Abstract
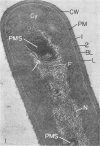
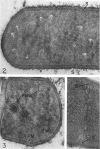
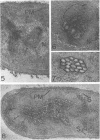
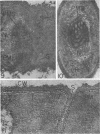
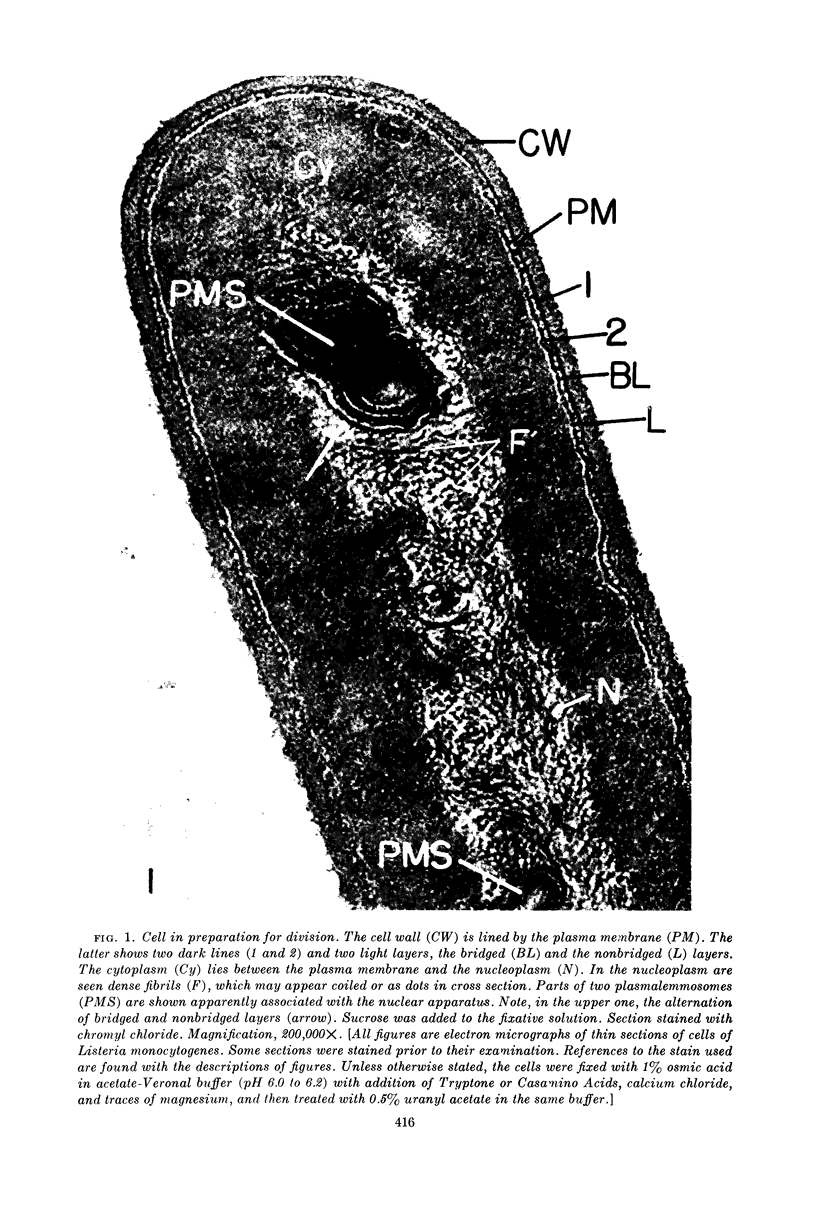
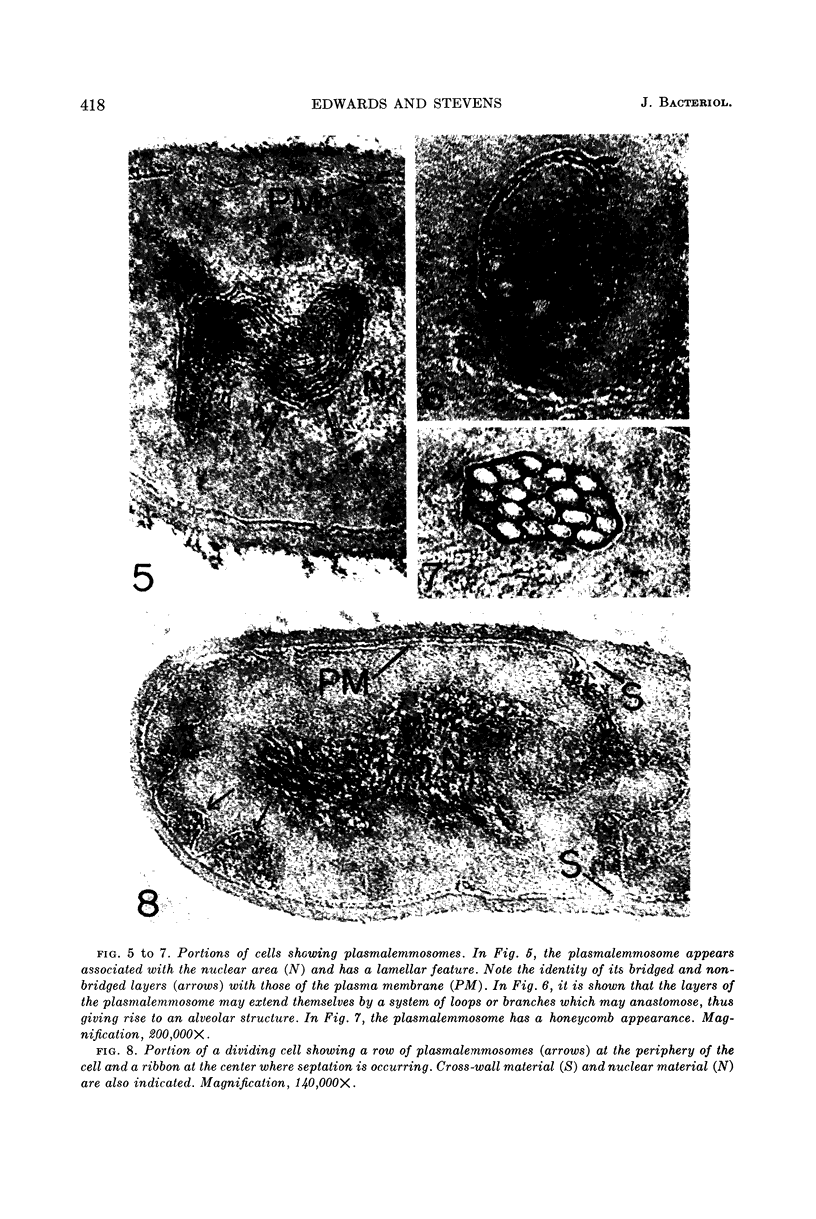
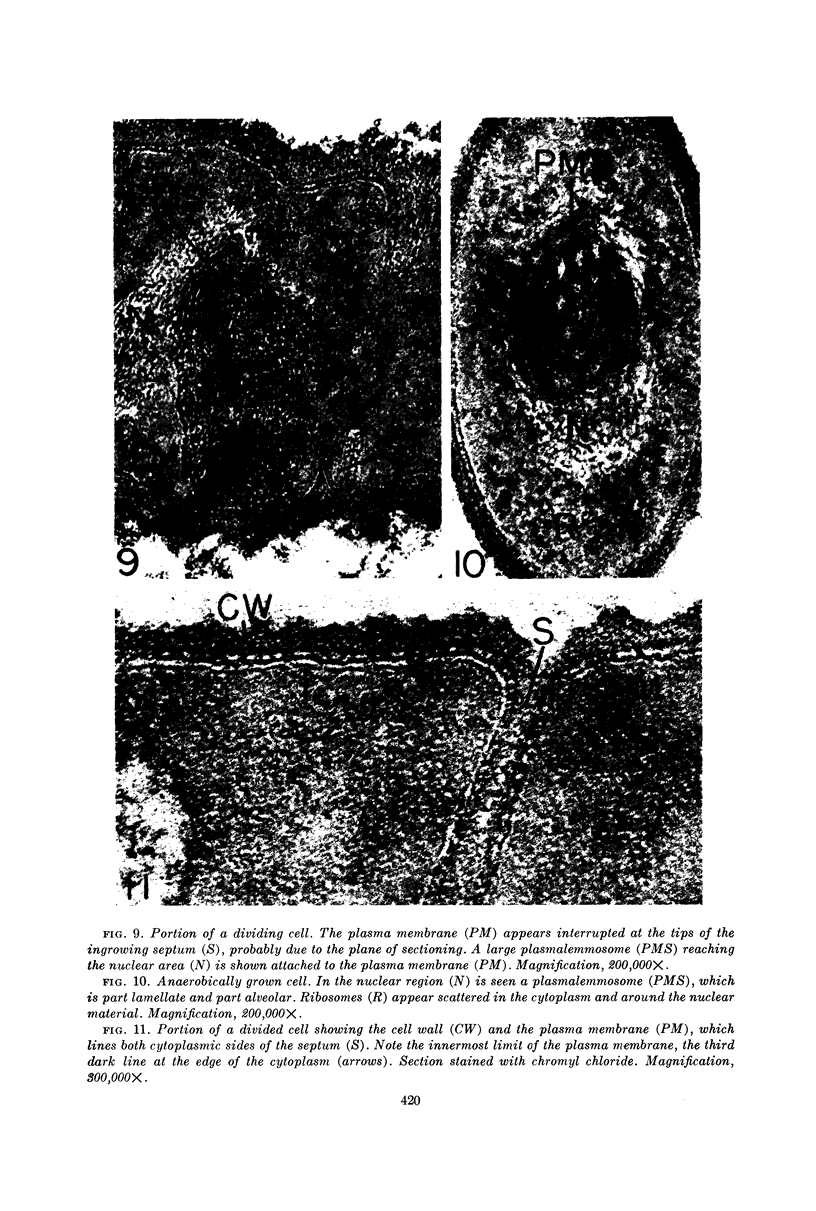
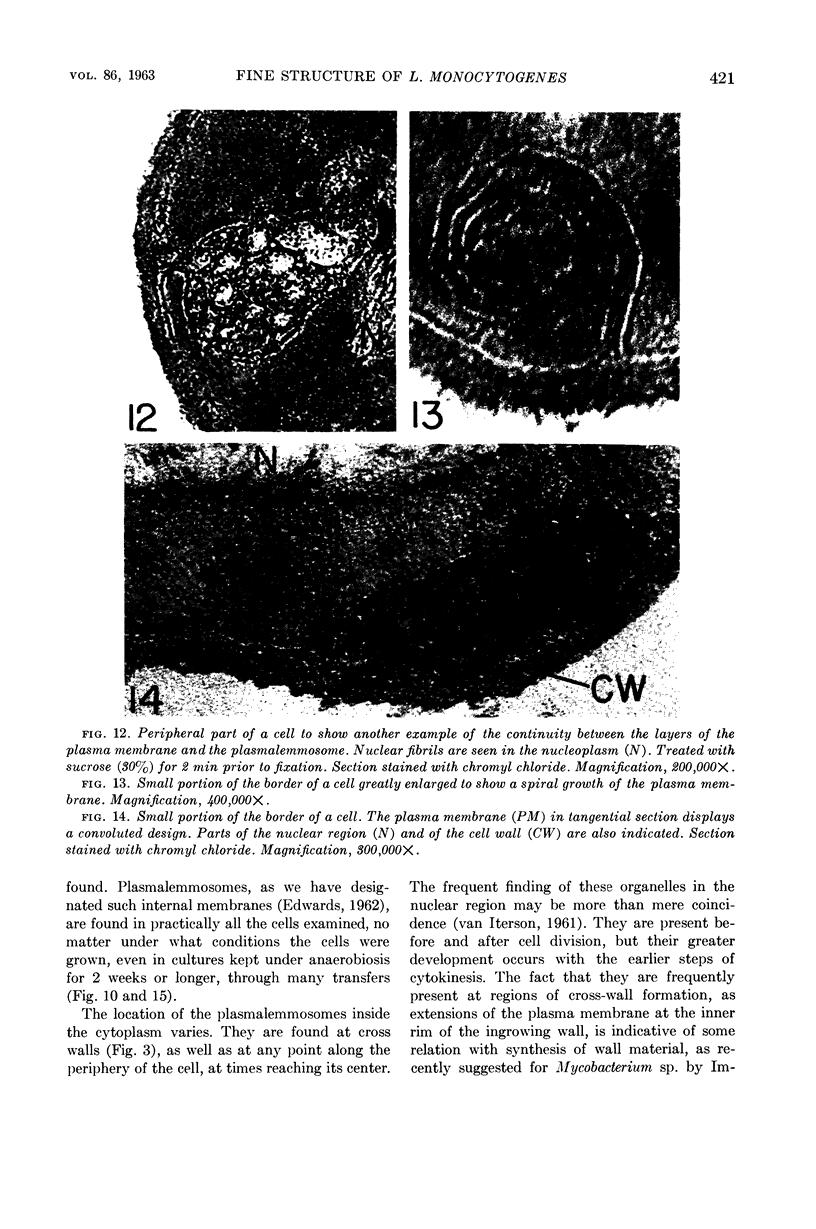
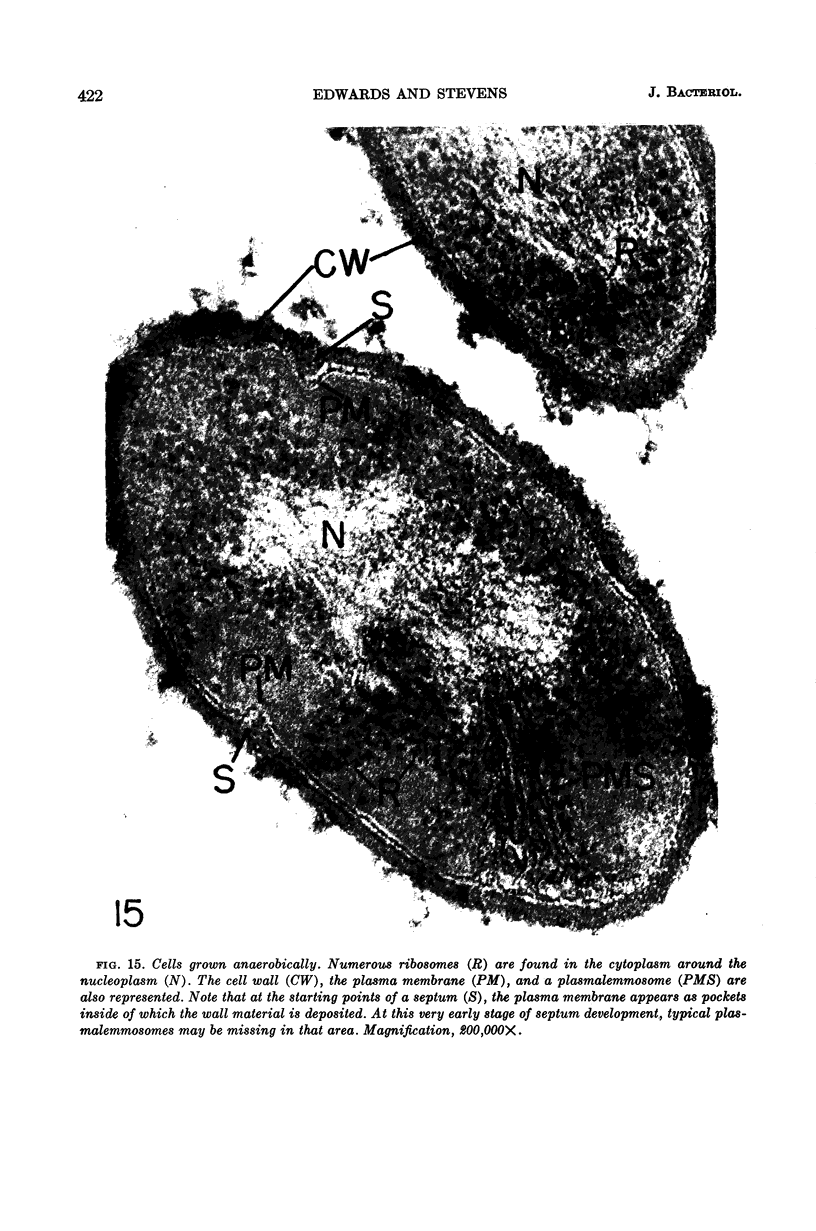
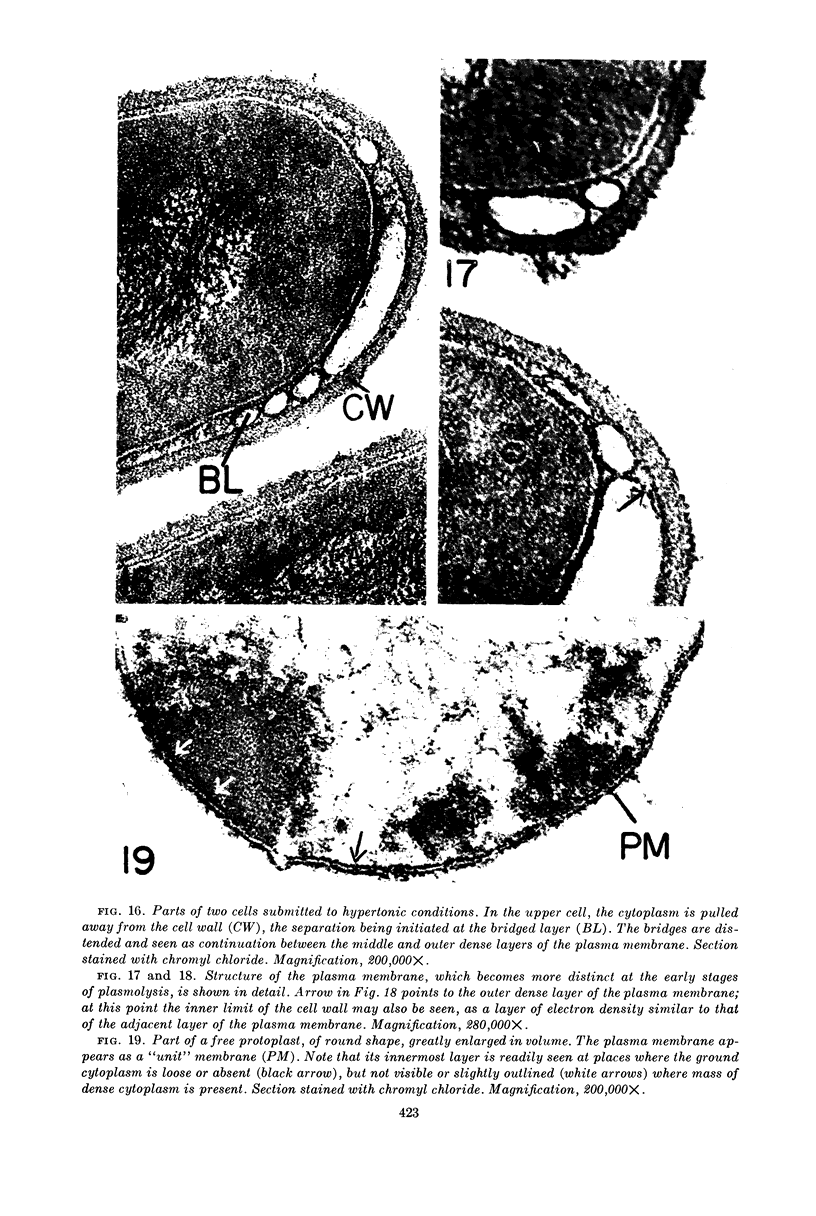
Edwards, Mercedes R. (New York State Department of Health, Albany) and Roy W. Stevens. Fine structure of Listeria monocytogenes. J. Bacteriol. 86:414–428. 1963.—Cells of Listeria monocytogenes, at different stages of growth, were fixed with osmium tetroxide and treated with uranyl acetate. The material was dehydrated in alcohol, embedded in prepolymerized methacrylate, and studied in thin sections. In most of the micrographs, the plasmalemma (or plasma membrane) showed a pattern of three dense lines, each ca. 25 A thick, alternating with two light zones, each ca. 30 A thick. The outer light zone was regularly bridged by strands of dense material, and the inner one was not. The dense line at the edge of the cytoplasm was not always discernible because of its similarity in density with the ground cytoplasm, although it could be easily demonstrated in lysed cells and in protoplasts. The latter were found to be limited by a pair of dense lines, each ca. 25 A thick, bounding a light core ca. 30 A thick. This structure corresponds to a “unit” membrane, but it represents only a part of the plasmalemma of the intact cell; it was therefore interpreted as being more complex than a single unit membrane. Intracytoplasmic membranes of various configurations were clearly shown to be extensions of the plasmalemma. They may branch repeatedly and anastomose to form a complicated honeycomb-like organelle or organelles of different appearances, sometimes lamellate. The lamellar bodies are envisioned as resulting from spiraled ingrowths. The various kinds of ingrowths of the plasmalemma were designated “plasmalemmosomes” to indicate their origin; however, some of these organelles in Listeria were similar to those described in different bacteria by other authors. Plasmalemmosomes have been found in both aerobically and anaerobically grown cells. Another outstanding feature in many micrographs was the nucleoid, which contains dense fibrils measuring 25 to 50 A in diameter. These fibrils frequently appeared to be coiled and were of the order of magnitude ascribed to deoxyribonucleic acid molecules.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- CHAPMAN G. B. Electron microscope observations on the behavior of the bacterial cytoplasmic membrane during cellular division. J Biophys Biochem Cytol. 1959 Oct;6:221–224. doi: 10.1083/jcb.6.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAPMAN G. B. Electron microscopy of ultrathin sections of bacteria. III. Cell wall, cytoplasmic membrane, and nuclear material. J Bacteriol. 1959 Jul;78(1):96–104. doi: 10.1128/jb.78.1.96-104.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAPMAN G. B., HANKS J. H., WALLACE J. H. An electron microscope study of the disposition and fine structure of Mycobacterium lepraemurium in mouse spleen. J Bacteriol. 1959 Feb;77(2):205–211. doi: 10.1128/jb.77.2.205-211.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAPMAN G. B., HILLIER J. Electron microscopy of ultra-thin sections of bacteria I. Cellular division in Bacillus cereus. J Bacteriol. 1953 Sep;66(3):362–373. doi: 10.1128/jb.66.3.362-373.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., KUNISAWA R. The fine structure of Rhodospirillum rubrum. J Cell Biol. 1963 Feb;16:401–419. doi: 10.1083/jcb.16.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COTA-ROBLES E. H. ELECTRON MICROSCOPY OF PLASMOLYSIS IN ESCHERICHIA COLI. J Bacteriol. 1963 Mar;85:499–503. doi: 10.1128/jb.85.3.499-503.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON I. M., STERN H. Structure in the bacterial cell-wall during cell division. Biochim Biophys Acta. 1954 Jan;13(1):31–40. doi: 10.1016/0006-3002(54)90267-x. [DOI] [PubMed] [Google Scholar]
- DREWS G. [Electron microscopic studies on Mycobacterium phlei. (Structure and formation of metachromatic granula)]. Arch Mikrobiol. 1960;35:53–62. [PubMed] [Google Scholar]
- FITZ-JAMES P. C. Participation of the cytoplasmic membrane in the growth and spore fromation of bacilli. J Biophys Biochem Cytol. 1960 Oct;8:507–528. doi: 10.1083/jcb.8.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLAUERT A. M., BRIEGER E. M., ALLEN J. M. The fine structure of vegetative cells of Bacillus subtilis. Exp Cell Res. 1961 Jan;22:73–85. doi: 10.1016/0014-4827(61)90087-8. [DOI] [PubMed] [Google Scholar]
- GLAUERT A. M., HOPWOOD D. A. A membranous component of the cytoplasm in Streptomyces coelicolor. J Biophys Biochem Cytol. 1959 Dec;6:515–516. doi: 10.1083/jcb.6.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLAUERT A. M., HOPWOOD D. A. The fine structure of Streptomyces coelicolor. I. The cytoplasmic membrane system. J Biophys Biochem Cytol. 1960 Jun;7:479–488. doi: 10.1083/jcb.7.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLAUERT A. M. The fine structure of bacteria. Br Med Bull. 1962 Sep;18:245–250. doi: 10.1093/oxfordjournals.bmb.a069988. [DOI] [PubMed] [Google Scholar]
- HAYES W. Conjugation in Escherichia coli. Br Med Bull. 1962 Jan;18:36–40. doi: 10.1093/oxfordjournals.bmb.a069932. [DOI] [PubMed] [Google Scholar]
- HOPWOOD D. A., GLAUERT A. M. The fine structure of Streptomyces coelicolor. II. The nuclear material. J Biophys Biochem Cytol. 1960 Sep;8:267–278. doi: 10.1083/jcb.8.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IMAEDA T., OGURA M. Formation of intracytoplasmic membrane system of mycobacteria related to cell division. J Bacteriol. 1963 Jan;85:150–163. doi: 10.1128/jb.85.1.150-163.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOIKE M., TAKEYA K. Fine structures of intracytoplasmic organelles of mycobacteria. J Biophys Biochem Cytol. 1961 Mar;9:597–608. doi: 10.1083/jcb.9.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUDD S. Cytology of bacteria. I. The bacterial cell. Annu Rev Microbiol. 1954;8:1–22. doi: 10.1146/annurev.mi.08.100154.000245. [DOI] [PubMed] [Google Scholar]
- MUDD S., WINTERSCHEID L. C., DeLAMATER E. D., HENDERSON H. J. Evidence suggesting that the granules of mycobacteria are mitochondria. J Bacteriol. 1951 Oct;62(4):459–475. doi: 10.1128/jb.62.4.459-475.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIKLOWITZ W. Mitochondrienäquivalente bei Escherichia Coli. Zentralbl Bakteriol Orig. 1958 Nov;173(1-2):12–24. [PubMed] [Google Scholar]
- OHYE D. F., MURRELL W. G. Formation and structure of the spore of Bacillus coagulans. J Cell Biol. 1962 Jul;14:111–123. doi: 10.1083/jcb.14.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERS A. The formation and structure of myelin sheaths in the central nervous system. J Biophys Biochem Cytol. 1960 Oct;8:431–446. doi: 10.1083/jcb.8.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTSON J. D. The ultrastructure of cell membranes and their derivatives. Biochem Soc Symp. 1959;16:3–43. [PubMed] [Google Scholar]
- ROBINOW C. F. Morphology of the bacterial nucleus. Br Med Bull. 1962 Jan;18:31–35. doi: 10.1093/oxfordjournals.bmb.a069931. [DOI] [PubMed] [Google Scholar]
- ROBINOW C. F. On the plasma membrane of some bacteria and fungi. Circulation. 1962 Nov;26:1092–1104. doi: 10.1161/01.cir.26.5.1092. [DOI] [PubMed] [Google Scholar]
- RYTER A., KELLENBERGER E., BIRCHANDERSEN A., MAALOE O. Etude au microscope électronique de plasmas contenant de l'acide désoxyribonucliéique. I. Les nucléoides des bactéries en croissance active. Z Naturforsch B. 1958 Sep;13B(9):597–605. [PubMed] [Google Scholar]
- SHINOHARA C., FUKUSHI K., SUZUKI J. Mitochondria-like structures in ultrathin sections of Mycobacterium avium. J Bacteriol. 1957 Sep;74(3):413–415. doi: 10.1128/jb.74.3.413-415.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH C. W., METZGER J. F. Demonstration of a capsular structure on Listeria monocytogenes. Pathol Microbiol (Basel) 1962;25:499–506. doi: 10.1159/000161305. [DOI] [PubMed] [Google Scholar]
- STUART D. C., Jr Fine structure of the nucleoid and internal membrane systems of Streptomyces. J Bacteriol. 1959 Aug;78:272–281. doi: 10.1128/jb.78.2.272-281.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN ITERSON W., ROBINOW C. F. Observations with the electron microscope on the fine structure of the nuclei of two spherical bacteria. J Biophys Biochem Cytol. 1961 Jan;9:171–181. doi: 10.1083/jcb.9.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN ITERSON W. Some features of a remarkable organelle in Bacillus subtilis. J Biophys Biochem Cytol. 1961 Jan;9:183–192. doi: 10.1083/jcb.9.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. II. Application of solutions containing lead and barium. J Biophys Biochem Cytol. 1958 Nov 25;4(6):727–730. doi: 10.1083/jcb.4.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]