Abstract
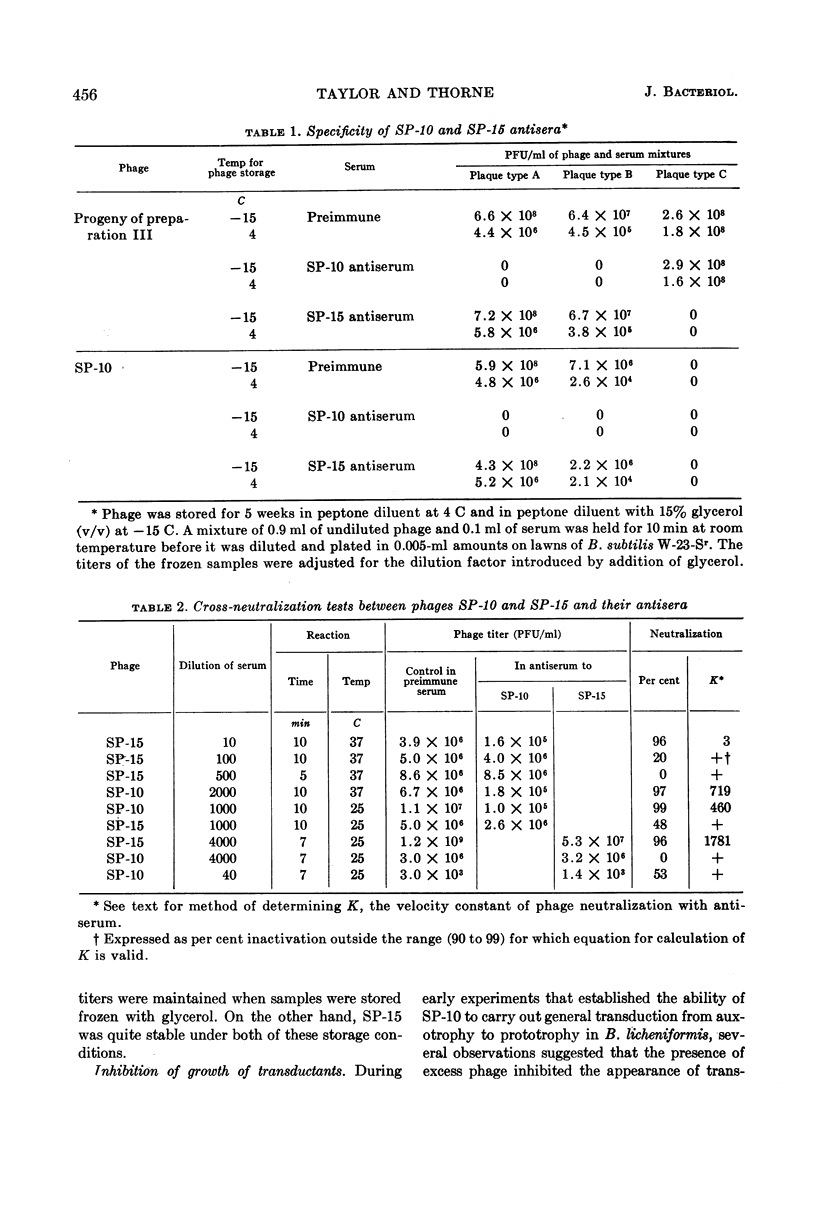
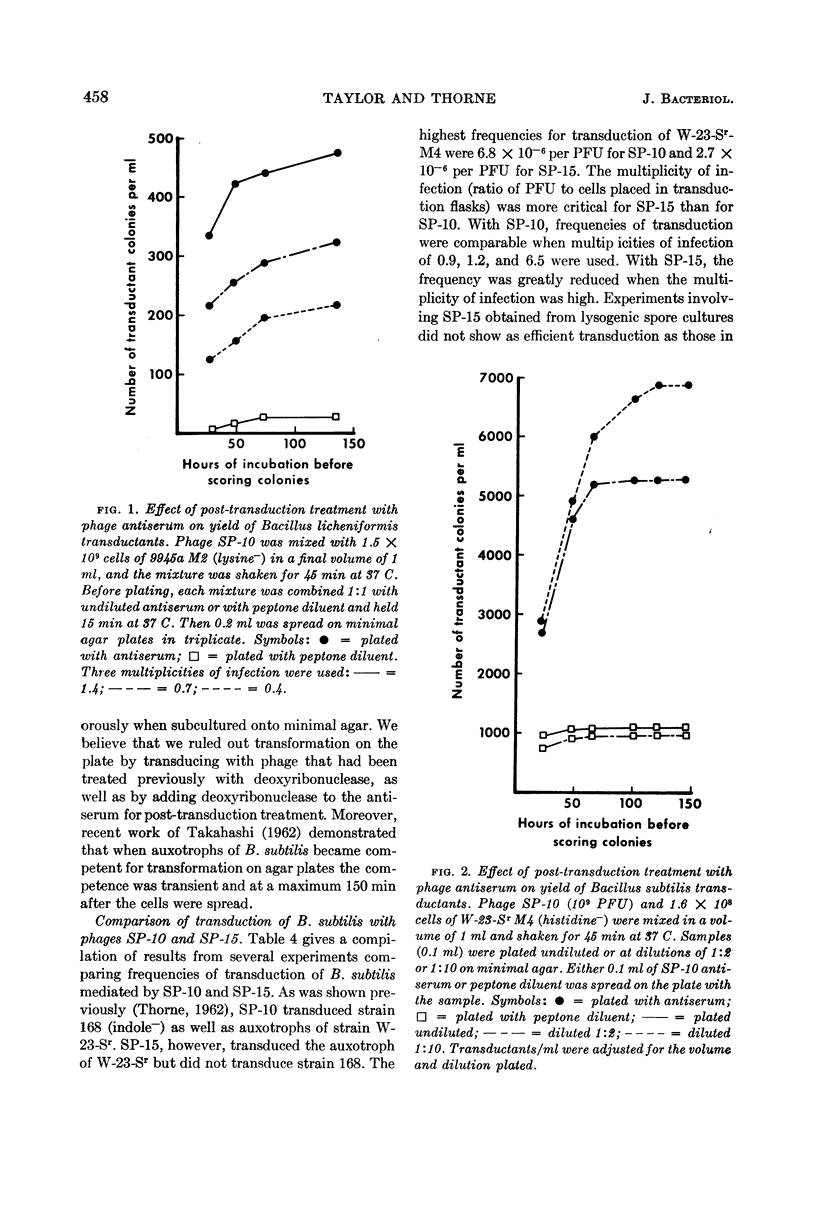
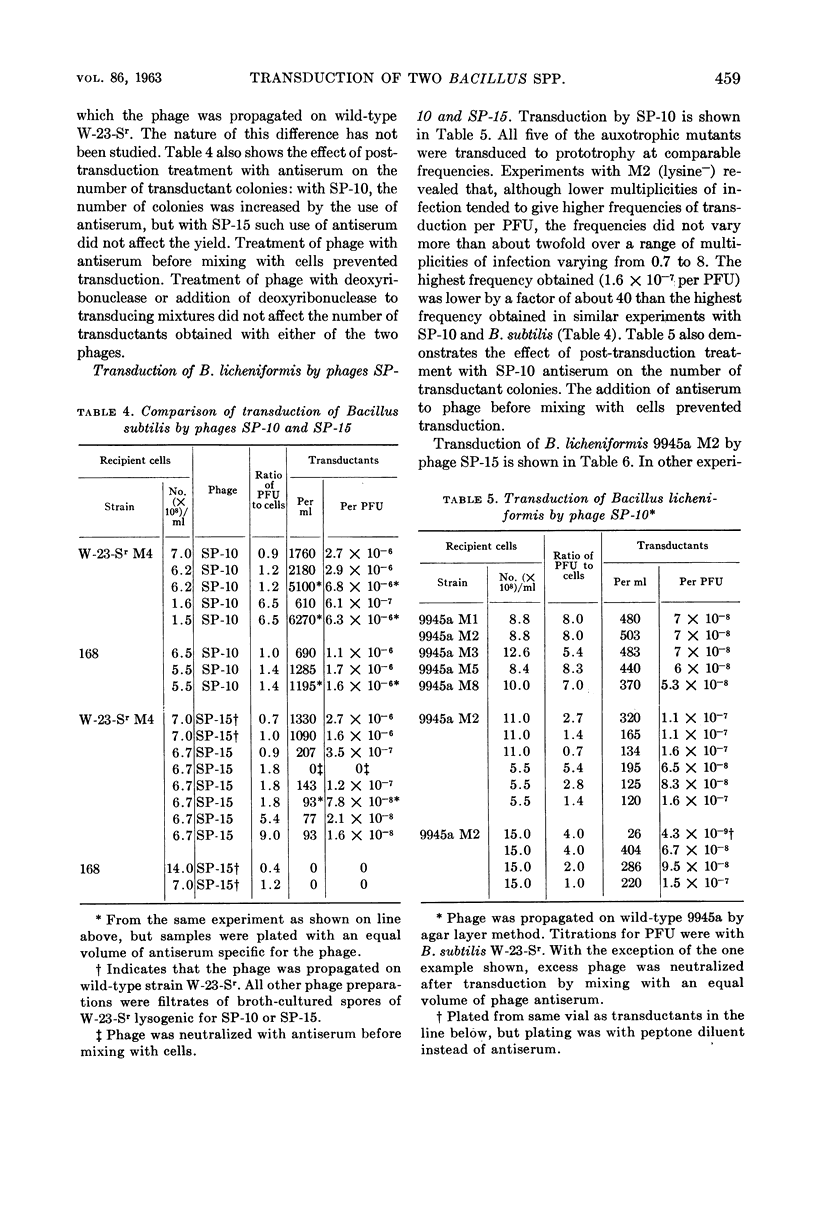
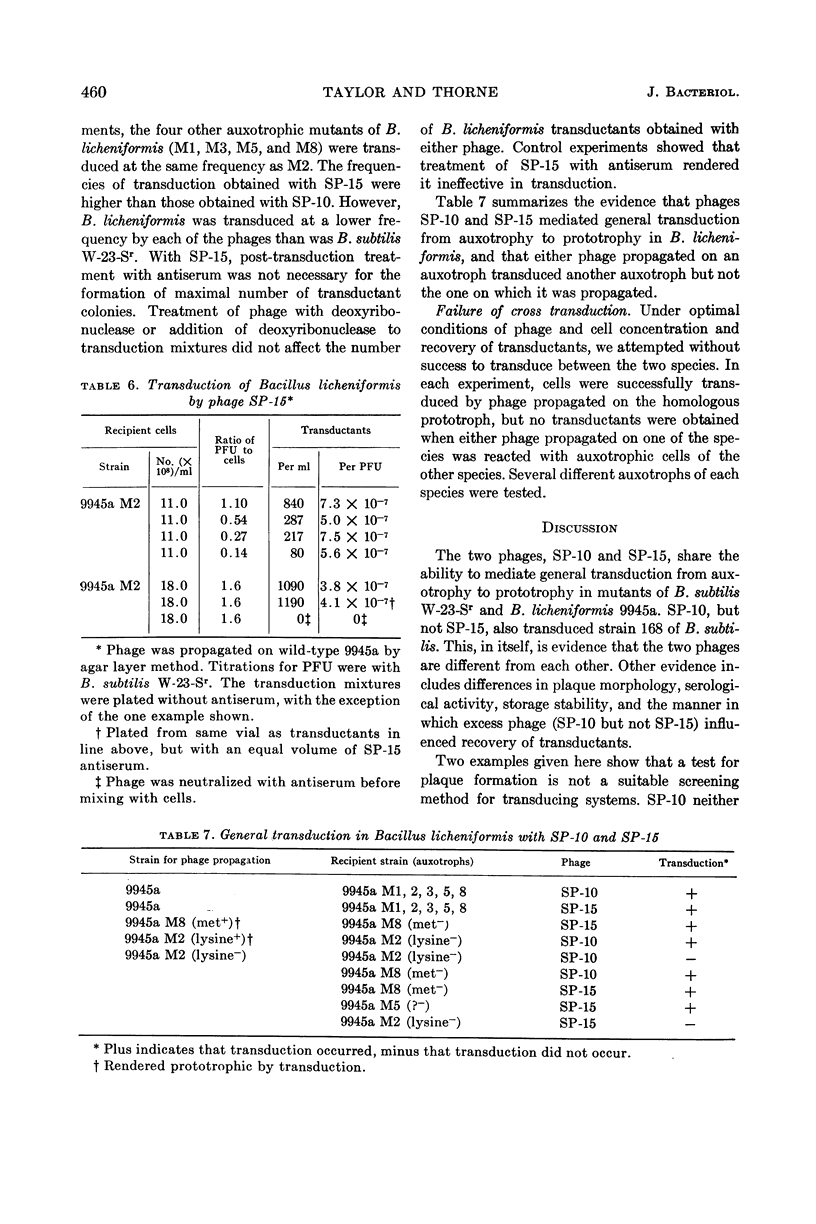
Taylor, Martha J. (U.S. Army Biological Laboratories, Fort Detrick, Frederick, Md.) and Curtis B. Thorne. Transduction of Bacillus licheniformis and Bacillus subtilis by each of two phages. J. Bacteriol. 86:452–461. 1963.—A second transducing bacteriophage, designated SP-15, was isolated from the same soil-sample culture filtrate that supplied the Bacillus subtilis transducing phage, SP-10, reported earlier from this laboratory. SP-10 and SP-15 differ serologically and in several other respects, but share the ability to propagate on B. subtilis W-23-Sr (streptomycin-resistant) and B. licheniformis ATCC 9945a, and to mediate general transduction in either species when propagated homologously. Attempts to transduce between the species have failed. SP-10 forms plaques readily on both W-23-Sr and 9945a; SP-15 forms minute plaques on W-23-Sr and has shown no evidence of any lytic activity on 9945a. Maximal recoveries of prototrophic colonies from mixtures of SP-10 with auxotrophs of either W-23-Sr or 9945a were obtained only when excess phage was neutralized by post-transduction treatment with specific phage antiserum. Such treatment was not necessary for maximal recovery of transductants effected by SP-15. Unlike SP-10, SP-15 propagated on W-23-Sr did not transduce B. subtilis 168 (indole−). SP-15 transduced B. licheniformis more efficiently than did SP-10. Neither phage was able to transduce B. licheniformis as efficiently as it transduced B. subtilis. The differing influences of multiplicity of infection were compared for the two phages in both species.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DUFF J. T., WYSS O. Isolation and classification of a new series of Azotobacter bacteriophages. J Gen Microbiol. 1961 Feb;24:273–289. doi: 10.1099/00221287-24-2-273. [DOI] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- PERRY H. M., Jr Chelation therapy in circulatory and sclerosing diseases. Fed Proc. 1961 Sep;20(3):254–257. [PubMed] [Google Scholar]
- TAKAGI J., IKEDA Y. Transformation of genetic traits by the DNA prepared from a lysogenic phage S-1. Biochem Biophys Res Commun. 1962 Jun 4;7:482–485. doi: 10.1016/0006-291x(62)90340-6. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI I. Genetic transduction in Bacillus subtilis. Biochem Biophys Res Commun. 1961 Jun 28;5:171–175. doi: 10.1016/0006-291x(61)90104-8. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI I. Genetic transformation of Bacillus subtilis by extracellular DNA. Biochem Biophys Res Commun. 1962 Jun 4;7:467–470. doi: 10.1016/0006-291x(62)90337-6. [DOI] [PubMed] [Google Scholar]
- THORNE C. B., GOMEZ C. G., NOYES H. E., HOUSEWRIGHT R. D. Production of glutamyl polypeptide by Bacillus subtilis. J Bacteriol. 1954 Sep;68(3):307–315. doi: 10.1128/jb.68.3.307-315.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THORNE C. B. Transduction in Bacillus subtilis. J Bacteriol. 1962 Jan;83:106–111. doi: 10.1128/jb.83.1.106-111.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]


