Abstract
Epoxyeicosatrienoic acids (EETs) are endothelium-derived metabolites of arachidonic acid. They relax vascular smooth muscle by membrane hyperpolarization. These actions are inhibited by the EET antagonist, 14,15-epoxyeicosa-5(Z)-enoic acid (14,15-EE5ZE). We synthesized 20-125iodo-14,15-EE5ZE (20-125I-14,15-EE5ZE), a radiolabeled EET antagonist, and characterized its binding to cell membranes. 14,15-EET (10−9-10−5M) caused a concentration-related relaxation of the preconstricted bovine coronary artery and phosphorylation of p38 in U937 cells that were inhibited by 20-125I-14,15-EE5ZE. Specific 20-125I-14,15-EE5ZE binding to U937 cell membranes reached equilibrium within 5 min and remained unchanged for 30 min. The binding was saturable and reversible, and it exhibited KD and Bmax values of 1.11 ± 0.13 nM and 1.13 ± 0.04 pmol/mg protein, respectively. Guanosine 5′-O-(3-thio)triphosphate (10 μM) did not change the binding, indicating antagonist binding of the ligand. Various EETs and EET analogs (10−10-10−5M) competed for 20-125I-14,15-EE5ZE binding with an order of potency of 11,12-EET = 14,15-EET > 8,9-EET = 14,15-EE5ZE > 15-hydroxyeicosatetraenoic acid = 14,15-dihydroxyeicosatrienoic acid. 8,9-Dihydroxyeicosatrienoic acid and 11-hydroxyeicosatetraenoic acid did not compete for binding. The soluble and microsomal epoxide hydrolase inhibitors (1-cyclohexyl-3-dodecyl-urea, elaidamide, and 12-hydroxyl-elaidamide) and cytochrome P450 inhibitors (sulfaphenazole and proadifen) did not compete for the binding. However, two cytochrome P450 inhibitors, N-methylsulfonyl-6-(2-propargyloxyphenyl)hexanamide (MS-PPOH) and miconazole competed for binding with Ki of 1558 and 315 nM, respectively. Miconazole and MS-PPOH, but not proadifen, inhibited 14,15-EET-induced relaxations. These findings define an EET antagonist's binding site and support the presence of an EET receptor. The inhibition of binding by some cytochrome P450 inhibitors suggests an alternative mechanism of action for these drugs and could lead to new drug candidates that target the EET binding sites.
Epoxyeicosatrienoic acids (EETs) are cytochrome P450 epoxygenase metabolites of arachidonic acid (Capdevila et al., 1981; Spector and Norris, 2007). Four EET regioisomers (14,15-, 11,12-, 8,9-, and 5,6-EET) are synthesized. They are actively metabolized by β-oxidation and epoxide hydration in mammalian cells and tissues (Spector et al., 2004). EETs function as endothelium-derived hyperpolarization factor in the cardiovascular system (Campbell et al., 1996; Fisslthaler et al., 1999; Campbell and Falck, 2007), but also have effects on the immune (Node et al., 1999; Liu et al., 2005) and neuronal systems (Inceoglu et al., 2007, 2008; Terashvili et al., 2008). They cause vasodilation, mitogenesis, angiogenesis, inhibition of inflammation, fibrinolysis, and antinociception (Spector and Norris, 2007). These functions are attributed, but not limited, to several signal transduction pathways including G protein coupling to large-conductance, calcium-activated potassium (BKCa) channels (Li and Campbell, 1997), nuclear factor κB (Node et al., 1999), epidermal growth factor receptor-Src-kinase (Chen et al., 1999), mitogen-activated protein (MAP) kinases (Fleming et al., 2001), and phosphatidylinositol 3-kinase (Chen et al., 2001).
Although many downstream molecules and pathways have been identified, the initiation step in EET signaling pathways is still not clear. Low-affinity EET-binding proteins have been proposed to mediate EET action. These binding proteins include fatty acid-binding protein (Widstrom et al., 2001), peroxisomal proliferator-activated receptor (PPAR)-α (Cowart et al., 2002), PPAR-γ (Liu et al., 2005), and ATP-sensitive K channels (Lu et al., 2006). Although EETs may exert some actions through some of these proteins, the micromolar affinity of EETs for these proteins cannot explain physiological responses that occur with nanomolar concentrations of EETs. High-affinity EET binding proteins or receptors still require identification.
Several lines of evidence suggest that EETs act through a specific binding site. Falck et al. (2003a) tested a series of 14,15-EET analogs for their ability to relax the bovine coronary artery. 14(S),15(R)-cis-Epoxyeicosa-8Z-enoic acid was the simplest structure with full agonist activity. The requirement for a specific stereoisomer of the epoxide suggested a specific binding site for the EET. On vascular smooth muscle, 14,15-EET that was tethered to silica beads could not enter the cell but inhibited aromatase activity to a similar extent as 14,15-EET (Snyder et al., 2002). Thus, 14,15-EET acted on the cell surface and not intracellularly. A high-affinity EET binding site was described in intact cells and membrane preparations from guinea pig mononuclear cells and human U937 cells. By use of [3H]14,15-EET as a radioligand, specific and saturable binding with a KD of 5.7 nM was determined in guinea pig monocytes and a KD of 13.84 nM in U937 cells (Wong et al., 1993, 1997, 2000). This binding site was further defined in the cell membranes by Yang et al. (2008) by use of 20-125iodo-14,15-epoxyeicosa-8Z-enoic acid (20-125I-14,15-EE8ZE). 20-125I-14,15-EE8ZE bound U937 membranes in a specific, saturable, and reversible manner with a KD of 11.8 nM. EET analogs, but not prostaglandins or lipoxygenase metabolites, displaced the 14,15-EET radioligands from their binding site. This binding site was down-regulated by cAMP-protein kinase A pathway activation and GTPγS suggesting a possible G protein-coupled receptor (GPCR) (Wong et al., 1997; Yang et al., 2008). These studies suggested that EETs act via a cell surface receptor. U937 cells are good model systems for studying a high-affinity EET binding site/receptor(s).
Radiolabeled ligands have been key tools for receptor identification, signal transduction pathway investigation, drug discovery, and mapping amino acid residues in ligand binding sites. 3H-Labeled ligands, in general, have low specific activity and are expensive to synthesize (Wong et al., 1993, 1997, 2000). 125I-Labeled EET agonist ligands have been synthesized (Yang et al., 2007, 2008), but antagonist radioligands are traditionally favored in drug screening. Furthermore, antagonists are proposed to occupy a different, but overlapping, binding pocket than agonists. An antagonist EET radioligand may be used to map an antagonist's binding pocket of the EET-binding protein(s). Here, we have modified the structure of the first EET antagonist, 14,15-epoxyeicosa-5Z-enoic acid (14,15-EE5ZE), synthesized, and characterized the first EET antagonist radioligand, 20-125I-14,15-EE5ZE (Gauthier et al., 2002).
Materials and Methods
Synthesis of 20-125I-14,15-EE5ZE.
20-125I-14,15-EE5ZE is synthesized from the corresponding 20-tosyl (OTs)-14,15-EE5ZE as reported previously (Prestwich et al., 1988; Yang et al., 2008). The syntheses of nonradiolabeled (cold) 20-I-14,15-EE5ZE and 20-OTs-14,15-EE5ZE are described in the Supplemental Data (Mosset et al., 1989; Cai et al., 2006; Yang et al., 2008). Here, the synthesis of 20-125I-14,15-EE5ZE is described. To 2 mCi in 20 μl of carrier-free Na125I (0.8 nmol; 17.4 Ci/mg) was added 20 μl of NaI in acetone (6.4 μg) and 40 μl of 20-OTs-14,15-EE5ZE in acetone (640 nmol). The reaction was carried out at 37°C for 4 days, with shaking 2 to 3 times daily, and stopped by 10 μl of a saturated Na2S2O3 solution. The reaction mixture was added to a Bio-Sil A (Bio-Rad Laboratories, Hercules, CA) silicic acid column. The column was then eluted by 2 volumes of hexane/ethyl acetate (90%:10%) and 2 volumes of hexane/ethyl acetate (80%:20%). The eluent was dried under N2 and purified by high-performance liquid chromatography with use of a C18 reverse-phase column (Nucleosil; 5 μM; 4.6 × 250 mm; Phenomenex, Torrance, CA). A linear gradient of 50 to 100% solvent B in solvent A (solvent B: acetonitrile/glacial acetic acid = 999:1; solvent A: water) over 40 min was used to elute 20-125I-14,15-EE5ZE. 20-I-14,15-EE5ZE was used as a chromatographic standard and detected in the column effluent by UV absorbance at 205 nm. The specific activity of 20-125I-14,15-EE5ZE was 47.69 Ci/mmol.
Culture of U937 Cells.
U937 cells were cultured in suspension in RPMI 1640 medium (Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum (HyClone Laboratories, Logan, UT), 25 mM HEPES, 2 mM l-glutamine, and 1 mM sodium pyruvate, 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin B (Yang et al., 2007, 2008). Culture medium was changed every 2 to 3 days. Cells were cultured at 37°C in a 5% CO2 in air-humidified atmosphere and harvested after reaching a density of 5 to 10 × 105 cells/ml.
Measurement of Phospho-p38 and p38 in U937 Cells.
U937 cells (106 cells/ml) were suspended in phosphate-buffered saline containing SKF525a (10 μM), triascin C (20 μM), and 12-(3-adamantan-1-yl-ureido)-dodecanoic acid (1 μM) to inhibit cytochrome P450, esterification, and epoxide hydrolase (EH), respectively (Yang et al., 2008). Cells were incubated for 10 min at 37°C with vehicle, 14,15-EET (100 nM), 20-I-14,15-EE5ZE (10–1000 nM), or 14,15-EET and 20-I-14,15-EE5ZE (10–1000 nM). Subsequently, the cell suspension was centrifuged for 5 min at 4°C. The cell pellet was resuspended in lysis buffer (150 mM NaCl, 10 mM HEPES, 1 mM EDTA, 1 mM EGTA, 1 mM Na2S2O5, pH 7.5, containing 1% Triton X-100 and Roche protease inhibitor mix) and incubated for 10 min on ice. Proteins were separated by electrophoresis, and phospho-p38 and p38 were detected by Western immunoblotting.
Western Blotting.
U937 cellular lysates were mixed with reducing buffer and heated at 95°C for 10 min to denature proteins (Yang et al., 2008). The above samples were separated by electrophoresis on a 12% polyacrylamide Redi-Gel (Bio-Rad Laboratories) and transferred to a nitrocellulose membrane (Bio-Rad Laboratories) for immunoblotting with anti-phospho-p38 antibody (Cell Signaling Technology, Danvers, MA). The nitrocellulose membrane was reprobed with anti-p38 antibody (Santa Cruz Biotechnology, Santa Cruz, CA).
Vascular Reactivity of Bovine Coronary Arteries.
Fresh bovine hearts were obtained from a local slaughterhouse. The left anterior descending branch of coronary artery was dissected, cleaned of connective tissue, and cut into 3-mm-long rings of 1.5- to 3.0-mm diameter (Campbell et al., 1996; Falck et al., 2003a). The arterial rings were suspended in a water-jacketed tissue chamber containing Krebs' buffer (119 mM NaCl, 4.8 mM KCl, 24 mM NaHCO3, 0.2 mM KH2PO4, 0.2 mM MgSO4, 11 mM glucose, 0.02 mM EDTA, and 3.2 mM CaCl2) in 5% CO2 and 95% O2 environment at 37°C. Ring tension was recorded with a model FT-03C force transducer (Grass Instruments, Milford, MA), ETH-400 bridge amplifier, and MacLab 8e A/D converter controlled by a Macintosh computer. The arterial rings were stretched gradually to a tension of 3.5g and equilibrated for 1.5 h. KCl (40–60 mM) was repeatedly added and washed away until reproducible stable contractions were reached. The thromboxane mimetic U46619 (20 nM) was added to increase basal contraction to 50 to 75% KCl. Increasing concentrations of 14,15-EET or the BKCa channel activator, NS1619, were added and relaxations recorded. To block the 14,15-EET effects, rings were preincubated with vehicle, 20-I-14,15-EE5ZE (10 μM), proadifen (20 μM), miconazole (20 μM), or MS-PPOH (20 μM) for 10 min, and the 14,15-EET relaxation was recorded. Similar experiments using miconazole (20 μM) and MS-PPOH (20 μM) were repeated with the BKCa channel opener NS1619 as the agonist (Gauthier et al., 2002). Results are expressed as the percentage of relaxation of the U46619-treated rings, with 100% relaxation representing basal tension.
U937 Membrane Preparation.
Cell and membrane preparations were kept in ice or in the cold room. Cells were pooled and centrifuged at 1000 rpm for 5 min (Yang et al., 2007, 2008). Cell pellets were combined, washed with 10 ml of phosphate-buffered saline, pH 7.4, twice, and resuspended with Hanks' balanced salt solution containing protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN). After sonicating for 20 s, the lysate was centrifuged at 1000g for 10 min. The supernatants were centrifuged at 110,000g for 45 min, and the pellet was resuspended in binding buffer consisting of 10 mM HEPES, 5 mM CaCl2. 5 mM MgCl2, and 5 mM EGTA, pH 7.4. Protein concentration was determined by the Bradford method (Bio-Rad Laboratories).
20-125I-14,15-EE5ZE Binding Assays.
20-125I-14,15-EE5ZE binding assays were performed with a Brandel 48-well harvester system (Brandel Inc., Gaithersburg, MD) at 4°C (Yang et al., 2007, 2008). Binding was determined in triplicate and repeated on three to four membrane preparations. Fifty micrograms of protein was incubated in binding buffer (see U937 Membrane Preparation for composition) with various concentrations of 20-125I-14,15-EE5ZE for various times. The binding was stopped by filtration through GF/A glass filter paper. After washing five times with 3 ml of binding buffer each, the radioactivity on the filter paper was counted by a γ-scintillation counter. Nonspecific binding was measured in the presence of 20 μM 14,15-EE5ZE. Specific binding was calculated from total binding minus nonspecific binding. The data were analyzed using Prism software as reported previously (Yang et al., 2007, 2008).
Time course of binding was determined by incubating 2.9 nM radioligand with the membranes for various times (0–30 min) (Yang et al., 2008). Saturation of binding was carried out by use of a 15-min incubation time with different concentrations of the radioligand. To determine the reversibility of ligand binding, 1 or 20 μM 11,12-EET was incubated with membranes for various times (0–60 min) after 10 min of preincubation with radioligand (2.9 nM). For ligand competition, 20-125I-14,15-EE5ZE (1–2 nM) was incubated in presence of different concentrations of competing ligands for 15 min. Binding obtained in the presence of vehicle was defined as 100%. To determine the effect of GTPγS on ligand binding, the membranes were preincubated with 10 μM GTPγS or vehicle for 15 min before incubation with various concentrations of the radioligand for 15 min.
Statistical Analysis.
The data are expressed as means ± S.E.M. Statistical evaluation of the data were performed by a one-way analysis of variance followed by the Student-Newman-Keuls multiple comparison test when significant differences were present. P < 0.05 was considered statistically significant.
Results
Chemical Structures of EETs, EET Analogs, Cytochrome P450 Inhibitors, and Epoxide Hydrolase Inhibitors.
Figure 1A shows the structures of EET regioisomers, EET analogs, cytochrome P450 inhibitors, and epoxide hydrolase inhibitors that were studied.
Fig. 1.
Chemical structures of EETs, EET analogs, cytochrome P450 inhibitors, and EH inhibitors. CDU, 1-cyclohexyl-3-dodecyl-urea.
Synthesis of 20-125I-14,15-EE5ZE.
Cumulative synthesis and structure-activity relationships have revealed the basic structural requirements for EET agonist and antagonist activity (Gauthier et al., 2002, 2003; Falck et al., 2003a, 2003b). 14,15-EE8ZE has all of the structural features of a full agonist whereas 14,15-EE5ZE is the first EET receptor antagonist. We have previously synthesized a 125I-labeled EET agonist, 20-125I-14,15-EE8ZE (Yang et al., 2008). In a similar manner, we synthesized 20-125I-14,15-EE5ZE as a radiolabeled antagonist.
Antagonist Activity of 20-I-14,15-EE5ZE.
We tested whether 20-I-14,15-EE5ZE is an antagonist similar to 14,15-EE5ZE in rings of bovine coronary arteries. 14,15-EET relaxed U46619 preconstricted bovine coronary artery rings with EC50 value of approximately 2 μM (Fig. 2A). Pretreatment with 10 μM 20-I-14,15-EE5ZE reduced 14,15-EET-induced relaxations. These results indicate that 20-I-14,15-EE5ZE inhibits the action of 14,15-EET.
Fig. 2.
Effect of 20-I-14,15-EE5ZE and cytochrome P450 inhibitors on 14,15-EET- and NS1619-induced relaxation of bovine coronary arteries. Bovine coronary artery rings were preconstricted with U46619 and treated with increasing concentrations of 14,15-EET (A, B, C, E) or NS-1619 (D, F) in the presence of vehicle or 20-I-14,15-EE5ZE (1 × 10−5 M) (A), proadifen (2 × 10−5 M) (B), MS-PPOH (2 × 10−5 M) (C, D) or miconazole (2 × 10−5 M) (E, F). Each value represents the mean ± S.E.M. *, p < 0.01.
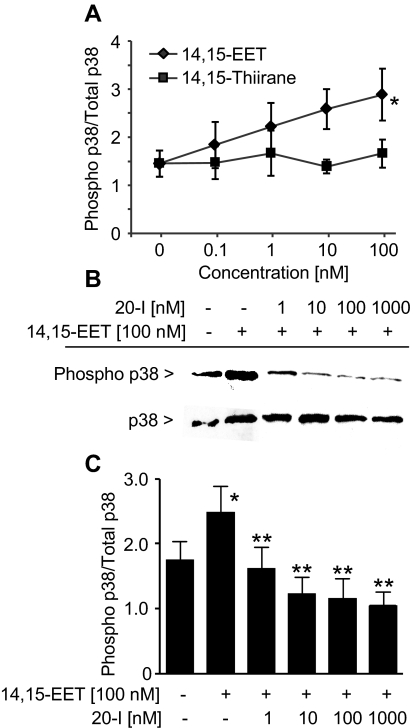
To further confirm the antagonist activity of 20-I-14,15-EE5ZE, 14,15-EET-induced p38 MAP kinase activity was monitored in U937 cells with immunoblotting. The phosphorylation of p38 in U937 cells was stimulated in concentration-dependent manner by 14,15-EET (0.1–100 nM) (Fig. 3A). In contrast, the inactive EET thiirane analog (0.1–100 nM) did not alter p38 phosphorylation, indicating specific activation of p38 by 14,15-EET (Falck et al., 2003a). The effect of 20-I-14,15-EE5ZE was tested on 14,15-EET-induced p38 phosphorylation (Fig. 3, B and C). 20-I-14,15-EE5ZE decreased 14,15-EET-stimulated p38 phosphorylation at concentrations from 1 to 1000 nM. This result indicates that 20-I-14,15-EE5ZE is an antagonist of 14,15-EET in U937 cells.
Fig. 3.
Effect of 20-I-14,15-EE5ZE on 14,15-EET-stimulated p38 phosphorylation in U937 cells. Western immunoblotting of phosphorylated p38 and total p38. A, U937 cells were treated with various concentrations of 14,15-EET or 14,15-thiirane. B, U937 cells were treated without 100 nM 14,15-EET (lanes 1 and 2) or with 100 nM 14,15-EET combined with different concentrations of 20-I-14,15-EE5ZE (lanes 3–6). The U937 cell proteins were separated through SDS-polyacrylamide gel electrophoresis, transferred onto nitrocellulose membrane, and immunoblotted with anti-phospho-p38 antibody (top row) or anti-p38 antibody (bottom row). C, summary of four independent experiments with the same experimental protocol as B. The results were expressed as ratio of phospho-p38 over total p38 (mean ± S.E.M., n = 4). *, p < 0.05 compared with no treatment; **, p < 0.05 compared with 14,15-EET.
Characterization of 20-125I-14,15-EE5ZE Binding on U937 Membranes.
Figure 4, A and C, shows the time- and concentration-dependent binding of 20-125I-14,15-EE5ZE to U937 cell membranes. The half-time of association was 0.9 min at 2.9 nM 20-125I-14,15-EE5ZE (Fig. 4A). The specific binding reached equilibrium within 5 min and remained unchanged up to 30 min. Equilibrium binding was performed at an incubation time of 15 min with increasing concentration of radioligand. Specific binding increased with radioligand and was saturable (Fig. 4, B and C). Nonspecific binding increased linearly with increasing concentrations of the radioligand. Scatchard analysis of the saturable binding suggested a single-site binding model (Fig. 4D) (r2 = 0.95). Binding affinity KD was 1.11 ± 0.13 nM, and Bmax was 1.13 ± 0.04 pmol/mg (n = 4). If we assume association rate constant kon = 6.4 × 106 M−1 s−1 for 20-125I-14,15-EE5ZE, the dissociation rate constant koff will be 0.007 s−1 calculated from koff = KD × kon and the t1/2 is 99 s from t1/2 = ln 2/koff. This antagonist radioligand has 10 times higher affinity than the agonist radioligand, 20-125I-14,15-EE8ZE (KD = 11.8 nM and Bmax = 5.8 pmol/mg, n = 5) (Yang et al., 2008). This difference in KD values between the agonist and antagonist ligands was statistically significant (p < 0.0057). The Bmax values also differ with the two ligands. The antagonist radioligand may bind different populations of receptors than the agonist radioligand.
Fig. 4.
Time- and concentration-dependent binding of 20-125I-14,15-EE5ZE to U937 membranes. A, time-dependent binding of 20-125I-14,15-EE5ZE to U937 membranes. 20-125I-14,15-EE5ZE (2.9 nM) was incubated with 50 μg of total U937 membrane for indicated times at 4°C (n = 4). Specific binding was determined in the presence of 20 μM 14,15-EE5ZE. B, effect of 20-125I-14,15-EE5ZE concentration on total, nonspecific, and specific binding (n = 4). C, specific binding of 20-125I-14,15-EE5ZE expanded from B. D, Scatchard analysis of data from B. Each value represents the mean ± S.E.M. (n = 4).
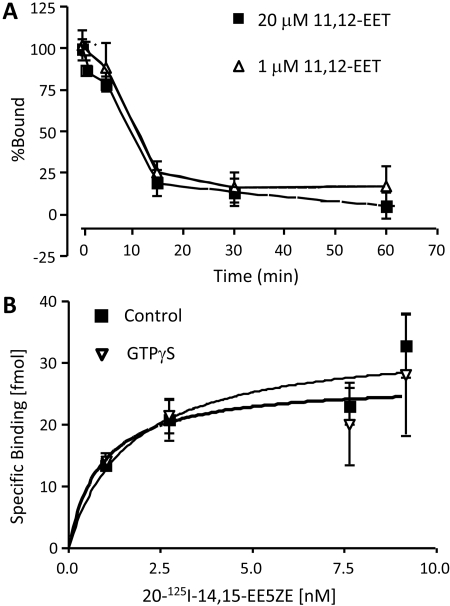
To test whether the binding is reversible, 20-125I-14,15-EE5ZE (2.9 nM) was incubated with U937 membranes for 10 min to establish equilibrium. 11,12-EET (1 or 20 μM) was then added to compete for binding. The incubations were stopped at different times from 20 s to 1 h. Figure 5A shows that 11,12-EET replaced the radioligand completely within 0.5 h and with 50% displacement of the ligand in less than 10 min. The rate of displacement of 20-125I-14,15-EE5ZE was slower than with 20-125I-14,15-EE8ZE, which was less than 1 min (Yang et al., 2008). The slower dissociation time contributes to the higher affinity for the antagonist radioligand. These data also indicate that 20-125I-14,15-EE5ZE binding to U937 membranes is reversible and the same binding site is occupied by 11,12-EET.
Fig. 5.
20-125I-14,15-EE5ZE binding to U937 membranes. A, reversibility: U937 membranes were incubated with 2.9 nM 20-125I-14,15-EE5ZE for 10 min to reach binding equilibrium. 11,12-EET (1 or 20 μM) was added, and binding was terminated at the indicated times. Specific binding was determined. B, effect of GTPγS. Membranes were preincubated with or without 10 μM GTPγS for 15 min. Indicated concentrations of 20-125I-14,15-EE5ZE were added, and the incubation was continued for 15 min. The specific binding was determined. Each value represents the mean ± S.E.M. (n = 4).
Previous experiments suggested that the EET receptor in U937 membranes might be a GPCR because GTPγS blocked the binding of the agonist radioligand 20-125I-14,15-EE8ZE (Yang et al., 2008). 20-125I-14,15-EE5ZE binding to U937 membranes was determined in the present or absence of 10 μM GTPγS. The specific binding did not differ in the presence or absence of GTPγS (Fig. 5B). This experiment further indicates that 20-I-14,15-EE5ZE is a antagonist.
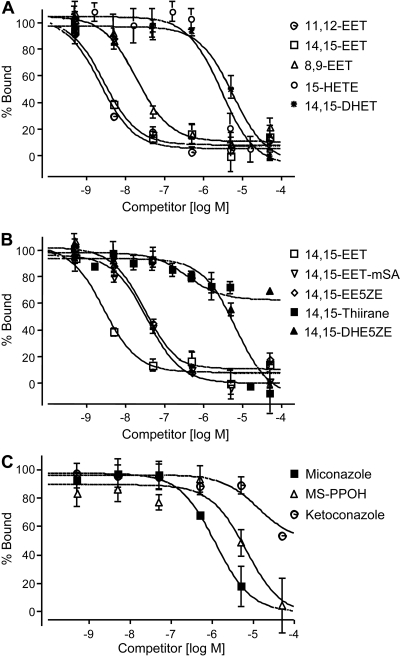
Competition for 20-125I-14,15-EE5ZE binding to U937 cell membranes was performed using three EETs, several EET structural analogs, cytochrome P450 inhibitors, and EH inhibitors (Zou et al., 1994; Harder et al., 1995; Wang et al., 1998; Morisseau et al., 1999; Falck et al., 2003a). Figure 6, A and B, and Table 1 show the rank order and Ki values of competing compounds for 20-125I-14,15-EE5ZE binding (11,12-EET = 14,15-EET > 8,9-EET = 14,15-EET-mSA = 14,15-EE5ZE > 15-HETE = 14,15-DHET = thiirane > 14,15-DHE5ZE). 8,9-DHET and 11-HETE did not displace 20-125I-14,15-EE5ZE from its binding site (Table 1). These results suggest that the binding site is specific for three EETs (8,9-EET, 11,12-EET, and 14,15-EET) but not for 15-HETE, 14,15-DHET, thiirane of 14,15-EET, or 14,15-DHE5ZE. This competition rank order of EETs and EET analogs is similar to the rank order previously report for 20-125I-14,15-EE8ZE suggesting the same binding site (Yang et al., 2008).
Fig. 6.
Inhibition of 20-125I-14,15-EE5ZE binding to U937 membranes by EETs (A), EET analogs (B), and cytochrome P450 inhibitors (C). 20-125I-14,15-EE5ZE (2.9 nM) was incubated with increasing concentrations of EETs, EET analogs or inhibitors, and U937 membrane for 15 min. Specific binding was determined in the presence or absence of 20 μM 14,15-EE5ZE. Specific binding obtained in the presence of vehicle represents 100% binding. Each value represents the mean ± S.E.M. (n = 4).
TABLE 1.
Comparison of Ki of different EETs, EET analogs, cytochrome P450 inhibitors, and EH inhibitors.
| Competitor | Ki (95% Confidence Intervals) |
|---|---|
| nM | |
| 11,12-EET | 0.990 (0.658–1.49) |
| 14,15-EET | 1.35 (0.76–2.39) |
| 8,9-EET | 9.26 (4.48–19.1) |
| 14,15-EET-mSA | 15.4 (11.2–21.2) |
| 14,15-EE5ZE | 15.5 (10.2–23.6) |
| 15-HETE | 1243 (680–2269) |
| 14,15-DHET | 2531 (1812–3534) |
| 14,15-Thiirane | 3091 (1868–5116) |
| 14,15-DHE5ZE | >5 × 104 |
| 8,9-DHET | >5 × 104 |
| 11-HETE | >5 × 104 |
| 1-Cyclohexyl-3-dodecyl-urea | >2 × 104 |
| Elaidamide | >2 × 104 |
| 12-Hydroxyl-elaidamide | >2 × 104 |
| Sulfaphenazole | >2 × 104 |
| Proadifen | >2 × 104 |
| Miconazole | 315 (145–685) |
| MS-PPOH | 1558 (659–3684) |
| Ketoconazole | >5 × 104 |
Cytochrome P450s metabolize arachidonic acid to EETs and EH metabolizes EETs to DHETs (Morisseau et al., 1999; Spector et al., 2004). Several of the inhibitors of these enzymes have structures similar to fatty acids and the EETs. For this reason, cytochrome P450 and EH inhibitors were also tested. The cytochrome P450 inhibitors sulfaphenazole and proadifen and the soluble and microsomal EH inhibitors 1-cyclohexyl-3-dodecyl-urea, elaidamide, and 12-hydroxyl-elaidamide did not compete with 20-125I-14,15-EET, indicating that this binding site is not a cytochrome P450 or EH (Table 1). Three cytochrome P450 inhibitors inhibited 20-125I-14,15-EE5ZE binding to U937 membranes. Miconazole and MS-PPOH inhibit with Ki of 315 and 1558 nM, respectively (Fig. 6B). In contrast, ketoconazole is less effective in inhibiting binding with approximately 50% inhibition at the highest concentration tested, 50 μM. These findings suggest that the three compounds are cytochrome P450 and EET receptor dual inhibitors with principle structures unrelated to EETs or EET analogs. To test this possibility, we examined their effects of 14,15-EET-induced relaxation of coronary arteries. 14,15-EET caused a concentration-related relaxation of preconstricted arterial rings (Fig. 2). Both miconazole (20 μM) and MS-PPOH (20 μM) inhibited the EET-induced relaxations (Fig. 2, E and C). In contrast, proadifen (20 μM) was without effect (Fig. 2B). NS1619, a BKCa channel opener, relaxed coronary arteries in a concentration-related manner (Gauthier et al., 2002) (Fig. 2, D and F). The relaxations to NS1619 were not altered by MS-PPOH but were reduced slightly, and significantly, by miconazole at the highest concentrations of NS1619. Thus, the blockade of 14,15-EET-induced relaxations by MS-PPOH is due to inhibition of EET binding to its receptor and not inhibition of the BKCa channel. The blockade of the EET relaxations by miconazole is predominantly due to inhibition of EET binding; however, a component is due to a reduction in BKCa channel activation.
Discussion
EETs are synthesized by the vascular endothelium and have a number of cardiovascular actions (Rosolowsky and Campbell, 1996; Campbell and Falck, 2007; Spector and Norris, 2007). They have been implicated as endogenous mediators of vasodilation, cardioprotection, and angiogenesis and inhibitors of inflammation, thrombosis, and platelet aggregation (Node et al., 1999; Krotz et al., 2003; Gauthier et al., 2007; Gross et al., 2008). Considering these diverse actions, it is important to understand the mechanism of action of the EETs.
In screening a series of 14,15-EET analogs for relaxation of bovine coronary artery rings, we discovered that 14,15-EE5ZE blocked the relaxations by all four regioisomeric EETs (Gauthier et al., 2002). However, this analog did not block the relaxations to iloprost, sodium nitroprusside, or the potassium channel openers, NS1619 or bimikalim, or the contractions to potassium chloride, the thromboxane mimetic U46619 or 20-HETE. Furthermore, 14,15-EE5ZE displaced 20-125I-14,15-EE8ZE from its binding site on membranes of U937 cells with a Ki similar to 14,15-EET (Ki = 37 nM for 14,15-EE5ZE and 40 nM for 14,15-EET) (Yang et al., 2008). These studies suggested that 14,15-EE5ZE was a selective EET antagonist. Because the iodo group approximates the size of a methyl group and previous studies permitted addition of a 20-iodo group to 14,15-EE8ZE without changing biological activity (Prestwich et al., 1988; Yang et al., 2008), we synthesized 20-I-14,15-EE5ZE as a possible EET antagonist and tested its activity. For this purpose, 14,15-EET relaxed the preconstricted bovine coronary artery. 20-I-14,15-EE5ZE, like 14,15-EE5ZE, inhibited 14,15-EET-induced relaxations. Activation of p38 MAP kinase by EETs was reported in endothelial and smooth muscle cells (Fleming et al., 2001). Likewise, we showed that 14,15-EET induces p38 MAP kinase phosphorylation in a concentration-related manner in U937 cells. The inactive thiirane analog of 14,15-EET did not alter the formation of phospho-p38 (Falck et al., 2003a). The 14,15-EET-induced increase in phospho-p38 was blocked in a concentration-related manner by 20-I-14,15-EE5ZE. The consequence of EET activating p38 MAP kinase in U937 cell was not studied further. These studies confirmed that 20-I-14,15-EE5ZE, like 14,15-EE5ZE, is an EET antagonist.
We synthesized and characterized 20-125I-14,15-EE5ZE as an antagonist radioligand. It showed specific, saturable binding to U937 membranes, and the specific binding was reversed by the addition of an excess of 11,12-EET. The antagonist radioligand bound with higher affinity than did the agonist radioligand. 20-125I-14,15-EE5ZE had a KD of 1.11 nM, whereas 20-125I-14,15-EE8ZE had a KD of 11.8 nM (Yang et al., 2008). The reason for the lower KD for the antagonist radioligand than the agonist radioligand is the faster kon (0.5 versus 6.4 × 106 M−1s−1 for agonist versus antagonist) and slower koff (0.06 versus0.007 s−1 for agonist versus antagonist) (Yang et al., 2008). The higher affinity of 20-125I-14,15-EE5ZE is an advantage over previously studied radioligands providing a higher sensitivity for ligand binding studies.
The binding of 20-125I-14,15-EE5ZE was displaced by EETs and some EET analogs. The active EET agonists and EET antagonist displaced the radioligand with a lower Ki than the inactive analogs or EET metabolites (Falck et al., 2003a, 2003b). Likewise, Wong et al. (1993) demonstrated that 14,15-EET displaced [3H]14,15-EET binding to monocytes, but the ligand was not displaced by thromboxane, platelet-activating factor, leukotriene B4, or leukotriene D4. Inceoglu et al. (2007) showed that the EETs did not alter binding to neurokinin, cannabinoid, benzodiazepine, or dopamine receptors. It is interesting that the ranking order of the Ki values of EETs and EET analogs was similar with 20-125I-14,15-EE8ZE and 20-125I-14,15-EE5ZE, suggesting that the agonist and antagonist radioligands label the same binding protein on U937 membranes.
Several lines of evidence suggest that the EET binding site is a GPCR. 11,12-EET increased KCa channel activity in cell-attached patches of coronary artery smooth muscle cells (Li and Campbell, 1997). However, in inside-out patches of the same cells, the EET was without effect unless GTP was added to the bath. The ability of 11,12-EET to increase KCa channel activity in inside-out patches with GTP was inhibited by the G protein antagonist GDPβS or an anti-Gsα antibody (Li and Campbell, 1997). These studies indicate that 11,12-EET activates KCa channels via a membrane-delimited mechanism involving activation of a G protein, possibly Gsα. Likewise, EETs increase GTPγ[35]S binding to Gs, but not Gi, to endothelial cell membranes (Node et al., 2001). Radioligand-binding studies confirmed these findings. The binding of 20-125I-14,15-EE8ZE to membranes of U937 was decreased in a concentration-related manner by the GTP analog, GTPγS (Yang et al., 2008). Because this ligand is an EET agonist, the decreased binding indicates that the EET binding site is coupled to a G protein. In contrast, 20-125I-14,15-EE5ZE is an EET antagonist, and, as would be predicted with an antagonist, GTPγS did not affect binding of the radioligand. These results further supported the notion that the EET receptor is coupled to a G protein.
Cytochrome P450 inhibitors and EH inhibitors are commonly used to estimate the contribution of endogenous EETs to physiological or pathological processes (Harder et al., 1995; Fisslthaler et al., 1999; Campbell and Falck, 2007; Gauthier et al., 2007; Spector and Norris, 2007). As a result, we wondered if these drugs altered binding of 20-125I-14,15-EE5ZE to its binding site. None of the EH inhibitors competed with 20-125I-14,15-EE5ZE for binding to U937 membranes. Thus, despite their lipid character and the ability of some EH inhibitors to activate PPARs (Liu et al., 2005), they are without effect on EET binding, which supports their activity as specific EH inhibitors. The cytochrome P50 inhibitors proadifen and sulfaphenazole also failed to alter binding. Thus, this high-affinity binding site for 20-125I-14,15-EE5ZE is unique from other previous known lipid receptors and EET-related enzymes.
Three cytochrome P450 inhibitors, miconazole, MS-PPOH, and ketoconazole, displace 20-125I-14,15-EE5ZE from the EET receptor. They represent the first group of ligands structurally unrelated to the EETs. Miconazole inhibits cytochrome P450 and EET binding with similar Ki values (300 and 315 nM, respectively) (Zou et al., 1994; Harder et al., 1995). MS-PPOH has a lower Ki value for inhibition of EET binding than for inhibition of cytochrome P450 (1.6 and 13 μM, respectively) (Wang et al., 1998). Ketoconazole was the least effective inhibitor of EET binding and inhibited binding by approximately 50% at 50 μM. The Ki for ketoconazole inhibition of cytochrome P450 epoxygenase is approximately 10 μM (Zou et al., 1994; Harder et al., 1995). Thus, these structures differ widely in their specificity for cytochrome P450 and EET binding. To determine whether binding predicts EET antagonist activity, we tested the effects of these inhibitors on 14,15-EET-induced relaxation of the bovine coronary artery. Both MS-PPOH and miconazole inhibited the relaxations to 14,15-EET, whereas proadifen, which did not affect binding, was without effect on EET relaxations. MS-PPOH did not alter the relaxations to the BKCa channel opener NS1619, indicating that it does not block BKCa channel activation. Although miconazole (10 μM) failed to alter BKCa channel activity (Campbell et al., 1996), miconazole (20 μM) partially inhibited the relaxations to NS1619. However, this degree of BKCa channel inhibition could not account for the blockade of EET-induced relaxation by miconazole. Thus, MS-PPOH acts as an EET antagonist, and miconazole acts predominantly as an EET antagonist. The structural differences in these nonlipid inhibitors may lead to the design of EET receptor ligands with improved water solubility and kinetic properties that are useful for future animal and human applications. These dual cytochrome P450 inhibitors and EET receptor ligands, and possibly other cytochrome P450 inhibitors, inhibit EET signaling pathways at two different sites of action. Careful interpretation of previous publications using these dual inhibitor/ligands may be needed. Variations on the structures of miconazole and/or MS-PPOH may lead to the identification of specific, noneicosanoid EET antagonists devoid of cytochrome P450 inhibition. Such antagonists may be useful for studies in vivo and provide new insights into the endogenous roles of the EETs.
Supplementary Material
Acknowledgments
We thank Daniel Goldman and Sarah Christian for technical assistance, Dr. Kathryn Gauthier for review of the manuscript and suggestions, Gretchen Barg for secretarial assistance, and Drs. Bruce Hammock and Christophe Morisseau of the University of California at Davis for the EH inhibitors.
This work was supported by the National Institutes of Health National Heart, Lung and Blood Institute [Grant HL-51055]; the National Institutes of Health National Institute of General Medical Sciences [Grant GM-31278]; and the Robert A. Welch Foundation.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.157818
 The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- EET
- epoxyeicosatrienoic acid
- 14,15-EE8ZE
- 14(S),15(R)-cis-epoxy-eicosa-8(Z)-enoic acid
- 14,15-EE5ZE
- 14(S),15(R)-cis-epoxyeicosa-5(Z)-enoic acid
- EH
- epoxide hydrolase
- 20-I-14,15-EE8ZE
- 20-iodo-14,15-epoxyeicosa-8(Z)-enoic acid
- OTs
- 20-tosyl
- 14,15-EET-mSA
- 14,15-Epoxyeicosatrienoyl-methylsulfonamide
- 15-HETE
- 15-hydroxyeicosatetraenoic acid
- 14,15-DHET
- 14,15-dihydroxyeicosatrienoic acid
- 14,15-DHE5ZE
- 14,15-dihydroxy-eicosa-5(Z)-enoic acid
- 8,9-DHET
- 8,9-dihydroxyeicosatrienoic acid
- 11-HETE
- 11-hydroxyeicosatetraenoic acid
- MS-PPOH
- N-methylsulfonyl-6-(2-propargyloxyphenyl)hexanamide
- GTPγS
- guanosine 5′-O-(3-thio)triphosphate
- MAP
- mitogen-activated protein
- PPAR
- peroxisomal proliferator-activated receptor
- U46619
- 9–11-dideoxy-11α,9a-epoxymethano-prostaglandin F2α
- NS1619
- 1,3-dihydro-1-[2-hydroxy-5-(trifluoromethyl)phenyl]-5-(trifluoromethyl)-2H-benzimidazol-2-one
- BKCa
- large-conductance Ca2+ -activated K+ channel
- KATP
- ATP-sensitive K+ channel.
References
- Cai G, Zhu W, Ma D. (2006) A sequential reaction process to assemble polysubstituted indolizidines, quinolzidines and quinolzidine analogues. Tetrahedron 62:5697–5708 [Google Scholar]
- Campbell WB, Falck JR. (2007) Arachidonic acid metabolites as endothelium-derived hyperpolarizing factors. Hypertension 49:590–596 [DOI] [PubMed] [Google Scholar]
- Campbell WB, Gebremedhin D, Pratt PF, Harder DR. (1996) Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res 78:415–423 [DOI] [PubMed] [Google Scholar]
- Capdevila J, Chacos N, Werringloer J, Prough RA, Estabrook RW. (1981) Liver microsomal cytochrome P-450 and the oxidative metabolism of arachidonic acid. Proc Natl Acad Sci U S A 78:5362–5366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JK, Capdevila J, Harris RC. (2001) Cytochrome P450 epoxygenase metabolism of arachidonic acid inhibits apoptosis. Mol Cell Biol 21:6322–6331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JK, Wang DW, Falck JR, Capdevila J, Harris RC. (1999) Transfection of an active cytochrome P450 arachidonic acid epoxygenase indicates that 14,15-epoxyeicosatrienoic acid functions as an intracellular second messenger in response to epidermal growth factor. J Biol Chem 274:4764–4769 [DOI] [PubMed] [Google Scholar]
- Cowart LA, Wei S, Hsu MH, Johnson EF, Krishna MU, Falck JR, Capdevila JH. (2002) The CYP4A isoforms hydroxylate epoxyeicosatrienoic acids to form high affinity peroxisome proliferator-activated receptor ligands. J Biol Chem 20:35105–35112 [DOI] [PubMed] [Google Scholar]
- Falck JR, Krishna UM, Reddy YK, Kumar PS, Reddy KM, Hittner SB, Deeter C, Sharma KK, Gauthier KM, Campbell WB. (2003a) Comparison of the vasodilatory properties of 14,15-EET analogs: Structural requirements for dilation. Am J Physiol Heart Circ Physiol 284:H337–H349 [DOI] [PubMed] [Google Scholar]
- Falck JR, Reddy LM, Reddy YK, Bondlela M, Krishna UM, Ji Y, Sun J, Liao JK. (2003b) 11,12-Epoxyeicosatrienoic acid (11,12-EET): Structural determinants for inhibition of TNF-alpha-induced VCAM-1 expression. Bioorg Med Chem Lett 13:4011–4014 [DOI] [PubMed] [Google Scholar]
- Fisslthaler B, Popp R, Kiss L, Potente M, Harder DR, Fleming I, Busse R. ( 1999) Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature 401:493–497 [DOI] [PubMed] [Google Scholar]
- Fleming I, Fisslthaler B, Michaelis UR, Kiss L, Popp R, Busse R. (2001) The coronary endothelium-derived hyperpolarizing factor (EDHF) stimulates multiple signalling pathways and proliferation of vascular cells. Pflugers Arch 442:511–518 [DOI] [PubMed] [Google Scholar]
- Gauthier KM, Deeter C, Krishna UM, Reddy YK, Bondlela M, Falck JR, Campbell WB. (2002) 14,15-Epoxyeicosa-5(Z)-enoic acid: a selective epoxyeicosatrienoic acid antagonist that inhibits endothelium-dependent hyperpolarization and relaxation in coronary arteries. Circ Res 90:1028–1036 [DOI] [PubMed] [Google Scholar]
- Gauthier KM, Jagadeesh SG, Falck JR, Campbell WB. (2003) 14,15-Epoxyeicosa-5(Z)-enoic-mSI: a 14,15- and 5,6-EET antagonist in bovine coronary arteries. Hypertension 42:555–561 [DOI] [PubMed] [Google Scholar]
- Gauthier KM, Yang W, Gross GJ, Campbell WB. (2007) Roles of epoxyeicosatrienoic acids in vascular regulation and cardiac preconditioning. J Cardiovasc Pharmacol 50:601–608 [DOI] [PubMed] [Google Scholar]
- Gross GJ, Gauthier KM, Moore J, Falck JR, Hammock BD, Campbell WB, Nithipatikom K. (2008) Effects of the selective EET antagonist, 14,15-EEZE, on cardioprotection produced by exogenous and endogenous EETs in the canine heart. Am J Physiol Heart Circ Physiol 294:H2838–H2844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder DR, Campbell WB, Roman RJ. (1995) Role of cytochrome P-450 enzymes and metabolites of arachidonic acid in the control of vascular tone. J Vasc Res 32:79–92 [DOI] [PubMed] [Google Scholar]
- Inceoglu B, Jinks SL, Ulu A, Hegedus CM, Georgi K, Schmelzer KR, Wagner K, Jones PD, Morisseau C, Hammock BD. (2008) Soluble epoxide hydrolase and epoxyeicosatrienoic acids modulate two distinct analgesic pathways. Proc Natl Acad Sci U S A 105:18901–18906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inceoglu B, Schmelzer KR, Morisseau C, Jinks SL, Hammock BD. (2007) Soluble epoxide hydrolase inhibition reveals novel biological functions of epoxyeicosatrienoic acids (EETs). Prostaglandins Other Lipid Mediat 82:42–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krotz F, Riexinger T, Buerkle MA, Nithipatikom K, Gloe T, Sohn HY, Campbell WB, Pohl U. (2003) Membrane potential-dependent inhibition of platelet adhesion to endothelial cells by epoxyeicosatrienoic acids. Arterioscler Thromb Vasc Biol 24:595–600 [DOI] [PubMed] [Google Scholar]
- Li PL, Campbell WB. (1997) Epoxyeicosatrienoic acids activate K+ channels in coronary smooth muscle through guanine nucleotide binding protein. Circ Res 80:877–884 [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhang Y, Schmelzer K, Lee TS, Fang X, Zhu Y, Spector AA, Gill S, Morisseau C, Hammock BD, et al. ( 2005) The antiinflammatory effect of laminar flow: the role of PPARγ epoxyeicosatrienoic acids and soluble epoxide hydrolase. Proc Natl Acad Sci U S A 102:16747–16752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T, Ye D, Wang X, Seubert JM, Graves JP, Bradbury JA, Zeldin DC, Lee HC. (2006) Cardiac and vascular KAPT channels in rats are activated by endogenous epoxyeicosatrienoic acids through different mechanisms. J Physiol 575:627–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisseau C, Goodrow MH, Dowdy D, Zheng J, Greene JF, Sanborn JR, Hammock BD. (1999) Potent urea and carbamate inhibitors of soluble epoxide hydrolases. Proc Natl Acad Sci U S A 96:8849–8854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosset P, Gree R, Falck JR. (1989) Synthesis of two intermediate phosphonium salts for 5,20- and 15,20-diHETEs. Synth Commun 19:645–658 [Google Scholar]
- Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, Liao JK. (1999) Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science 285:1276–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Node K, Ruan XL, Dai J, Yang SX, Graham L, Zeldin DC, Liao JK. (2001) Activation of Gαs mediates induction of tissue-type plasminogen activator gene transcription by epoxyeicosatrienoic acids. J Biol Chem 276:15983–15989 [DOI] [PubMed] [Google Scholar]
- Prestwich GD, Eng WS, Robles S, Vogt RG, Wiśniewski JR, Wawrzeńczyk C. (1988) Synthesis and binding affinity of an iodinated juvenile hormone. J Biol Chem 263:1398–13404 [PubMed] [Google Scholar]
- Rosolowsky M, Campbell WB. (1996) Synthesis of hydroxyeicosatetraenoic acids (HETEs) and epoxyeicosatrienoic acids (EETs) by cultured bovine coronary artery endothelial cells. Biochim Biophys Acta 1299:267–277 [DOI] [PubMed] [Google Scholar]
- Snyder GD, Krishna UM, Falck JR, Spector AA. (2002) Evidence for a membrane site of action for 14,15-EET on expression of aromatase in vascular smooth muscle. Am J Physiol Heart Circ Physiol 283:H1936–H1942 [DOI] [PubMed] [Google Scholar]
- Spector AA, Fang X, Snyder GD, Weintraub NL. (2004) Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Prog Lipid Res 43:55–90 [DOI] [PubMed] [Google Scholar]
- Spector AA, Norris AW. (2007) Action of epoxyeicosatrienoic acids on cellular function. Am J Physiol Cell Physiol 292:C996–C1012 [DOI] [PubMed] [Google Scholar]
- Terashvili M, Tseng LF, Wu HE, Narayanan J, Hart LM, Falck JR, Pratt PF, Harder DR. (2008) Antinociception produced by 14,15-epoxyeicosatrienoic acid is mediated by the activation of beta-endorphin and met-enkephalin in the rat ventrolateral periaqueductal gray. J Pharmacol Exp Ther 326:614–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MH, Brand-Schieber E, Zand BA, Nguyen X, Falck JR, Balu N, Schwartzman ML. (1998) Cytochrome P450-derived arachidonic acid metabolism in the rat kidney: characterization of selective inhibitors. J Pharmacol Exp Ther 284:966–973 [PubMed] [Google Scholar]
- Widstrom RL, Norris AW, Spector AA. (2001) Binding of cytochrome P450 monooxygenase and lipoxygenase pathway products by heart fatty acid-binding protein. Biochemistry 40:1070–1076 [DOI] [PubMed] [Google Scholar]
- Wong PY, Lai PS, Falck JR. (2000) Mechanism and signal transduction of 14(R),15(S)-epoxyeicosatrienoic acid (14,15-EET binding in guinea pig monocytes. Prostaglandins Other Lipid Med. 62:321–333 [DOI] [PubMed] [Google Scholar]
- Wong PY, Lai PS, Shen SY, Belosludtsev YY, Falck JR. (1997) Post-receptor signal transduction and regulation of 14(R), 15(S)-epoxyeicosatrienoic acid (14,15-EET) binding in U-937 cells. J Lipid Med Cell Signal 16:155–169 [DOI] [PubMed] [Google Scholar]
- Wong PY, Lin KT, Yan YT, Ahern D, Iles J, Shen YS, Bhatt RK, Falck JR. (1993) 14(R), 15(S)-Epoxyeicosatrienoic acid receptor in guinea pig mononuclear cell membranes. J Lipid Mediat 6:199–208 [PubMed] [Google Scholar]
- Yang W, Holmes BB, Gopal VR, Kishore RV, Sangras B, Yi XY, Falck JR, Campbell WB. (2007) Characterization of 14,15-epoxyeicosatrienoyl-sulfonamides as 14,15-epoxyeicosatrienoic acid agonists: Use for studies of metabolism and ligand binding. J Pharmacol Exp Ther 321:1023–1031 [DOI] [PubMed] [Google Scholar]
- Yang W, Tuniki VR, Anjaiah S, Falck JR, Hillard CJ, Campbell WB. (2008) Characterization of epoxyeicosatrienoic acid binding site in U937 membranes using a novel radiolabeled agonist, 20–125I-14,15-epoxyeicosa-8(Z)-enoic acid. J Pharmacol Exp Ther 324:1019–1027 [DOI] [PubMed] [Google Scholar]
- Zou AP, Ma YH, Sui ZH, Ortiz de Montellano PR, Clark JE, Masters BS, Roman RJ. (1994) Effects of 17-octadecynoic acid, a suicide-substrate inhibitor of cytochrome P450 fatty acid omega-hydroxylase, on renal function in rats. J Pharmacol Exp Ther 268:474–481 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.