Abstract
The nociceptin receptor (NOPr), a member of the opioid receptor family, is a target for the treatment of pain and drug abuse. Nociceptin/orphanin FQ (N/OFQ), the endogenous peptide for NOPr, not only modulates opioid antinociception, but also blocks the rewarding effects of several abused drugs, such as morphine, cocaine, and amphetamine. We hypothesized that NOPr agonists, with bifunctional activity at the μ-opioid receptor (MOPr), may function as nonaddicting analgesics or as drug abuse medications. Bifunctional small-molecule NOPr agonists possessing different selectivities and efficacies at MOPr were evaluated in an acute thermal antinociception assay, and for their ability to induce conditioned place preference (CPP) and their effect on morphine-induced CPP. 1-(1-Cyclooctylpiperidin-4-yl)-indolin-2-one) (SR14150), a high-affinity NOPr partial agonist, with low MOPr affinity and efficacy, produced analgesia that was naloxone-reversible. SR14150 did not induce CPP alone, nor did it attenuate morphine-induced CPP. 3-Ethyl-1-(1-(4-isopropylcyclohexyl)piperidin-4-yl)-indolin-2-one (SR16507), which has high affinity for both NOPr and MOPr, full agonist activity at NOPr, and partial agonist activity at MOPr, was also a potent analgesic and produced CPP alone, but also modestly attenuated morphine CPP. 1-(1-(2,3,3a,4,5,6-hexahydro-1H-phenalen-1-yl)piperidinl-4-yl)-indolin-2-one (SR16835), a NOPr full agonist and low-affinity MOPr partial agonist, was not antinociceptive, did not produce CPP alone, but attenuated morphine CPP. Our results suggest that NOPr full-agonist activity is required to modulate opioid-induced reward, whereas a bifunctional NOPr/MOPr partial agonist profile may be suitable as a nonaddicting analgesic. The opioid-modulating effects of the NOPr ligands may be used effectively to produce better medications for treatment of drug abuse and pain.
Nociceptin/orphanin FQ (N/OFQ), the endogenous peptide for the NOP receptor, has been shown to be intimately involved in both pain and addiction. NOPr and N/OFQ are found in pain pathways in both brain and spinal cord (Neal et al., 1999, 2001). However, the effects of exogenously administered N/OFQ and NOP agonists in antinociceptive assays are quite complex and seem to be region- and assay-specific. Initial observations that N/OFQ, when injected intracerebroventricularly into mice, led to a decrease in hot-plate (Meunier et al., 1995) and tail-flick latency (Reinscheid et al., 1995), suggested that this peptide is pronociceptive. Ultimately, it was demonstrated that N/OFQ injected intracerebroventricularly blocked the stress-induced analgesia produced by the intracerebroventricular injection procedure, as well as antinociception induced by exogenously applied opioid agonists (Mogil et al., 1996a,b). However, when given intrathecally, N/OFQ produces acute antinociception in the tail-flick assay (Xu et al., 1996; Yamamoto et al., 1997). The small-molecule NOP agonist Ro 64-6198, when given intraperitoneally, and presumably activating NOPr in both the brain and spinal cord, reversed the antinociceptive effect of morphine in the tail-withdrawal assay (Kotlinska et al., 2003b). In the hot-plate assay, however, Ro 64-6198 was shown to be antinociceptive and produced additive effects with morphine (Reiss et al., 2008).
The N/OFQ-NOPr system also plays a significant role in the reward process and drug abuse, in particular. There is a high density of NOP receptors in areas implicated in drug abuse and reward (Neal et al., 1999). N/OFQ has been shown to block the rewarding properties of several common drugs of abuse. In particular, N/OFQ blocks conditioned place preference (CPP) induced by morphine, cocaine, amphetamines, and alcohol, and the self-administration of alcohol (Murphy et al., 1999; Kotlinska et al., 2003a; Kuzmin et al., 2003; Ciccocioppo et al., 2004; Sakoori and Murphy, 2004). However, unlike dynorphin and other κ agonists, N/OFQ is not dysphoric and does not induce a place aversion (Bals-Kubik et al., 1989; Devine et al., 1996). The reward-attenuating activity of the N/OFQ-NOP system may be effectively harnessed to provide therapeutic benefit for treating drug abuse. Indeed, the small-molecule NOPr agonist Ro 64-6198 was shown to block both the acquisition and reinstatement of morphine and alcohol CPP in mice and alcohol self-administration in rats (Kuzmin et al., 2003; Shoblock et al., 2005; Kuzmin et al., 2007).
MOPr agonists such as morphine are used extensively for the treatment of severe acute and chronic pain; however, their use is plagued with side effects such as dependence and tolerance. Because N/OFQ and NOPr agonists modulate opioid antinociception and opioid-induced reward, dual-targeted NOPr/MOPr ligands may provide a novel approach for developing nonaddicting analgesics and drug abuse pharmacotherapy. The concept of using mixed-action opioids for the treatment of pain and drug abuse is clinically validated. Mixed-action MOPr/KOPr opioids such as nalbuphine are used for the treatment of pain, without the abuse liability of pure MOPr agonists (Hoskin and Hanks, 1991). MOPr/KOPr opioids also decrease cocaine self-administration in rhesus monkeys, and produce fewer side effects compared with pure KOPr agonists (Mello et al., 1993; Bowen et al., 2003). NOPr ligands with multifunctional activity at other opioid receptors have not been actively investigated thus far. However, buprenorphine, a MOPr partial agonist and KOPr antagonist has been shown to have low efficacy at NOPr (Huang et al., 2001; Spagnolo et al., 2008). Its NOPr agonist activity has been reported to be responsible for the attenuation of its antinociceptive activity at high doses (Lutfy et al., 2003), and for the attenuation of alcohol consumption (Ciccocioppo et al., 2007). Buprenorphine's NOPr agonist activity is also implicated in the reduction of cocaine use in dually addicted cocaine-alcohol addicts (Montoya et al., 2004).
In a continuing effort to develop novel analgesics and drug abuse medications targeting the NOP receptor, we have focused our effort on discovering bifunctional NOPr/MOPr ligands, with the hypothesis that if NOPr agonist-MOPr agonist activities are present in the same molecule, the NOPr agonist activity may modulate the rewarding effects of the MOPr activity, thereby producing opioid analgesics that have a reduced addiction liability (nonaddicting analgesics). NOPr/MOPr agonists may also function as drug abuse medications with diminished dependence and withdrawal tendencies. We recently reported that SR16435, a nonselective NOPr and MOPr partial agonist, had potent antinociceptive activity in acute thermal pain. However, it also produced a CPP on its own, an effect mediated by its MOPr agonist activity and reversible by naloxone (Khroyan et al., 2007). It is possible that the partial agonist efficacy of SR16435 at NOPr may not be sufficient to attenuate the rewarding effect of its MOPr activity. To explore the effect of NOPr versus MOPr selectivity and efficacy on the overall behavioral profile of such bifunctional ligands, we investigated three other NOPr/MOPr agonist ligands, with varying degrees of NOPr and MOPr efficacy and selectivity. We report here the acute antinociceptive activity of SR14150, SR16507, and SR16835. We also characterized their rewarding effects alone and their ability to attenuate morphine-induced reward. Our data show that the overall antinociceptive and antirewarding profile of these ligands depends on their selectivity between NOPr and MOPr, as well as intrinsic activity at these receptors. Although NOPr full-agonist activity is required to attenuate morphine-induced reward, a NOPr-selective NOPr/MOPr partial agonist has antinociceptive activity without rewarding effects.
Materials and Methods
Animals.
Male ICR mice weighing 20 to 25 g at the start of the experiment were used. We have used this mouse strain previously, in antinociceptive and place-conditioning assays (Khroyan et al., 2007). Animals were group-housed under standard laboratory conditions and were kept on a 12:12 h day-night cycle (lights on at 8:00 AM). Animals were handled for at least 2 to 3 days before conducting the experiments. For thermal nociception experiments, animals were transported to the testing room and acclimated to the environment for 1 h. Mice were maintained in accordance with the guidelines of SRI International and of the 2003 National Research Council's Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research.
Drugs.
SR14150, SR16507, and SR16835 were synthesized in our laboratory, as hydrochloride salts (Zaveri et al., 2004). The NOPr antagonist SB-612111 was also synthesized in our laboratory using reported methodology (Barlocco et al., 2004). Morphine hydrochloride (Eli Lilly & Co., Indianapolis, IN), and naloxone (Sigma-Aldrich, St. Louis, MO) were dissolved in water. SR14150, SR16507, SR16835, and SB-612111 were dissolved in 1 to 2% Dulbecco's modified Eagle's medium and 0.5% aqueous hydroxypropyl cellulose. Drugs were injected in a volume of 0.1 ml/25g s.c. Controls received 0.1 ml/25 g of the appropriate vehicle.
In Vitro Characterization
Cell Culture.
All receptors were individually expressed in CHO cells stably transfected with human receptor cDNA, developed in our laboratory. Receptor expression levels were 1.2, 1.6, and 1.8 pmol/mg protein for the NOPr, MOPr, and KOPr receptors, respectively. The cells were grown in Dulbecco's modified Eagle's medium with 10% fetal bovine serum, in the presence of 0.4 mg/ml G418 and 0.1% penicillin/streptomycin, in 100-mm plastic culture dishes. For binding assays, the cells were scraped off the plate at confluence.
Receptor Binding.
Binding to cell membranes was conducted in a 96-well format, as described previously (Dooley et al., 1997). Cells were removed from the plates by scraping with a rubber policeman, homogenized in 50 mM Tris, pH 7.5, by use of a Polytron homogenizer, then centrifuged once, and washed by an additional centrifugation at 27,000g for 15 min. The pellet was resuspended in Tris, and the suspension incubated with [3H][d-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin (120 Ci/mmol, 0.2 nM), [3H][d-Pen2,d-Pen5]-enkephalin (120 Ci/mmol, 0.2 nM), [3H]U69593 (120 Ci/mmol, 0.2 nM), or [3H]N/OFQ (120 Ci/mmol, 0.2 nM) for binding to MOPr, DOPr, KOPr, and NOPr, respectively. Nonspecific binding was determined with 1 μM unlabeled version of the radioligand. Total volume of incubation was 1.0 ml, and samples were incubated for 60 min at 25°C. The amount of protein in the binding assay was 15 μg. The reaction was terminated by filtration with use of a Tomtec 96 harvester (Tomtec, Orange, CT) through glass fiber filters. Bound radioactivity was counted on a β-plate liquid scintillation counter (PerkinElmer Life and Analytical Sciences, Waltham, MA) and expressed in counts per minute. IC50 values were determined by use of at least six concentrations of each peptide analog, and calculated by use of Prism (GraphPad Software, Inc., La Jolla, CA). Ki values were determined by the method of Cheng and Prusoff (1973).
[35S]GTPγS Binding.
[35S]GTPγS binding was conducted basically as described by Traynor and Nahorski (1995). Cells were scraped from tissue culture dishes into 20 mM HEPES, 1 mM EDTA, then centrifuged at 500g for 10 min. Cells were resuspended in this buffer and homogenized by use of a Polytron homogenizer. The homogenate was centrifuged at 27,000g for 15 min, and the pellet was resuspended in buffer A, containing 20 mM HEPES, 10 mM MgCl2, 100 mM NaCl, pH 7.4. The suspension was recentrifuged at 27,000g and suspended once more in buffer A. For the binding assay, membranes (8–15 μg of protein) were incubated with [35S]GTPγS (50 pM), GDP (10 μM), and the appropriate compound, in a total volume of 1.0 ml, for 60 min at 25°C. Samples were filtered over glass fiber filters and counted as described for the binding assays. Statistical analysis was conducted by use of Prism.
In Vivo Characterization
Assessment of Acute Thermal Nociception.
Tail-flick assay.
Acute nociception was assessed by use of the tail-flick assay with an analgesia instrument (Stoelting Co., Wood Dale, IL) that uses radiant heat. This instrument is equipped with an automatic quantification of tail-flick latency, and a 15-s cutoff to prevent damage to the animal's tail. During testing, the focused beam of light was applied to the lower half of the animal's tail, and tail-flick latency was recorded. Baseline values for tail-flick latency were determined before drug administration in each animal. The mean basal tail-flick latency was 4.11 + 0.08 S.E.M.
After baseline measures, animals received a subcutaneous injection of their assigned dose of drug and were tested for tail-flick latencies at 10, 30, and 60 min after injection. Controls received an injection of vehicle before testing.
Drug regimen.
Animals (n = 8–14/group) received injections of SR14150 (3.0–15.0 mg/kg s.c.), SR16507 (0.3–1.0 mg/kg s.c.), or SR16835 alone (10.0–56.0 mg/kg s.c.). A group of animals also served as positive controls and received 10 mg/kg morphine, whereas a second group of animals served as vehicle controls. SR14150 and SR16507 produced antinociceptive effects, so in follow-up experiments, these compounds were coadministered with 1 mg/kg naloxone to examine whether the antinociceptive effects could be reversed, and with 10 mg/kg the selective high-affinity NOPr antagonist SB-612111, (Zaratin et al., 2004) to examine whether the antinociceptive effects could be potentiated. This dose of naloxone has been shown to reverse the effects of morphine, whereas the dose of SB-612111 was chosen based on previous experiments in our laboratory (Khroyan et al., 2007).
Statistical Analyses.
Antinociception (% maximum potential effect; % MPE) was quantified by the following formula: % MPE = 100 * [(test latency − baseline latency)/(15 − baseline latency)]. If the animal did not respond before the 15-s cutoff, the animal was assigned a score of 100%. Behavioral results were analyzed by use of repeated measures ANOVAs with drug treatment (SR14150, SR16507, SR16835, morphine, SB-612111, and naloxone) as between group variables and after drug-injection time (10, 30, and 60 min) as the repeated measure followed by Student-Newman-Keuls post hoc tests where appropriate. The level of significance was set at P < 0.05.
Assesment of the Rewarding and Behavioral Effects of NOP Ligands Using the Place Conditioning (PC) Paradigm.
PC apparatus.
The apparatus consisted of rectangular Plexiglas chambers divided into two distinct equal-sized compartments (19 cm × 22.8 cm × 18 cm high; Lafayette Instruments, Lafayette, IN). One compartment had cedar-scented bedding underneath a bar grid floor, and all but the front walls were black. The other compartment had pine-scented bedding beneath a mesh floor, and all but the front wall were white. The front walls were transparent so that the animal's behavior could be monitored. A removable partition divided the two compartments. During conditioning, the compartments were divided by a solid partition. On the PC test day, the solid partition was replaced with a partition that had an opening, allowing the animal free access to both compartments. A video camera that was linked to a computer was mounted above the chambers and tracked the animals' movement. We have previously used this setup in several published studies (e.g., see Khroyan et al., 2007). Previous experiments using this setup have indicated that the apparatus is unbiased, as untreated animals do not show a preference for one compartment over the other (unpublished observation).
PC training.
Conditioning was carried out in four trials run over eight consecutive days. Each PC trial was composed of two sessions conducted over two consecutive days. During the drug session, animals received a subcutaneous injection of their respective drug and were confined to one of the compartments for 20 min. On the other day, the vehicle session, animals received an injection of vehicle and were confined to the alternate compartment for 20 min. A group of mice received vehicle in both compartments and served as controls. These two sessions were repeated over eight consecutive days such that animals received four drug sessions and four vehicle sessions. The particular compartment paired with the drug and the order of placement into the drug-paired versus saline-paired compartment was counterbalanced across groups.
Acute and repeated measures of global activity.
During conditioning, overall activity of the animals after acute and repeated drug injection was recorded. These data were captured by the Spontaneous Motor Recording and Tracking software system (SMART; Panlab, Barcelona, Spain), a color image-capturing system that works in real time and tracks all the movements of the animal, for a given amount of time via a video camera connected to the computer. This system tracks and records all behavior that results in any movement/positional changes, as a result of drug treatment. We term this as “global activity,” because it encompasses fine movement, movement due to rearing, grooming, sniffing, and locomotor activity.
PC test day.
Twenty-four hours after the last conditioning session, the animals were given access to both compartments simultaneously for 15 min, and the amount of time the animals spent in each compartment was recorded. No drug or vehicle was administered on the PC test day.
Drug regimen.
Animals were assigned to groups (n = 8–14/group) receiving injections of SR14150 (1.0–30.0 mg/kg s.c.), SR16507 (1.0–3.0 mg/kg s.c.), or SR16835 (3.0–10.0 mg/kg s.c.) alone. Animals were then immediately placed in the drug-paired compartment for 20 min. Positive controls received an injection of 15 mg/kg s.c. morphine immediately before placement in the drug-paired compartment. A separate group of animals served as controls and received vehicle injections before placement into either compartment. PC testing was carried out as described above.
To study the effects of SR compounds on morphine CPP, animals (n = 7–8/ group) received a injection of SR14150 (1.0 and 10.0 mg/kg s.c.), SR16507 (1.0 and 3.0 mg/kg s.c.), or SR16835 (3.0–30.0 mg/kg s.c.) 10 min before an injection of 15.0 mg/kg s.c. morphine, after which the animals were immediately placed into the drug-paired compartment. During the vehicle sessions, animals received two injections of vehicle (10 min apart) and were placed into the other compartment. PC testing was carried out as outlined above. The 10-min pretreatment time point is consistent with those reported in other similar studies (Shoblock et al., 2005). Furthermore, from the tail-flick assay, we know that the behavioral effects of these compounds occur within this time frame.
To examine whether SR16835 attenuated morphine-induced reward via activation of the NOPr, a group of animals were given 10 mg/kg the NOP antagonist SB-612111 5 min before a injection of 10 mg/kg s.c. SR16835. Ten minutes later they received an injection of 15 mg/kg s.c. morphine and were placed immediately into their drug-paired compartment. During the vehicle sessions, animals received three injections of vehicle spaced similarly in time to mimic the drug sessions. PC testing was carried out as outlined above.
Statistical analyses.
Drug-induced behaviors were analyzed by use repeated-measures ANOVAs with drug treatment (SR14150, SR16507, SR16835, morphine, naloxone, and SB-612111) as between subjects measures and injection day (first versus fourth) as a repeated measure. Significant interactions were further analyzed with one-way ANOVAs and post hoc tests. To examine sensitization effects, following a significant overall ANOVA, t tests were used to compare data after the fourth injection relative to the first. For the PC test day data, the percentage time animals spent in their drug-paired compartment was analyzed using one way ANOVAs and significant effects were further analyzed with post-hoc tests. A CPP was evident if animals spent significantly more time in their drug-paired compartment relative to control animals, whereas a conditioned place aversion (CPA) was evident if animals spent significantly less time in their drug-paired compartment. The level of significance was set at P < 0.05.
Results
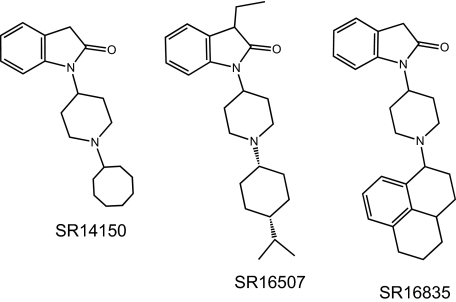
Chemical structures of the NOPr agonists SR14150, SR16507, and SR16835 are shown in Fig. 1. Table 1 shows in vitro binding affinities and functional activities of N/OFQ and the SR compounds at the NOPr, MOPr, and KOPr. SR14150 has 20-fold selectivity for NOPr over MOPr, and has partial agonist activity at both sites, as determined by stimulation of [35S]GTPγS binding. SR16835 is 7-fold selective for NOPr, is a full agonist at NOPr and a weak partial agonist at MOPr. SR16507 has approximately equal binding affinity at both NOPr and MOPr and functions as a full agonist at NOPr and a partial agonist at the MOPr.
Fig. 1.
Chemical structures of bifunctional NOPr/MOPr ligands SR14150, SR16507, and SR16835.
TABLE 1.
In vitro binding affinities and functional activities of NOP ligands
Binding was conducted as described in Materials and Methods. Values shown represent mean ± S.D.
| Receptor Binding Ki |
[35S]GTPγS NOP |
[35S]GTPγS MOP |
|||||
|---|---|---|---|---|---|---|---|
| NOP | MOP | KOP | EC50 | Stimulation | EC50 | Stimulation | |
| nM | nM | % | nM | % | |||
| N/OFQ | 0.2 ± 0.04 | 133 ± 30 | 247 ± 3.4 | 4.0 ± 0.1 | 100 | >10,000 | |
| Morphine | >10,000 | 1.1 ± 0.1 | 46.9 ± 14.5 | 0 | 5.2 ± 1.6 | 93 ± 2.8 | |
| SR14150 | 1.39 ± 0.42 | 29.9 ± 2.1 | 42.7 ± 1.0 | 20.8 ± 3.1 | 54.2 ± 10.9 | 99 ± 12 | 23.4 ± 3.2 |
| SR16507 | 5.22 ± 0.65 | 1.07 ± 0.17 | 82.4 ± 16.4 | 8.5 ± 0.8 | 95 ± 12 | 5.2 ± 1.6 | 47 ± 1.5 |
| SR16835 | 11.4 ± 0.9 | 79.9 ± 3.9 | 681.3 ± 61.6 | 46.1 ± 20.5 | 106.6 ± 7.4 | 129 ± 48 | 18 ± 1.6 |
Effect of SR14150 and SR16507 on Tail-Flick Latency, Potentiation by SB-612111, and Reversal by Coadministration of Naloxone.
The effect of the moderately selective NOPr agonist SR14150 on tail-flick latency is shown in Fig. 2A. The overall ANOVA indicated that there was a significant interaction effect [F(6,60) = 3.25, P < 0.05], suggesting that different doses of SR14150 produced different effects at the three post-drug-injection times (10, 30, and 60 min). The positive control morphine produced the anticipated increase in %MPE at all time points. The 3.0 mg/kg dose of SR14150 produced significant antinociception only at the 60-min postinjection time point. Animals that received the 10 mg/kg SR14150 showed an increase in tail-flick latency relative to vehicle controls at the 30- and 60-min postinjection time points, whereas 30 mg/kg SR14150 produced an increase in tail-flick latency at all postinjection time points. 100% MPE was observed at 30 mg/kg, the highest dose of SR14150. It should be noted that, at 30 mg/kg, the animals were not moving around and had a tendency to stay curled up, but they still had muscle tone and responded very rapidly to touch (qualitative observations immediately before testing).
Fig. 2.
Acute thermal antinociceptive effect of SR14150 (A) and SR16507 (B) alone in the tail-flick assay. Data are mean %MPE (± S.E.M.). *, significant difference from vehicle control (P < 0.05).
The effect of the nonselective NOPr/MOPr agonist SR16507 on thermal antinociception is shown in Fig. 2B. The overall ANOVA indicated that the interaction effect was not significant, but that there was a main effect of dose [F(3,28) = 14.97, P < 0.05]. Regardless of the postinjection time, the 1.0 and 3.0 mg/kg doses of SR16507 produced an increase in tail-flick latency relative to vehicle controls, whereas 0.3 mg/kg SR16507 only produced a modest increase in tail-flick latency at the 60-min postinjection time point. The effects of SR16507 were dose-dependent because 3.0 mg/kg produced a significantly greater increase in tail-flick latency relative to 1.0 mg/kg. Qualitatively, neither the 0.3 nor the 1.0 mg/kg doses of SR16507 produced a decrease in global activity at any of the time points before testing. However, the 3 mg/kg dose produced a pronounced decrease in activity, and the animals preferred to be still, but would still respond to touch. This effect was mainly visible at the 10- and 30-min postinjection time points, whereas at the 60-min time point, seven or eight animals were completely active and had activity levels similar to vehicle controls.
To determine the relative involvement of the MOPr and NOPr in the antinociceptive efficacies of SR14150 and SR16507, the animals were cotreated with the opioid antagonist naloxone and the NOPr antagonist SB-612111. The opioid antagonist naloxone (1 mg/kg) completely blocked the antinociceptive activity of SR14150 at both the 10 and 30 mg/kg doses (Fig. 3A). Naloxone completely blocked the antinociceptive activity of the 1 mg/kg dose of SR16507, and partially blocked antinociception produced by 3 mg/kg (Fig. 3B).
Fig. 3.
Attenuation of SR14150 (A) and SR16507 (B) antinociception by coadministration of 1 mg/kg naloxone, and potentiation of SR14150 antinociception by coadministration of SB-612111 (C). Data are mean %MPE (± S.E.M.). †, significant difference from SR14150 or SR16507 alone (P < 0.05).
We also examined the relative involvement of NOPr in mediating antinociception of these bifunctional compounds. When the NOP antagonist SB-612111 was given as a pretreatment to SR14150, the antinociceptive effects of the 3 and 10 mg/kg doses were potentiated (Fig. 3C). The antinociceptive effect of SR16507 is also potentiated by SB-612111 as we have shown recently (Khroyan et al., 2009). Together these findings suggest that the antinociceptive effects of systemically administered SR14150 and SR16507 are mediated by their MOPr agonist activity and are attenuated by their NOP agonist component.
Effect of SR16835 on Tail-Flick Latency.
The overall ANOVA indicated that the 7-fold selective NOPr agonist SR16835 (10.0–56.0 mg/kg) did not have any analgesic effects on its own (data not shown). Doses higher than 56.0 mg/kg were not used because five of seven animals tested were less active and only moved if poked.
Effect of SR14150 on PC, Global Activity, and Morphine-Induced Behaviors.
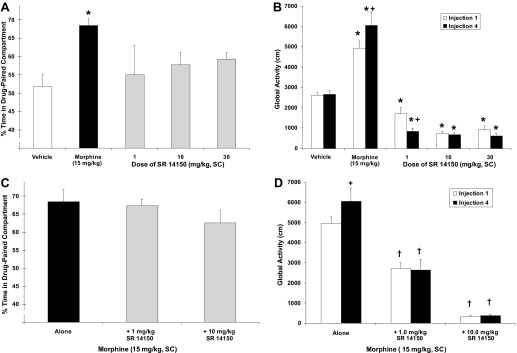
The effect of SR14150 on place conditioning is shown in Fig. 4A. None of the doses of SR14150 (1.0–30.0 mg/kg) produced a significant CPP or CPA. However, animals that received 15 mg/kg morphine exhibited a significant CPP (P < 0.05), as expected.
Fig. 4.
Effects of SR14150 on PC (A), global activity (B), morphine-induced CPP (C), and morphine-induced activity (D). Data are mean (± S.E.M.) percentage of time spent in drug-paired compartment (A, C) or activity (cm) after first and last drug injection (B, D). *, significant difference from vehicle controls; +, significant difference from first injection; †, significant difference from morphine alone (P < 0.05).
The effect of SR14150 on global activity after the first and fourth injections is shown in Fig. 4B. The overall ANOVA indicated that there was a significant dose by injection day interaction [F(4,52) = 5.3, P < 0.05]. SR14150 (1.0–10.0 mg/kg) produced a decrease in global activity, relative to vehicle controls, that was evident acutely and after repeated administration (P > 0.05). Furthermore, sensitization to the hypoactivity effects of the 1.0 mg/kg dose of SR14150 was evident as a further decrease in activity after the fourth administration relative to the first injection (P < 0.05). As expected, morphine administration produced an increase in global activity after the first injection, relative to vehicle controls (P < 0.05). Furthermore, sensitization of morphine-induced global activity was also evident as an increase in activity after the fourth injection relative to the first injection (P < 0.05).
The effect of SR14150 on morphine-induced behaviors is shown in Fig. 4, C and D. The 1.0 and 10.0 mg/kg doses of SR14150 did not have a significant effect on morphine-induced CPP (Fig. 4C). However, SR14150 attenuated morphine-induced hyperactivity as shown in Fig. 4D. Behavioral sensitization was also not evident in the groups receiving SR14150 and morphine [F(2,24) = 52.6, P < 0.05]. SR14150 produced a dose-dependent decrease in morphine-induced hyperactivity such that 10.0 mg/kg SR14150 coadministered with morphine produced a significant decrease in morphine-induced activity relative to the 1.0 mg/kg dose (P < 0.05).
Effect of SR16507 on PC, Global Activity, and Morphine-Induced Behaviors.
The effect of SR16507 on place conditioning is shown in Fig. 5A. The overall ANOVA indicated a significant effect [F(3,33) = 13.2, P < 0.05]. The group of animals that received 15 mg/kg morphine exhibited a significant CPP (P < 0.05). The 1.0 and 3.0 mg/kg doses of SR16507 also produced a significant CPP, similar to morphine (P < 0.05).
Fig. 5.
Effects of SR16507 on PC (A), global activity (B), morphine-induced CPP (C), and morphine-induced activity (D). Data are mean (± S.E.M.) percentage of time spent in drug-paired compartment (A, C) or activity (cm) after first and last drug injection (B, D). *, significant difference from vehicle controls; +, significant difference from first injection; †, a significant difference from morphine alone (P < 0.05).
The effect of SR16507 on global activity after the first and fourth injections is shown in Fig. 5B. The overall ANOVA indicated that there was a significant dose by injection day interaction [F(3,33) = 19.1, P < 0.05]. As shown previously, morphine administration produced an increase in global activity after the first injection relative to vehicle controls (P < 0.05). Furthermore, sensitization of morphine-induced global activity was also evident as an increase in activity after the fourth injection relative to the first injection (P < 0.05). Conversely, but similar to SR14150, the 1.0 and 3.0 mg/kg doses of SR16507 produced a decrease in global activity relative to vehicle controls (P > 0.05). After the first drug injection, both 1.0 and 3.0 mg/kg SR16507 produced a decrease in global activity relative to vehicle controls, whereas only the 3.0 mg/kg dose produced a decrease after the fourth injection.
The effect of SR16507 on morphine-induced behaviors is shown in Fig. 5, C and D. The 1.0 and 3.0 mg/kg doses of SR16507 modestly attenuated morphine-induced CPP ([F(2,22) = 7.2, P < 0.05]; Fig. 5C). As shown in Fig. 5D, SR16507 also attenuated morphine-induced hyperactivity [F(2,22) = 8.4, P < 0.05]. Both the 1.0 and 3.0 mg/kg doses of SR16507 decreased morphine-induced hyperactivity after the first and fourth drug injections (P < 0.05). However, morphine-induced sensitization was not blocked by the 1.0 mg/kg dose because there was an increase in activity after the fourth injection relative to the first injection in animals given morphine and 1.0 mg/kg SR16507 (P < 0.05).
Effect of SR16835 on PC, Global Activity and Morphine-Induced Behaviors.
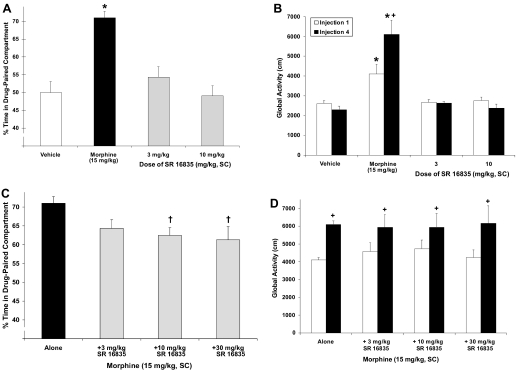
The behavioral effects of SR16835 alone are shown in Fig. 6, A and B. SR16835 alone did not produce a CPP or CPA relative to controls (Fig. 6A). Likewise, the 3.0 and 10.0 mg/kg doses of SR16835 did not produce any changes in global activity relative to vehicle controls (Fig. 6B).
Fig. 6.
Effects of SR16835 on PC (A), global activity (B), morphine-induced CPP (C), and morphine-induced activity (D). Data are mean (± S.E.M.) percentage of time spent in drug-paired compartment (A, C) or activity (cm) after first and last drug injection (B, D). *, significant difference from vehicle control; +, significant difference from first injection; †, significant difference from morphine alone (P < 0.05).
The effect of SR16835 on morphine-induced behaviors is shown in Fig. 6, C and D. SR16835 modestly attenuated morphine-induced CPP ([F(3,26) = 10.1, P < 0.05] (Fig. 6C). Post hoc tests indicated that only the two highest doses of SR16835 (10.0 and 30.0 mg/kg) produced a significant decrease in morphine-CPP relative to morphine alone (P < 0.05). In contrast, SR16835 did not alter morphine-induced hyperactivity nor did it alter morphine-induced sensitization at any of the doses tested (Fig. 6D). The attenuating effect of 10 mg/kg SR16835 on morphine CPP was blocked by the coadministration of NOP antagonist SB-612111 (10 mg/kg), indicating that the inhibitory effect of SR16835 on morphine-induced reward is mediated through the NOP receptor (Fig. 7).
Fig. 7.
Effect of the NOP antagonist SB-612111 (10 mg/kg) on the attenuation of morphine CPP by 10 mg/kg SR16835. *, significant difference from vehicle control; †, significant difference from morphine alone (P < 0.05).
Discussion
In the present study, we have determined the in vivo pharmacological profile of three NOPr agonists with differing selectivity and efficacy for the NOPr and MOPr, in a model of acute nociception (the tail-flick assay) and a model of reward (PC paradigm), in mice. The behavioral effects of all three compounds are summarized in Table 2.
TABLE 2.
Summary of in vitro and in vivo findings with NOP agonists SR14150, SR16507, and SR16835
| NOP Binding | NOP Selectivity (versus MOP) | Behavioral Effects of SR Compounds Alone |
Changes in Morphine-Induced Behaviors |
||||
|---|---|---|---|---|---|---|---|
| Antinociception | Activity | CPP | Activity | CPP | |||
| nM | -fold | ||||||
| SR14150 | 1.4 ± 0.4 | 21 | ↑ | ↓ | 0 | ↓ | N/C |
| SR16507 | 5.2 ± 0.6 | ↑ | ↓ | + | ↓ | ↓ | |
| SR16835 | 11.4 ± 0.6 | 7 | N/C | N/C | 0 | N/C | ↓ |
N/C, No change.
SR14150 is a high-affinity NOPr ligand that has 20-fold selectivity over MOPr and partial agonist activity at both MOPr and NOPr. SR14150 has antinociceptive activity in the tail-flick assay that seems to be caused by activation of MOPr, because it is completely blocked by coadministration of naloxone. This suggests that the MOPr partial agonist efficacy of SR14150 is sufficient to produce an acute antinociceptive effect. Furthermore, SR14150's antinociceptive effect was potentiated by cotreatment with the NOPr antagonist SB-612111, suggesting that the NOPr component in SR14150 is diminishing its MOPr-mediated antinociception. This result is not surprising, because the NOPr agonist Ro 64-6198 has been shown to decrease morphine antinociception (Kotlinska et al., 2003b). A similar potentiation of buprenorphine's antinociceptive activity was also observed in acute pain assays, after cotreatment with the NOPr antagonist J-113397 (Lutfy et al., 2003), and with NOPr antagonist SB-612111, as we demonstrated recently (Khroyan et al., 2009)
Even though SR14150 shows MOPr-mediated antinociception, it is not rewarding in the place-conditioning paradigm (Fig. 4A). One plausible explanation could be that the low efficacy of SR14150 at MOPr may not be sufficient to produce reward. However, other MOPr partial agonists with similar efficacies at MOPr, such as pentazocine and buprenorphine, have been shown to produce CPP comparable with that produced by morphine (Suzuki et al., 1991; Tzschentke, 2004; Marquez et al., 2007). It is possible that CPP could be induced with higher doses of SR14150; however, significantly higher doses were impossible to test because of loss of muscle tone at higher doses of SR14150. Alternatively, given that N/OFQ and Ro 64-6198 block CPP of a number of abused drugs (Murphy et al., 1999; Kotlinska et al., 2003a; Kuzmin et al., 2003; Ciccocioppo et al., 2004; Sakoori and Murphy, 2004), a more likely explanation is that the NOPr agonist activity present in SR14150 is attenuating MOPr-mediated reward. In these experiments, NOPr agonist activity is clearly present, because SR14150 administration produces hypolocomotor effects rather than hyperlocomotion, as do MOPr agonists such as morphine. Reduction in locomotor activity has been reported previously for the NOPr agonist Ro 64-6198 and with N/OFQ (Reinscheid et al., 1995; Higgins et al., 2001).
SR14150 did not affect the acquisition of morphine CPP (Fig. 4), even though, at the same doses, it attenuated morphine-induced hyperactivity and behavioral sensitization. SR14150 has only partial agonist activity at NOPr, which may not be sufficient to reduce CPP produced by a high efficacy MOPr agonist, such as morphine.
The bifunctional ligand SR16507 has high binding affinity for both NOPr and MOPr and is a full agonist at NOPr and a partial agonist at MOPr. We hypothesized that when administered systemically, the increased agonist efficacy at NOPr would reduce both its overall antinociceptive activity and its rewarding properties. However, SR16507 has potent antinociceptive activity in the tail-flick assay at low doses. At higher doses, a loss of muscle tone confounds the assay; an effect mediated by its NOPr activity, because SB-612111 administration allows the animal to recover from the hypolocomotor effects of SR16507 (unpublished observation). Similar to SR14150, the antinociceptive activity of SR16507 was blocked by naloxone and, as we have shown previously (Khroyan et al., 2009), potentiated by SB-612111.
SR16507 produced CPP alone but decreased global activity (Fig. 5B). However, unlike the NOP partial agonist SR14150, SR16507 also modestly attenuated morphine CPP (Fig. 5A). Therefore, functionally, the NOPr full agonist activity of SR16507 is sufficient to attenuate CPP induced by morphine.
Our third compound, SR16835 is a modestly (7-fold) selective NOPr full agonist, of slightly lower NOPr affinity than the other two compounds described here. It is noteworthy, however, that it has very low efficacy at the MOPr (Table 1). Consequently, SR16835 does not produce MOPr-mediated behavioral effects such as antinociception and CPP. However, the full NOPr agonist activity of SR16835 is apparently sufficient to attenuate morphine CPP, similar to other full NOPr agonists SR16507, Ro 64-6198 (Shoblock et al., 2005), and N/OFQ itself (Murphy et al., 1999). Furthermore, the inhibition of morphine CPP by SR16835 is reversed by the selective NOPr antagonist SB-612111 (Fig. 7), indicating that the reduction in morphine CPP by SR16835 is due to its NOPr agonist activity.
Our findings confirm and extend our previous studies on NOPr/MOPr mixed ligands (Khroyan et al., 2007; Spagnolo et al., 2008). We had previously hypothesized that a compound that binds to both NOPr and MOPr would maintain antinociceptive activity via the MOPr, but the NOPr activity in the same molecule would attenuate the reward and reduce tolerance development. SR16435, a partial agonist at MOPr and NOPr, with high affinity to both receptors, is a potent analgesic with reduced tolerance development, but exhibits a CPP to the same extent as morphine (Khroyan et al., 2007). We further hypothesized that either an increase in efficacy for the NOPr and/or a decrease in efficacy and/or affinity for the MOPr would be necessary to attenuate the rewarding effects of a bifunctional, nonselective NOPr/MOPr compound such as SR16435. This was borne out with SR14150 and SR16835, both of which have higher selectivity and efficacy at NOPr compared with SR16435, and lower affinity and efficacy at MOPr. The functional balance of NOPr partial agonist activity and reduced MOPr agonist activity present in SR14150 may provide a profile of antinociceptive activity without rewarding effects. Higher doses of SR14150 produce hypolocomotor effects. Nevertheless, this type of compound could still be considered potentially viable as a nonaddicting analgesic, especially in light of the recent report on the selective NOPr agonist Ro 64-6198, which showed that Ro 64-6198 had significant antinociceptive activity without hypolocomotor effects when adminstered systemically in monkeys (Ko et al., 2009). This suggests that NOPr agonists show antinociceptive activity in higher species. Therefore, a compound like SR14150 may prove to be a successful analgesic with reduced or no addiction liability in humans.
NOPr agonists are effective in decreasing the rewarding effects of drugs of abuse. Therefore, it was important to determine to what extent a reduction in MOPr affinity and an increase in NOPr efficacy was needed to produce a compound that attenuated the rewarding effects of morphine. SR14150, a partial agonist at NOPr was not rewarding on its own, but was unable to attenuate morphine CPP. However, NOPr full agonists SR16835 and SR16507 were both able to attenuate morphine CPP to some extent, even though the nonselective NOPr/MOPr agonist SR16507 produces CPP on its own. It seems, from our results, that full agonist activity at NOPr is required to reduce morphine CPP. Furthermore, because SR16835 is somewhat selective for NOPr in both binding and functional assays (Table 1) and has very low efficacy at MOPr, it selectively attenuates morphine CPP without having rewarding effects on its own. Therefore, it seems that a bifunctional NOPr-selective full agonist may be useful to treat addiction, even though it may have low MOPr agonist efficacy. In fact, a small amount of MOPr agonist activity may actually decrease withdrawal and improve compliance in addicts, as does buprenorphine.
Although several selective NOPr agonists and antagonists have been reported, they have not been effective on their own to treat either pain or addiction. Consequently, the ultimate clinical indications that may be remedied by “selective” NOPr agonists and antagonists have not yet been established. However, several “mixed” opioid ligands such as MOPr/KOPr agonists (e.g., nalbuphine and TRK-820) have been developed that have potent antinociceptive effects through the MOPr, but are less rewarding because of their activity at the KOPr (Hoskin and Hanks, 1991; Hasebe et al., 2004). The KOPr has been shown to have an antiopioid effect with respect to mesolimbic dopamine release and reward. Because the NOP-N/OFQ system has also been shown to have a similar antiopioid effect, dual-targeted NOPr/MOPr ligands may provide a good combination for producing antinociceptive activity and balancing the side-effect profile of reward and tolerance. Our results demonstrate that the behavioral and pharmacological profile of NOPr/MOPr compounds depends on both intrinsic activity and selectivity at NOPr and MOPr, and such compounds have potential utility as medications for both pain and addiction. However, it should be noted that these compounds are not totally selective for NOPr and MOPr. Although the DOPr affinity of these compounds is very low (data not shown), they do have measurable affinity for KOPr, albeit significantly lower than for MOPr. Although unlikely based on the low affinity and efficacy at KOPr, one cannot completely rule out the possibility that KOPr activation may contribute to some of the pharmacological actions of our compounds.
MOPr partial agonists like buprenorphine have been used successfully to treat chronic pain and drug addiction. In fact, buprenorphine was recently shown to be efficacious in decreasing alcohol self-administration in rats (Ciccocioppo et al., 2007) and has been observed to decrease cocaine intake in dually addicted alcohol and opioid addicts (Montoya et al., 2004). This effect of buprenorphine has been attributed to its NOPr agonist activity (Ciccocioppo et al., 2007) and is consistent with the effect of NOPr agonists on alcohol- and opioid-induced reward in rats and mice (Kuzmin et al., 2003; Shoblock et al., 2005; Kuzmin et al., 2007). Increasing the balance of NOPr agonist activity, along with MOPr partial agonist activity, may provide compounds with an overall profile similar to or preferable to that of buprenorphine, and effective in treating drug addiction. The NOPr/MOPr balance of the bifunctional ligands reported here may be further modulated to explore this possibility. Our continuing studies to optimize the balance between the stimulation of the NOPr and MOPr may lead to the identification of clinically useful treatments for both pain and drug abuse.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grants R01DA14026, R01DA023281] (to N.T.Z. and L.T., respectively).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.157446
- NOPr
- nociceptin/orphanin FQ receptor
- ORL1
- opioid receptor-like receptor
- N/OFQ
- nociceptin/orphanin FQ
- MOPr
- μ-opioid receptor
- KOPr
- κ-opioid receptor
- DOPr
- δ-opioid receptor
- PC
- place conditioning
- CPP
- conditioned place preference
- CPA
- conditioned place aversion
- ANOVA
- analysis of variance
- GTPγS
- guanosine 5′-O-(3-thiotriphosphate)
- SR14150
- 1-(1-cyclooctylpiperidin-4-yl)-indolin-2-one
- SR16507
- 3-ethyl-1-(1-(4-isopropylcyclohexyl)piperidin-4-yl)-indolin-2-one
- SR16835
- 1-(1-(2,3,3a,4,5,6-hexahydro-1H-phenalen-1-yl)piperidinl-4-yl)-indolin-2-one
- SR16435
- 1-(1-(bicyclo[3.3.1]nonan-9-yl)piperidin-4-yl)indolin-2-one
- SB-612111
- (5S,7S)-7-{[4-(2,6-dichlorophenyl)piperidin-1-yl]methyl}-1-methyl-6,7,8,9-tetrahydro-5H-benzo[7]annulen-5-ol
- TRK-820
- nalfurafine
- G418
- (2R,3S,4R,5R,6S)-5-amino-6-[(1R,2S,3S,4R,6S)-4,6-diamino-3-[(2R,3R,4R,5R)-3,5-dihydroxy-5-methyl-4-methylaminooxan-2-yl]oxy-2-hydroxycyclohexyl]oxy-2-(1-hydroxyethyl)oxane-3,4-diol
- U69593
- (+)-(5α,7α,8β)-N-methyl-N-[7-(1-pyrrolidinyl)-1-oxaspiro[4.5]dec-8-yl]-benzeneacetamide
- J-113397
- 1-[(3R,4R)-1-cyclooctylmethyl-3-hydroxymethyl-4-piperidyl]-3-ethyl-1,3-dihydro-2H-benzimidazol-2-one.
References
- Bals-Kubik R, Herz A, Shippenberg TS. (1989) Evidence that the aversive effects of opioid antagonists and kappa-agonists are centrally mediated. Psychopharmacology (Berl) 98:203–206 [DOI] [PubMed] [Google Scholar]
- Barlocco D, Cignarella G, Giuseppe G, Grugni M, Ronzoni S. (2004) inventors; Smithkline Beecham Corporation, assignee. Benzosuberonylpiperidine compounds as analgesics. U.S. patent 20040029917A1 [Google Scholar]
- Bowen CA, Negus SS, Zong R, Neumeyer JL, Bidlack JM, Mello NK. (2003) Effects of mixed-action kappa/mu opioids on cocaine self-administration and cocaine discrimination by rhesus monkeys. Neuropsychopharmacology 28:1125–1139 [DOI] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. (1973) Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 percent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 22:3099–3108 [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Fedeli A, Angeletti S, Weiss F, Heilig M, Massi M. (2004) Attenuation of ethanol self-administration and of conditioned reinstatement of alcohol-seeking behaviour by the antiopioid peptide nociceptin/orphanin FQ in alcohol-preferring rats. Psychopharmacology (Berl) 172:170–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Rimondini R, Sommer W, Massi M, Heilig M. (2007) Buprenorphine reduces alcohol drinking through activation of the nociceptin/orphanin FQ-NOP receptor system. Biol Psychiatry 61:4–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine DP, Reinscheid RK, Monsma FJ, Jr, Civelli O, Akil H. (1996) The novel neuropeptide orphanin FQ fails to produce conditioned place preference or aversion. Brain Res 727:225–229 [DOI] [PubMed] [Google Scholar]
- Dooley CT, Spaeth CG, Berzetei-Gurske IP, Craymer K, Adapa ID, Brandt SR, Houghten RA, Toll L. (1997) Binding and in vitro activities of peptides with high affinity for the nociceptin/orphanin FQ receptor, ORL1. J Pharmacol Exp Ther 283:735–741 [PubMed] [Google Scholar]
- Hasebe K, Kawai K, Suzuki T, Kawamura K, Tanaka T, Narita M, Nagase H, Suzuki T. (2004) Possible pharmacotherapy of the opioid kappa receptor agonist for drug dependence. Ann N Y Acad Sci 1025:404–413 [DOI] [PubMed] [Google Scholar]
- Higgins GA, Grottick AJ, Ballard TM, Richards JG, Messer J, Takeshima H, Pauly-Evers M, Jenck F, Adam G, Wichmann J. (2001) Influence of the selective ORL1 receptor agonist, Ro64–6198, on rodent neurological function. Neuropharmacology 41:97–107 [DOI] [PubMed] [Google Scholar]
- Hoskin PJ, Hanks GW. (1991) Opioid agonist-antagonist drugs in acute and chronic pain states. Drugs 41:326–344 [DOI] [PubMed] [Google Scholar]
- Huang P, Kehner GB, Cowan A, Liu-Chen LY. (2001) Comparison of pharmacological activities of buprenorphine and norbuprenorphine: norbuprenorphine is a potent opioid agonist. J Pharmacol Exp Ther 297:688–695 [PubMed] [Google Scholar]
- Khroyan TV, Zaveri NT, Polgar WE, Orduna J, Olsen C, Jiang F, Toll L. (2007) SR 16435 [1-(1-(bicyclo[3.3.1]nonan-9-yl)piperidin-4-yl)indolin-2-one], a novel mixed nociceptin/orphanin FQ/mu-opioid receptor partial agonist: analgesic and rewarding properties in mice. J Pharmacol Exp Ther 320:934–943 [DOI] [PubMed] [Google Scholar]
- Khroyan TV, Polgar WE, Jiang F, Zaveri NT, Toll L. (2009) Nociceptin/orphanin FQ receptor activation attenuates antinociception induced by mixed nociceptin/orphanin FQ/μ-opioid receptor agonists. J Pharmacol Exp Ther 331:946–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Woods JH, Fantegrossi WE, Galuska CM, Wichmann J, Prinssen EP. (2009) Behavioral effects of a synthetic agonist selective for nociceptin/orphanin FQ peptide receptors in monkeys. Neuropsychopharmacology 34:2088–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlinska J, Rafalski P, Biala G, Dylag T, Rolka K, Silberring J. (2003a) Nociceptin inhibits acquisition of amphetamine-induced place preference and sensitization to stereotypy in rats. Eur J Pharmacol 474:233–239 [DOI] [PubMed] [Google Scholar]
- Kotlinska J, Wichmann J, Rafalski P, Talarek S, Dylag T, Silberring J. (2003b) Non-peptidergic OP4 receptor agonist inhibits morphine antinociception but does not influence morphine dependence. Neuroreport 14:601–604 [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Kreek MJ, Bakalkin G, Liljequist S. (2007) The nociceptin/orphanin FQ receptor agonist Ro 64–6198 reduces alcohol self-administration and prevents relapse-like alcohol drinking. Neuropsychopharmacology 32:902–910 [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Sandin J, Terenius L, Ogren SO. (2003) Acquisition, expression, and reinstatement of ethanol-induced conditioned place preference in mice: effects of opioid receptor-like 1 receptor agonists and naloxone. J Pharmacol Exp Ther 304:310–318 [DOI] [PubMed] [Google Scholar]
- Lutfy K, Eitan S, Bryant CD, Yang YC, Saliminejad N, Walwyn W, Kieffer BL, Takeshima H, Carroll FI, Maidment NT, et al. ( 2003) Buprenorphine-induced antinociception is mediated by mu-opioid receptors and compromised by concomitant activation of opioid receptor-like receptors. J Neurosci 23:10331–10337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez P, Baliram R, Kieffer BL, Lutfy K. (2007) The mu opioid receptor is involved in buprenorphine-induced locomotor stimulation and conditioned place preference. Neuropharmacology 52:1336–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Kamien JB, Lukas SE, Drieze J, Mendelson JH. (1993) The effects of nalbuphine and butorphanol treatment on cocaine and food self-administration by rhesus monkeys. Neuropsychopharmacology 8:45–55 [DOI] [PubMed] [Google Scholar]
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B. (1995) Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature 377:532–535 [DOI] [PubMed] [Google Scholar]
- Mogil JS, Grisel JE, Reinscheid RK, Civelli O, Belknap JK, Grandy DK. (1996a) Orphanin FQ is a functional anti-opioid peptide. Neuroscience 75:333–337 [DOI] [PubMed] [Google Scholar]
- Mogil JS, Grisel JE, Zhangs G, Belknap JK, Grandy DK. (1996b) Functional antagonism of mu-, delta- and kappa-opioid antinociception by orphanin FQ. Neurosci Lett 214:131–134 [DOI] [PubMed] [Google Scholar]
- Montoya ID, Gorelick DA, Preston KL, Schroeder JR, Umbricht A, Cheskin LJ, Lange WR, Contoreggi C, Johnson RE, Fudala PJ. (2004) Randomized trial of buprenorphine for treatment of concurrent opiate and cocaine dependence. Clin Pharmacol Ther 75:34–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy NP, Lee Y, Maidment NT. (1999) Orphanin FQ/nociceptin blocks acquisition of morphine place preference. Brain Res 832:168–170 [DOI] [PubMed] [Google Scholar]
- Neal CR, Jr, Akil H, Watson SJ., Jr (2001) Expression of orphanin FQ and the opioid receptor-like (ORL1) receptor in the developing human and rat brain. J Chem Neuroanat 22:219–249 [DOI] [PubMed] [Google Scholar]
- Neal CR, Jr, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Akil H, Watson SJ., Jr (1999) Opioid receptor-like (ORL1) receptor distribution in the rat central nervous system: comparison of ORL1 receptor mRNA expression with (125)I-[(14)Tyr]-orphanin FQ binding. J Comp Neurol 412:563–605 [PubMed] [Google Scholar]
- Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ, Jr, Civelli O. (1995) Orphanin FQ: a neuropeptide that activates an opioid-like G protein-coupled receptor. Science 270:792–794 [DOI] [PubMed] [Google Scholar]
- Reiss D, Wichmann J, Tekeshima H, Kieffer BL, Ouagazzal AM. (2008) Effects of nociceptin/orphanin FQ receptor (NOP) agonist, Ro64–6198, on reactivity to acute pain in mice: comparison to morphine. Eur J Pharmacol 579:141–148 [DOI] [PubMed] [Google Scholar]
- Sakoori K, Murphy NP. (2004) Central administration of nociceptin/orphanin FQ blocks the acquisition of conditioned place preference to morphine and cocaine, but not conditioned place aversion to naloxone in mice. Psychopharmacology (Berl) 172:129–136 [DOI] [PubMed] [Google Scholar]
- Shoblock JR, Wichmann J, Maidment NT. (2005) The effect of a systemically active ORL-1 agonist, Ro 64–6198, on the acquisition, expression, extinction, and reinstatement of morphine conditioned place preference. Neuropharmacology 49:439–446 [DOI] [PubMed] [Google Scholar]
- Spagnolo B, Calo G, Polgar WE, Jiang F, Olsen CM, Berzetei-Gurske I, Khroyan TV, Husbands SM, Lewis JW, Toll L, et al. ( 2008) Activities of mixed NOP and mu-opioid receptor ligands. Br J Pharmacol 153:609–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Masukawa Y, Shiozaki Y, Misawa M. (1991) Potentiation of pentazocine conditioned place preference by tripelennamine in rats. Psychopharmacology (Berl) 105:9–12 [DOI] [PubMed] [Google Scholar]
- Traynor JR, Nahorski SR. (1995) Modulation by μ-opioid agonists of guanosine-5′-O-(3-[35S]thio)triphosphate binding to membranes from human neuroblastoma SH-SY5Y cells. Mol Pharmacol 47:848–854 [PubMed] [Google Scholar]
- Tzschentke TM. (2004) Reassessment of buprenorphine in conditioned place preference: temporal and pharmacological considerations. Psychopharmacology (Berl) 172:58–67 [DOI] [PubMed] [Google Scholar]
- Xu XJ, Hao JX, Wiesenfeld-Hallin Z. (1996) Nociceptin or antinociceptin: potent spinal antinociceptive effect of orphanin FQ/nociceptin in the rat. Neuroreport 7:2092–2094 [PubMed] [Google Scholar]
- Yamamoto T, Nozaki-Taguchi N, Kimura S. (1997) Analgesic effect of intrathecally administered nociceptin, an opioid receptor-like1 receptor agonist, in the rat formalin test. Neuroscience 81:249–254 [DOI] [PubMed] [Google Scholar]
- Zaratin PF, Petrone G, Sbacchi M, Garnier M, Fossati C, Petrillo P, Ronzoni S, Giardina GA, Scheideler MA. (2004) Modification of nociception and morphine tolerance by the selective opiate receptor-like orphan receptor antagonist (−)-cis-1-methyl-7-[[4-(2,6-dichlorophenyl)piperidin-1-yl]methyl]-6,7,8,9-tetrahydro-5H-benzocyclohepten-5-ol (SB-612111). J Pharmacol Exp Ther 308:454–461 [DOI] [PubMed] [Google Scholar]
- Zaveri NT, Jiang F, Olsen CM, Deschamps JR, Parrish D, Polgar W, Toll L. (2004) A novel series of piperidin-4-yl-1,3-dihydroindol-2-ones as agonist and antagonist ligands at the nociceptin receptor. J Med Chem 47:2973–2976 [DOI] [PMC free article] [PubMed] [Google Scholar]