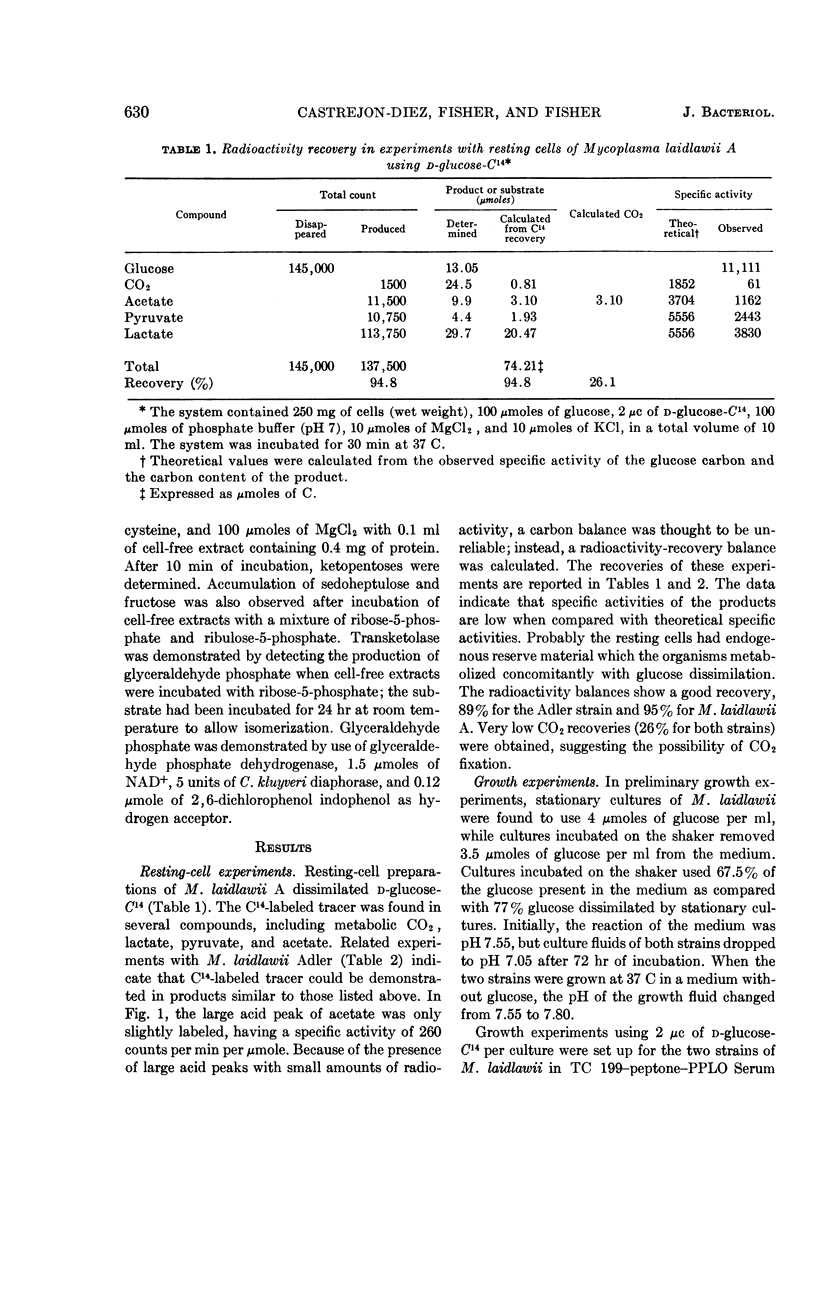
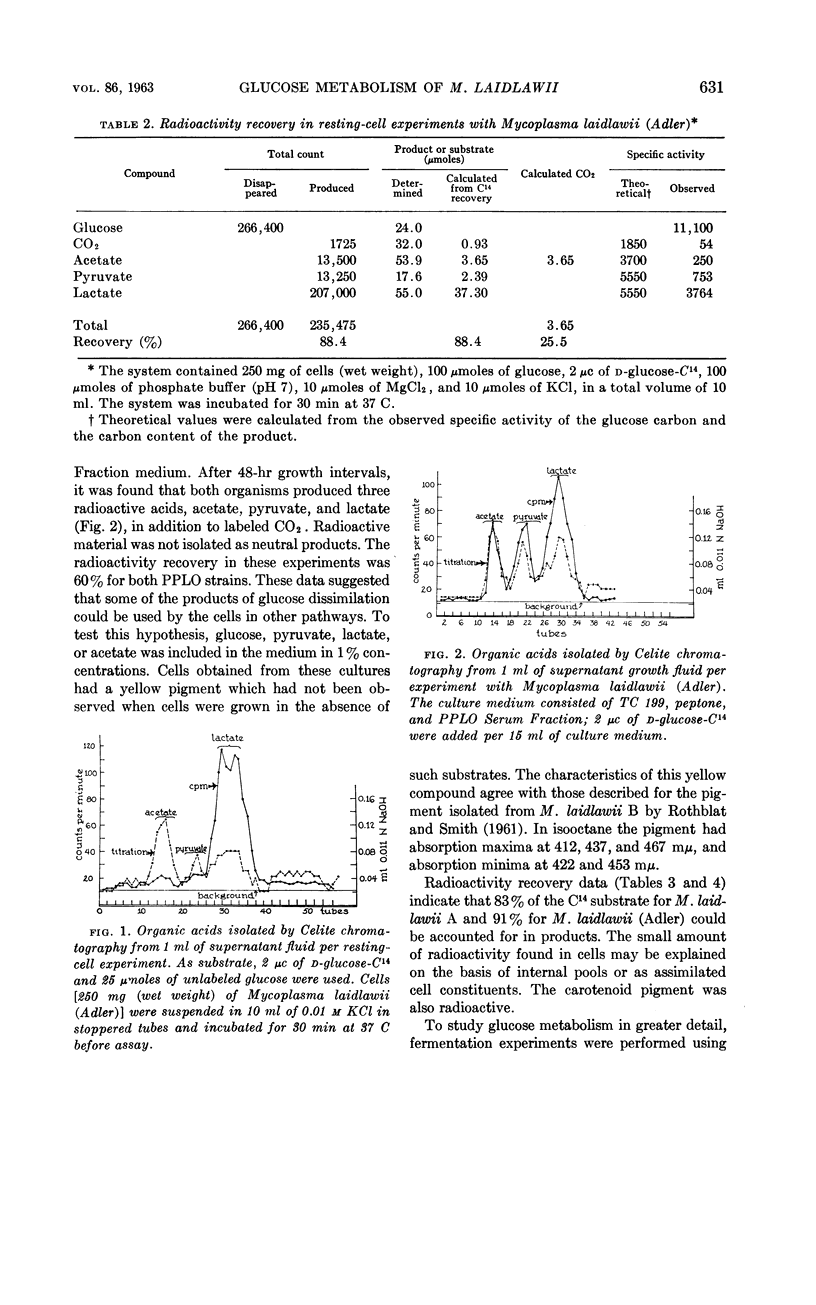
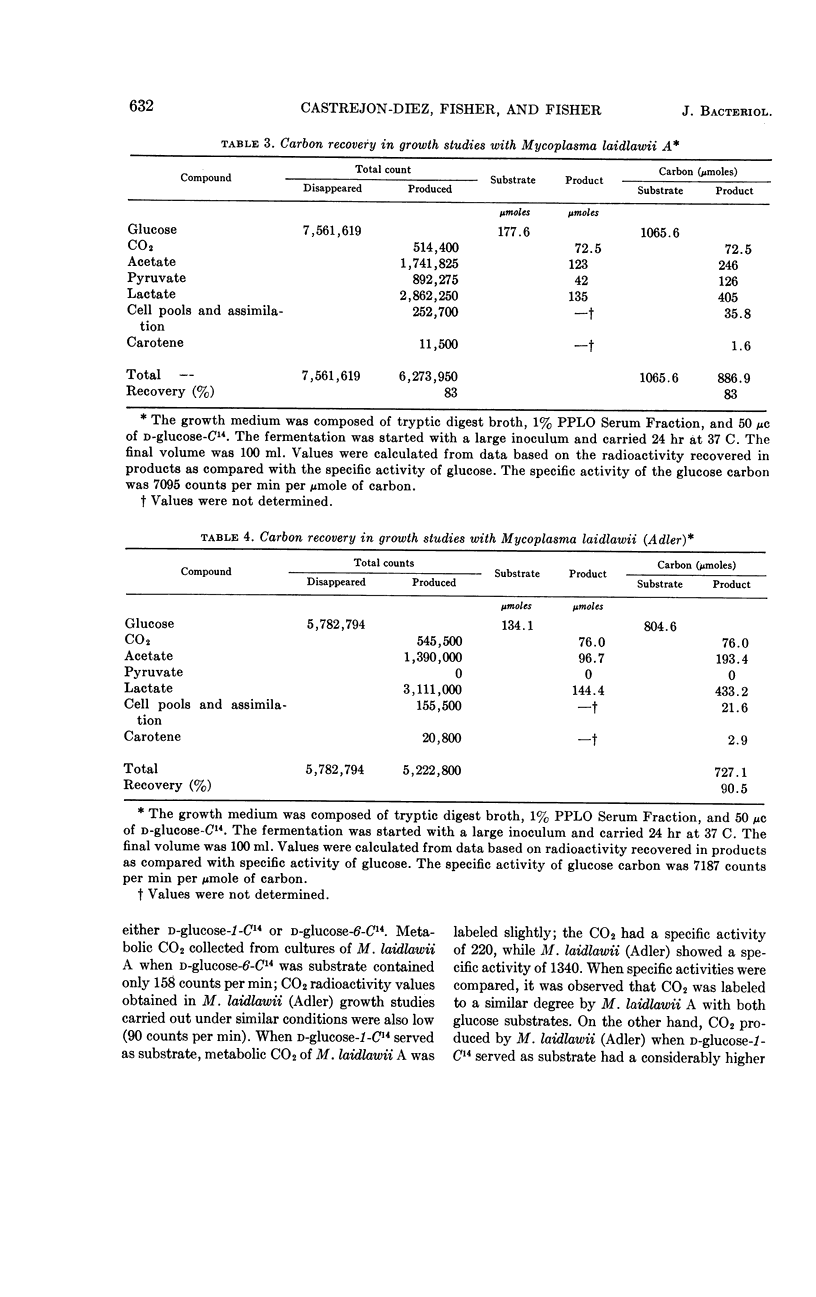
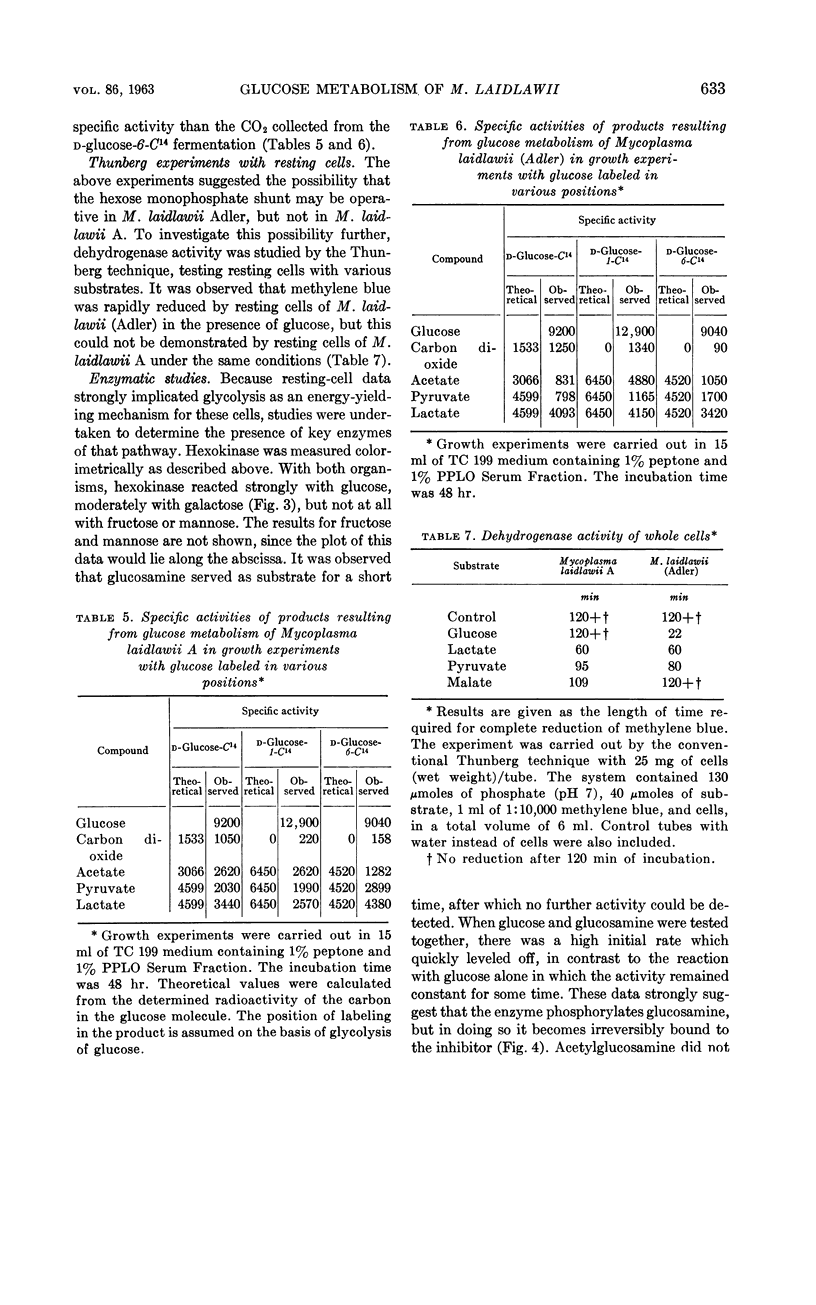
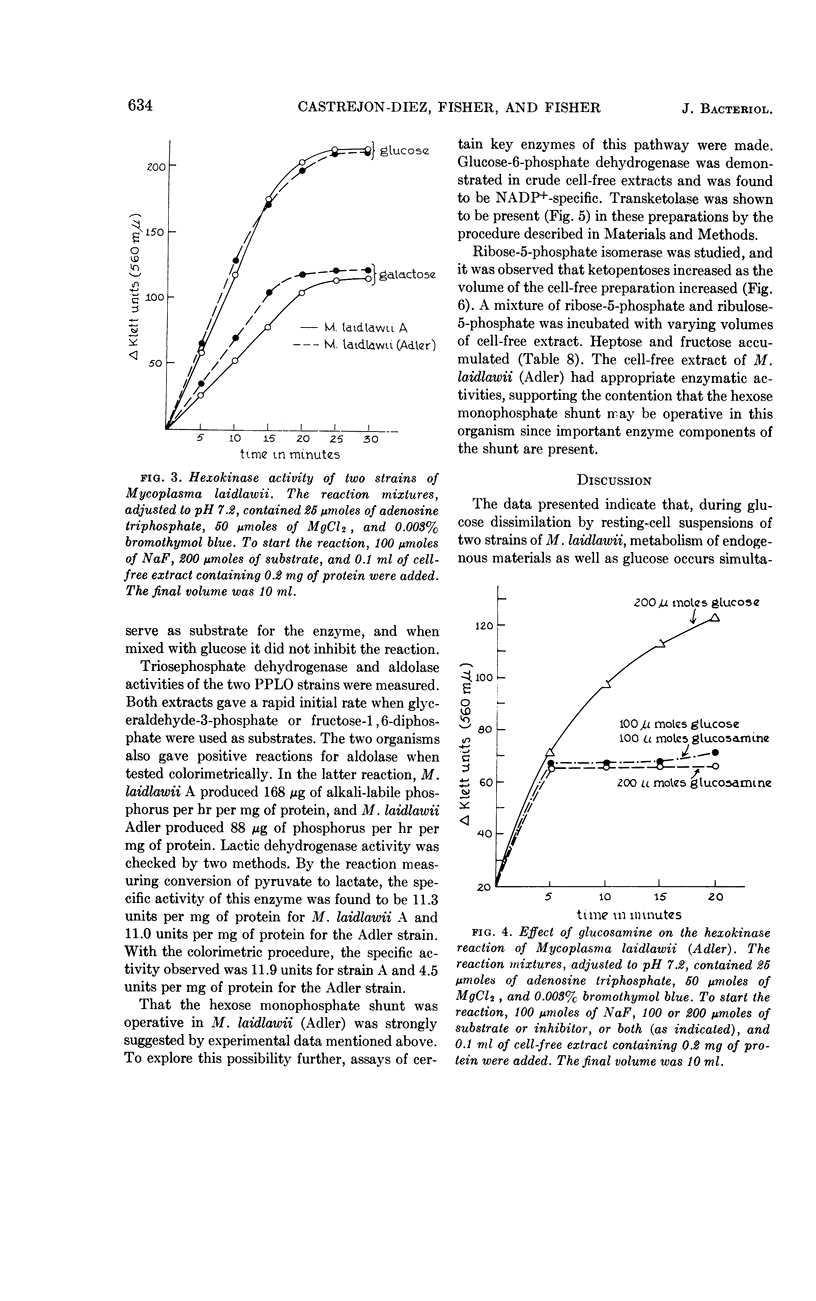
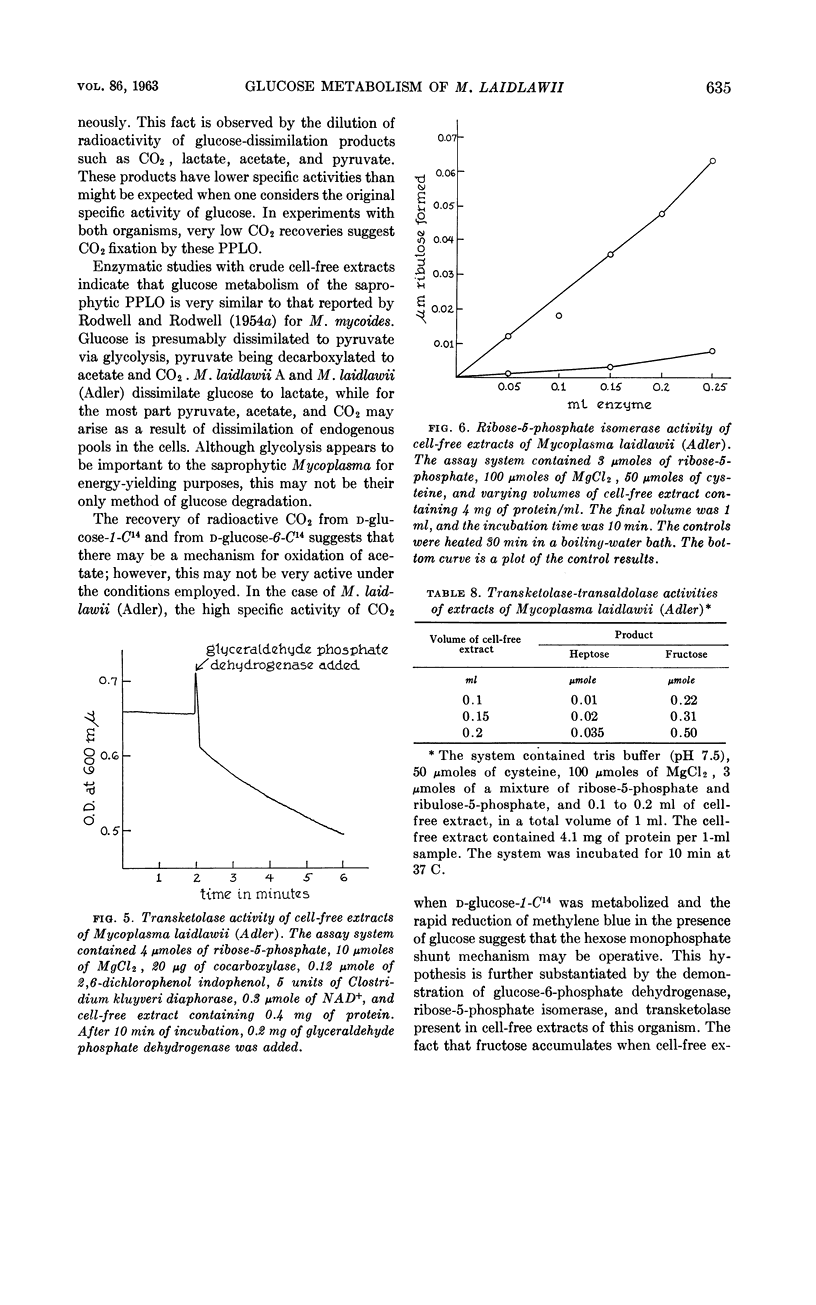
Abstract
Castrejon-Diez, Jaime (Tulane University School of Medicine, New Orleans, La.), Thelma N. Fisher, and Earl Fisher, Jr. Glucose metabolism of two strains of Mycoplasma laidlawii. J. Bacteriol. 86:627–636. 1963.—Two strains of Mycoplasma laidlawii were incubated in systems containing d-glucose-C14; carbon dioxide, acetate, pyruvate, and lactate were isolated from appropriate fluids after resting-cell and growth experiments. In resting-cell experiments, radioactivity recoveries were shown to be 95% for M. laidlawii A and 89% for M. laidlawii (Adler). By growth studies, the radioactivity recovery for M. laidlawii A was 83% and for M. laidlawii (Adler) was 90.5%. Low specific activities of the products as compared with the specific activity of glucose suggested cellular pools, or that the dissimilation of other substances present in the complex growth medium yielded products which contributed to the dilution factors. Enzyme studies added support to the hypothesis that glycolysis is operative in these organisms. Experiments with d-glucose-1-C14 or d-glucose-6-C14 as substrate suggested that the hexose monophosphate shunt may be functional in M. laidlawii (Adler), particularly since glucose-6-phosphate dehydrogenase, ribose-5-phosphate isomerase, and transketolase were demonstrated. This pathway is absent in M. laidlawii A.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARD R. C., GUNSALUS I. C. Glucose metabolism of Clostridium perfringens: existence of metallo-aldolase. J Bacteriol. 1950 Mar;59(3):387–400. doi: 10.1128/jb.59.3.387-400.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DISCHE Z., BORENFREUND E. A new spectrophotometric method for the detection and determination of keto sugars and trioses. J Biol Chem. 1951 Oct;192(2):583–587. [PubMed] [Google Scholar]
- DISCHE Z. Qualitative and quantitative colorimetric determination of heptoses. J Biol Chem. 1953 Oct;204(2):983–997. [PubMed] [Google Scholar]
- FISHER T. N., FISHER E., Jr Effects of cortisone and herpes simplex virus on metabolic processes. I. Alterations in HeLa cell metabolism. Proc Soc Exp Biol Med. 1959 Apr;100(4):780–786. doi: 10.3181/00379727-100-24777. [DOI] [PubMed] [Google Scholar]
- HOCKENHULL D. J. D., FLOODGATE G. D. o-Phenylenediamine and 1:2-diamino-4-nitrobenzene as reagents for a-keto acids. Biochem J. 1952 Sep;52(1):38–40. doi: 10.1042/bj0520038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HULLIN R. P., NOBLE R. L. The determination of lacic acid in microgram quantities. Biochem J. 1953 Sep;55(2):289–291. doi: 10.1042/bj0550289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEIMARK H. C., PICKETT M. J. Products of glucose metabolism by pleuropneumonialike organisms. Ann N Y Acad Sci. 1960 Jan 15;79:531–537. doi: 10.1111/j.1749-6632.1960.tb42719.x. [DOI] [PubMed] [Google Scholar]
- RODWELL A. W. Nutrition and metabolism of Mycoplasma mycoides var. mycoides. Ann N Y Acad Sci. 1960 Jan 15;79:499–507. doi: 10.1111/j.1749-6632.1960.tb42716.x. [DOI] [PubMed] [Google Scholar]
- RODWELL A. W., RODWELL E. S. The breakdown of carbohydrates by Asterococcus mycoides, the organism of bovine pleuropneumonia. Aust J Biol Sci. 1954 Feb;7(1):18–30. [PubMed] [Google Scholar]
- RODWELL A. W., RODWELL E. S. The breakdown of pyruvate by Asterococcus mycoides, the organism of bovine pleuropneumonia. Aust J Biol Sci. 1954 Feb;7(1):31–36. [PubMed] [Google Scholar]
- RODWELL A. W., RODWELL E. S. The pathway for glucose oxidation by Asterococcus mycoides, the organism of bovine pleuropneumonia. Aust J Biol Sci. 1954 Feb;7(1):37–46. [PubMed] [Google Scholar]
- ROTHBLAT G. H., SMITH P. F. Nonsaponifiable lipids of representative pleuropneumonia-like organisms. J Bacteriol. 1961 Oct;82:479–491. doi: 10.1128/jb.82.4.479-491.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEUBERT W. Degradation of isoprenoid compounds by micro-organisms. I. Isolation and characterization of an isoprenoid-degrading bacterium, Pseudomonas citronellolis n. sp. J Bacteriol. 1960 Mar;79:426–434. doi: 10.1128/jb.79.3.426-434.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOLS A., CRANE R. K. Substrate specificity of brain hexokinase. J Biol Chem. 1954 Oct;210(2):581–595. [PubMed] [Google Scholar]
- SWIM H. E., KRAMPITZ L. O. Acetic acid oxidation by Escherichia coli; evidence for the occurrence of a tricarboxylic acid cycle. J Bacteriol. 1954 Apr;67(4):419–425. doi: 10.1128/jb.67.4.419-425.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOURTELLOTTE M. E., JACOBS R. E. Physiological and serologic comparisons of PPLO from various sources. Ann N Y Acad Sci. 1960 Jan 15;79:521–530. doi: 10.1111/j.1749-6632.1960.tb42718.x. [DOI] [PubMed] [Google Scholar]


