Abstract
Compared to conventional drug therapy, autologous hematopoietic stem cell transplantation (HSCT) 3 can induce very long-term remission in refractory lupus patients. Herein, we show that in post-transplant patients, both CD4+CD25highFoxP3+, and an unusual CD8+FoxP3+ Treg subset return to levels seen in normal subjects; accompanied by almost complete inhibition of pathogenic T cell response to critical peptide autoepitopes from histones in nucleosomes, the major lupus autoantigen from apoptotic cells. In addition to a stably sustained elevation of FoxP3, post-transplant CD8 T cells also maintained markedly higher expression levels of LAP, CD103, PD-1, PD-L1 and CTLA-4, as compared to pre-transplant CD8 T cells that were identically treated by a one-time activation and rest in short-term culture. The post-transplant CD8 Treg cells have autoantigen-specific and nonspecific suppressive activity, which is contact-independent and predominantly TGF-β-dependent. By contrast, the pre-transplant CD8 T cells have helper activity, which is cell-contact dependent. Although CD4+CD25high Treg cells are known to return during clinical “remission” of conventional drug treated lupus, the post-transplant patient's CD8 Treg are considerably more potent, and they are absent in drug treated patients in whom CD4 T cell autoreactivity to nucleosomal epitopes persists even during “clinical remission”. Therefore, unlike conventional drug therapy, HSCT generates a newly differentiated population of LAPhighCD103high CD8TGF-β Treg cells, which repairs the Treg deficiency in human lupus to maintain patients in true immunological remission.
Keywords: Systemic Lupus Erythematosus, Human, T cells, Peptide epitopes, Tolerance, Autoimmunity
Introduction
Systemic lupus erythematosus (SLE) is characterized by autoantigen-driven interactions between autoimmune T helper (Th) cells and B cells leading to production of somatically mutated IgG autoantibodies against apoptotic nuclear antigens (1-6). Nucleosomes and their core histones peptides are major lupus immunogens, and five critical autoepitopes that trigger interaction between lupus T and B cells in patients and mice with SLE are in histone (H) regions, H1′22-42, H385-102, H3115-135, H416-39 and H471-94 (7-11). These histone peptide epitopes accelerate lupus nephritis upon immunization, but they delay or even reverse disease upon tolerization at high or low doses, in mice with spontaneous SLE (10, 11).
Although the immunology of SLE is complex, defect/s in immunoregulation probably plays a crucial role in expansion of autoimmune cells. Most work has focused on CD4+CD25+ Treg cells (12-19). However, CD8+ Treg, originally called T suppressors (20), have not been well defined. CD8+CD28 Treg cells that inhibit APCs by contact (21), or by cytokines, IFN-γ (22) and/or IL-10 (23) in allogeneic-transplant situations, as well as cytotoxic CD8 T cells that are restricted to examples of organ-specific autoimmunity (24) have been reported. Furthermore, TGF-β + IL-2 treatment, or TGF-β treated APC can induce CD8 Treg cells, which may produce IL-4, TGF-β or IL-10 (19, 25).
The percentage of CD4+CD25+T cells is significantly decreased in patients with lupus (26-28), and in lupus prone BWF1 and SNF1 mice (29), but autoantigen specificity of such cells was not determined. Low dose tolerance in SNF1 mice with nucleosomal histone peptide epitope, H471-94, H416-39 or H1′22-42 that contain both MHC class II and class I binding motifs, induced CD8+, as well as CD4+CD25+ Treg subsets containing autoantigen-specific Treg cells which suppress responses of pathogenic lupus T cells to nucleosomal epitopes, and reduce autoantibody production by inhibiting the T cell help to nuclear autoantigen-specific B cells (11, 30). High doses of an anti-DNA or other Ig related peptide (pConsensus or “hCDR1”) could suppress lupus in BWF1 mice and expand CD4 Treg cells in vivo; pConsensus could also induce human functional CD4+CD25high Treg cells in vitro (31-33). On the other hand, in lupus-susceptible MRL mice, CD4+CD25− T cells show reduced sensitivity to suppression by CD4+CD25+ Treg cells (34), as found in some patients with active lupus (35). CD4+CD25− T cells also resist inhibition by CD4+CD25+ Treg or TGF-β in Cbl-b deficient (Cbl-b−/−) C57BL/6 mice (36). Indeed, human lupus CD4+ T cells are resistant to anergy, associated with reduction of Cbl/Cbl-b (37, 38), and they also resist activation induced apoptosis by increasing COX-2 and c-FLIP expression (39). The CD8+ T cells are also abnormal in lupus patients, being less competent in cytotoxic activity, and they actually help B cells to make autoantibodies (40-42). CD8+ T cells from healthy subjects, derived by culturing with IL-2 and GMCSF, mediated non-specific suppression by IFN-γ and IL-6, and this Treg function was found to be impaired in active lupus (43). However, pathogenic IgG autoantibodies belong to Th1- or IFN-γ-dependent subclasses, and IL-6 production is actually increased in lupus, which might contribute to differentiation of autoimmune Th cells into CD40Lhigh, IL-17/IL-21 producing inflammatory Th17 and follicular T helper (TFH) cells with concomitant decrease in Treg cells (30, 44-49). Thus, very little is known about how CD8+ Treg cells could control human lupus, although animal models indicate their importance (11, 19, 50). Moreover, it was reported CD4+CD25+ Treg cells increase after stem cell transplant in patients with JIA, but no studies on CD8+ Treg were done (51).
Compared to conventional drug treatment, autologous hematopoietic stem cell transplantation (HSCT) can induce very long – term remission in refractory lupus patients. There are over 70 patients that have undergone autologous hematopoietic stem cell transplantation (HSCT) at Northwestern University for refractory SLE with promising outcomes in terms of treatment safety and disease remission as reported previously (52-54). Stem cell transplantation for autoimmune disease is a three step procedure (55, 56): 1) harvesting of autologous hematopoietic / immune stem cells from a patient's blood, 2) ablation or near ablation of a patients immune system (T and B cells) with chemotherapy (cyclophosphamide) and antilymphocyte antibodies, and 3) reinfusion of autologous immune stem cells to hasten hematopoietic and immune recovery. The rationale of an autologous hematopoietic (immune cell) transplant is to immune ablate and then immune reset by regenerating T and B cells from the stem cell compartment (55). Importantly, immune ablation is achieved by using a non-myeloablative regimen that specifically targets only the immune compartment and does not destroy the entire bone marrow compartment, which makes the therapy safer than autologous HSCT performed with myeloablative intent to destroy any leukemia containing bone marrow compartment in leukemic patients. The exact mechanism/s by which self-tolerance returns after HSCT therapy still remains undefined.
In this study, we focus on the role of CD8 Treg cells in HSCT therapy. We studied 15 pre- and 15 post-transplant patients with SLE, and some of them were prospectively followed for up to 8 years after receiving HSCT. Our results provide evidence that TGF-β producing CD8+LAPhighCD103highFoxP3+ Treg subset return in post-transplant patients and play very important role of controlling autoimmune responses in SLE.
Materials and Methods
Subjects
We enrolled 15 pre- and 15 post-transplant (non-myeloablative HSCT), as well as 10 conventional drug treated lupus patients (mean age 34 years, range 16-58) who fulfilled the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus (57), and 15 healthy donors (age 23-57 years) who had no history of autoimmune disease (normal controls). Disease activity was scored based on the SLE disease activity index (SLEDAI) (58). Patients with SLEDAI < 3 were considered to have inactive disease (remission) and those with SLEDAI ≥ 3 were considered to have active disease. Clinically relevant demographic profile of the patients is shown in Table I and II. The study was approved by the Institutional Review Board of Northwestern University.
Table I.
HSCT patient demographic: Clinical and treatment status of pre- and post-transplant SLE patients in the study.
| Patients Code | Status | Age/Sex/Race | SLEDAI | Current Treatment | |
|---|---|---|---|---|---|
| 1 | pre-t | 24/ F/ W | 28 | Pred., CTX, MMF, HCQ | |
| 1-B | post-t | 3 months | 0* | Pred., MMF | |
| 2 | pre-t | 40/ F/ W | 12 | Pred., CTX, HCQ | |
| 2-B | post-t | 3 years | 1 | Pred., MMF | |
| 3 | pre-t | 26/ F/ W | 6 | MMF | |
| 3-B | post-t | 2 years | 0 | none | |
| 4 | pre-t | 51/ F/ W | 26 | Pred., MMF | |
| 4-B | post-t | 2 years | 0 | HCQ | |
| 5 | pre-t | 42/ F/ W | 35 | Pred., MMF | |
| 5-B | post-t | 2 years | 4 | Pred., MMF | |
| 6 | pre-t | 40/ F/ H | 24 | MMF | |
| 6-B | post-t | 2 years | 0 | MMF | |
| 7 | pre-t | 31/ F/ W | 18 | CTX | |
| 7-B | post-t | 1 year | 0 | none | |
| 8 | pre-t | 47/ F/ W | 13 | Pred., MMF | |
| 8-B | post-t | 6 months | 0 | Pred., | |
| 9 | pre-t | 20/ F/ H | 38 | Pred., CTX, MMF | |
| 9-B | post-t | 1 year | 0 | none | |
| 10 | pre-t | 21/ F/ W | 11 | Pred., CTX, MMF, RTX | |
| 10-B | post-t | 1 year | 0 | none | |
| 11 | pre-t | 40/ F/ W | 23 | Pred., MMF, RTX | |
| 12 | pre-t | 27/ F/ | 8 | Pred., CTX, | |
| 13 | pre-t | 16/ F/ W | 15 | Pred., AZA, CTX, RTX | |
| 14 | pre-t | 54/ F/ W | 26 | Pred., MMF, CTX | |
| 15 | pre-t | 22/ M | 11 | Pred., MMF, CTX, RTX | |
| 16 | post-t | 5 yrs | 27/ F | 0* | none |
| 17 | post-t | 8 years | 48/ F/ W | 1 | MMF, HCQ |
| 18 | post-t | 5 years | 51/ F | 0 | MMF |
| 19 | post-t | 2 years | 46/ F | 0 | Pred. |
| 20 | post-t | 8 years | 34/F/W | 0 | none |
no score compiled, but clinically in remission.
SLE = systemic lupus erythematosus; SLEDAI = SLE disease activity index; Pre-t = pre-transplant; Post-t = post-transplant; W = white/caucasian; H = Hispanic; Pred = Prednisone or Steroids; HCQ = Hydroxycholoquine (Plaquenil); MMF = Mycophenolate Mofetil (CellCept). AZA = Azathioprine(lmuran), CTX = Cytophosphamide (Cytoxan), RTX = Rituximab.
Table II.
Conventionally treated patient demographic: Clinical and treatment status of conventional drug treated SLE patients in the study.
| Patient Code | Age/Sex | SLEDAI score | Current Treatment |
|---|---|---|---|
| 1 | 42/ F | 0 | HCQ |
| 2 | 36/F | 0 | MTX, HCQ |
| 3 | 53/F | 0 | HCQ |
| 4 | 58/F | 0 | Pred., HCQ |
| 5 | 54/F | 0 | Pred., HCQ |
| 6 | 38/F | 0 | none |
| 7 | 47/F | 2 | AZA, LEF |
| 8 | 51/F | 0 | none |
| 9 | 53/F | 6 | HCQ |
| 10 | 34/F | 2 | none |
SLE = systemic lupus erythematosus; SLEDAI = SLE disease activity index; Pre-t = pre-transplant; Post-t = post-transplant; Pred = Prednisone or Steroids; HCQ = Hydroxycholoquine (plaquenil); AZA = Azathioprine (imuran); MTX = Methotrexate; LEF = Leflunomide (Arava).
Monoclonal antibodies and cytokines
For immunostaining, mouse PE-, FITC-, PerCP-, and APC- conjugated mAbs against Human CD3(UCHT1), CD28(L293), CD4(SK3), CD8(SK1), CD25(M-A053), CD103(Ber-ACT8), CD56(B159), CD27(M-T271), CD62L(Dreg56), CTLA-4(BNI3), IFN-γ (25723.11), IL-13 (JES10-5A2), CD127 (HIL-7R-M21), and corresponding mouse isotype controls (obtained from BD Biosciences or BD PharMingen, San Jose, CA) were used. FITC conjugated PD-1(MIH4) and APC conjugate PD-L1 (MIH1) as well as corresponding isotype control were purchased from eBioscience. PE conjugated LAP (27232) and FITC conjugated IL-10 (127107) with corresponding isotype controls, and IL-2, IL-7 and IL-15 were purchased from R&D systems (Minneapolis MN) for cell culture. Anti-TGF-β, anti-IL-13 and anti-IL-10 (R&D systems) neutralization antibodies were used to test for blocking suppression.
Peptides and Nucleosomes
All peptides were synthesized by F-moc Chemistry and their purity was checked by amino acid analysis by the manufacturers (Chiron Mimotopes, San Diego, CA and New England peptide, Gardner, MA). Nucleosomes were prepared as described (6, 7).
Cell preparation
PBMCs from patients or healthy donors were isolated as described (7, 9). Aliquots of these cells were used to make short-term T cells lines and others cells were used directly for stimulation with antigens. After an initial period of rest and recovery with IL-2 (10 units/ml), short-term CD4 and CD8 T cell lines were derived from PBMC of Lupus and normal subjects under identical conditions by one-time stimulation with anti-CD3 and anti-CD28 antibodies, with interleukins-2, 7, and 15 in culture, and then resting for 10 days to remove any confounding effects of cytokines, anti-T cell autoantibodies, autoantigenic stimulation and drugs (5, 9, 37, 39). The short-term line T cells from lupus patients retain autoimmune function and other immune abnormalities characteristic of lupus (9, 37, 39, 41). To get CD4+CD25− T cells, CD4 line T cells were stained with anti-CD4-PerCP and anti-CD25-PE or the isotype control antibody conjugated with PE for 30 min at 4°C, CD4+CD25− and CD4+CD25+ T cells were purified using a MoFlo high-speed cell sorter (DakoCytomation, Carpintena, CA) to a purity of >98%. In some experiments CD4+CD25high T cells, or CD4+CD127−CD25high T cells were purified from PBMC or short-term CD4 lines by cell sorting using published methods (59).
Flow cytometry
T cells from patients and healthy donors were stained with CD4-PerCP plus CD25-FITC, or CD8-APC plus CD28-FITC and PE-conjugated anti-CD103, CD56, CD27, CD62L, PD-1, PD-L1 at 4 °C for 30 min in the dark. Matched PE-conjugated IgG isotype controls were used. To stain for PD-1, PD-L1 and CTLA-4, T cells were stimulated with anti-CD3/CD28 for 24 hours in the presence of 20 units/ml of IL-2. For maximal (surface and intracellular) staining of CTLA-4, T cells were cultured with 0.1 mM pervanadate (phosphatase inhibitor) for 15 min at room temperature in the dark (37), washed once in complete RPMI, and then stained first for surface antigens. Next, they were fixed and permeabilizaed, and then incubated with anti-CTLA-4 or the isotype control Ab at 37 °C for one hour. To detect CD4 and CD8 T cell's intracellular levels of FoxP3, the cells were first stained for surface markers by anti- CD4-PerCP, CD8-APC, CD25-FITC or CD28-FITC, and then the cells were stained with FoxP3-PE or the isotype control (PCH101; eBioscience, San Diego, CA) according to the manufacturers after fixation and permeabilization. Data 20,000 cells were collected using FACS Calibur or LSR II flow cytometer (BD Biosciences), and analyzed by BD CellQuest or Tree Star FlowJo.
Detection of CD4 T cells response to autoepitopes
Fresh PBMC samples were cultured with nucleosomal histone peptide epitopes (H1′22-42, H385-102, H3115-135, H416-39, H471-94) or whole nucleosomes (Nuc.) in the presence of IL-7, IL-15 and anti-CD28/CD49d (BD biosciences) for 3 days, and golgistop Brefeldin A (BFA, final concentration 1ug/ml; Sigma Chemical Co., Louis, Missouri, USA) was added to the wells for the last 17 hours of incubation, and then surface-stained with anti-CD4, anti-CD8 and intracellularly with anti- IFN-γ or IL-13. Cytokine Response Index (CRI) ratios were calculated by dividing values for corresponding staining of resting control (without peptide or Nucleosome stimulation). CRI below 2 is considered to be at background level (9). Viable cells gated for being CD4+ or CD8+ were analyzed for IFN-γ or IL-13 production by flow cytometry (CFC, Becton Dickinson). We did not study IL-4 production, because available reagents are not suitable for this assay.
Suppressor assays
To washout confounding effects of extrinsic factors influencing lupus patient's T cells (5, 9, 39); short-term T cell lines were derived from PBMC by one time stimulation by anti-CD3, anti-CD28 and IL-2, and then rested for 10 days before starting the suppression assays. We used these short-term CD4+ and CD8+ T cell lines, derived under identical conditions, from lupus patients or healthy donors to measure CD8+ Treg suppressor functions. CD4+CD25− or total CD4+ T cells from the lines were used as target (responder) cells that were stimulated with anti-CD3/CD28 for three days in the presence of 20 units/ml IL-2, then rested for 10 days with IL-2 before starting the suppression assay. CFSE (2 μM; Molecular Probes, Carlsbad, CA) labeled target (responder) cells (5 × 105 cells/well) were stimulated with 50 units/ml IL-2, or an equal number of irradiated allogeneic APC (3000 rad), and co-cultured with different numbers of autologous CD8 line T cells (the ratios of target T cells : CD8 T cells being 1 : 1, 1 : 0.5, 1 : 0.25, 1 : 0.125, 1 : 0.0625 and 1 : 0.03125), either in contact or separated by cell-impermeable membranes of transwell plate (Corning Costar, Lowell, MA. 0.4 μm pore size), and proliferation (CFSE dilution) of gated CD4 T cells was assessed by flow cytometry after culturing 4 days. The % of inhibition was calculated as (% of proliferated target T cells cultured alone – % of proliferated target T cells in coculture with CD8 T cells) / (% of proliferated target T cells cultured alone) × 100. In experiments to block post-CD8 T cells' suppressive effect, XViVo-20 serum-free medium (Lonza, Allendale, NJ) supplemented with penicillin-streptomycin was used and different concentrations of anti-TGF-β, anti-IL-10, or anti-IL-13 neutralization Ab, or isotype control were added at the day 0 and maintained throughout the experiment. The % blocking of suppression was calculated as (% of proliferated target T cells in cultures with blocking antibody – % of proliferated target T cells cultured with respective isotype control) / (% of proliferated target T cells cultured in medium – % of proliferation target T cells cultured with isotype control) × 100.
To measure the autoantigen-specific helper or suppression function of CD8 T cells, post-transplant PBMC were co-cultured with autologous pre-transplant or post-transplant CD8 T cells at ratios of 1:0.5 and stimulated with nucleosomal histone peptide epitopes or whole nucleosomes (Nuc.). In addition, PBMC from drug treated lupus patients were co-cultured with allogeneic pre- or post- transplant CD8 T cells and stimulated with autoantigens. Intracellular cytokine response of target CD4 T cells in the PBMC was measured by flow cytometry (CFC), as mentioned above.
Statistical analysis
Data analyses were performed using Prism 4.0 software (GraphPad Softwear, San Diego, CA). Comparisons were performed by Student's t-tests. Results are expressed as mean ± SD, P values less than 0.05 were considered significant.
Results
Autologous hematopoietic stem cell transplantation (HSCT) reduced IFN-γ response and increased immunoregulatory IL-13 response of CD4 T cells in fresh PBMC to major peptide autoepitopes from nucleosomes
Most pathogenic autoantibodies in lupus that fix complement and bind to activating FcγR on inflammatory cells belong to the IgG subclasses that are Th1 or IFN-γ dependent (7). Although T cells in freshly obtained lupus PBMC proliferate poorly when tested immediately ex vivo for reasons mentioned above (4, 5, 9, 37, 39), the CD4 T cells in lupus patients respond strongly to the critical histone peptides in nucleosomes by producing IFN-γ, and those peptide epitopes also accelerate lupus in mouse models upon immunization (7-9). Herein, we wanted to know whether the CD4 T cells in fresh PBMC from post-transplant lupus patients can still respond to those autoantigens. The results (Figure 1) show that Th1 type IFN-γ response of CD4 T cells to critical peptide epitopes (H1′22-42, H385-102, H3115-135, H416-39, H471-94) and nucleosomes goes down to background (normal) levels in the post-transplant lupus patients who were in remission. On the other hand, counter-inflammatory IL-13 response of those T cells goes up (Post-t vs. Normal, p <0.008 - <0.01; Post-t vs. Pre-t, p <0.05 - <0.01). By contrast, the IFN-γ response of CD4 T cells in pre-transplant lupus patients' PBMCs to the peptide autoepitopes was elevated (Pre-t vs. Normal, p <0.001 - <0.01; Pre-t vs. Post-t, p <0.05 - <0.002), similar to patient with active lupus that we had previously reported (9), but they had low IL-13 response (Fig. 1A). In this regard, we had previously found that CD4+ T cells in lupus patients, who were in clinical “remission” after conventional drug therapy, still retained IFN-γ response to the peptide epitopes (9). Therefore, the post-transplant remission patients are different with regard to mechanisms downregulating the autoimmune response.
Figure 1.
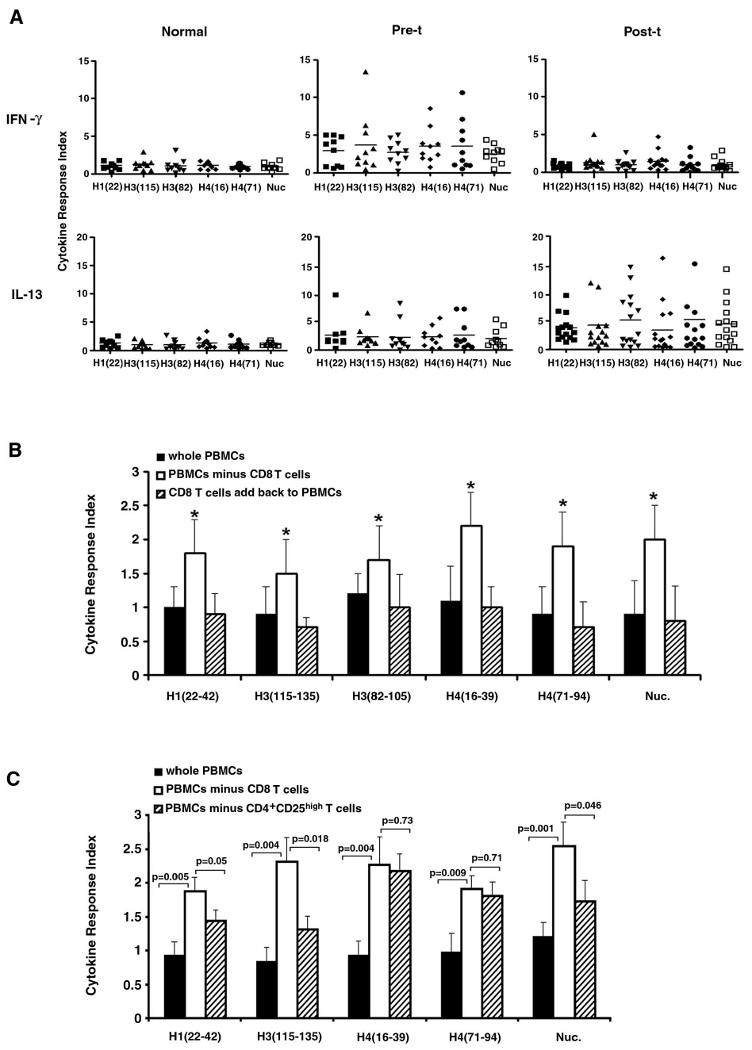
Autologous hematopoietic stem cell transplantation (Post-t) reduced IFN-γ, but increased IL-13 responses of CD4+ T cell in fresh PBMC to nuclesomal autoepitopes. A, Fresh PBMC samples were cultured with nucleosomal histone peptide epitopes, or whole nuclesomes (Nuc.) in the presence of IL-2, IL-7, IL-15 and anti-CD28/CD49d for 3 days, and then stained for surface CD4 and intracellular IFN-γ or IL-13 for flow cytometry analysis of gated CD4+ T cells. Peptide epitope designations are abbreviated to fit X-axis, for example H1′22-42 is H1, H3115-135 is H3(115), and so on. The baseline values of IFN-γ response of CD4 T in PBMC (cultured in medium only) were 0.06-0.27, and the baseline values of IL-13 response of CD4 T (medium only) were 0.07-0.34. Cytokine Response Index (CRI) ratios were calculated by dividing experimental values by corresponding values of resting control CD4 cells (without autoantigen stimulation). CRI below 2 is considered to be at background. The horizontal line indicates the mean. We used a BRN-3 transcription factor related peptide, called Eluted Peptide-2 (EP-2) (sequence DWMEEEEGAQREKE) as a negative control peptide for the histone peptide epitopes (8) and OVA323-339 as an irrelevant control antigen for nucleosomes; and the positive control stimulation was done with anti-CD3. The CRI for IFN-γ response of CD4 T cells to EP-2 were 0.5-1.6, to OVA were 0.4-1.2, and to anti-CD3 were 12.7-51. The CRI for IL-13 response of CD4 T cells to EP-2 were 0.4-1.8, to OVA were 0.6-1.5, and to anti-CD3 were 9.3-46. B, Post-transplant PBMC were depleted of CD8 T cells and then cultured with nucleosomal autoepitopes to measure IFN-γ response of CD4 T cells as in panel A, along with two other groups: whole PBMC group and a group with CD8 T cells added back to PBMC which were depleted of CD8 T cells. Experiments were repeated five times and the bars show the mean ± SD. * p < 0.01. C, CD4+CD25high T cells or CD8 T cells were removed from post-transplant PBMC by sorting, then the whole PBMC, or PBMC without CD4+CD25high or without CD8 T cells were cultured with nucleosomal autoepitopes to measure IFN-γ response of CD4 T cells as we did in panel A. Experiments were repeated three times and bars show the mean ± SD.
CD8 T cells are the major contributors to the suppression of autoreactive response in post- transplant patients
To address the question whether the reduced IFN-γ response of CD4+ T cells to the peptide epitopes in the post-transplant lupus patients is because of the suppressive function of CD8 Treg cells or because HSCT caused lower responsiveness of the CD4+ T cells, or both, CD8+ T cells were deleted from the post-transplant PBMC, and then CD4+ T cells' intracellular IFN-γ response to peptide epitopes were measured in three groups: (a) total PBMC, (b) PBMCs deleted of CD8+ T cells, and (c) CD8+ T cells added back to group (b). The IFN-γ response of CD4+ T cells in the group of PBMCs deleted of CD8+ T cells (group b) to peptide epitopes was significantly higher than that of CD4+ T cells of other two groups (Fig. 1B), indicating that CD8 Treg cells play critical role in suppressing the autoreactive CD4 T cells response. CD4+CD25high Treg cells in the PBMC might also have contributed to suppression because the response was not restored to pre-transplant levels after CD8 T cell removal, which we address below using short-term line T cells. Indeed, when CD4+CD25high cell subset (ranging between 0.9 and 1.25%) was deleted from post-t PBMC, the IFN-γ response of CD4 T cells to some histone epitopes was also significantly restored, but not as strongly as deletion of total CD8 T cells did in the case of some of the peptides and whole nucleosomes (Fig. 1C).
Increase in percentage of CD4+CD25highFoxP3+ and CD8+FoxP3+ and CD8+CD103+ T cells in short-term T cell lines from post-transplant lupus patients
Human peripheral blood contains Treg cells, and the transcription factor FoxP3 is critical for Treg cells. To compare the numbers of Treg cells in pre- and post- transplant, as well as conventional drug treated lupus patients' peripheral blood, we used 3-color flow cytometry to distinguish two types of CD8 Treg cell subsets based on the expression of CD28, namely CD8+CD28−FoxP3+ and CD8+CD28+FoxP3+ cells; and we also examined the CD4+CD25highFoxP3+ Treg subset. We stained fresh PBMC directly ex vivo, as well as short-term CD4 and CD8 line T cells. To washout confounding effects of extrinsic factors influencing lupus patient's T cells (5, 9, 39); short-term T cell lines were derived from PBMC by one time stimulation by anti-CD3, anti-CD28 and IL-2, and then rested for 10 days. Such short-term line T cells retain the autoimmune characteristics of lupus T cells (5, 9, 39). Figure 2A, shows that the % FoxP3+ cells in short-term line CD8+ (CD28− or CD28+) T cells from post-transplant patients were increased considerably, as compared to pre-transplant samples, however, this difference can not be seen in CD8+CD28− cells in fresh PBMC (Figure 2C), probably because the differences in FoxP3 expression become evident after T cell activation. Nevertheless, the short-term culture T cells were rested following stimulation for 10 days, demonstrating a stable and sustained elevation of FoxP3 expression only in the post-transplant and normal T cells. Although it was believed that CD28− phenotype can be used as a Ts cells marker (60), our result shows that the % FoxP3+ cells in the CD8+ T cells from post-transplant patients were significantly increased not only in CD28− subpopulations but also in CD28+; and in contrast to pre-transplant patient samples and drug treated patient samples, FoxP3+ cells were also significantly increased in the CD4+ CD25high T cell lines from post-transplant patients.
Figure 2.
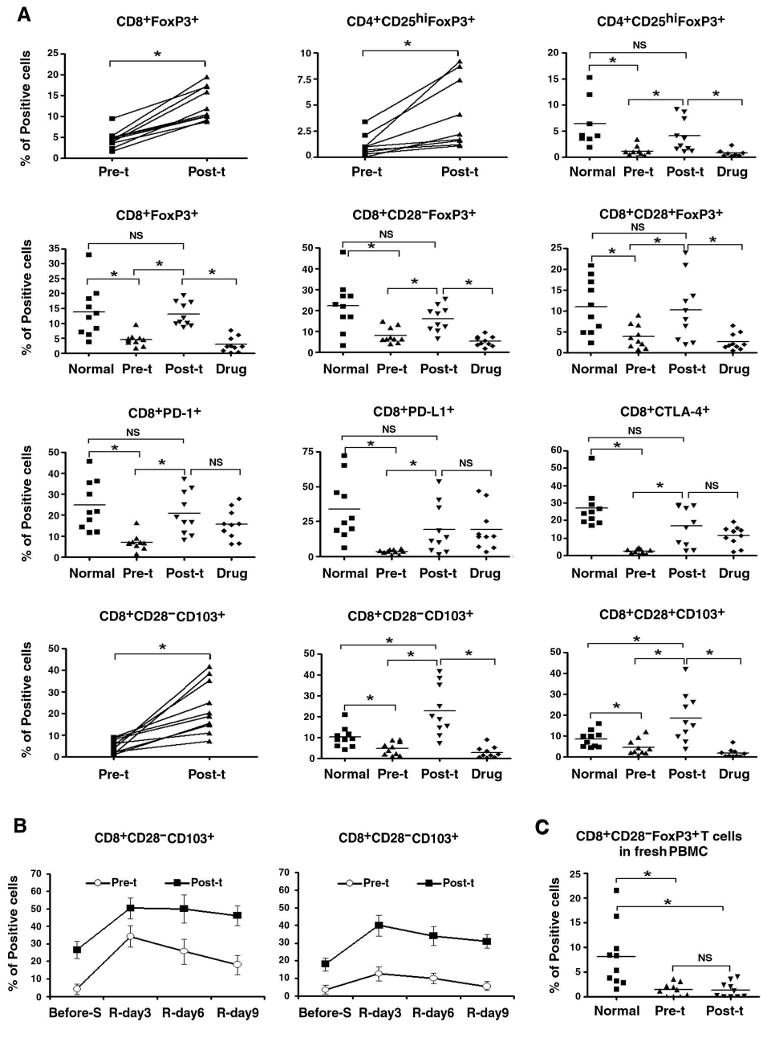
Increase in CD8+ Foxp3+ and CD4+CD25hiFOXP3+ Treg cells after stem cells transplant. A, Short-term CD4 and CD8+ T cell line cells from normal subjects, and pre- and post-transplant (pre-t and post-t), as well as conventional drug-treated (drug) lupus patients were stained for CD4, CD25; or CD8, CD28, CD103, PD-1, PD-L1 and intracellular FoxP3, CTLA-4 (to stain for PD-1, PD-L1 and CTLA-4, the CD8 T cells were stimulated with anti-CD3 for 24 hours). Where samples were available from the same patient, connected plots are shown first for pre- and post- transplant samples, in addition to the collective data. Y-axes show % of positive cells among viable T cells gated for being CD4+ or CD8+. n=10, the horizontal line indicates the mean, NS = not significant, * p < 0.01. B, Pre- and post-transplant CD8 line T cells were stained with CD8, CD28 and CD103 before cells were stimulated with anti-CD3/CD28 for 24 hours in the presence of 20 units/ml IL-2, and then cells were rested for 9 days, and levels of CD8, CD28 and CD103 expression were measured at day 3, day 6 and day 9 of resting. Results from three experiments are shown, and the bars represent the mean ± SD, * P ≤ 0.05. C, % of CD8+CD28− FoxP3+ cell subset was measured in fresh PBMC from pre- and post-transplant lupus patients, as well as normal subjects. n=10, the horizontal line indicates the mean, NS = not significant, * p < 0.01.
We wanted to define the surface marker/s which is/are co-expressed in FoxP3 positive CD8+ T cells by flow cytometry. We detected that CD103, PD1, PDL-1 and CTLA-4 (61-63) were all significantly increased in post-transplant CD8 line T cells compared to pre-transplant samples (Figure 2A). Although PD-1, PD-L1 and CTLA-4 expression in CD8 line T cells from conventional drug-induced remission patients were not significantly different from those in post-transplant CD8 line T cells, expression of CD103 and intracellular Foxp3 were significantly lower in CD4 and CD8 line T cells from drug-induced remission lupus patients. However, none of PD-1, PD-L1, CTLA-4 or CD103 markers showed correlated expression with FoxP3 in CD8 T cells at the same time point (data not shown). When CD103 expression was compared in pre- and post-transplant patients' CD8 line T cells after rest periods following TCR activation, different kinetics were observed in both the CD28+ and CD28− subsets. Results showed that although the CD103 expression were high in CD8 line T cells from post-transplant patients and low in CD8 line T cells from pre-transplant patients before anti-CD3/CD28 stimulation, the CD103 level in both pre- and post-transplant CD8 line T cells increased markedly after TCR activation. However, post-transplant CD8 T cells retained high level of CD103 even after cells were rested for 9 days, but pre-transplant CD8 T cells' CD103 level decreased rapidly after cells were rested for only 6 days. These temporal changes in CD103 level showed same trend in both CD28+ and CD28− subsets (Figure 2B).
HSCT, but not conventional drug therapy restored suppressive activity in CD8+ T cells of lupus patients
To determine if the CD8 T cells had suppressor activity, CD4 line T cells were used as target (responding) cells. In most experiments, CD25+ cells were removed from the CD4+ target (responder) T cell population, in case the CD4+CD25high cells confound results of CD8+ Treg activity. In our suppression assay system, we used IL-2 to induce proliferation of the rested CD4 line T cells (target cells), as well as to activate any CD8 line Treg cells, because that is a more physiological stimulus than overwhelming and artificial anti-CD3 stimulation. Allogeneic irradiated APC were also potent stimulators for T cells, but not consistently, as in some lines, they could not stimulate CD4 or CD8 T cells very well. This might have been due to some degrees of MHC–match between the allogeneic APC and the T cells by coincidence. As it is well known that high dose of IL-2 alone can induce proliferation of TCR-activated and subsequently rested T cells (64, 65), we searched for the optimal IL-2 dose and found that 20, 50 and 100 units/ml of IL-2 all can activate CD4 and CD8 line T cells and the suppression results were consistent even at the highest level of IL-2 (dose data not shown), indicating that IL-2 hogging by CD8 T cells was not the cause of suppression. Therefore, 50 units/ml of IL-2 was chosen in our suppression assays. Since there is no unique cell surface marker to isolate CD8 Treg cells, total CD8 line T cells were used in coculture with autologous target CD4 T cells at different ratios (target CD4T : Treg at 1 : 1, 1 : 0.5, 1: 0.25, 1 : 0.125, 1 : 0.0625, and 1 : 0.03125). We found that ratios of 1 : 1, 1 : 0.5 and even 1 : 0.25 could show strong suppressive effect (Fig. 3C) and the 1 : 0.5 ratio was used in most of our experiments, which showed that the CD8 T cells from post-transplant patients significantly decreased the proliferative responses of autologous CD4 T cells to high dose of IL-2 (50 units/ml), or to allogeneic APC (Fig. 3D). Collectively, in the presence of post-transplant CD8 T cells, % suppression of proliferation of CD4 target T cells was 35-48%, but this suppressive effect could not be found with conventional drug-induced remission patients' CD8+ T cells. CD8+ T cells from pre-transplant lupus patients could not suppress their autologous CD4 T cells proliferation. Instead, those patients' CD8 T cells actually enhanced autologous CD4 T cell proliferation that was induced by IL-2.
Figure 3.
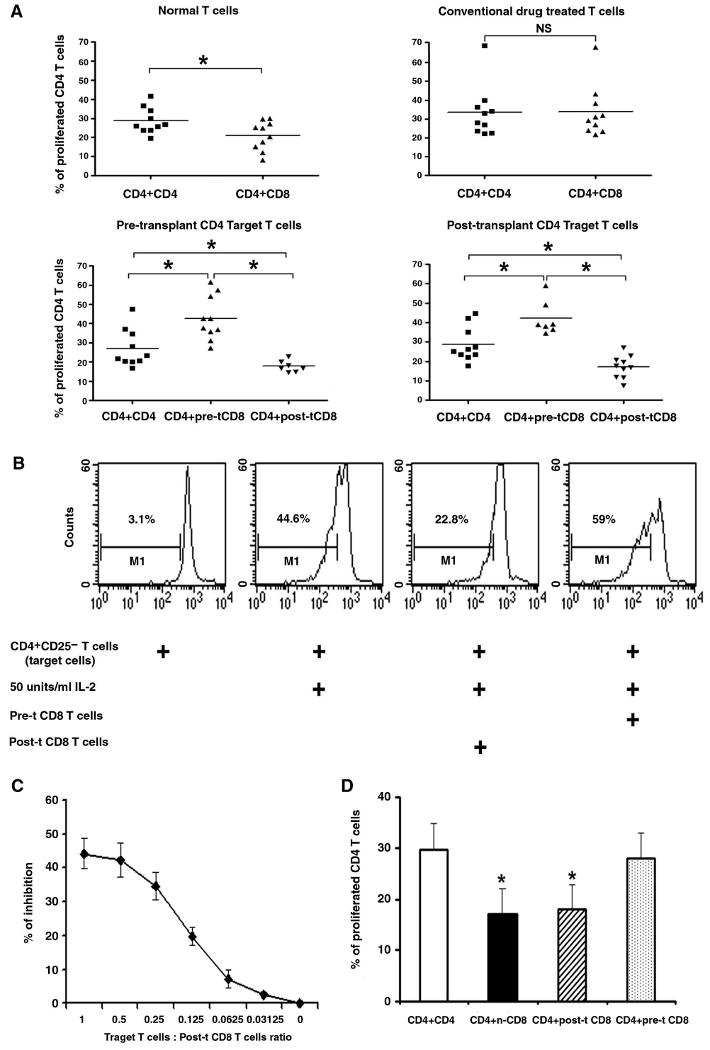
Restoration of suppressive activity in CD8+ T cells of lupus patients after stem cell transplantation. CFSE labeled target cells (short-term line CD4+T cells or CD4+CD25− T cells) at 5 × 105 cell/well were cocultured with autologous pre- or post-transplant CD8 line T cells at ratio of 1 : 0.5 for 4 days either in contact or separated by transwell membrane, and 50 units /ml IL-2, or 105/well irradiated allogeneic APC were used as stimulator in the culture system.
A, Target CD4 T cells and autologous CD8 line T cells from normal donors, or pre- and post-transplant (pre-t and post-t), as well as conventional drug treated lupus patients were cocultured in contact in the presence of 50 units/ml IL-2, n = 7-10, the horizontal line indicates the mean, NS = not significant. B, Representative histograms, showing CFSE-labeled post-transplant target CD4+CD25− T cells' proliferation with IL-2 by themselves or cocultured with autologous pre- or post-transplant CD8 T cells. C, Titration of post-transplant CD8 Treg cells in suppression assay; results from three experiments are shown. The % of inhibition was determined by comparing proliferation of target T cells alone to the % of proliferation of target T cells in coculture with CD8 T cells. D, Post-transplant target CD4 cells were cocultured with autologous pre- and post-transplant CD8 line T cells, or with allogeneic normal CD8 T cells in transwell membrane system, and both stimulated by irradiated allogeneic APC; n-CD8 = normal CD8. In Figure 3 D, results from five experiments are shown, and the bars represent the mean ± SD. * p < 0.01.
Post-transplant CD8 T cells even without Treg enrichment, have stronger suppressive function than autologous CD4+CD25high Treg subset
We compared the suppressive effects of post-transplant, short-term line, unfractionated CD8+ T cells with CD4+CD25+ T cells purified from CD4+ line T cells on autologous target cells (short-term line CD4+ T cells as a whole or CD4+CD25− T cells purified from it) side by side. CD4+CD25+ T cells or CD8 T cells were cocultured with the target T cells in cell contact or separated by transwell membrane, and results showed that the post-transplant CD4+CD25+ T cells had minor suppressive effect on target T cells proliferation in both coculture conditions (the percentage of suppression were 16% on CD4+CD25− target T cells and 18% on total CD4 target T cells), while the autologous CD8 Treg cells showed significantly higher inhibitory function (the percentage of suppression were 40% on CD4+CD25− target T cells and 36% on total CD4 target T cells) (Figure 4A). We found that post-t CD4 line T cells were 90% CD4+CD127− following the one-time activation and rest, and therefore, most the CD4+CD25+ T cells used above were CD127−. We next compared the suppressive effects of post-transplant, short-term line CD8+ T cells as a whole with CD4+CD127−CD25high Treg cells sorted from the CD4+ line T cells on autologous CD4+CD25− target cells in cell contact, and found that the suppressive effect of those post-t CD4+CD127−CD25high T cells were not as strong as the post-t CD8 T cells, even though the latter could not be fractionated for further enrichment of putative CD8 Treg (Figure 4B). These results suggest that CD8 Treg subset generated by HSCT play the major role in restoring immunoregulation.
Figure 4.
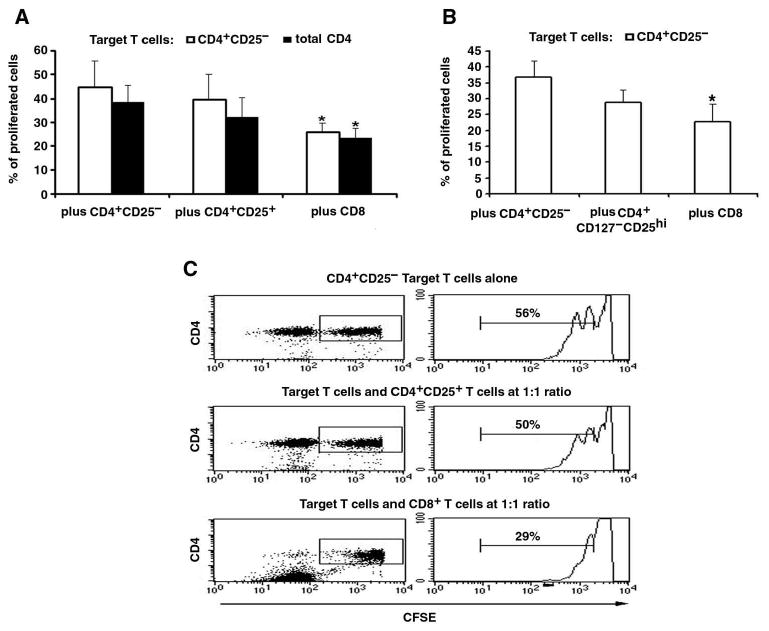
Post-transplant CD8 Treg cells have stronger suppressive function than their autologous CD4+CD25+ Treg cells.
A, As we did in Figure 3, post-transplant CD8 line T cells or CD4+CD25+ T cells sorted from autologous post-transplant CD4 line T cells were cocultured in contact, at the ratio of 1:1, with IL-2 stimulated autologous CD4 line T cells as a whole or CD4+CD25− T cells purified from it, as target. Results from three experiments are shown, and the bar represent the mean ± SD, * p < 0.05. B, As in panel A, post-transplant CD8 line T cells as a whole, or CD4+CD127−CD25high T cells that were sorted from autologous post-transplant CD4 line T cells, were cocultured in contact with IL-2 stimulated target cells (sorted autologous CD4+CD25− T cells), at the ratio of 1:1. * p <0.05. C, Representative histograms showing CFSE-labeled post-transplant, target CD4+CD25− T cells' proliferation when cocultured with autologous CD4+CD25+ T cells or CD8 T cells. Proliferation (CFSE dilution) of gated CD4 T cells was assessed by flow cytometry after co-culturing for 4 days (see Methods).
CD8 Treg cell–mediated suppression of CD4 T cells is contact independent
A transwell system was used to explore whether post-transplant CD8 Treg cells down-regulate CD4 T cells response via cytokine production. CD8 T cells and CD4 T cells were separated by a membrane with 0.4-μm pores, and cultured with irradiated allogeneic APC or 50 units/ml IL-2. The proliferative response of CD4 T cells was inhibited to the same degree when a membrane was placed between the target CD4 and normal or post-transplant CD8 T cells (Figure 5). These experiments indicated soluble suppressive factors are the main mechanism of suppression excluding any cytotoxic contact effect. On the other hand, the “helper” activity of pre-transplant lupus CD8 T cells enhancing CD4 T cell proliferation disappeared when the CD4 T cells were separated from pre-transplant CD8 T cells by a transwell membrane (Figure 5), suggesting that the helper function of pre-transplant CD8 T cells needed cell to cell contact.
Figure 5.
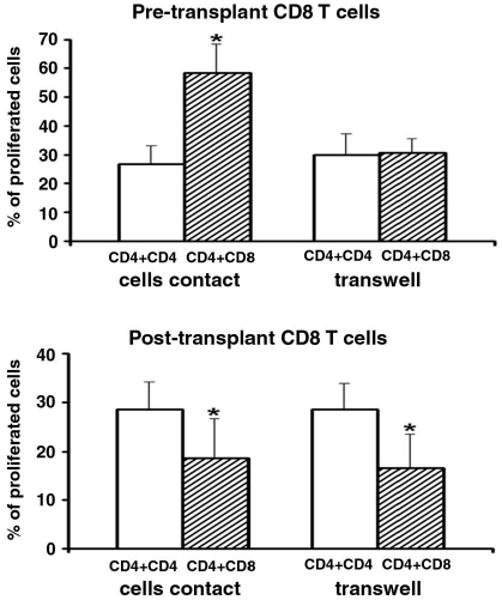
Post-transplant CD8 Treg cells mediate contact-independent suppressive activity, whereas pre-transplant CD8 T cells show contact-dependent helper function. Pre-transplant or post-transplant CD8 cells were cocultured with autologous IL-2 stimulated CD4 line T cells in contact or separated by transwell membrane by the ratio of 1 : 0.5 (target T cells : CD8 T cells). Culture conditions were similar to that in Figure 3, results from five experiments are shown, and the bars represent the mean ± SD. * p < 0.01.
CD8+ Treg cells from post-transplant lupus patients also have autoantigen-specific suppressive activity
As mentioned in the methods, we derived CD4 and CD8 short-term T cell lines from PBMC. Such short-term T cells from patients with lupus retain specificity for nuclear autoantigens (9, 37). To explore whether post- or pre- transplant CD8+ T cells also have lupus autoantigen-specific suppressive or helper activity, freshly obtained whole PBMC has to be used as target, because live APCs are needed for presentation of lupus autoantigen epitopes. However, post-transplant lupus PBMC do not respond significantly to autoepitopes (Figure 1A). Therefore, in an autologous combination, where matched pre-transplant CD8 line T cells and post-transplant PBMC (fresh) from the same patient were available, they could be cocultured to determine the activity of pre-transplant CD8 T cells. Unfortunately, the converse experiment could not be done because pre-transplant PBMC lose APC activity in storage, by the time post-transplant CD8 T cells could be derived from the same patients months later after stem cell transplantation. Therefore, we did attempt to study any suppressive effect of post-transplant CD8 T cells in allogeneic combinations using PBMC from non-autologous lupus patient.
First, pre-transplant CD8 line T cells were cocultured with their autologous post-transplant whole PBMC in the presence of nucleosomes or one of the histone peptide epitopes, and responses of CD4 T cells in the target PBMC were measured by CFC (Figure 6A), using procedures as in Figure 1A. Then, in the same way, pre- or post-transplant CD8 line T cells were cocultured with the allogeneic, drug-treated lupus patients' PBMC that retain autoimmune responsiveness to nucleosome autoepitopes. The peptide epitopes have both class II and class I motifs for stimulation of CD4+ target responder and CD8+ Treg cells (30). The low level of IFN-γ response to the autoepitopes in post-transplant CD4 T cells was reversed to pathogenic high levels when cultured with autologous pre-transplant CD8 T cells (Figure 6A). On the other hand, response to the autoepitopes in drug treated patients' CD4 T cells was reduced when cultured with allogeneic post-transplant lupus CD8 T cells, but it increased when cultured with allogeneic pre-transplant CD8 T cells (Figure 6B). In the latter case, the increased response in this short 3-day assay was not related to allogeneic MHC stimulation, because the control wells of coculturing the target PBMC with the allogeneic CD8 T cells, without peptide or nucleosome, did not show any disproportionate increase in IFN-γ response as compared to controls in Figure 1A containing only autologous cells. Moreover, the same allogeneic post-transplant CD8 T cells showed suppressive effect on IFN-γ response (Figure 6B). These results along with those in Figure 1B indicate that pre- and post-transplant CD8 T cells can exhibit autoantigen-specific help or suppressive activity respectively.
Figure 6.
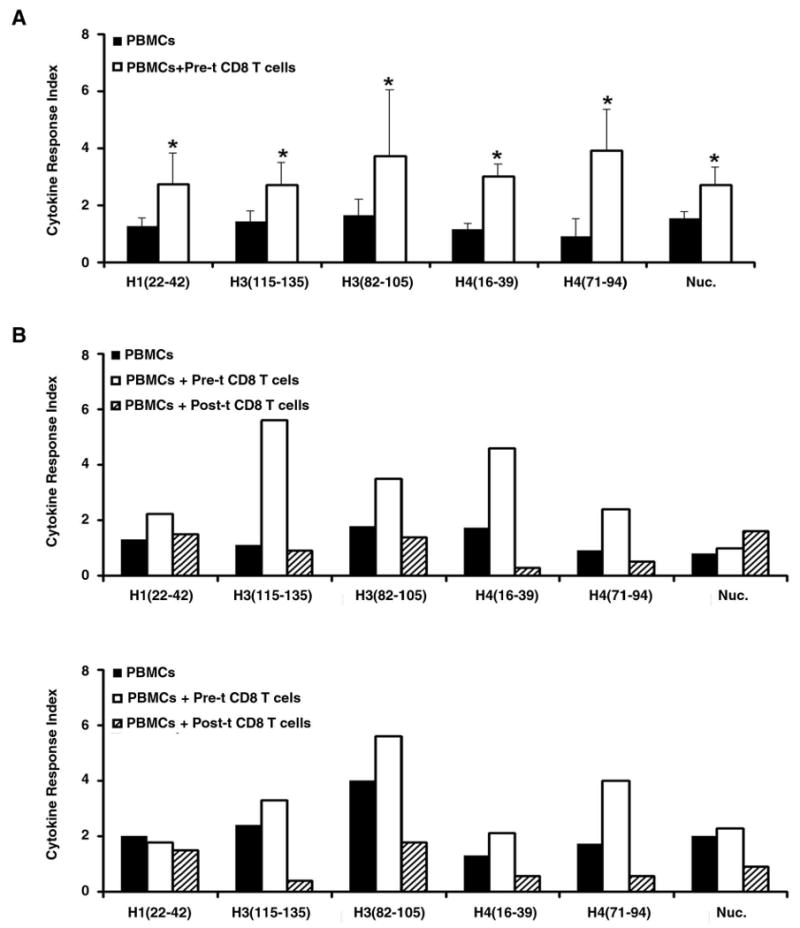
Pre- and post-transplant CD8 T cells show autoantigen-specific help or suppression function respectively. A, Post-transplant PBMCs were co-cultured with autologous pre-transplant CD8 T cells. B, Drug treated lupus patients' PBMCs were co-cultured with allogeneic pre- or post-transplant lupus CD8 T cells, in a ratio of 1 : 0.5 for three days. Gated viable CD4+ T cells were then analyzed for IFN-γ production by CFC, as in Figure 1. Experiments were repeated five times and the bars show the mean ± SD. * p < 0.01, in panel A. Because of the complexity of using allogeneic combinations, the experiments in panel B were done only with two separate sets of samples.
Blockade of TGF-β predominantly abrogates the suppressive function of post-transplant CD8 T cells
Having shown that post-transplant CD8 T cells' suppression effect on CD4 T cells is mediated by soluble suppressive factor (Fig. 5) and that post-transplant CD4 T cells produce higher levels of IL-13 in response to nucleosomal autoepitopes (Fig 1A), we asked whether CD8 line T cells from post-transplant lupus patient (post-t) expressed higher levels of the TGF-β latency-associated peptide (LAP), or IL-10, and whether blocking TGF-β, IL-10 or IL-13 abrogates the suppression function. As shown in figure 7A and B, IL-2 treated post-t CD8 T cells expressed markedly higher levels of LAP than IL-2 treated CD8 T cells from pre-transplant (pre-t) and conventional drug (drug) treated lupus patients. Although the levels of IL-10 in post-t CD8 T cells were much lower than LAP, it was still significantly higher than that in pre-t and conventional drug treated CD8 T cells. Anti-TGF-β neutralization antibody significantly blocked the ability of post-transplant CD8 T cell's suppressive effect, the lowest effective concentration of anti-TGF-β being 10 ng/ml. Blocking IL-10 could also reduce the suppressive function of post-t CD8 Treg cells, however, the blocking effect was not as strong as with blocking of TGF-β (Figure 7 C and D), indicating TGF-β plays a more important role in post-t CD8 Treg suppressive function. On the other hand, anti-IL-13 neutralization antibody could not abrogate the CD8 T cells' suppressive effect, suggesting IL-13 may not play a role in CD8 Treg function here.
Figure 7.
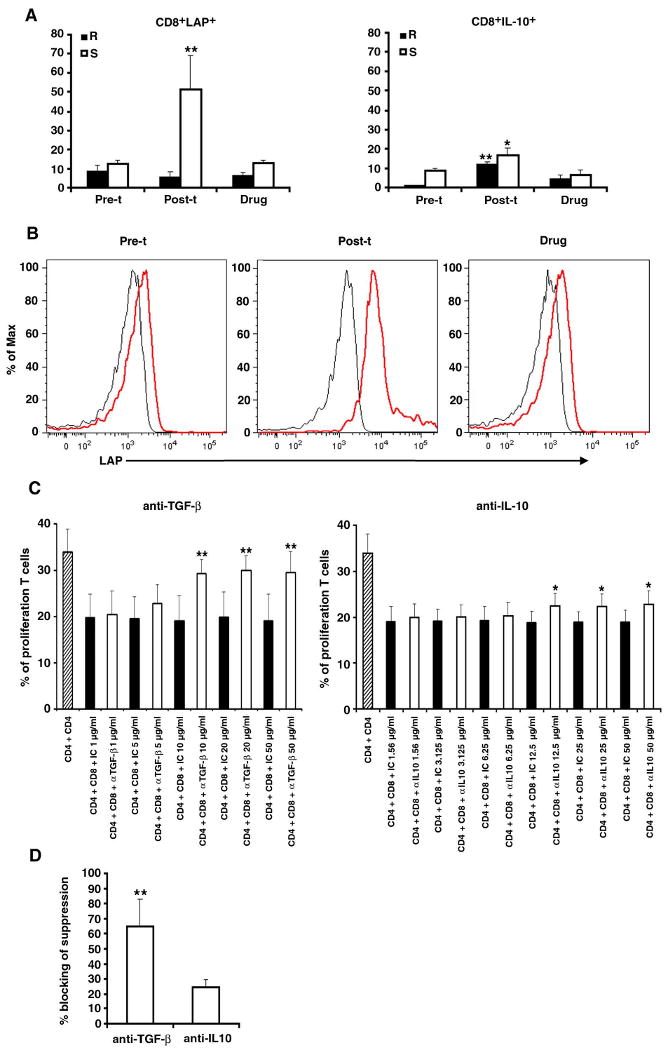
Post-transplant CD8 T cells suppress autologous CD4 T cells proliferation by secreting TGF-β mainly, and IL-10 to a lesser extent. A, Rested, short-term CD8+ T cell line cells from pre- and post-transplant (pre-t and post-t), as well as conventional drug-treated (drug) lupus patients were stained for CD8 and LAP or intracellular IL-10 before (R) or after (S) culturing with 20 units/ml IL-2 for 48 hours, Y-axes show % of LAP or IL-10 positive cells among viable T cells gated for being CD8+. Results from four experiments are shown, and the bars represent the mean ± SD. * p < 0.05 and ** p < 0.01. B, Examples of histograms of LAP expression (red line) in pre- and post-transplant, as well as conventional drug treated CD8 T cells after culturing with IL-2 (20 units/ml) for 48 hr (black line for isotype control). C, Different concentrations (numbers represent μg/ml) of anti-TGF-β or anti-IL-10 neutralizing antibody or the relevant isotype control (IC) were added to the post-transplant CD4+ target T cells cocultured with the autologous CD8 T cells in XViVo-20 serum free medium. Results show that 10 to 50 μg/ml anti-TGF-β neutralizing antibody could significantly block the suppression function of post-t CD8 on CD4 proliferation, and 12.5-50 μg/ml anti-IL-10 neutralizing antibody also showed blocking of the CD8 T cells' suppressive effect. D, For comparison, data in panel C is shown as “blocking of suppression” by anti-TGF-β or anti-IL-10 neutralizing antibody at the highest optimal concentration in the suppression assays. The % blocking of suppression was calculated as stated in materials and methods. For C and D, results from five experiments are shown, and the bars represent the mean ± SD, *p < 0.05 and ** p < 0.01.
Discussion
Although aggressive immunosuppressive therapy can control disease activity and reduce organ damage, it is impossible to achieve the goal of drug-free remission or “cure”, as patients with organ threatening active SLE still have 20% 2-year and 35% 5-year disease related mortalities with conventional treatment (66, 67). The approach of hematopoietic stem cell transplantation (HSCT) was first reported for patients with SLE in 1997 showing encouraging responses and inducing long-term remission and low (about 1.5%) mortalities. HSCT thus became a great opportunity to offer patients with refractory SLE long-term remission. Even if the disease returned in some patients later, it was mild and easy to treat (55, 66).
There are three hypotheses formulated for the mechanisms of stem cell transplantation therapy: (1) immune ablation eliminates autoreactive T-cell clones; (2) autoreactive T-cell clones are rendered tolerant; and (3) regulatory networks controlling the autoreactive T cells are restored. It was suggested that an “immune-reset” occurs following autologous HSCT in patients with autoimmune disease (56), in fact, upon immune reconstitution the default mechanism of the immune system is self-tolerance, but the exact mechanism by which self-tolerance returns following a HSCT remains undefined. In an attempt to address this issue, we studied both Treg function and nucleosomal autoantigen-specific T cell response before and after HSCT in lupus patients who were in remission after HSCT and compared them with lupus patients who received conventional drug treatment and were in “clinical remission”, and healthy controls as well.
Most studies on Treg cells in human SLE have focused on CD4+ Treg cells, but results are very conflicted. Valencia et al (28) described reduced numbers of suppressive CD4+CD25highFoxP3 Treg cells in the peripheral blood of patients with active SLE (aSLE), compared with patients with inactive SLE (iSLE) and normal donors, and those aSLE CD4 Treg cells failed to suppress CD4+CD25− T cell proliferation while iSLE CD4 Treg showed normal suppressive function. However, Yan et al (68) found that the number of CD4+CD25highFoxP3 Treg cells in aSLE patient's fresh PBMC was actually higher than that in iSLE and normal controls, but aSLE CD4+CD25high Treg cells showed impaired suppressive function when in the presence of lupus APC. Venigalla et al (35) also reported the number of CD4+CD25highFoxP3 Treg cells in aSLE fresh PBMC were higher than that in iSLE, but both aSLE and iSLE CD4 Treg cells' suppressive function were impaired. Although those three groups utilized very similar methods, they got different results, most likely because Treg studies were done with patient's blood samples immediately ex vivo. We have shown previously that lupus T cells need to rest and recover in vitro, as many extrinsic factors may affect immune cell function in lupus in vivo, such as excessive IFNα, IL-6, IL-10 production, recurrent autoantigenic stimulation, autoantibodies against immune cell surface molecules, and global immunosuppressant drugs, such as steroids, cytoxan, etc (4, 5, 9, 37, 39). In our study comparing the autologous pre- and post-transplant lupus patients' CD4 line T cells that had been rested, we found the number of pre-transplant CD4+CD25highFoxP3+ Treg cell to be significantly lower, and the frequency of this subset in post-transplant lupus patients was restored to levels seen in non-lymphopenic, normal subject's short-term lines derived by identical procedure. We also analyzed the suppressive function of CD4+CD25high Treg cells which come from post-transplant lupus patients; but those cells had minor suppressive function, nowhere as strong as their autologous post-transplant CD8 Treg cells (Figure 4).
Although very little is known about CD8 Treg cells in human SLE, observations indicated the important role of CD8 Treg in human organ transplantation, murine SLE (11, 50) and experimental autoimmune encephalomyelitis (EAE), but these CD8 Treg are different in different situations (69). Our study found that the number of CD8+FoxP3+ Treg (CD28− or CD28+) cells significantly increased in post-transplant CD8 line T cells as compared with pre-transplant and drug treated lupus patients, moreover, those cells showed strong suppressive effect on CD4 T cells proliferation. However, our data did not show any significant difference in the numbers of CD8+CD28 FoxP3+ Treg cells in fresh PBMC from pre-and post-transplant lupus patients, although those CD8 Treg cell numbers are much higher in normal PBMCs (Figure 2C), indicating that unknown in vivo influences still exists between post-transplant lupus and normal control subjects. The one time TCR stimulation with IL-2 in vitro followed by resting probably removes the extrinsic in vivo influences mentioned above and brings out these Treg cells in post-transplant lupus patients to the levels seen in normal subjects. Nevertheless, the short-term culture T cells were rested following stimulation for 10 days, demonstrating a stable and sustained elevation of FoxP3 expression only in the post-transplant and normal T cells. The CD8 Treg activity in the post-transplant patients and normal subjects reported here is mediated by soluble factors, such as TGF-β, and not through cytotoxic activity. Filaci et al (43), used different conditions to generate CD8 Treg cells in vitro by first culturing CD8 T cells with irradiated autologous monocytes in the presence of IL-2 and GM-CSF for 7 days, and then purified CD8 T cells to study Treg activity. They reported that these cytokine-induced CD8 Treg cells from conventional drug-treated, inactive lupus patients mediated suppressive function by secreting IL-6 and IFN-γ, which cytokines paradoxically may aggravate lupus. By contrast, our results show that the CD8 cells from lupus patients with conventional drug-induced remission, actually have no suppressive function on CD4 T cell proliferation. The results indicate different mechanisms in HSCT and conventional drug treatment, and understanding those mechanisms may be helpful to search for better therapy for lupus. As table I shows, most post-transplant lupus patients in our study were in remission (SLEDAI < 3), and the CD8 Treg cells from these patients showed strong suppressive function. We have one post-transplant lupus patient in our study (patient 5-B) with a SLEDAI = 4, and the CD8 Treg from this patient didn't show strong suppressive function (the % of inhibition was only 8%). In the future, we will compare autoimmune responses and CD8 Treg suppressive function in rare post-transplant lupus patients who have relapsed years after transplantation. It was also reported that target CD4+CD25− T cells from active SLE patients show low sensitivity to the suppressive function of their autologous CD4+CD25highCD127−/low Treg cells (35). We could not confirm this result (Figure 4). Indeed, the post-transplant CD8 Treg cells could suppress to a similar degree both pre- and post-transplant CD4 T target cells proliferation, just like normal CD8 Treg cells. The mechanisms by which transplanted stem cells can induce more functional CD8 Treg cells to grow up in SLE is unclear; it may be related to the fact that immune ablation in HSCT eliminates autoreactive T and B cells giving more space for homeostatic proliferation of Treg cells (70), but this is not the case in non-lymphopenic normal subjects where these Treg cells are also prevalent. In contrast to post-transplant patient's CD8 Treg cells, our results show that pre-transplant CD8 line T cells markedly augmented proliferation of autologous CD4 target T cells in contact cultures and helped in CD4 T cells response to histone peptide or nucleosomes (Figures 5 and 6). Horwitz et al (40) also reported that deleting CD8 T cells from active lupus PBMC can reduce the polyclonal IgG production and adding autologous CD8 T cells back to PBMC can reconstitute this antibody production, but the underlying mechanisms of this helper activity are still not clear.
Nucleosomes and their core histones peptides have been identified to be the major immunogens that initiate cognate interactions between autoimmune Th and B cells for the production of pathogenic antinuclear autoantibodies in lupus. Previous studies have shown that in contrast to normal T cells, conventional drug treated lupus patients' T cells responded strongly to the nucleosomal histone peptide epitopes by producing intracellular IFN-γ, irrespective of the patient's disease status (9). Remarkably, these peptides can also induce anti-DNA autoantibodies and nephritis in lupus-prone mice on immunization (7, 8). Our study established here that after HSCT, IFN-γ production response of CD4+ T cell in fresh PBMC to nucleosomal autoepitopes was reduced to background levels in the post-transplant lupus patients, while immunoregulatory IL-13 response was increased, and this reduced IFN-γ response went up when CD8 T cells were deleted from the PBMC, considerably more than when CD4+CD25high subset was deleted. Moreover, IFN-γ response to the autoepitopes in post-transplant PBMC-CD4 T cells was reversed to pathogenic high levels when cultured with autologous pre-transplant CD8 T cells. Similarly, in an allogeneic culture combination, the IFN-γ response of PBMC-CD4 T cells from conventional drug treated patients to nucleosomes and its peptide epitopes was suppressed when cultured with post-transplant CD8 T cells, but this response was increased when cultured with pre-transplant CD8 T cells. Overall, the observations indicate that HSCT-induced CD8 Treg cells play very important role in restoring self tolerance in post-transplant lupus patients, and the mechanisms of HSCT induced remission are different from conventional drug induced remission.
Ts or CD8+ Treg cells have been studied in other systems, but no unique surface marker has been identified so far which can be used to distinguish them. Although the transcription factor FoxP3 is critical for Treg cells, it is expressed intracellularly. CD28 Ag was believed to be poorly expressed on CD8 Treg cells, however, our results show that the % of CD8+CD28− cells are not markedly different in pre- and post-transplant lupus patient's fresh PBMC and CD8 line T cells. In fact, the % of CD8+CD28− was higher than 90% in both pre- and post-transplant CD8 line T cells, but the former showed a helper function and the latter showed a suppressive function. In additional, the % FoxP3+ cells in the CD8+ T cell lines from post-transplant patients as compared to pre-transplant samples, were significantly increased not only in CD28− subpopulations, but also in CD28+ subset. Therefore, CD28 may not be a suitable marker for CD8 Treg cells of lupus. In our study, the short-term post-transplant CD8 line T cells highly express LAP, CD103, PD-1, PD-L1 and intracellular CTLA-4, as compared pre-transplant CD8 line T cells. Moreover, CD103 expression remained at high level in post-transplant CD8 line T cells even after the cells had been rested for 9 days after TCR stimulation, while CD103 expression decreased rapidly in pre-transplant CD8 line T cells under same conditions, but again, those molecular markers are not always co-expressed with FoxP3 at the same time point. We also measured CD27, CD56, and CD62L expression level in CD8 line T cells, but we could not find significant differences for those in pre and post-transplant CD8 T cells (data not shown). To explore unique marker(s) for CD8 Treg cells, further work is being done.
In conclusion, HSCT can induce immunologic self tolerance in refractory SLE by restoring the CD8 TGF-β FoxP3+ regulatory network in particular, accompanied by almost complete inhibition of pathogenic T cell response to autoepitopes from nucleosomes. The CD8 Treg cells generated in post-transplant lupus patients are much more potent than CD4+25high Treg in suppressing lupus autoimmunity. These CD8+FoxP3+ TGFβ producing Treg are more relevant for controlling lupus, as shown in stem cell transplant patients here and in peptide-treated murine models (11, 19, 30, 50), and they are different from cytotoxic, contact-dependent CD8+ Treg described in other situations, such as transplantation and organ-specific autoimmune disease (20-24, 69,71).
Footnotes
This work was supported by National Institutes of Health Grant RO1 AR39157 (to S.K.D.) and Lupus Foundation of America (to R.K.B.) and NIH P60 AR30692 (to R.R.G.).
Abbreviations: SLE, systemic lupus erythematosus; Treg, Regulatory T cells; HSCT, hematopoietic stem cell transplantation; Post-t, post-transplant; Pre-t, pre-transplant; CRI, cytokine response index; LAP, latency associated peptide.
Disclosures: The authors have no financial conflict of interest.
References
- 1.Kotzin BL. Systemic lupus erythematosus. Cell. 1996;85:303–306. doi: 10.1016/s0092-8674(00)81108-3. [DOI] [PubMed] [Google Scholar]
- 2.Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008;358:929–939. doi: 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- 3.Datta SK. Major peptide autoepitopes for nucleosome-centered T and B cell interaction in human and murine lupus. Ann NY Acad Sci. 2003;987:79–90. doi: 10.1111/j.1749-6632.2003.tb06035.x. [DOI] [PubMed] [Google Scholar]
- 4.Krishnan S, Farber DL, Tsokos GC. T cell rewiring in differentiation and disease. J Immunol. 2003;171:3325–3331. doi: 10.4049/jimmunol.171.7.3325. [DOI] [PubMed] [Google Scholar]
- 5.Datta SK, Zhang L, Xu L. T-helper cell intrinsic defects in lupus that break peripheral tolerance to nuclear autoantigens. J Mol Med. 2005;83:267–278. doi: 10.1007/s00109-004-0624-2. [DOI] [PubMed] [Google Scholar]
- 6.Mohan C, Adams S, Stanik V, Datta SK. Nucleosome: A major immunogen for the pathogenic autoantibody-inducing T cells of lupus. J Exp Med. 1993;177:1367–1381. doi: 10.1084/jem.177.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaliyaperumal A, Mohan C, Wu W, Datta SK. Nucleosomal peptide epitopes for nephritis-inducing T helper cells of murine lupus. J Exp Med. 1996;183:2459–2469. doi: 10.1084/jem.183.6.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaliyaperumal A, Michaels MA, Datta SK. Naturally processed chromatin peptides reveal a major autoepitope that primes pathogenic T and B cells of lupus. J Immunol. 2002;168:2530–2537. doi: 10.4049/jimmunol.168.5.2530. [DOI] [PubMed] [Google Scholar]
- 9.Lu L, Kaliyaperumal A, Boumpas DT, Datta SK. Major peptide autoepitopes for nucleosome-specific T cells of human lupus. J Clin Invest. 1999;104:345–355. doi: 10.1172/JCI6801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaliyaperumal A, Michaels MA, Datta SK. Antigen-specific therapy of murine lupus nephritis using nucleosomal peptides: Tolerance spreading impair pathogenic function of autoimmune T and B cells. J Immunol. 1999;162:5775–5783. [PubMed] [Google Scholar]
- 11.Kang HK, Michaels MA, Berner BR, Datta SK. Very low-dose tolerance with nucleosomal peptides controls lupus and induces potent regulatory T cell subsets. J Immunol. 2005;174:3247–3255. doi: 10.4049/jimmunol.174.6.3247. [DOI] [PubMed] [Google Scholar]
- 12.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 13.Shevach EM. Regulatory T cells in autoimmunity. Annu Rev Immunol. 2000;18:423–449. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 14.Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T cell subset inhibits antigen-specific T cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 16.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 17.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 18.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng SG, Wang JH, Koss MN, Quismorio F, Jr, Gray JD, Horwitz DA. CD4+ and CD8+ regulatory T cells generated ex vivo with IL-2 and TGF-beta suppress a stimulatory graft- versus-host disease with a lupus-like syndrome. J Immunol. 2004;172:1531–1539. doi: 10.4049/jimmunol.172.3.1531. [DOI] [PubMed] [Google Scholar]
- 20.Gershon RK, Kondo K. Infectious immunological tolerance. Immunology. 1971;21:903–914. [PMC free article] [PubMed] [Google Scholar]
- 21.Filaci G, Suciu-Foca N. CD8+ T suppressor cells are back to the game: are they players in autoimmunity? Autoimmunity Rev. 2002;1:279–283. doi: 10.1016/s1568-9972(02)00065-4. [DOI] [PubMed] [Google Scholar]
- 22.Balashov KE, Khoury SJ, Hafler DA, Weiner HL. Inhibition of T cell responses by activated human CD8+ T cells is mediated by interferon-gamma and is defective in chronic progressive multiple sclerosis. J Clin Invest. 1995;95:2711–2719. doi: 10.1172/JCI117973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilliet M, Liu YJ. Generation of human CD8 T regulatory cells by CD40 ligand-activated plasmacytoid dendritic cells. J Exp Med. 2002;195:695–704. doi: 10.1084/jem.20011603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang H, Braunstein NS, Yu B, Winchester R, Chess L. CD8+ T cells control the TH phenotype of MBP-reactive CD4+ T cells in EAE mice. Proc. Natl Acad Sci USA. 2001;98:6301–6306. doi: 10.1073/pnas.101123098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kosiewicz MM, Alard P, Liang S, Clark SL. Mechanisms of tolerance induced by transforming growth factor-beta-treated antigen-presenting cells: CD8 regulatory T cells inhibit the effector phase of the immune response in primed mice through a mechanism involving Fas ligand. Int Immunel. 2004;16:697–706. doi: 10.1093/intimm/dxh067. [DOI] [PubMed] [Google Scholar]
- 26.Mellor-Pita S, Citores MJ, Castejon R, Tutor-Ureta P, Yebra-Bango M, Andreu JL, Vargas JA. Decrease of regulatory T cells in patients with systemic lupus erythematosus. Ann Rheum Dis. 2006;65:553–554. doi: 10.1136/ard.2005.044974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu MF, Wang CR, Fung LL, Wu CR. Decreased CD4+CD25+ T cells in peripheral blood of patients with systemic lupus erythematosus. Scand J Immunol. 2004;59:198–202. doi: 10.1111/j.0300-9475.2004.01370.x. [DOI] [PubMed] [Google Scholar]
- 28.Valencia X, Yarboro C, Illei G, Lipsky PE. Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol. 2007;178:2579–2588. doi: 10.4049/jimmunol.178.4.2579. [DOI] [PubMed] [Google Scholar]
- 29.Wu HY, Staines NA. A deficiency of CD4+CD25+ T cells permits the development of spontaneous lupus-like disease in mice, and can be reversed by induction of mucosal tolerance to histone peptide autoantigen. Lupus. 2004;13:192–200. doi: 10.1191/0961203303lu1002oa. [DOI] [PubMed] [Google Scholar]
- 30.Kang HK, Liu M, Datta SK. Low-Dose peptide tolerance therapy of lupus generates plasmacytoid dendritic cells that cause expansion of autoantigen-specific Treg cells and contraction of Inflammatory TH17 cells. J Immunol. 2007;178:7849–7858. doi: 10.4049/jimmunol.178.12.7849. Cover page figure. [DOI] [PubMed] [Google Scholar]
- 31.La Cava A, Ebling FM, Hahn BH. Ig-reactive CD4+CD25+ T cells from tolerized (New Zealand Black × New Zealand White) F1 mice suppress in vitro production of antibodies to DNA. J Immunol. 2004;173:3542–3548. doi: 10.4049/jimmunol.173.5.3542. [DOI] [PubMed] [Google Scholar]
- 32.Sharabi A, Zinger H, Zborowsky M, Sthoeger ZM, Mozes E. A peptide based on the complementarity-determining region 1 of an autoantibody ameliorates lupus by up-regulating CD4+CD25+ cells and TGF-beta. Proc Natl Acad Sci U S A. 2006;103:8810–8815. doi: 10.1073/pnas.0603201103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hahn BH, Anderson M, Le E, La Cava A. Anti-DNA Ig peptides promote Treg cell activity in systemic lupus erythematosus patients. Arthritis Rheum. 2008;58:2488–2497. doi: 10.1002/art.23609. [DOI] [PubMed] [Google Scholar]
- 34.Monk CR, Spachidou MF, Rovis F, Leung E, Botto M, Lechler RI, Garden OA. MRL/Mp CD4+,CD25- T cells show reduced sensitivity to suppression by CD4+CD25+ regulatory T cells in vitro: a novel defect of T cell regulation in systemic lupus erythematosus. Arthritis Rheum. 2005;52:1180–1184. doi: 10.1002/art.20976. [DOI] [PubMed] [Google Scholar]
- 35.Venigalla RK, Tretter T, Krienke S, Max R, Eckstein V, Blank N, Fiehn C, Ho AD, Lorenz HM. Reduced CD4+,CD25- T cell sensitivity to the suppressive function of CD4+,CD25high,CD127 -/low regulatory T cells in patients with active systemic lupus erythematosus. Arthritis Rheum. 2008;58:2120–2130. doi: 10.1002/art.23556. [DOI] [PubMed] [Google Scholar]
- 36.Wohlfert EA, Callahan MK, Clark RB. Resistance to CD4+CD25+ regulatory T cells to TGF-beta in Cbl-b-/- mice. J Immunol. 2004;173:1059–1065. doi: 10.4049/jimmunol.173.2.1059. [DOI] [PubMed] [Google Scholar]
- 37.Yi Y, McNerney M, Datta SK. Regulatory defects in Cbl and mitogen-activated protein kinase (extracellular signal-related kinase) pathways cause persistent hyperexpression of CD40 ligand in human lupus T cells. J Immunol. 2000;165:6627–6634. doi: 10.4049/jimmunol.165.11.6627. [DOI] [PubMed] [Google Scholar]
- 38.Jury EC, Kabouridis PS, Flores-Borja F, Mageed RA, Isenberg DA. Altered lipid raft-associated signaling and ganglioside expression in T lymphocytes from patients with systemic lupus erythematosus. J Clin Invest. 2004;113:1176–1187. doi: 10.1172/JCI20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu L, Zhang L, Yi Y, Kang HK, Datta SK. Human lupus T cells resist inactivation and escape death by upregulating COX-2. Nat Med. 2004;10:411–415. doi: 10.1038/nm1005. [DOI] [PubMed] [Google Scholar]
- 40.Linker-Israeli M, Quisimoro FP, Horwitz DA. CD8+ lymphocytes from patients with systemic lupus erythematosus sustain, rather than suppress, spontaneous polyclonal IgG production and synergize with CD4+ cells to support autoantibody synthesis. Arthritis Rheum. 1990;33:1216–1225. doi: 10.1002/art.1780330823. [DOI] [PubMed] [Google Scholar]
- 41.Desai-Mehta A, Lu L, Ramsey-Goldman R, Datta SK. Hyperexpression of CD40 ligand by B and T cells in human lupus and its role in pathogenic autoantibody production. J Clin Invest. 1996;97:2063–2073. doi: 10.1172/JCI118643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stohl W, Elliott JE, Li L, Podack ER, Lynch DH, Jacob CO. Impaired nonrestricted cytolytic activity in systemic lupus erythematosus: involvement of a pathway independent of Fas, tumor necrosis factor, and extracellular ATP that is associated with little detectable perforin. Arthritis Rheum. 1997;40:1130–1137. doi: 10.1002/1529-0131(199706)40:6<1130::AID-ART17>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 43.Filaci G, Bacilieri S, Fravega M, Monetti M, Contini P, Ghio M, Setti M, Puppo F, Indiveri F. Impairment of CD8+ T suppressor cell function in patients with active systemic lupus erythematosus. J Immunol. 2001;166:6452–6457. doi: 10.4049/jimmunol.166.10.6452. [DOI] [PubMed] [Google Scholar]
- 44.Crispín JC, Oukka M, Bayliss G, Cohen RA, Van Beek CA, Stillman IE, Kyttaris VC, Juang VT, Tsokos GC. Expanded Double Negative T Cells in Patients with Systemic Lupus Erythematosus Produce IL-17 and Infiltrate the Kidneys. J Immunol. 2008;181:8761–8766. doi: 10.4049/jimmunol.181.12.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hsu HC, Yang P, Wang J, Wu Q, Myers R, Chen J, Yi J, Guentert T, Tousson A, Stanus AL, Le TV, Lorenz RG, Xu H, Kolls JK, Carter RH, Chaplin DD, Williams RW, Mountz JD. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat Immunol. 2008;9:166–175. doi: 10.1038/ni1552. [DOI] [PubMed] [Google Scholar]
- 46.Bauquet AT, Jin H, Paterson AM, Mitsdeorffer MI, Ho IC, Sharpe AH, Kuchroo VK. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat Immunol. 2009;10:167–175. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu HY, Quintana FJ, Weiner HL. Nasal anti-CD3 antibody ameliorates lupus by inducing an IL-10-secreting CD4+CD25− LAP+ regulatory T cell and is associated with down-regulation of IL-17+CD4+ICOS+CXCR5+ follicular helper T cells. J Immunol. 2008;181:6038–6050. doi: 10.4049/jimmunol.181.9.6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malissen B. Revisiting the follicular helper T cell paradigm. Nature Immunol. 2009;10:371–372. doi: 10.1038/ni0409-371. [DOI] [PubMed] [Google Scholar]
- 49.Mohan C, Shi Y, Laman JD, Datta SK. Interction between CD40 and its ligand gp39 in the development of murine lupus nephritis. J Immunol. 1995;154:1470–1480. [PubMed] [Google Scholar]
- 50.Singh RP, La Cava A, Wong M, Ebling F, Hahn BH. CD8+ T cell-mediated suppression of autoimmunity in a murine lupus model of peptide-induced immune tolerance depends on Foxp3 expression. J Immunol. 2007;178:7649–7657. doi: 10.4049/jimmunol.178.12.7649. [DOI] [PubMed] [Google Scholar]
- 51.de Kleer I, Vastert B, Klein M, Teklenburg G, Arkesteijn G, Yung GP, Albani S, Kuis W, Wulffraat N, Prakken B. Autologous stem cell transplantation for autoimmunity induces immunologic self-tolerance by reprogramming autoreactive T cells and restoring the CD4+CD25+ immune regulatory network. Blood. 2006;107:1696–1702. doi: 10.1182/blood-2005-07-2800. [DOI] [PubMed] [Google Scholar]
- 52.Burt RK, Traynor A, Ramsey-Goldman R. Hematopoietic stem-cell transplantation for systemic lupus erythematosus. N Engl J Med. 1997;337:1777–1778. doi: 10.1056/NEJM199712113372416. [DOI] [PubMed] [Google Scholar]
- 53.Traynor AE, Schroeder J, Rosa RM, Cheng D, Stefka J, Mujais S, Baker S, Burt RK. Treatment of severe systemic lupus erythematosus with high-dose chemotherapy and haemopoietic stem-cell transplantation: a phase I study. Lancet. 2000;356:701–707. doi: 10.1016/S0140-6736(00)02627-1. [DOI] [PubMed] [Google Scholar]
- 54.Burt RK, Traynor A, Statkute L, Barr WG, Rosa R, Schroeder J, Verda L, Krosnjar N. Nonmyeloablative hematopoietic stem cell transplantation for systemic lupus erythematosus. JAMA. 2006;295:527–535. doi: 10.1001/jama.295.5.527. [DOI] [PubMed] [Google Scholar]
- 55.Burt RK, Marmont A, Oyama Y, Slavin S, Arnold R, Hiepe F, Fassas A, Snowden J. Randomized controlled trials of autologous hematopoietic stem cell transplantation for autoimmune diseases: the evolution from myeloablative to lymphoablative transplant regimens. Arthritis Rheum. 2006;54:3750–3760. doi: 10.1002/art.22256. [DOI] [PubMed] [Google Scholar]
- 56.Muraro PA, Douek DC, Packer A, Chung K, Guenaga FJ, Cassiani-Ingoni R, Campbell C, Memon S, Nagle JW, Hakim FT, Gress RE, McFarland HF, Burt RK, Martin R. Thymic output generates a new and diverse TCR repertoire after autologous stem cell transplantation in multiple sclerosis patients. J Exp Med. 2005;201:805–816. doi: 10.1084/jem.20041679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 58.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35:630–640. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 59.Baecher-Allan CM, Hafler DA. The purification and functional analysis of human CD4+CD25high regulatory T cells. In: Coligan JE, Bierer BE, Margulies DH, Shevach EM, Strober W, editors. Current Protocols in Immunology. John Wiley & Sons, Inc.; 2006. pp. 7.4B.1–7.4B12. [DOI] [PubMed] [Google Scholar]
- 60.Cortesini R, LeMaoult J, Ciubotariu R, Cortesini NS. CD8+CD28- T suppressor cells and the induction of antigen-specific, antigen-presenting cell-mediated suppression of Th reactivity. Immunol Rev. 2001;182:201–206. doi: 10.1034/j.1600-065x.2001.1820116.x. [DOI] [PubMed] [Google Scholar]
- 61.Keino H, Masli S, Sasaki S, Streilein JW, Stein-Streilein J. CD8+ T Regulatory Cells Use a Novel Genetic Program that Includes CD103 to Suppress Th1 Immunity in Eye-Derived Tolerance. Invest Ophthalmol Vis Sci. 2006;47:1533–1542. doi: 10.1167/iovs.04-1454. [DOI] [PubMed] [Google Scholar]
- 62.Okazaki T, Iwai Y, Honjo T. New regulatory co-receptors: inducible costimulator and PD-1. Curr Opin Immunol. 2002;14:779–782. doi: 10.1016/s0952-7915(02)00398-9. [DOI] [PubMed] [Google Scholar]
- 63.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 Control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 64.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–679. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 65.Gaffen AL, Lie KD. Overview of interleukin-2 function, production and clinical applications. Cytokine. 2004;28:109–123. doi: 10.1016/j.cyto.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 66.Burt RK, Traynor AE. SLE - hematopoietic stem cell transplantation for systemic lupus erythematosus. Arthritis Res Ther. 2003;5:207–209. doi: 10.1186/ar786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pavletic SZ, Illei GG. The role of immune ablation and stem cell transplantation in severe SLE. Best Pract Res Clin Rheumatol. 2005;19:839–858. doi: 10.1016/j.berh.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 68.Yan B, Ye S, Chen G, Kuang M, Shen N, Chen S. Dysfunctional CD4+,CD25+ regulatory T cells in untreated active systemic lupus erythematosus secondary to interferon-alpha-producing antigen-presenting cells. Arthritis Rheum. 2008;58:801–812. doi: 10.1002/art.23268. [DOI] [PubMed] [Google Scholar]
- 69.Lu L, Kim HJ, Werneck MB, Cantor H. Regulation of CD8+ regulatory T cells: Interruption of the NKG2A-Qa-1 interaction allows robust suppressive activity and resolution of autoimmune disease. Proc Natl Acad Sci. 2008;105:19420–19425. doi: 10.1073/pnas.0810383105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barthlott T, Kassiotis G, Stockinger B. T cell regulation as a side effect of homeostasis and competition. J Exp Med. 2003;197:451–460. doi: 10.1084/jem.20021387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shustov A, Luzina I, Nguyen P, Papadimitriou JC, Handwerger B, Elkon KB, Via CS. Role of perforin in controlling B-cell hyperactivity and humoral autoimmunity. J Clin Invest. 2000;106:R39–R47. doi: 10.1172/JCI8876. [DOI] [PMC free article] [PubMed] [Google Scholar]


