrifuged at 200 × g at RT for 10 min, the supernatant removed, and the cells resuspended in 1 mL FWB. Primary antibodies (100 μL each) were then added, gently mixed, and incubated for 60 min at RT in the dark. Then, 2 mL HBS was added and the cell suspension centrifuged at 200 × g for 10 min. The supernatant was aspirated and the cells were washed two additional times in 2 mL HBS, finally resuspending them in 1 mL FWB. Each cell suspension was filtered to 50 μm in a sterile CellTrics disposable filter (Partec, Germany).
Flow cytometry assays and fluorescent activated cell sorting (FACs) were performed on a MoFlo flow cytometer and cell sorter (Beckman Coulter). The CD11c expression profiles were determined using Summit 4.0 software (Beckman Coulter). The entire cell suspension was sorted for each experiment and sorted cells (CD11c+ and CD11c-) sterilely collected into tubes containing 1 mL of FBS. Both samples and sorted populations of cells were maintained at RT during the entire process.
To ensure recovery of exposed CD40 on the cell surface, cells were allowed to recover for 4 h prior to transduction with Ad vectors. Following transduction, cells were collected by centrifugation, and brought into culture in RPMI-1640 (Gibco/BRL) containing 10% FBS, penicillin/streptomycin/fungizone (Gibco/BRL) and 25% lymphocyte conditioned media (CM). CM was obtained from cultures of freshly prepared canine lymphocytes which had been stimulated for 24-48 hr with phytohemagglutinin (PHA, 10 μg/ml, Sigma-Aldrich) [35, 36].
2.5. Recombinant protein purification and western blot analysis
The 6-HIS-tagged soluble human CD40L protein and its derivatives were expressed in E. coli BL21(DE3)(pLysS) as previously described [21]. The concentrations of the proteins in purified preparations and in cell lysate were determined using the BioRad DC Protein Assay.
2.6. Gene transfer experiments
293, 293/hCD40 and 293/cCD40 cell lines and canine DCs were plated in 24-well plates at 1 × 105 cells/well. Prior to transduction, cells were washed with serum-free growth medium and incubated on ice with 0.2 ml of either medium or medium containing a blocking agent. In the latter instance, recombinant Ad5 fiber knob [37] or soluble hCD40L proteins were added to the medium at concentrations of 100 μg/ml for 1 h on ice. Cells were transduced at a multiplicity of infection (MOI) of 10, 100 or 1000 vp per cell with Ad5 vectors in medium containing 2% FCS. After incubation on ice for 1 h, the medium containing the virus and the inhibitor was removed, and cells were washed with medium containing 10% FCS. Fresh medium was added, and incubation was continued at 37°C for 22 h to allow reporter gene expression. Cells were then washed with PBS and lysed in Luciferase Reporter Lysis Buffer (Promega). The luciferase activity in the cell lysates was measured according to the manufacturer's protocol. Each data point was assessed in triplicate and calculated as the mean of three determinations. In instances where transduction was performed without the addition of a blocking agent, the virus was added to the cells in 0.4 ml aliquots of medium containing 2% FCS.
All incubation and washing steps in gene transfer experiments involving DCs were performed in cell suspensions since these cells are only loosely adherent. To minimize variation in the data, which could result from the loss of cells during the washing steps, the luciferase activity measured in the cell lysates was normalized to the protein concentration of resulting cell lysates.
2.7. In vivo canine vaccinations
All experiments were conducted under the oversight of the Auburn University IACUC committee in AAALAC approved animal and clinical care facilities. Outbred beagle dogs from two litters were used for vaccination. The dogs were 10 and 15 weeks of age at the start of the experiment and weighed approximately 10 to 15 kg each. Additional adult beagle and beagle-corgi crosses were used to provide blood for isolation of PBMCs. 1 × 109 vp of Ad5CEA.FF/hCD40L (5 dogs) or control Ad5CEA (5 dogs) vectors were delivered in 0.5 ml sterile phosphate-buffered saline (PBS) to each dog. Intradermal (i.d.) injections were performed in the right lower abdomen using a 25 gauge needle. The dogs were re-injected with the same dose of each Ad5 vector and by the same route as their original injection on weeks 4, 8 and 12. Peripheral blood was collected each week and used for ELISA detection of anti-CEA antibodies. At week 14, lymphocytes were purified from collected peripheral blood for lymphoproliferation assays.
2.8. Lymphoproliferation assays
PBMCs were obtained by density gradient centrifugation using lymphocyte separation media (Cellgro, Mediatech, Inc, Herndon, VA) and were resuspended in complete medium consisting of RPMI 1640 supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 50 μM 2-mercaptoethanol, and antibiotics. Cells were added at 1 × 105 cells per well in round bottom 96 well plates. Stimulated cells were incubated in triplicate wells with recombinant human CEA protein (Vitro Diagnostics, Inc. Aurora, CO) over a range of concentrations (1–30 μg/ml). Additionally, BSA (30 μg/ml) or ovalbumin (OVA) (25 μg/ml) wells were included as negative control antigens and Con-A wells (0.5 μg/ml) were included as positive control mitogens. Control cells were cultured in complete medium alone. All cells were incubated at 37°C in a humidified atmosphere of 5% CO2 in air for two days, followed by an overnight pulse with 1 μCi/well of tritiated thymidine diluted at 50 μCi/ml. Cells were harvested and incorporated radioactivity was quantified using a solid-phase beta scintillation counter (Matrix 9600; Packard Instrument Co., Downers Grove, IL). The Stimulation Index (S.I.) was calculated as the mean counts per million (cpm) of the stimulated cells divided by the mean cpm of the control (OVA-stimulated) cells. A positive response was defined as a post-vaccination S.I. >3.0. The assay was performed in triplicate for each sample and results are presented as means.
2.9. ELISA for detection of anti-CEA antibodies
For CEA antibody detection, 96 well EIA plates (Costar 3590) were coated with recombinant CEA protein (Vitro Diagnostics, Inc. Aurora, CO) at 100 ng/well in borate saline (BS) buffer, pH 8.4, for 4 h at RT, and then blocked with borate saline plus 1% (w/v) bovine serum albumin (BS-BSA). Serial three-fold dilutions of dog serum in BS-BSA (1:50 - 1:109,350) were added to the wells and incubated overnight at 4°C. Plates were washed with PBS containing 0.05% (v/v) Tween-20 and incubated with AP conjugated rabbit anti-dog (IgG H+L) antibodies (Jackson Immunoresearch Laboratories, Inc. West Grove, PA) diluted 1:2000 in BS-BSA for 4 hr at RT. After washing, AP substrate (p-nitrophenyl phosphate, Sigma St. Louis, MO) in diethanolamine buffer, pH 9.0, was added and incubated for 20 min at RT. Absorbance was measured at 405 nm on a Multiskan Ascent microplate reader using Ascent software (Labsystems OY, Helsinki, Finland). Absorbance on CEA coated plates was corrected for absorbance on parallel plates coated with ovalbumin (Sigma–Aldrich Chemical Co., St. Louis, MO). COL-1 mouse monoclonal antibody to CEA (NeoMarkers, Fremont, CA) followed with AP conjugated goat anti-mouse IgG (Southern Biotechnology, Birmingham, AL) was used as a positive control. For detection of IgG isotypes goat anti-dog IgG1 and sheep anti-dog IgG2 antibodies conjugated to horseradish peroxidase (Bethyl Laboratories Inc., Montgomery, TX) were applied to the wells at a dilution of 1:10,000. After incubation and wash 3, 3′, 5′5 tetramethyl benzidine substrate (Sigma) was applied for colorimetric development. The reaction was stopped by adding 0.5 M H2S04 and absorbance was read at 450 nm. Statistical analysis was performed by using two-tailed Student's t-test and values are presented as mean ± standard error of the mean.
3. Results
3.1. Generation of CD40-targeted Ad5
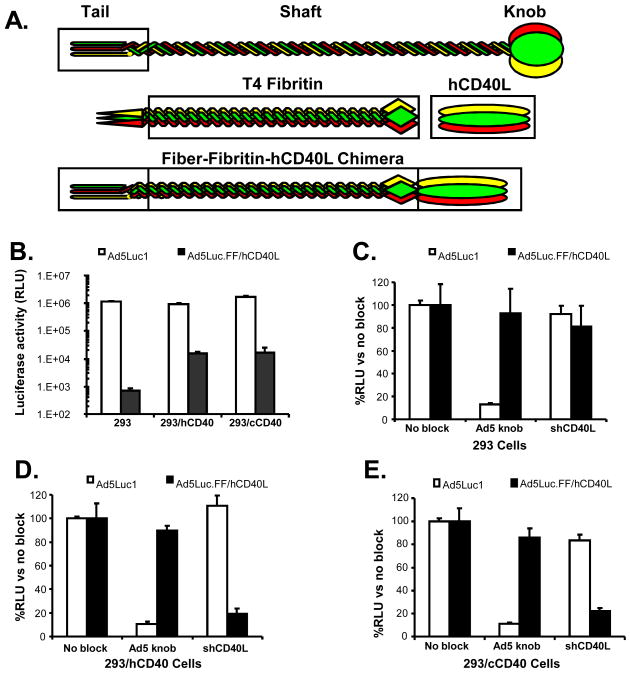
Human, rhesus and murine DCs have been successfully transduced with CD40-targeted Ad5 in vitro, resulting in selective transduction and simultaneous activation of DCs, indicating CD40 is an effective target in many species [17-24, 38, 39]. To establish the principle that CD40-targeted Ad5 can also mediate gene transfer to canine DCs, we employed a genetically engineered Ad5 vector encoding firefly luciferase, designated Ad5Luc.FF/hCD40L (Fig. 1) [19, 21]. This vector was previously constructed so that the shaft and knob domains of the native fiber protein are replaced by phage T4 fibritin (as a trimerization motif) genetically fused to the TNF-Like (TNF-L) ectodomain of human CD40L (Fig. 1A). The presence of the phage T4 fibritin structure allows the TNF-L ectodomain of CD40L to retain its functional tertiary structure, required for activation of CD40 [21]. Human and canine CD40 are 83% similar, and amino acids predicted to be involved in CD40-CD40L binding are conserved [40, 41], suggesting an Ad5 vector expressing human CD40L may interact effectively to allow transduction of CD40-expressing canine cells.
Fig. 1.
Efficiency of gene transfer and receptor specificity of CD40-targeted Ad5. (A) Schematic diagram of CD40-targeted Ad5. The shaft and knob domains of native Ad5 fiber were replaced by phage T4 fibritin genetically fused to the TNF-L ectodomain of human CD40L. (B) 293, 293/hCD40 and 293/cCD40 cells were transduced with untargeted Ad5Luc1 (white bars) or CD40-targeted Ad5Luc.FF/hCD40L (black bars) at an MOI of 10 vp/cell. To determine receptor specificity, 293 (C), 293/hCD40 (D) and 293/cCD40 (E) cells were transduced with untargeted Ad5Luc1 (white bars) or CD40-targeted Ad5Luc.FF/hCD40L (black bars) at an MOI of 10 vp/cell. Prior to infection with virus, cells were incubated with recombinant Ad5 fiber knob or shCD40L to block transduction through CAR or CD40, respectively. 24 hr after transduction, luciferase activity was determined as a measure of Ad5-mediated gene transfer. Luciferase activity is expressed as relative light units. Luciferase activity detected in the presence of blocking agents is normalized against luciferase activity in absence of blocking agents. Mean ± SD are shown from three replicates performed simultaneously.
3.2. Gene transfer analysis in 293 cells expressing CD40
To confirm that the human CD40L ectodomain of Ad5Luc.FF/hCD40L mediates vector targeting to canine CD40, luciferase transgene expression was analyzed as a measure of vector transduction potential in 293 cells and cell lines stably expressing CD40. 293 cells, which do not express endogenous CD40, or 293 cells stably transfected with either human CD40 (293/hCD40) or canine CD40 (293/cCD40) were incubated with either untargeted Ad5Luc1 or CD40-targeted Ad5Luc.FF/hCD40L (10 vp/cell) (Fig. 1B). All 293 cell lines, which express the Ad5 receptor, CAR, were efficiently transduced by untargeted Ad5Luc1. In contrast, wild-type 293 cells incubated with Ad5Luc.FF/hCD40L expressed very low levels of luciferase, indicating this CD40-targeted vector does not transduce cells efficiently through CAR. However, Ad5Luc.FF/hCD40L mediated luciferase expression in both 293/hCD40 and 293/cCD40 cells (Fig. 1B), suggesting Ad5Luc.FF/hCD40L capably transduces cells expressing either human or canine CD40.
3.3 Validation of CD40-mediated transduction
To further investigate the targeting specificity of Ad5Luc.FF/hCD40L, viral transduction was performed in the presence of specific blocking agents. Prior to transduction, 293, 293/hCD40 and 293/cCD40 cells were incubated with either soluble recombinant Ad5 fiber knob or soluble human CD40L (shCD40L) to block CAR or CD40, respectively, on the cell surface. Luciferase expression under these blocking conditions was compared to luciferase expression without blocking (normalized for each vector to 100%). As shown in Fig. 1 (C-E), transduction of all 293 cell lines by Ad5Luc1 was inhibited by soluble knob (87% block in 293 cells, and 90% block in 293/hCD40 and 293/cCD40), indicating transduction by untargeted Ad5 requires CAR, as expected. The very low level of luciferase expression in 293 cells incubated with Ad5Luc.FF/hCD40L was not affected by soluble Ad5 knob (Fig. 1C). The high luciferase expression levels in 293/hCD40 and 293/cCD40 cells transduced by Ad5Luc.FF/hCD40L were not affected when CAR was blocked with soluble Ad5 knob (Fig. 1D and E), further demonstrating that Ad5Luc.FF/hCD40L does not require CAR for transduction of CD40-expressing cells. In contrast, pre-incubation with shCD40L decreased luciferase expression levels by 81% in 293/hCD40 cells, and by 78% in 293/cCD40 cells (Fig. 1D and E), but did not affect luciferase expression levels following Ad5Luc1 transduction in 293 cells (Fig. 1C). These results demonstrate that transduction by Ad5Luc.FF/hCD40L specifically requires cellular expression of CD40.
3.4. Targeted gene transfer to canine cells
To determine if CD40-targeted Ad5 also transduces canine DCs, as observed with DCs from other species, transduction experiments were conducted with canine DCs cultured from isolated PBMCs. Flow cytometry with anti-CD40 and -CD11c antibodies indicated CD40 expression on 43.5% of CD11c+ PBMCs (DCs), while only 1.7% of CD11c- cells were CD40+ (Fig. 2A). Based on CD40 expression, DCs were sorted into two groups: CD11c+/CD40- and CD11c+/CD40+. Cells from each group were incubated with either Ad5Luc1 or Ad5Luc.FF/hCD40L for analysis of transduction efficiency as measured by luciferase expression levels. As observed previously with human DCs [17], canine CD11c+/CD40+ DCs were refractory to transduction by untargeted Ad5, even at a very high vector concentration of 1000 vp/cell (Fig. 2B). In contrast, luciferase transgene expression was 15-fold higher in CD11c+/CD40+ DCs incubated with Ad5Luc.FF/hCD40L. Transgene expression was only slightly increased in CD11c+/CD40- DCs following incubation with Ad5Luc.FF/hCD40L, suggesting this vector specifically transduces cells via CD40. This minimal increase in Ad5Luc.FF/hCD40L-mediated gene transfer in CD11c+/CD40- DCs may be due to low-level expression of CD40 in this CD11c+ subset that could not be detected by flow analysis. Supporting this, luciferase expression was 84% lower in CD11c+/CD40+ cells pre-incubated with shCD40L and transduced with Ad5Luc.FF/hCD40L, but only 30% lower in CD11c+/CD40- cells pre-incubated with shCD40L (Fig. 2C and D). Furthermore, when transductions were performed with vectors encoding GFP, GFP expression was detected only in CD11c+/CD40+ cells incubated with the CD40-targeted vector. GFP expression was not detected in CD11c+/CD40- cells incubated with CD40-targeted vector or in either DC subset incubated with untargeted vector (Supplementary Fig. 1). These results again confirm the specificity of CD40-mediated transduction by this CD40-targeted Ad5 vector. Thus, the efficient and specific delivery of transgenes to canine DCs by Ad5Luc.FF/hCD40L in vitro validates the examination of this vector for in vivo delivery of transgenes to CD40-expressing cells, particularly DCs.
Fig. 2.
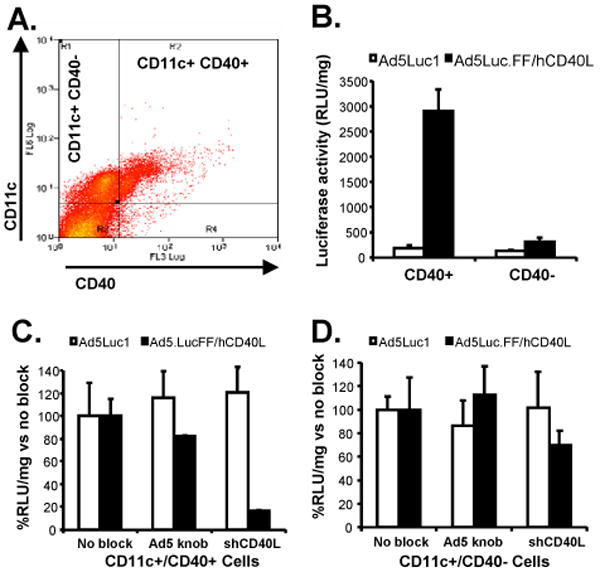
Receptor specificity of cell transduction and efficiency of gene transfer mediated by CD40-targeted Ad5 in canine DCs. (A) Anti-CD40 antibodies detected expression of CD40 on 43.5% CD11c+ cells, and cells were sorted cells based on CD40 expression levels. (B) CD11c+/CD40+ and CD11c+/CD40- cells were transduced with untargeted Ad5Luc1 (white bars) or CD40-targeted Ad5Luc.FF/hCD40L (black bars) at an MOI of 1000 vp/cell to determine the receptor specificity of vector transduction. (C) CD11c+/CD40- and (D) CD11c+/CD40+ canine cells were transduced with untargeted Ad5Luc1 (white bars) or CD40-targeted Ad5Luc.FF/hCD40L (black bars) at an MOI of 1000 vp/cell in the presence of recombinant Ad5 fiber knob or soluble human CD40L to further confirm the receptor specificity of vector transduction. Luciferase activity detected in the presence of blocking agents is normalized against luciferase activity in absence of blocking agents. Mean ± SD are shown from three replicates performed simultaneously.
3.5. Antigen-specific immune responses following vaccination in healthy dogs
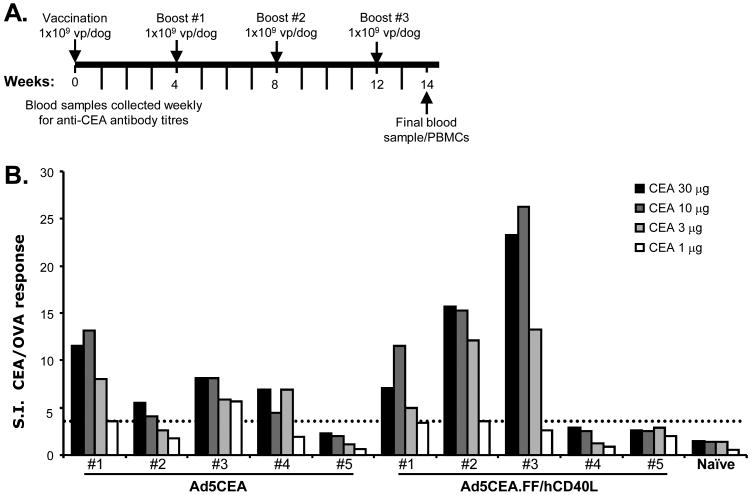
The ultimate goal is the development of a vaccine that can deliver tumor-specific transgenes to DCs in vivo in order to generate a TAA-specific immune response. Therefore, it was critical to investigate the capacity of untargeted versus CD40-targeted Ad5 vectors to induce TAA-specific immune responses. We have previously demonstrated an enhanced immune response in mice following injection of CD40-targeted Ad5 by various routes [25]. To determine if similar immune responses are detected in dogs, we performed a pilot experiment with healthy dogs injected with Ad5 vectors engineered to express human carcinoembryonic antigen (CEA) as a model tumor antigen (Ad5CEA and Ad5CEA.FF/hCD40L). CEA orthologues have been identified in dogs; however xenoantigens are more likely to overcome immunotolerance and induce a more robust antigen-specific immune response [42-45]. Five dogs received i.d. injections of Ad5CEA (1 × 109 vp/injection) and five dogs received injections of Ad5CEA.FF/hCD40L (1 × 109 vp/injection) according to the schedule outlined in Fig. 3A. One dog served as a negative control, receiving no injections. At week 14, PBMCs were harvested and lymphocyte proliferation was quantified as a measure of TAA-specific T cell activation. A stimulation index (S.I.) of greater than 3.0 was considered positive. A positive lymphoproliferative response was observed in three of five dogs immunized with Ad5CEA.FF/hCD40L and in four of five dogs immunized with Ad5CEA, although the average S.I. tended to be greater in responding animals immunized with Ad5CEA.FF/hCD40L (Fig. 3B). These results suggest that while vaccination with either untargeted or CD40-targeted Ad5 induces an antigen-specific T cell response, this response may be magnified in individuals responding to the CD40-targeted vector.
Fig. 3.
Antigen-specific lymphocyte responses in healthy dogs vaccinated with CD40-targeted Ad5. (A) On Day 0, five dogs were vaccinated i.d. with Ad5CEA (1 × 109 vp/dog) and five dogs were vaccinated with Ad5CEA.FF/hCD40L (1 × 109 vp/dog). At 4, 8 and 12 weeks post-vaccination, each dog received a booster injection (1 × 109 vp/dog) of the same vector. Blood was drawn at 14 weeks post-vaccination for lymphoproliferation assays. (B) Harvested PBMCs were stimulated for two days with recombinant human CEA at a range of concentrations (1-30μg) or ovalbumin (25 μg) followed by an overnight pulse with tritiated thymidine. Cells were harvested and incorporated radioactivity was quantified. The S.I. was calculated as the mean cpm of the stimulated cells divided by the mean cpm of the ovalbumin-stimulated cells. A positive response was defined as a post-vaccination S.I. >3.0 (denoted by dotted line).
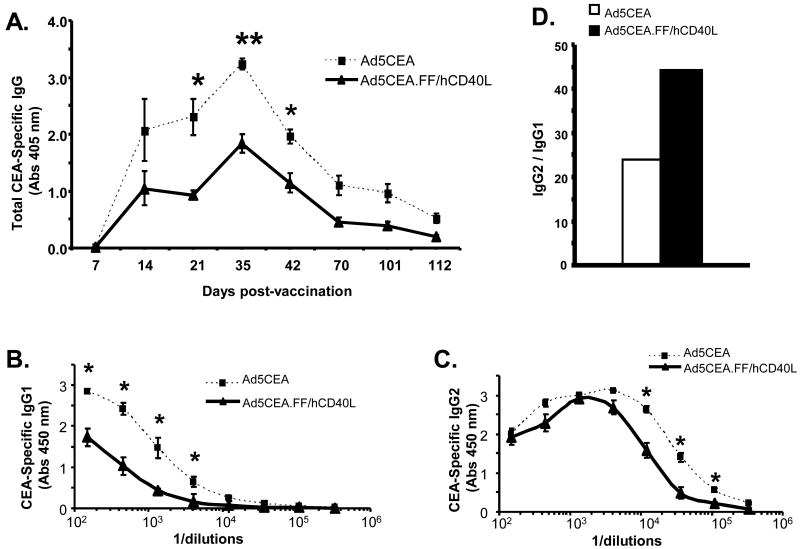
To analyze antigen-specific humoral immune responses, CEA-specific IgG serum concentrations were quantified. A strong anti-CEA humoral immune response was induced in both groups of dogs by 14 days post-immunization, though the antibody titer of dogs immunized with untargeted Ad5CEA was significantly higher as measured on days 21, 35 and 42 (Fig. 4A). We further examined the isotype of the antibody response on day 35, the day with the most significant difference in antibody responses between the groups. The concentration of IgG1 was significantly lower in dogs immunized with Ad5CEA.FF/hCD40L (Fig. 4B and C), resulting in a higher ratio of IgG2 to IgG1 (Fig. 4D).
Fig. 4.
Antigen-specific humoral responses in healthy dogs vaccinated with CD40-targeted Ad5. (A) Serum was drawn weekly from each dog following vaccination with Ad5CEA (squares) or Ad5CEA.FF/hCD40L (triangles). ELISAs were performed to detect human CEA specific canine IgGs. Serum from each time point was incubated with recombinant human CEA, and detected with anti-dog IgG secondary antibody to determine total IgG concentrations. (B) IgG1 and (C) IgG2 isotypes were detected with goat anti-dog IgG1 and sheep anti-dog IgG2 antibodies, respectively. (A, B and C) Absorbance was read at 450 nm. Statistical analysis for was performed by using two-tailed Student's t-test and values are presented as mean ± SE. *, p< 0.05; **, p< 0.0001. (D) Average ratio of IgG2:IgG1 following vaccination with Ad5CEA (white) or Ad5CEA.FF/hCD40L (black).
4. Discussion
DC-based vaccination can overcome tumor-associated suppression of immune responses, although the full potential of this strategy has yet to be realized in the context of a reliable cancer therapy [4-8]. In order to overcome the potential limitations associated with autologous DCs transduced and matured ex vivo, our lab has investigated methods to directly transduce and activate DCs in vivo in a an animal model highly likely to provide clinically relevant information.
Several strategies to accomplish in vivo delivery of immunotherapeutic antigens to DCs have now been reported, including the use of free antigen, protein fusions and viral gene therapy [46-51]. However, complete success depends on overcoming biological delivery challenges. In this regard, Arthur et al. previously found that Ad5 vectors are superior to other non-viral methods for delivering antigens to DCs in vitro [10], and other groups have since demonstrated highly efficient Ad5-mediated gene transfer to DCs ex vivo, using high concentrations of Ad5 [52, 53]. Additionally, Ad5 capsid protein is a potent adjuvant that enhances CTL response [54]. Therefore, TAA transfer by Ad5 vectors is likely to enhance production of tumor cell-specific CTL [55]. Thus, an Ad5 vector retargeted to bind a specific protein expressed on the surface of DCs seems likely to provide an enhanced in vivo immunotherapeutic strategy.
In addition to targeting DCs, proper activation and maturation of DCs is crucial for stimulating an antigen-specific immune response. It is now obvious that the collective term “DC” actually refers to a heterogeneous population of cells, derived from different lineages, in different maturation states, and likely displaying distinct functional features [8]. In general, antigen presentation by mature DCs leads to an antigen-specific immune response, while antigen presentation by immature DCs has been implicated in the induction of immune tolerance through activation of regulatory T cells [56]. CD40 is an attractive candidate for targeting DCs as it is expressed on the cell surface of DCs, and, while it is also expressed on endothelial cells and other immune cells, CD40 expression is far less ubiquitous than CAR expression. Additionally, localized dermal injection of CD40-targeted virus delivers virus to a location that is rich in CD40-positive DCs and lacks substantial numbers of other CD40 expressing cells, such as B cells. Binding of CD40L to CD40 induces DC maturation, enabling these cells to migrate to draining lymph nodes and activate antigen-specific T cells. This complete activation of DCs is critical for tumor rejection in vivo [57].
Results from the analysis of our in vitro gene transfer in model cell lines and canine DCs demonstrates CD40-targeting dramatically enhances Ad5-mediated transgene expression in cells expressing CD40, and in DCs in particular. Furthermore, CD40-targeted Ad5 requires cell expression of CD40 for transduction, and is incapable of transducing cells through CAR. This specificity will likely allow in vivo transduction of DCs with a much lower vector dose than is required for untargeted Ad5 transduction. Studies are currently underway to confirm the in vivo cell specificity of CD40-targeting.
In order to obtain clinically relevant information regarding the immune response generated in vivo following vaccination with CD40-targeted Ad5, we immunized healthy dogs with CD40-targeted or untargeted Ad5 encoding human CEA as a model tumor antigen. A key issue in the successful development of effective cancer immunotherapies is the design of vaccines that can overcome immune tolerance and induce a T cell response to autologous TAA, which are also expressed by normal cells. In this regard, experiments in mouse models suggested that vaccination with xenogeneic TAAs (xenoantigens) encoding slight differences in sequence overcome immune tolerance by improving MHC I and II epitope presentation, evoking tumor immunity [42-45]. Thus, using Ad5 vectors encoding human CEA allowed us to more thoroughly investigate the potential of eliciting TAA-specific immune responses in dogs via a CD40-targeted Ad5 vector.
Antigen-specific T cell responses were generated in dogs receiving either the CD40-targeted vector or the untargeted vector. Interestingly, the extent of lymphoproliferation appeared to be enhanced in responders immunized with CD40-targeted Ad5 as compared to untargeted Ad5, though these responses will need to be investigated with a larger number of dogs in order to thoroughly evaluate statistical significance. Further, as observed with previous studies in mice [25], an antigen-specific humoral response was also observed following vaccination with either CD40-targeted or untargeted vectors in all dogs. However, a clear difference in the quality of the response was detected in the ratio of IgG1 to IgG2 isotypes generated by the two vectors. Though the relationship between Th type responses and IgG subclass has not been completely characterized in dogs [58], previous reports suggest that increased IgG2 production correlates with a Th1 type immune response that is characterized by a cytokine profile similar to that in humans [59-62]. Also similar to human patients, Th1 inducing cells, as determined by cytokine production, are diminished in dogs with metastatic cancers [63]. Suppression of DC-activated Th1 immunity has been implicated in the progression of cancers such as melanoma, thus an enhanced antigen-specific Th1 cellular immune response is likely to result in a more effective anti-tumor response. Future studies measuring cytokine release by T cells will be required for complete evaluation of the immune response generated, however.
In summary, these results from a pilot vaccination study in dogs confirm in a clinically relevant animal model that re-targeting Ad5 to bind CD40 circumvents the requirement for CAR expression, allowing efficient transgene expression in DCs in vitro, and subsequent antigen-specific immune responses in vivo. CD40-targeted Ad5 may in turn provide more effective cancer therapies. Most importantly, immune responses in these dogs are comparable to expected responses in humans, and establish that dogs provide a reliable intermediate model system for investigating potential immunotherapies for cancers such as osteosarcoma, lymphoma, breast cancer and melanoma. Thus, these experiments have provided the critical groundwork necessary to allow further evaluation of targeted immunotherapies in canine cancer patients in order to provide information for development of successful translational therapies.
Supplementary Material
Supplementary Fig. 1. Specificity and transduction efficiency of untargeted and CD40-targeted Ad5. Cd11c+/CD40+ and CD11c+/CD40- canine DCs (A, B) were isolated and transduced with vectors encoding GFP for visualization of cells expressing the transgene. 293 cells (C) were similarly transduced as controls. Cells were transduced with untargeted Ad5GFPLuc (A, C upper panels) or CD40-targeted Ad5GFPCEA.FF/hCD40L (B, C lower panels) at an MOI of 1000 vp/cell for DCs and 10 vp/cell for 293 cells. Cells were imaged for bright field (left panels) or GFP fluorescence (correlative right panels) 30 h later.
Acknowledgments
The authors thank A. Church Bird for valuable consultations on FACs and flow cytometry, Dr. Sandra Ewald for critical advice for optimizing DC culture conditions, and Dr. Maaike Everts for critical reading of the manuscript. This work was supported by: 1R01 CA113454 to BFS, NIAMS P30-AR050948 to LT and NIH training grants T32 CA075930-09 and T32 AR053458-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chen L. Immunological ignorance of silent antigens as an explanation of tumor evasion. Immunol Today. 1998;19(1):27–30. doi: 10.1016/s0167-5699(97)01180-8. [DOI] [PubMed] [Google Scholar]
- 2.Geijtenbeek TB, van Vliet SJ, Engering A, t Hart BA, van Kooyk Y. Self- and nonself-recognition by C-type lectins on dendritic cells. Annu Rev Immunol. 2004;22:33–54. doi: 10.1146/annurev.immunol.22.012703.104558. [DOI] [PubMed] [Google Scholar]
- 3.O'Hagan DT, Valiante NM. Recent advances in the discovery and delivery of vaccine adjuvants. Nat Rev Drug Discov. 2003;2(9):727–35. doi: 10.1038/nrd1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mosca PJ, Lyerly HK, Clay TM, Morse MA, Lyerly HK. Dendritic cell vaccines. Front Biosci. 2007;12:4050–60. doi: 10.2741/2371. [DOI] [PubMed] [Google Scholar]
- 5.Cerundolo V, Hermans IF, Salio M. Dendritic cells: a journey from laboratory to clinic. Nat Immunol. 2004;5(1):7–10. doi: 10.1038/ni0104-7. [DOI] [PubMed] [Google Scholar]
- 6.den Brok MH, Nierkens S, Figdor CG, Ruers TJ, Adema GJ. Dendritic cells: tools and targets for antitumor vaccination. Expert Rev Vaccines. 2005;4(5):699–710. doi: 10.1586/14760584.4.5.699. [DOI] [PubMed] [Google Scholar]
- 7.Tuyaerts S, Aerts JL, Corthals J, Neyns B, Heirman C, Breckpot K, et al. Current approaches in dendritic cell generation and future implications for cancer immunotherapy. Cancer Immunol Immunother. 2007;56(10):1513–37. doi: 10.1007/s00262-007-0334-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lesterhuis WJ, Aarntzen EH, De Vries IJ, Schuurhuis DH, Figdor CG, Adema GJ, et al. Dendritic cell vaccines in melanoma: from promise to proof? Crit Rev Oncol Hematol. 2008;66(2):118–34. doi: 10.1016/j.critrevonc.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Proudfoot O, Apostolopoulos V, Pietersz GA. Receptor-mediated delivery of antigens to dendritic cells: anticancer applications. Mol Pharm. 2007;4(1):58–72. doi: 10.1021/mp0601087. [DOI] [PubMed] [Google Scholar]
- 10.Arthur JF, Butterfield LH, Roth MD, Bui LA, Kiertscher SM, Lau R, et al. A comparison of gene transfer methods in human dendritic cells. Cancer Gene Ther. 1997;4(1):17–25. [PubMed] [Google Scholar]
- 11.Lotem M, Zhao Y, Riley J, Hwu P, Morgan RA, Rosenberg SA, et al. Presentation of tumor antigens by dendritic cells genetically modified with viral and nonviral vectors. J Immunother (1997) 2006;29(6):616–27. doi: 10.1097/01.cji.0000211312.36363.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Leeuwen EB, Cloosen S, Senden-Gijsbers BL, Germeraad WT, Bos GM. Transduction with a fiber-modified adenoviral vector is superior to non-viral nucleofection for expressing tumor-associated Ag mucin-1 in human DC. Cytotherapy. 2006;8(1):36–46. doi: 10.1080/14653240500508166. [DOI] [PubMed] [Google Scholar]
- 13.Zhong L, Granelli-Piperno A, Choi Y, Steinman RM. Recombinant adenovirus is an efficient and non-perturbing genetic vector for human dendritic cells. Eur J Immunol. 1999;29(3):964–72. doi: 10.1002/(SICI)1521-4141(199903)29:03<964::AID-IMMU964>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Tang Y, Akbulut H, Zelterman D, Linton PJ, Deisseroth AB. An adenoviral vector cancer vaccine that delivers a tumor-associated antigen/CD40-ligand fusion protein to dendritic cells. Proc Natl Acad Sci U S A. 2003;100(25):15101–6. doi: 10.1073/pnas.2135379100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho HI, Kim HJ, Oh ST, Kim TG. In vitro induction of carcinoembryonic antigen (CEA)-specific cytotoxic T lymphocytes by dendritic cells transduced with recombinant adenoviruses. Vaccine. 2003;22(2):224–36. doi: 10.1016/s0264-410x(03)00569-3. [DOI] [PubMed] [Google Scholar]
- 16.Noureddini SC, Curiel DT. Genetic targeting strategies for adenovirus. Mol Pharm. 2005;2(5):341–7. doi: 10.1021/mp050045c. [DOI] [PubMed] [Google Scholar]
- 17.Tillman BW, de Gruijl TD, Luykx-de Bakker SA, Scheper RJ, Pinedo HM, Curiel TJ, et al. Maturation of dendritic cells accompanies high-efficiency gene transfer by a CD40-targeted adenoviral vector. J Immunol. 1999;162(11):6378–83. [PubMed] [Google Scholar]
- 18.Pereboev AV, Nagle JM, Shakhmatov MA, Triozzi PL, Matthews QL, Kawakami Y, et al. Enhanced gene transfer to mouse dendritic cells using adenoviral vectors coated with a novel adapter molecule. Mol Ther. 2004;9(5):712–20. doi: 10.1016/j.ymthe.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Izumi M, Kawakami Y, Glasgow JN, Belousova N, Everts M, Kim-Park S, et al. In vivo analysis of a genetically modified adenoviral vector targeted to human CD40 using a novel transient transgenic model. J Gene Med. 2005;7(12):1517–25. doi: 10.1002/jgm.806. [DOI] [PubMed] [Google Scholar]
- 20.Korokhov N, Noureddini SC, Curiel DT, Santegoets SJ, Scheper RJ, de Gruijl TD. A single-component CD40-targeted adenovirus vector displays highly efficient transduction and activation of dendritic cells in a human skin substrate system. Mol Pharm. 2005;2(3):218–23. doi: 10.1021/mp050002w. [DOI] [PubMed] [Google Scholar]
- 21.Belousova N, Korokhov N, Krendelshchikova V, Simonenko V, Mikheeva G, Triozzi PL, et al. Genetically targeted adenovirus vector directed to CD40-expressing cells. J Virol. 2003;77(21):11367–77. doi: 10.1128/JVI.77.21.11367-11377.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clement A, Pereboev A, Curiel DT, Dong SS, Hutchings A, Thomas JM. Converting nonhuman primate dendritic cells into potent antigen-specific cellular immunosuppressants by genetic modification. Immunol Res. 2002;26(13):297–302. doi: 10.1385/ir:26:1-3:297. [DOI] [PubMed] [Google Scholar]
- 23.de Gruijl TD, Luykx-de Bakker SA, Tillman BW, van den Eertwegh AJ, Buter J, Lougheed SM, et al. Prolonged maturation and enhanced transduction of dendritic cells migrated from human skin explants after in situ delivery of CD40-targeted adenoviral vectors. J Immunol. 2002;169(9):5322–31. doi: 10.4049/jimmunol.169.9.5322. [DOI] [PubMed] [Google Scholar]
- 24.Pereboev AV, Asiedu CK, Kawakami Y, Dong SS, Blackwell JL, Kashentseva EA, et al. Coxsackievirus-adenovirus receptor genetically fused to anti-human CD40 scFv enhances adenoviral transduction of dendritic cells. Gene Ther. 2002;9(17):1189–93. doi: 10.1038/sj.gt.3301767. [DOI] [PubMed] [Google Scholar]
- 25.Huang D, Pereboev AV, Korokhov N, He R, Larocque L, Gravel C, et al. Significant alterations of biodistribution and immune responses in Balb/c mice administered with adenovirus targeted to CD40(+) cells. Gene Ther. 2008;15(4):298–308. doi: 10.1038/sj.gt.3303085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paoloni M, Khanna C. Translation of new cancer treatments from pet dogs to humans. Nat Rev Cancer. 2008;8(2):147–56. doi: 10.1038/nrc2273. [DOI] [PubMed] [Google Scholar]
- 27.Khanna C, Lindblad-Toh K, Vail D, London C, Bergman P, Barber L, et al. The dog as a cancer model. Nat Biotechnol. 2006;24(9):1065–6. doi: 10.1038/nbt0906-1065b. [DOI] [PubMed] [Google Scholar]
- 28.Porrello A, Cardelli P, Spugnini EP. Oncology of companion animals as a model for humans. an overview of tumor histotypes. J Exp Clin Cancer Res. 2006;25(1):97–105. [PubMed] [Google Scholar]
- 29.Waters DJ, Wildasin K. Cancer clues from pet dogs. Sci Am. 2006;295(6):94–101. doi: 10.1038/scientificamerican1206-94. [DOI] [PubMed] [Google Scholar]
- 30.Smith BF, Baker HJ, Curiel DT, Jiang W, Conry RM. Humoral and cellular immune responses of dogs immunized with a nucleic acid vaccine encoding human carcinoembryonic antigen. Gene Ther. 1998;5(7):865–8. doi: 10.1038/sj.gt.3300675. [DOI] [PubMed] [Google Scholar]
- 31.Casal M, Haskins M. Large animal models and gene therapy. Eur J Hum Genet. 2006;14(3):266–72. doi: 10.1038/sj.ejhg.5201535. [DOI] [PubMed] [Google Scholar]
- 32.Seki T, Dmitriev I, Kashentseva E, Takayama K, Rots M, Suzuki K, et al. Artificial extension of the adenovirus fiber shaft inhibits infectivity in coxsackievirus and adenovirus receptor-positive cell lines. J Virol. 2002;76(3):1100–8. doi: 10.1128/JVI.76.3.1100-1108.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krasnykh V, Belousova N, Korokhov N, Mikheeva G, Curiel DT. Genetic targeting of an adenovirus vector via replacement of the fiber protein with the phage T4 fibritin. J Virol. 2001;75(9):4176–83. doi: 10.1128/JVI.75.9.4176-4183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graham FL, Prevec L. Methods for construction of adenovirus vectors. Mol Biotechnol. 1995;3(3):207–20. doi: 10.1007/BF02789331. [DOI] [PubMed] [Google Scholar]
- 35.Oster W, Lindemann A, Mertelsmann R, Herrmann F. Regulation of gene expression of M-, G-, GM-, and multi-CSF in normal and malignant hematopoietic cells. Blood Cells. 1988;14(23):443–62. [PubMed] [Google Scholar]
- 36.Lu L, Srour EF, Warren DJ, Walker D, Graham CD, Walker EB, et al. Enhancement of release of granulocyte- and granulocyte-macrophage colony-stimulating factors from phytohemagglutinin-stimulated sorted subsets of human T lymphocytes by recombinant human tumor necrosis factor-alpha. Synergism with recombinant human IFN-gamma. J Immunol. 1988;141(1):201–7. [PubMed] [Google Scholar]
- 37.Krasnykh VN, Mikheeva GV, Douglas JT, Curiel DT. Generation of recombinant adenovirus vectors with modified fibers for altering viral tropism. J Virol. 1996;70(10):6839–46. doi: 10.1128/jvi.70.10.6839-6846.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perreau M, Mennechet F, Serratrice N, Glasgow JN, Curiel DT, Wodrich H, et al. Contrasting effects of human, canine, and hybrid adenovirus vectors on the phenotypical and functional maturation of human dendritic cells: implications for clinical efficacy. J Virol. 2007;81(7):3272–84. doi: 10.1128/JVI.01530-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Korokhov N, Mikheeva G, Krendelshchikov A, Belousova N, Simonenko V, Krendelshchikova V, et al. Targeting of adenovirus via genetic modification of the viral capsid combined with a protein bridge. J Virol. 2003;77(24):12931–40. doi: 10.1128/JVI.77.24.12931-12940.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bajorath J, Chalupny NJ, Marken JS, Siadak AW, Skonier J, Gordon M, et al. Identification of residues on CD40 and its ligand which are critical for the receptor-ligand interaction. Biochemistry. 1995;34(6):1833–44. doi: 10.1021/bi00006a003. [DOI] [PubMed] [Google Scholar]
- 41.Bajorath J. Detailed comparison of two molecular models of the human CD40 ligand with an x-ray structure and critical assessment of model-based mutagenesis and residue mapping studies. J Biol Chem. 1998;273(38):24603–9. doi: 10.1074/jbc.273.38.24603. [DOI] [PubMed] [Google Scholar]
- 42.Weber LW, Bowne WB, Wolchok JD, Srinivasan R, Qin J, Moroi Y, et al. Tumor immunity and autoimmunity induced by immunization with homologous DNA. J Clin Invest. 1998;102(6):1258–64. doi: 10.1172/JCI4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gold JS, Ferrone CR, Guevara-Patino JA, Hawkins WG, Dyall R, Engelhorn ME, et al. A single heteroclitic epitope determines cancer immunity after xenogeneic DNA immunization against a tumor differentiation antigen. J Immunol. 2003;170(10):5188–94. doi: 10.4049/jimmunol.170.10.5188. [DOI] [PubMed] [Google Scholar]
- 44.Naftzger C, Takechi Y, Kohda H, Hara I, Vijayasaradhi S, Houghton AN. Immune response to a differentiation antigen induced by altered antigen: a study of tumor rejection and autoimmunity. Proc Natl Acad Sci U S A. 1996;93(25):14809–14. doi: 10.1073/pnas.93.25.14809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hawkins WG, Gold JS, Dyall R, Wolchok JD, Hoos A, Bowne WB, et al. Immunization with DNA coding for gp100 results in CD4 T-cell independent antitumor immunity. Surgery. 2000;128(2):273–80. doi: 10.1067/msy.2000.107421. [DOI] [PubMed] [Google Scholar]
- 46.Mahnke K, Qian Y, Fondel S, Brueck J, Becker C, Enk AH. Targeting of antigens to activated dendritic cells in vivo cures metastatic melanoma in mice. Cancer Res. 2005;65(15):7007–12. doi: 10.1158/0008-5472.CAN-05-0938. [DOI] [PubMed] [Google Scholar]
- 47.Hauser H, Shen L, Gu QL, Krueger S, Chen SY. Secretory heat-shock protein as a dendritic cell-targeting molecule: a new strategy to enhance the potency of genetic vaccines. Gene Ther. 2004;11(11):924–32. doi: 10.1038/sj.gt.3302160. [DOI] [PubMed] [Google Scholar]
- 48.Bonifaz LC, Bonnyay DP, Charalambous A, Darguste DI, Fujii S, Soares H, et al. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med. 2004;199(6):815–24. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang L, Yang H, Rideout K, Cho T, Joo KI, Ziegler L, et al. Engineered lentivector targeting of dendritic cells for in vivo immunization. Nat Biotechnol. 2008;26(3):326–34. doi: 10.1038/nbt1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kretz-Rommel A, Qin F, Dakappagari N, Torensma R, Faas S, Wu D, et al. In Vivo Targeting of Antigens to Human Dendritic Cells Through DC-SIGN Elicits Stimulatory Immune Responses and Inhibits Tumor Growth in Grafted Mouse Models. J Immunother (1997) 2007;30(7):715–26. doi: 10.1097/CJI.0b013e318135472c. [DOI] [PubMed] [Google Scholar]
- 51.Pereira CF, Torensma R, Hebeda K, Kretz-Rommel A, Faas SJ, Figdor CG, et al. In Vivo Targeting of DC-SIGN-positive Antigen-presenting Cells in a Nonhuman Primate Model. J Immunother (1997) 2007;30(7):705–14. doi: 10.1097/CJI.0b013e31812e6256. [DOI] [PubMed] [Google Scholar]
- 52.Mossoba ME, Medin JA. Cancer immunotherapy using virally transduced dendritic cells: animal studies and human clinical trials. Expert Rev Vaccines. 2006;5(5):717–32. doi: 10.1586/14760584.5.5.717. [DOI] [PubMed] [Google Scholar]
- 53.Basak SK, Kiertscher SM, Harui A, Roth MD. Modifying adenoviral vectors for use as gene-based cancer vaccines. Viral Immunol. 2004;17(2):182–96. doi: 10.1089/0882824041310603. [DOI] [PubMed] [Google Scholar]
- 54.Molinier-Frenkel V, Lengagne R, Gaden F, Hong SS, Choppin J, Gahery-Segard H, et al. Adenovirus hexon protein is a potent adjuvant for activation of a cellular immune response. J Virol. 2002;76(1):127–35. doi: 10.1128/JVI.76.1.127-135.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Banchereau J. The long arm of the immune system. Sci Am. 2002;287(5):52–9. doi: 10.1038/scientificamerican1102-52. [DOI] [PubMed] [Google Scholar]
- 56.Finkelman FD, Lees A, Birnbaum R, Gause WC, Morris SC. Dendritic cells can present antigen in vivo in a tolerogenic or immunogenic fashion. J Immunol. 1996;157(4):1406–14. [PubMed] [Google Scholar]
- 57.Mackey MF, Gunn JR, Maliszewsky C, Kikutani H, Noelle RJ, Barth RJ., Jr Dendritic cells require maturation via CD40 to generate protective antitumor immunity. J Immunol. 1998;161(5):2094–8. [PubMed] [Google Scholar]
- 58.Day MJ. Immunoglobulin G subclass distribution in canine leishmaniosis: a review and analysis of pitfalls in interpretation. Vet Parasitol. 2007;147(12):2–8. doi: 10.1016/j.vetpar.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 59.Carvalho LH, Sano G, Hafalla JC, Morrot A, Curotto de Lafaille MA, Zavala F. IL-4-secreting CD4+ T cells are crucial to the development of CD8+ T-cell responses against malaria liver stages. Nat Med. 2002;8(2):166–70. doi: 10.1038/nm0202-166. [DOI] [PubMed] [Google Scholar]
- 60.Boag PR, Parsons JC, Presidente PJ, Spithill TW, Sexton JL. Characterisation of humoral immune responses in dogs vaccinated with irradiated Ancylostoma caninum. Vet Immunol Immunopathol. 2003;92(12):87–94. doi: 10.1016/s0165-2427(03)00006-0. [DOI] [PubMed] [Google Scholar]
- 61.Fujiwara RT, Loukas A, Mendez S, Williamson AL, Bueno LL, Wang Y, et al. Vaccination with irradiated Ancylostoma caninum third stage larvae induces a Th2 protective response in dogs. Vaccine. 2006;24(4):501–9. doi: 10.1016/j.vaccine.2005.07.091. [DOI] [PubMed] [Google Scholar]
- 62.Breathnach RM, Fanning S, Mulcahy G, Bassett HF, Jones BR, Daly P. Evaluation of Th1-like, Th2-like and immunomodulatory cytokine mRNA expression in the skin of dogs with immunomodulatory-responsive lymphocytic-plasmacytic pododermatitis. Vet Dermatol. 2006;17(5):313–21. doi: 10.1111/j.1365-3164.2006.00534.x. [DOI] [PubMed] [Google Scholar]
- 63.Horiuchi Y, Hanazawa A, Nakajima Y, Nariai Y, Asanuma H, Kuwabara M, et al. T-helper (Th)1/Th2 imbalance in the peripheral blood of dogs with malignant tumor. Microbiol Immunol. 2007;51(11):1135–8. doi: 10.1111/j.1348-0421.2007.tb03999.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. Specificity and transduction efficiency of untargeted and CD40-targeted Ad5. Cd11c+/CD40+ and CD11c+/CD40- canine DCs (A, B) were isolated and transduced with vectors encoding GFP for visualization of cells expressing the transgene. 293 cells (C) were similarly transduced as controls. Cells were transduced with untargeted Ad5GFPLuc (A, C upper panels) or CD40-targeted Ad5GFPCEA.FF/hCD40L (B, C lower panels) at an MOI of 1000 vp/cell for DCs and 10 vp/cell for 293 cells. Cells were imaged for bright field (left panels) or GFP fluorescence (correlative right panels) 30 h later.