Abstract
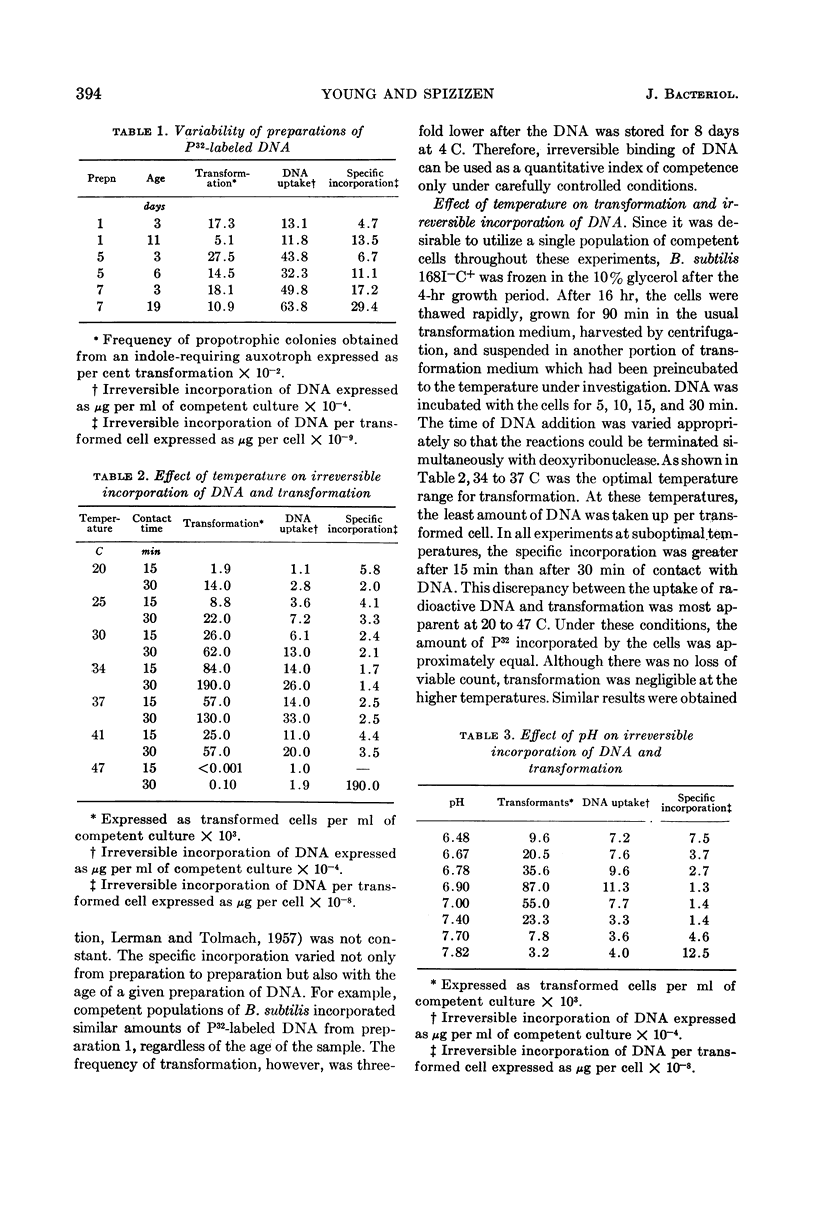
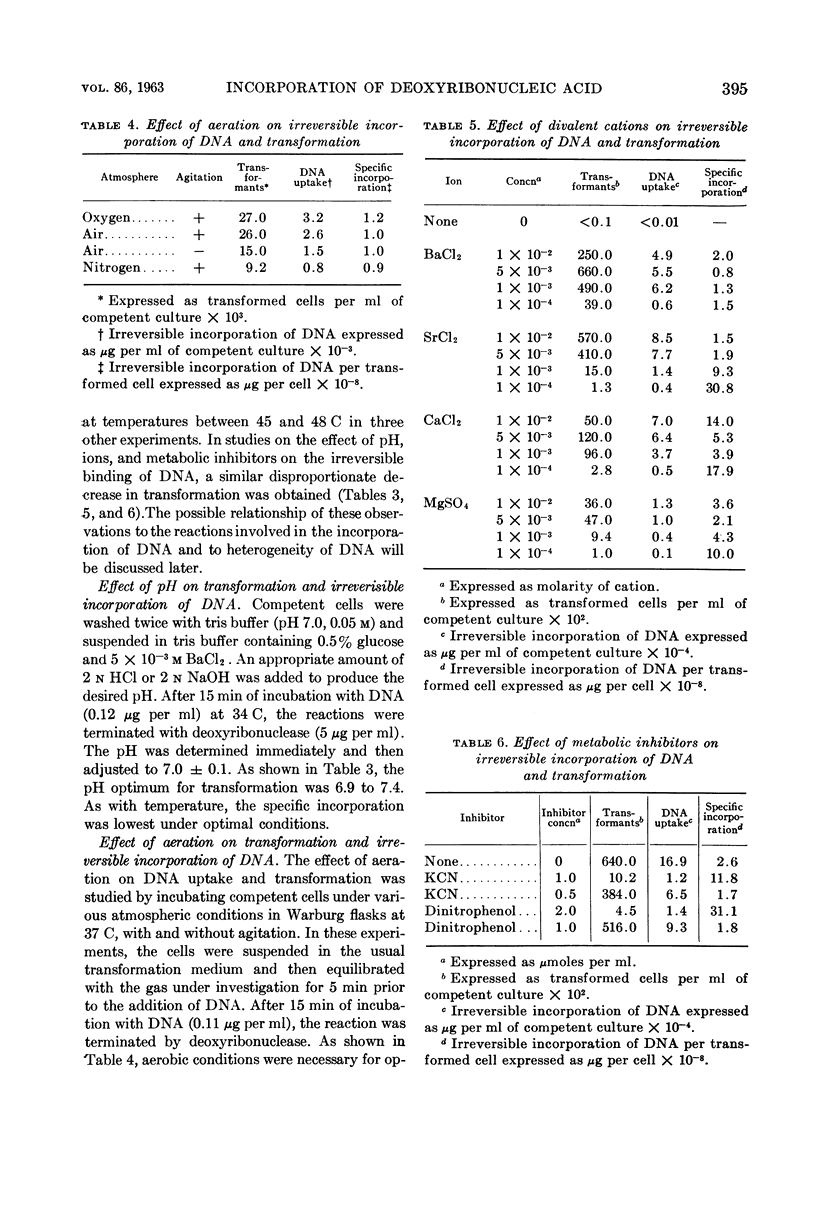
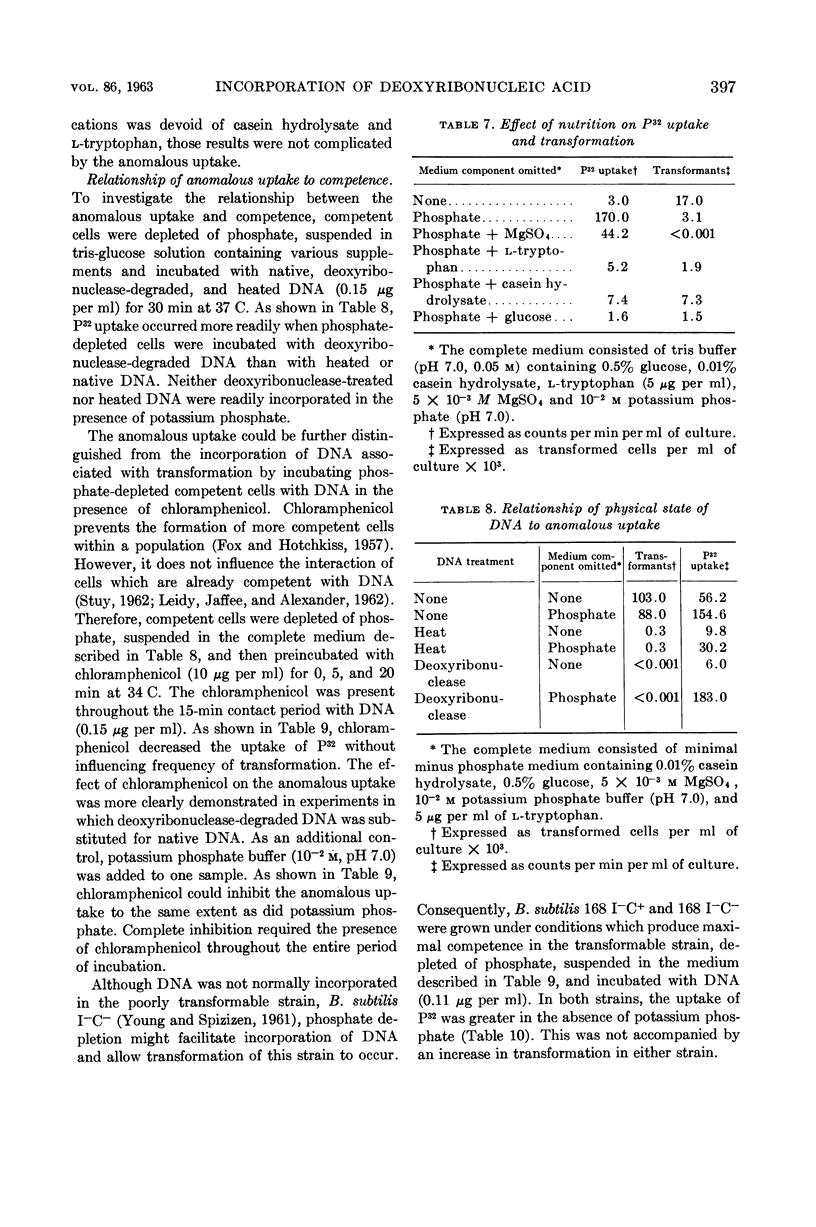
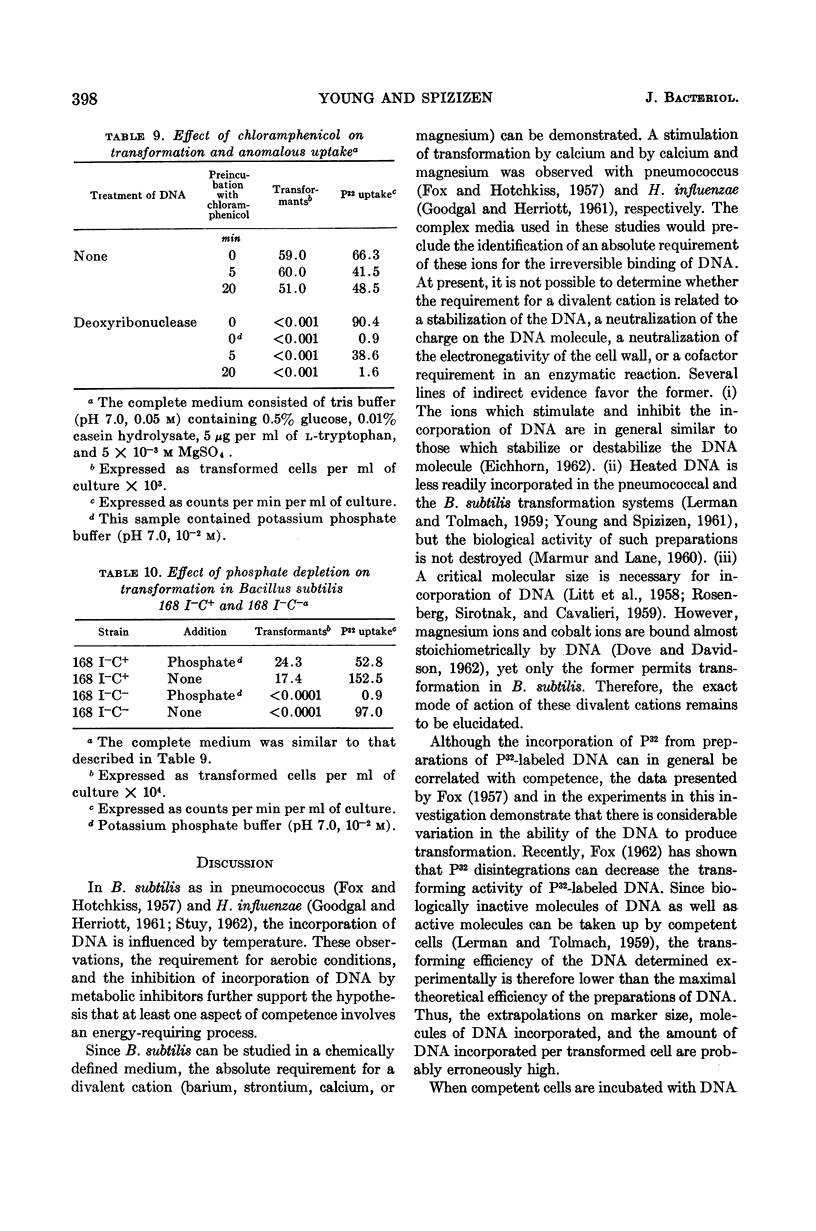
Young, F. E. (Western Reserve University Cleveland, Ohio) and John Spizizen. Incorporation of deoxyribonucleic acid in the Bacillus subtilis transformation system. J. Bacteriol. 86:392–400. 1963.—The optimal conditions for the incorporation of deoxyribonucleic acid (DNA) were studied. In competent cells, the irreversible binding of DNA was influenced by temperature, hydrogen ion concentration, and aeration. Divalent cations, such as barium, strontium, calcium, or magnesium, were required. Under suboptimal environmental conditions and with metabolic inhibitors, the process of transformation was decreased to a greater extent than was incorporation of DNA. Under conditions of phosphate depletion, the incorporation of P32 increased. However, the frequency of transformation decreased. This inducible process was not related to competence.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EICHHORN G. L. Metal ions as stabilizers or destabilizers of the deoxyriboucleic acid structure. Nature. 1962 May 5;194:474–475. doi: 10.1038/194474a0. [DOI] [PubMed] [Google Scholar]
- EPHRUSSI-TAYLOR H. [The status of the transforming DNA during the 1st phases of bacterial transformation]. C R Seances Soc Biol Fil. 1960;154:1951–1955. [PubMed] [Google Scholar]
- FOX M. S. Deoxyribonucleic acid incorporation by transformed bacteria. Biochim Biophys Acta. 1957 Oct;26(1):83–85. doi: 10.1016/0006-3002(57)90056-2. [DOI] [PubMed] [Google Scholar]
- FOX M. S., HOTCHKISS R. D. Fate of transforming deoxyribonucleate following fixation by transformable bacteria. Nature. 1960 Sep 17;187:1002–1006. doi: 10.1038/1871002a0. [DOI] [PubMed] [Google Scholar]
- FOX M. S., HOTCHKISS R. D. Initiation of bacterial transformation. Nature. 1957 Jun 29;179(4574):1322–1325. doi: 10.1038/1791322a0. [DOI] [PubMed] [Google Scholar]
- FOX M. S. The fate of transforming deoxyribonucleate following fixation by transformable bacteria. III. Proc Natl Acad Sci U S A. 1962 Jun 15;48:1043–1048. doi: 10.1073/pnas.48.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODGAL S. H., HERRIOTT R. M. Studies on transformations of Hemophilus influenzae. I. Competence. J Gen Physiol. 1961 Jul;44:1201–1227. doi: 10.1085/jgp.44.6.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOHIYAMA M., SAITO H. Stimulation of genetic transformation in Bacillus subtilis by biologically inactive DNA and polyphosphate. Biochim Biophys Acta. 1960 Jun 17;41:180–181. doi: 10.1016/0006-3002(60)90396-6. [DOI] [PubMed] [Google Scholar]
- LACKS S. Molecular fate of DNA in genetic transformation of Pneumococcus. J Mol Biol. 1962 Jul;5:119–131. doi: 10.1016/s0022-2836(62)80067-9. [DOI] [PubMed] [Google Scholar]
- LEIDY G., JAFFEE I., ALEXANDER H. E. Emergence of competence (for transformation) of three Hemophilus species in a chemically defined environment. Proc Soc Exp Biol Med. 1962 Dec;111:725–731. doi: 10.3181/00379727-111-27904. [DOI] [PubMed] [Google Scholar]
- LERMAN L. S., TOLMACH L. J. Genetic transformation. I. Cellular incorporation of DNA accompanying transformation in Pneumococcus. Biochim Biophys Acta. 1957 Oct;26(1):68–82. doi: 10.1016/0006-3002(57)90055-0. [DOI] [PubMed] [Google Scholar]
- LERMAN L. S., TOLMACH L. J. Genetic transformation. II. The significance of damage to the DNA molecule. Biochim Biophys Acta. 1959 Jun;33(2):371–387. doi: 10.1016/0006-3002(59)90127-1. [DOI] [PubMed] [Google Scholar]
- Litt M., Marmur J., Ephrussi-Taylor H., Doty P. THE DEPENDENCE OF PNEUMOCOCCAL TRANSFORMATION ON THE MOLECULAR WEIGHT OF DEOXYRIBOSE NUCLEIC ACID. Proc Natl Acad Sci U S A. 1958 Feb;44(2):144–152. doi: 10.1073/pnas.44.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmur J., Lane D. STRAND SEPARATION AND SPECIFIC RECOMBINATION IN DEOXYRIBONUCLEIC ACIDS: BIOLOGICAL STUDIES. Proc Natl Acad Sci U S A. 1960 Apr;46(4):453–461. doi: 10.1073/pnas.46.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRY D., SLADE H. D. Transformation of streptococci to streptomycin resistance. J Bacteriol. 1962 Mar;83:443–449. doi: 10.1128/jb.83.3.443-449.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAVIN A. W. The genetics of transformation. Adv Genet. 1961;10:61–163. doi: 10.1016/s0065-2660(08)60116-9. [DOI] [PubMed] [Google Scholar]
- Rosenberg B. H., Sirotnak F. M., Cavalieri L. F. ON THE SIZE OF GENETIC DETERMINANTS IN PNEUMOCOCCUS AND THE NATURE OF THE VARIABLES INVOLVED IN TRANSFORMATION. Proc Natl Acad Sci U S A. 1959 Feb;45(2):144–156. doi: 10.1073/pnas.45.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUY J. H. Transformability of Haemophilus influenzae. J Gen Microbiol. 1962 Nov;29:537–549. doi: 10.1099/00221287-29-3-537. [DOI] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOLL M. J., GOODGAL S. H. Recombination during transformation in Hemophilus influenzae. Proc Natl Acad Sci U S A. 1961 Apr 15;47:505–512. doi: 10.1073/pnas.47.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUNG F. E., SPIZIZEN J. Physiological and genetic factors affecting transformation of Bacillus subtilis. J Bacteriol. 1961 May;81:823–829. doi: 10.1128/jb.81.5.823-829.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]


