Abstract
The solution phase synthesis of a 167-member library of isocoumarins is described. The key intermediates for library generation, 4-iodoisocoumarins, are easily prepared by iodocyclization of the corresponding 2-(1-alkynyl)arenecarboxylate esters. The 4-iodoisocoumarins undergo palladium-catalyzed Sonogashira, Suzuki-Miyura and Heck reactions to yield a diverse set of isocoumarins. Alternatively, isocoumarins, bearing hydroxyl or bromine functionalities, have been prepared by ZnCl2 and Pd(PPh3)4 mediated cyclization of the corresponding o-iodobenzoic acid and appropriate terminal alkynes. The resulting isocoumarins were further diversified by derivatization of the hydroxyl or bromine groups. A small set of isoquinolinones were also prepared from the corresponding isocoumarins.
Introduction
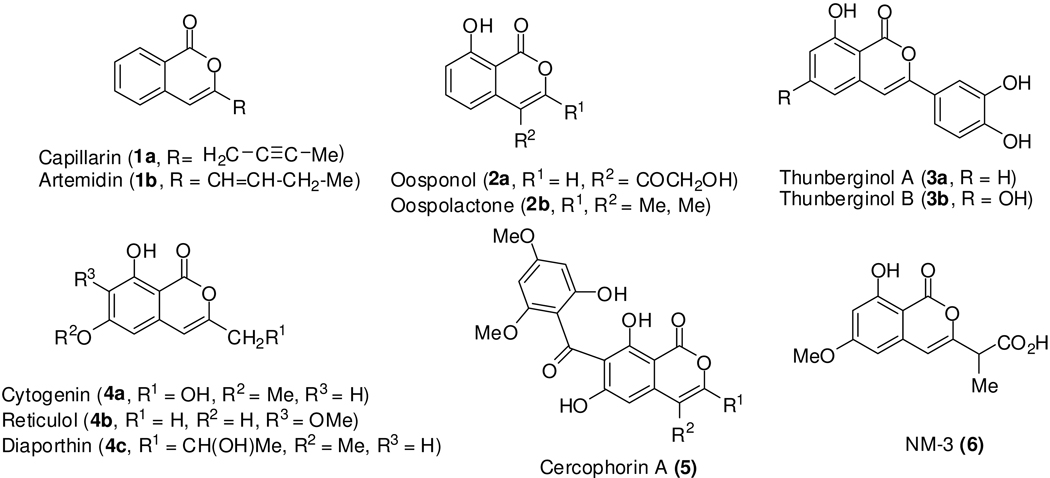
Isocoumarins are an important class of naturally-occurring lactones.1 They exhibit a wide range of pharmacological properties, including anti-fungal, anti-tumor, anti-allergic, anti-microbial, anti-inflammatory, anti-diabetic, phytotoxic and immunomodulatory activities.2 Selected simple, biologically active isocoumarins are shown in Figure 1. Among these, capillarin (1a), artemidin (1b) and cercophorin A (5) have been shown to possess antifungal activity.3 Capillarin (1a) also acts as an insect anti-feedant.4 Oosponol (2a) and oospolactone (2b) exhibit anti-fungal, as well as antibiotic, activities.5 Thunberginol A and B (3a and 3b) are known for their anti-allergic, anti-microbial and immunomodulatory activities.6 Cytogenin (4a) is known as an immunomodulator, anti-tumor and anti-arthritic agent.7 Reticulol (4b) also exhibits anti-tumor activity.8 Diaporthin (4c) is a phytotoxin.9 The synthetic isocoumarin NM-3 (6) has been found to be highly effective in the treatment of solid tumors and has entered clinical trials.10 Due to substantial biological activity, our group has long been interested in the synthesis of isocoumarins and several new methodologies have been developed in our laboratories.11 Many other useful methods have also been reported for the synthesis of the isocoumarin scaffold12–14.
Figure 1.
Selected biologically active isocoumarins.
High throughput screening of selected chemical libraries, having a heterocyclic or carbocyclic ring at their core, is one of the most expeditious ways to search for useful medicinal activity. The heteroatoms improve binding and the rigid cyclic framework imparts rigidity, enhancing the selectivity and further improving the binding. In a continuation of our efforts to adapt heterocyclization chemistry to a high throughput format,15 we herein report the first solution phase library synthesis of isocoumarins using alkyne cyclization chemistry as the key step. As shown in Scheme 1–Scheme 5, we have constructed a diverse 167 membered library of isocoumarins. The presence of the isocoumarin scaffold in both synthetic and natural, biologically active compounds justifies the preparation of this discovery library of isocoumarins.16 These compounds will be subjected to various high throughput screening (HTS) assays for their potential medicinal activity.
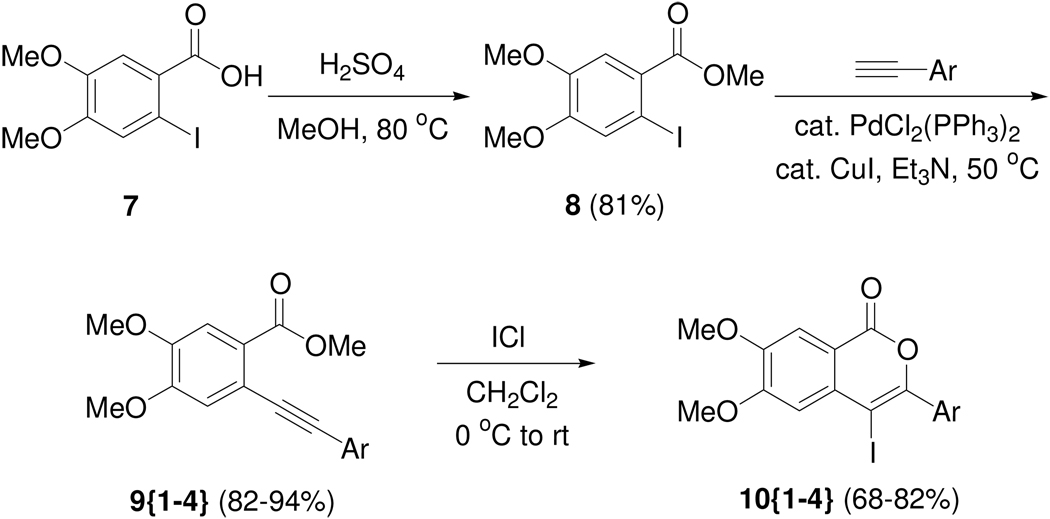
Scheme 1.
Synthesis of 4-iodoisocoumarins.
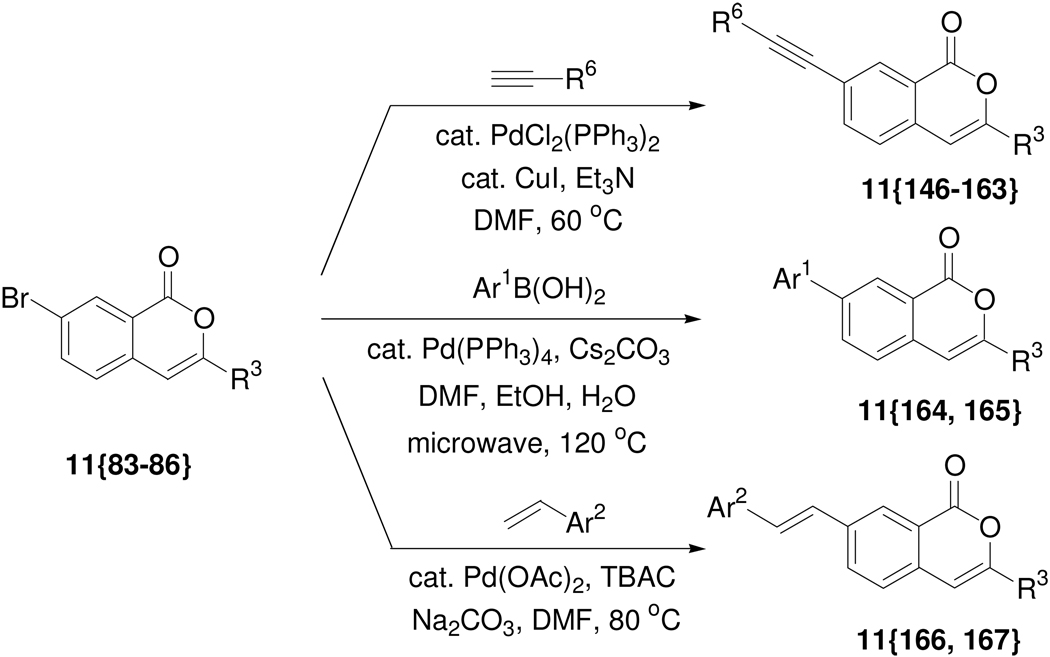
Scheme 5.
Synthesis of 7-substituted isocoumarins by further diversification.
Result and Discussion
Our initial strategy for the library generation is outlined in Scheme 1 and Scheme 2. We planned to utilize the 4-iodoisocoumarins as key intermediates that can be efficiently prepared using our alkyne iodocyclization chemistry.11a Subsequent diversification by various palladium-catalyzed cross-coupling reactions should afford a diverse set of isocoumarins.
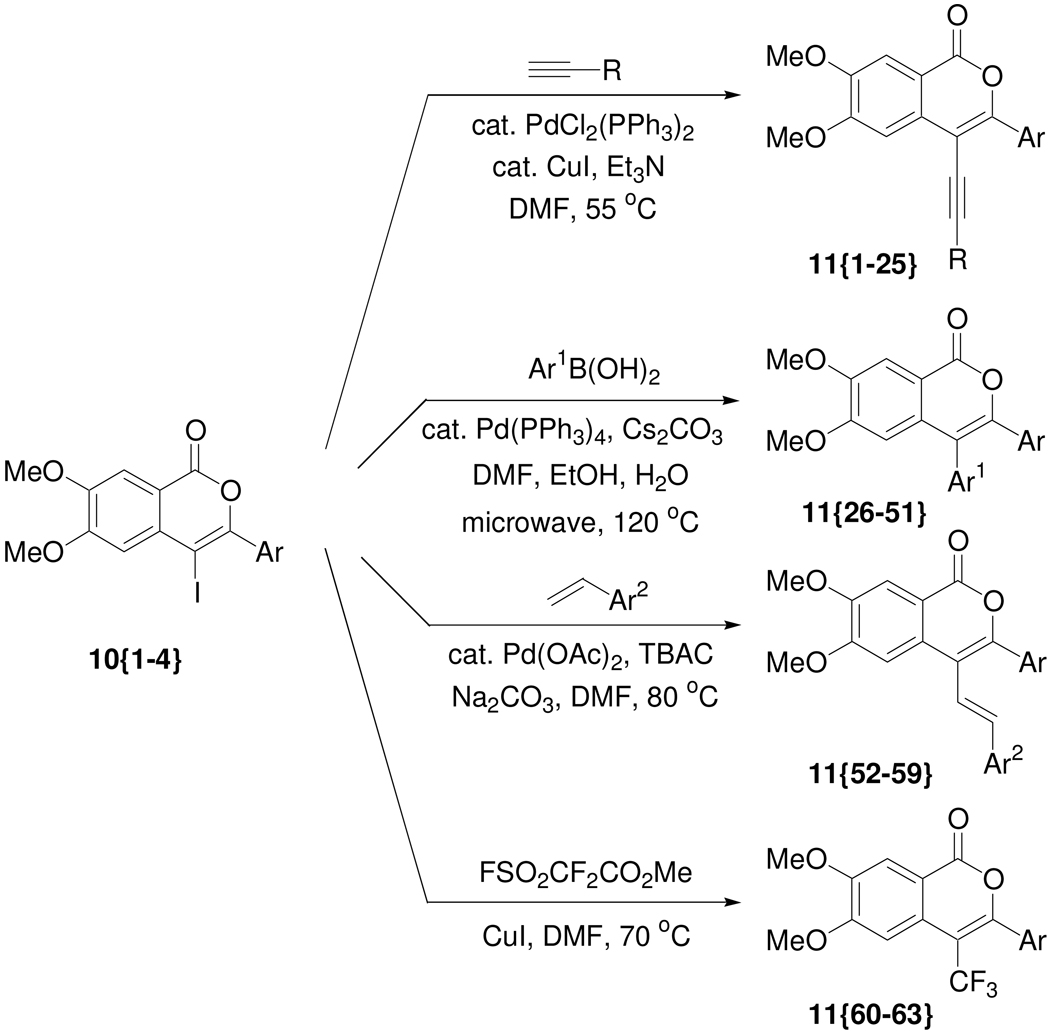
Scheme 2.
Library generation from the 4-iodoisocoumarins.
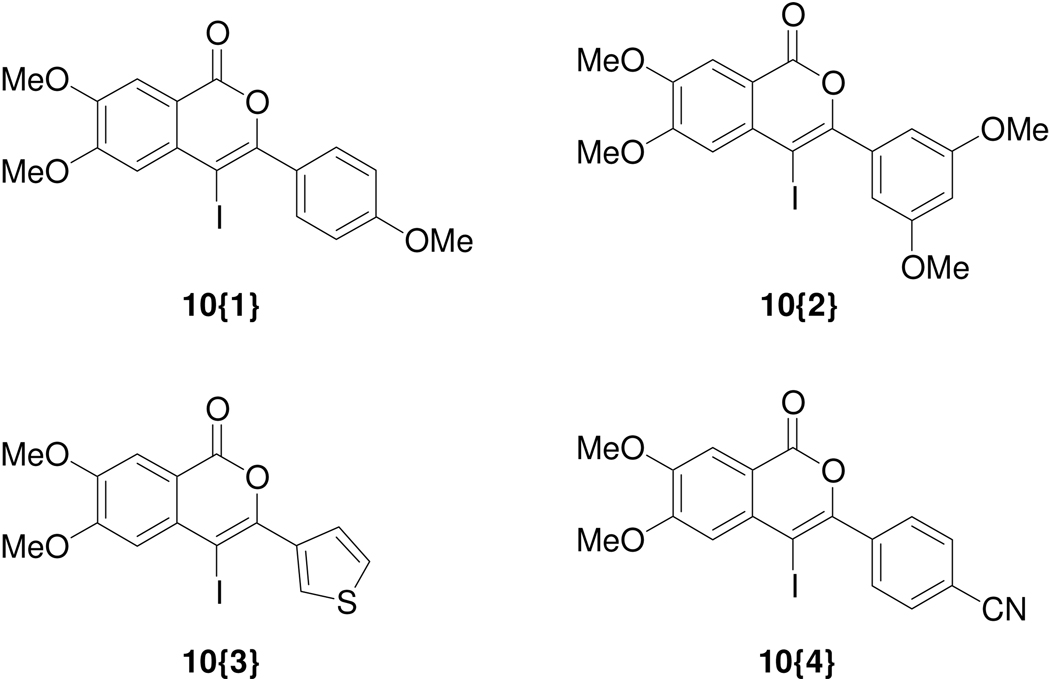
Initially, the 3,4-dimethoxybenzoic acid ester 8 was chosen as the starting material, because it was envisaged that the methoxy groups would provide desired polarity in the resulting library members. Compound 8 was prepared from the corresponding commercially available 2-iodo-4,5-dimethoxybenzoic acid (7). Sonogashira coupling of 8 with appropriate terminal alkynes 12{1–4} afforded the prerequisite starting materials for the iodocyclization chemistry (Scheme 1). Accordingly, a set of four 4-iodoisocoumarins 10{1–4} (Figure 2) were efficiently prepared on a gram scale by electrophilic cyclization of the o-(1-alkynyl)benzoates 9{1–4} using iodine monochloride.
Figure 2.
Key 4-iodoisocoumarin intermediates 10{1–4}.
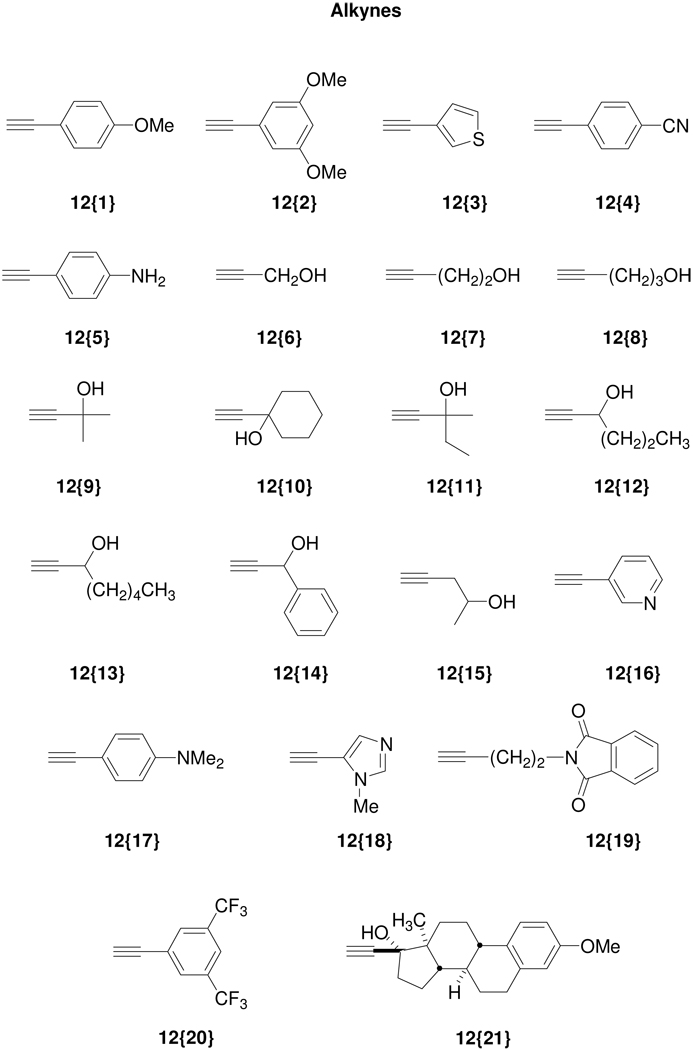
According to the plan, the 4-iodoisocoumarins 10{1–4} were used as key components for library generation and subsequently elaborated to produce a wide variety of isocoumarins. Thus, the palladium-catalyzed Sonogashira cross-coupling of the 4-iodoisocoumarins with various terminal acetylenes afforded isocoumarins 11{1–25}. The alkyne sublibrary (Figure 3) was chosen to include commercially available heteroatom-and heterocycle-containing (such as pyridine, thiophene, imidazole) alknes that could provide hydrogen bond donors and/or acceptors. Mestranol was chosen as a representative steroid with the ability to diffuse readily across cell membranes.
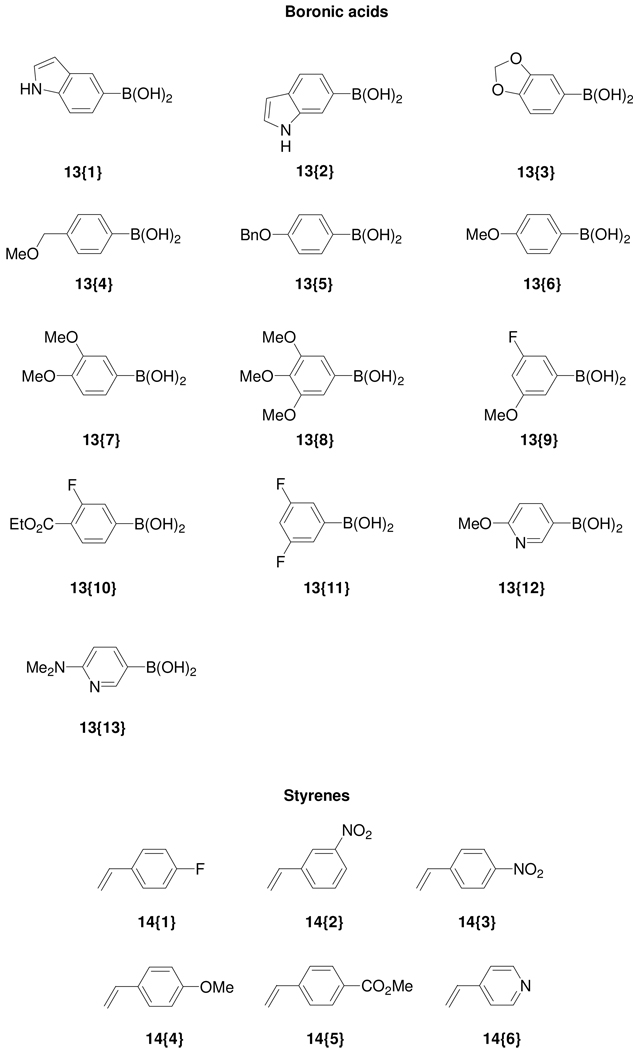
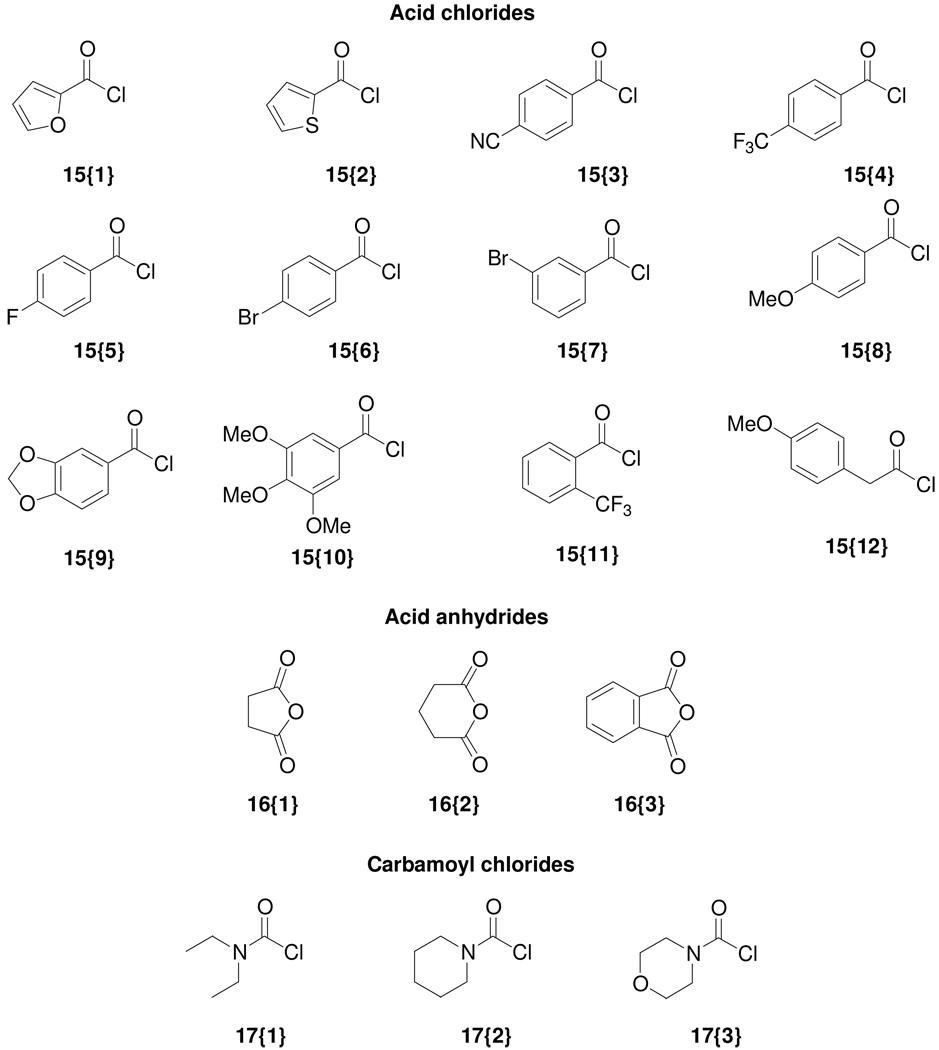
Figure 3.
Diverse terminal alkynes 12{1–21}, boronic acids 13{1–13}, styrenes 14{1–6}, acid chlorides 15{1–12}, acid anhydrides 16{1–3}, and carbamoyl chlorides 17{1–3} used for library synthesis.
Similarly, we obtained isocoumarins 11{26–51} by the palladium-catalyzed Suzuki-Miyaura reaction of the iodoisocoumarin intermediates 10{1–4} with various boronic acids (Figure 3). Commercially available boronic acids were chosen to include diverse functionality. Thus, boronic acids containing an indole scaffold, fluorine atoms, and polar functional groups were chosen in order to incorporate drug-like moieties into the resulting cross-coupling products. A brief examination of the reaction conditions suggested that microwave heating in these Suzuki-Miyaura cross-couplings is more effective than conventional heating and affords the desired isocoumarins in better yields. Microwave heating also allowed much shorter reaction times, which is desirable for library synthesis. However, the overall low yields in the Suzuki-Miyaura reactions are partially attributed to the instability of the lactone ring in the basic reaction medium used for cross-coupling, due to possible ring opening of the lactone starting materials.12n
The isocoumarins 11{52–59} have also been prepared by the Heck reaction of the 4-iodoisocoumarins. A small styrene sublibrary (Figure 3) for the Heck reaction was chosen to demonstrate vinylic functionalization as only a few functionally diverse styrenes were available from commercial sources. We avoided using well-known Michael acceptors, such as acrylates or acrylonitriles, in this Heck chemistry, since the resulting Heck products would also be good Michael acceptors and therefore undesirable for pharmaceutical applications. Due to the increasing importance of fluorine in pharmacologically active molecules due to its ability to enhance binding interactions and metabolic stability or to alter the physical and/or biological properties,17 we have prepared a small set of (4-trifluoromethyl)isocoumarins 11{60–63} from the 4-iodoisocoumarins. The strongly electron-withdrawing nature of the trifluoromethyl group generally significantly increases the binding interactions, thus improving oral bioavailability, as well as enhancing CNS penetration in certain cases.18 We have also utilized several fluorinated building blocks, such as 12{20}, 13{9–11}, and 14{1}, in order to incorporate fluorine atoms in our final library members. For synthesis of the trifluoromethyl analogs, the 4-iodoisocoumarins were treated with fluorosulfonylfluoroacetate,19 which generates the trifluoromethyl species in situ to provide compounds 11{60–63}. Thus, a total of 63 compounds have been prepared in unoptimized yields from the 4-iodoisocoumarin scaffold.
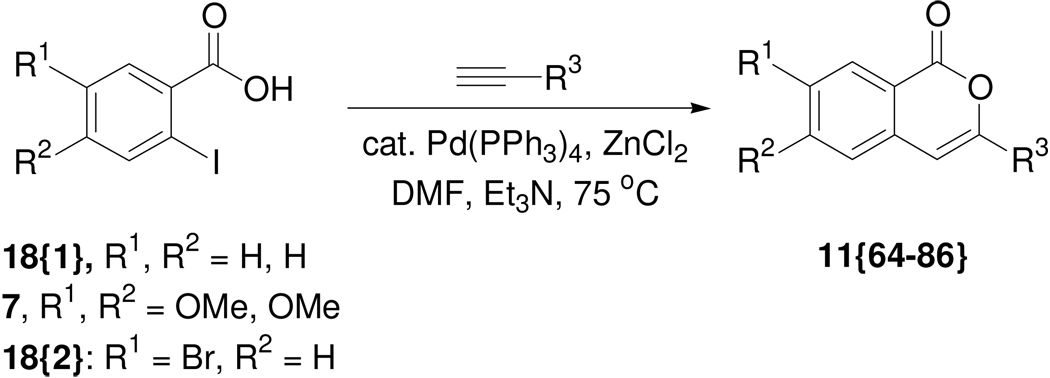
Next, we utilized the reaction of 2-iodobenzoic acids with terminal alkynes in the presence of a Pd(PPh3)4–ZnCl2–Et3N system12a in DMF to produce another set of diverse isocoumarins 11{64–86} (Scheme 3). We considered this reaction to be an attractive option for library synthesis, because it not only affords the final library members quite directly, but it also permits easy access to the isocoumarin scaffold containing handles that could be very useful for further modifications. Thus, the reaction of 2-iodobenzoic acid with appropriate terminal alkynes readily afforded the desired isocoumarins. The results are summarized in Table 2. We also briefly examined a similar method of isocoumarin synthesis from 2-iodobenzoic acid and terminal alkynes using 10% Pd/C, CuI, PPh3 and Et3N in ethanol.12b However, we found that the reaction employing the palladium-zinc chloride system afforded much cleaner reactions, yielding the desired products in better yield and purity. Benzoic acids 18{2} and alkynes 12{7–10} were chosen to maximize the utility of the resulting isocoumarin scaffold and allow for further derivatization of the embedded bromo or hydroxyl functionalities.
Scheme 3.
Synthesis of 3-substituted isocoumarins.
Table 2.
Library data for compounds 11{64–86}.
| compound | 2-iodobenzoic acid | alkyne | yielda (%) | purityc (%) |
|---|---|---|---|---|
| 11{64} | 18{1} | 12{1} | 64b | 92 |
| 11{65} | 18{1} | 12{2} | 43 | >99 |
| 11{66} | 18{1} | 12{3} | 42 | >99 |
| 11{67} | 18{1} | 12{5} | 33b | >99 |
| 11{68} | 18{1} | 12{7} | 68b | 97 |
| 11{69} | 18{1} | 12{8} | 67b | >99 |
| 11{70} | 18{1} | 12{9} | 64b | >99 |
| 11{71} | 18{1} | 12{10} | 51b | >99 |
| 11{72} | 18{1} | 12{11} | 33 | >99 |
| 11{73} | 18{1} | 12{12} | 28 | >99 |
| 11{74} | 18{1} | 12{13} | 27 | 92 |
| 11{75} | 18{1} | 12{14} | 31 | 92 |
| 11{76} | 18{1} | 12{15} | 54b | >99 |
| 11{77} | 18{1} | 12{17} | 23 | 95 |
| 11{78} | 7 | 12{7} | 52b | >99 |
| 11{79} | 7 | 12{8} | 56b | >99 |
| 11{80} | 7 | 12{9} | 51 | 98 |
| 11{81} | 7 | 12{12} | 35 | 97 |
| 11{82} | 7 | 12{15} | 39 | >99 |
| 11{83} | 18{2} | 12{7} | 42b | 99 |
| 11{84} | 18{2} | 12{8} | 41b | >99 |
| 11{85} | 18{2} | 12{9} | 61b | 90 |
| 11{86} | 18{2} | 12{10} | 46b | >99 |
Isolated yield after preparative HPLC.
Isolated yield after column chromatography.
UV purity determined at 214 nm after preparative HPLC.
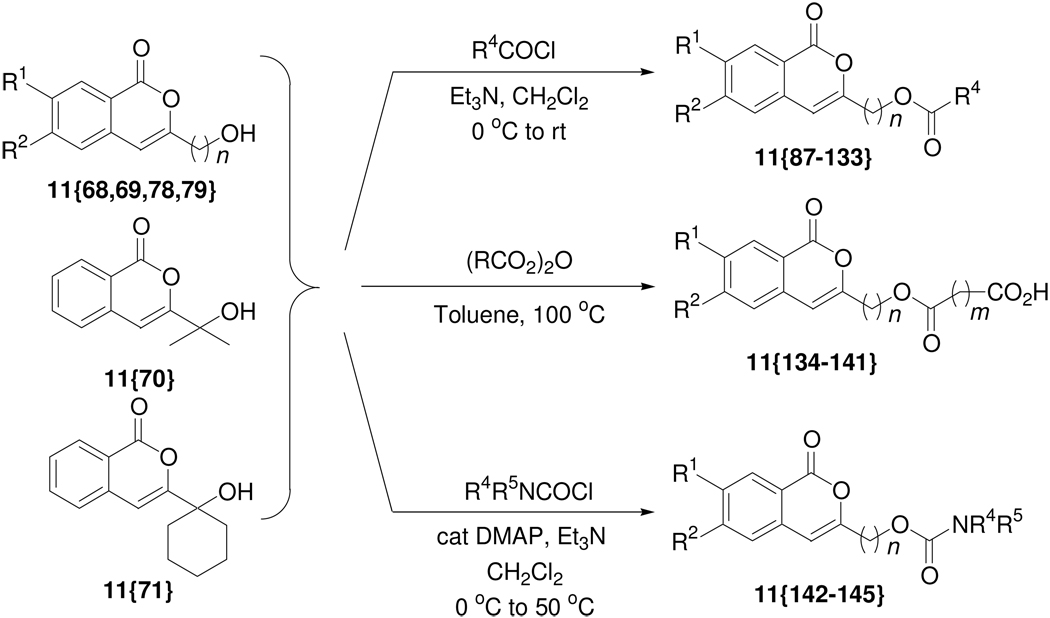
We have speculated previously that the presence of alcohol functionality in the isocoumarins would be an ideal point for further diversification, because such alcohols could be readily elaborated to more complex isocoumarins using a wide variety of commercially available carboxylic acid derivatives. Thus, isocoumarins 11{68–71, 78, 79, 83–86}, prepared in gram quantities for further derivatization, were subjected to an additional diversity step. Consequently, these hydroxyl-bearing isocoumarins were subjected to acylation reaction using various acid chlorides 15{1–12}, acid anhydrides 16{1–3} and carbamoyl chlorides 17{1–3} to generate a wide variety of isocoumarins 11{87–145}. The cyclic anhydrides 16{1–3} were chosen in order to have a polar carboxylic acid functionality present in the final molecule. In general, the reactions with carbamoyl chlorides were less efficient than those with acid chlorides and acid anhydrides. Although triethylamine was found to be sufficient in most acylation reactions with acid chlorides, DMAP was used in combination with triethylamine in some instances, especially for the more sluggish reactions. However, as shown in Table 3, the products were obtained in excellent purity (>99%) in most cases for this diversification of the hydroxyl functionality.
Table 3.
Library data for compounds 11{87–145}.
| compound | isocoumarin | reactant | methoda | yieldb (%) | purityd (%) |
|---|---|---|---|---|---|
| 11{87} | 11{68} | 15{1} | A | 70 | >99 |
| 11{88} | 11{68} | 15{2} | A | 66c | >99 |
| 11{89} | 11{68} | 15{3} | A | 59 | >99 |
| 11{90} | 11{68} | 15{4} | A | 59 | >99 |
| 11{91} | 11{68} | 15{5} | A | 49 | >99 |
| 11{92} | 11{68} | 15{6} | A | 74 | >99 |
| 11{93} | 11{68} | 15{7} | A | 74 | >99 |
| 11{94} | 11{68} | 15{8} | A | 75 | 99 |
| 11{95} | 11{68} | 15{9} | A | 76 | >99 |
| 11{96} | 11{68} | 15{10} | A | 55 | >99 |
| 11{97} | 11{68} | 15{11} | A | 77 | >99 |
| 11{98} | 11{69} | 15{1} | A | 64c | >99 |
| 1{99} | 11{69} | 15{3} | A | 88 | >99 |
| 11{100} | 11{69} | 15{4} | A | 89 | >99 |
| 11{101} | 11{69} | 15{5} | A | 45 | >99 |
| 11{102} | 11{69} | 15{8} | A | 52 | >99 |
| 11{103} | 11{69} | 15{9} | A | 76 | >99 |
| 11{104} | 11{69} | 15{10} | A | 45 | >99 |
| 11{105} | 11{69} | 15{11} | A | 62 | >99 |
| 11{106} | 11{70} | 15{1} | A | 37 | 94 |
| 11{107} | 11{70} | 15{2} | A | 37 | 97 |
| 11{108} | 11{70} | 15{3} | A | 35 | 97 |
| 11{109} | 11{70} | 15{4} | A | 38 | 99 |
| 11{110} | 11{70} | 15{5} | A | 33 | >99 |
| 11{111} | 11{70} | 15{6} | A | 38 | >99 |
| 11{112} | 11{71} | 15{1} | A | 36 | >99 |
| 11{113} | 11{71} | 15{2} | A | 23 | 98 |
| 11{114} | 11{71} | 15{3} | A | 29 | >99 |
| 11{115} | 11{71} | 15{4} | A | 14 | >99 |
| 11{116} | 11{71} | 15{5} | A | 20 | >99 |
| 11{117} | 11{71} | 15{6} | A | 21 | >99 |
| 11{118} | 11{78} | 15{1} | A | 90c | >99 |
| 11{119} | 11{78} | 15{2} | A | 21 | >99 |
| 11{120} | 11{78} | 15{3} | A | 65 | >99 |
| 11{121} | 11{78} | 15{4} | A | 45 | >99 |
| 11{122} | 11{78} | 15{5} | A | 60 | >99 |
| 11{123} | 11{78} | 15{7} | A | 40 | >99 |
| 11{124} | 11{78} | 15{12} | A | 14 | 95 |
| 11{125} | 11{79} | 15{1} | A | 58c | 99 |
| 11{126} | 11{79} | 15{2} | A | 80 | >99 |
| 11{127} | 11{79} | 15{3} | A | 58 | >99 |
| 11{128} | 11{79} | 15{4} | A | 79 | >99 |
| 11{129} | 11{79} | 15{5} | A | 72 | >99 |
| 11{130} | 11{79} | 15{6} | A | 67 | 99 |
| 11{131} | 11{79} | 15{7} | A | 82 | >99 |
| 11{132} | 11{79} | 15{9} | A | 20 | 97 |
| 11{133} | 11{79} | 15{10} | A | 8 | 99 |
| 11{134} | 11{68} | 16{1} | B | 86c | >99 |
| 11{135} | 11{68} | 16{2} | B | 42 | 99 |
| 11{136} | 11{68} | 16{3} | B | 68 | 98 |
| 11{137} | 11{69} | 16{1} | B | 39 | >99 |
| 11{138} | 11{69} | 16{2} | B | 17 | >99 |
| 11{139} | 11{78} | 16{2} | B | 61 | >99 |
| 11{140} | 11{79} | 16{1} | B | 68 | >99 |
| 11{141} | 11{79} | 16{2} | B | 67 | >99 |
| 11{142} | 11{68} | 17{1} | C | 17 | >99 |
| 11{143} | 11{68} | 17{3} | C | 12 | >99 |
| 11{144} | 11{69} | 17{3} | C | 13 | >99 |
| 11{145} | 11{78} | 17{2} | C | 37 | 95 |
Method A: RCOCl (1.3 equiv), Et3N (2 equiv), CH2Cl2, 0 °C to rt; Method B: (RCO2)2O (4 equiv), toluene, 110 °C; Method C: R2NCOCl (2 equiv), cat. DMAP, Et3N (3 equiv), CH2Cl2, 0 °C to 50 °C.
Isolated yield after preparative HPLC.
Isolated yield after column chromatography.
UV purity determined at 214 nm after preparative HPLC.
In order to further expand the diversity present in the aromatic core of the isocoumarin ring, 7-bromoisocoumarins 11{83–86} were subsequently modified using palladium-catalyzed cross-coupling reactions to generate the more complex isocoumarins 11{146–167} (Scheme 5). The new isocoumarins prepared by further diversification through Sonogashira, Suzuki-Miyaura and Heck reactions are summarized in Table 4.
Table 4.
Library data for compound 11{146–167}.
| compound | isocoumarin | reactant | methoda | yieldb (%) | purityc (%) |
|---|---|---|---|---|---|
| 11{146} | 11{83} | 12{2} | A | 80 | >99 |
| 11{147} | 11{83} | 12{3} | A | 79 | >99 |
| 11{148} | 11{83} | 12{17} | A | 69 | 95 |
| 11{149} | 11{83} | 12{18} | A | 45 | 92 |
| 11{150} | 11{83} | 12{19} | A | 62 | 99 |
| 11{151} | 11{84} | 12{2} | A | 77 | >99 |
| 11{152} | 11{84} | 12{3} | A | 58 | 91 |
| 11{153} | 11{84} | 12{10} | A | 70 | >99 |
| 11{154} | 11{84} | 12{16} | A | 14 | 92 |
| 11{155} | 11{84} | 12{17} | A | 54 | >99 |
| 11{156} | 11{84} | 12{18} | A | 59 | 96 |
| 11{157} | 11{84} | 12{19} | A | 21 | >99 |
| 11{158} | 11{85} | 12{2} | A | 58 | >99 |
| 11{159} | 11{85} | 12{3} | A | 53 | >99 |
| 11{160} | 11{85} | 12{18} | A | 58 | 94 |
| 11{161} | 11{85} | 12{19} | A | 58 | >99 |
| 11{162} | 11{86} | 12{18} | A | 45 | 99 |
| 11{163} | 11{86} | 12{19} | A | 58 | 99 |
| 11{164} | 11{83} | 13{1} | B | 47 | >99 |
| 11{165} | 11{86} | 13{1} | B | 10 | >99 |
| 11{166} | 11{84} | 14{2} | C | 26 | >99 |
| 11{167} | 11{86} | 14{1} | C | 69 | >99 |
Method A: alkyne (1.5 equiv), PdCl2(PPh3)2 (3 mol %), CuI (2 mol %), 3:1 DMF/Et3N, 60 °C; Method B: boronic acid (1.5 equiv), Pd(PPh3)4 (5 mol %), Cs2CO3 (2 equiv), 1.5:0.3:0.2 DMF/EtOH/H2O, microwave irradiation, 120 °C; Method C: styrene (2 equiv), Pd(OAc)2 (15 mol %), Na2CO3 (2.5 equiv), TBAC (1 equiv), DMF, 80 °C.
Isolated yield after preparative HPLC.
UV purity determined at 214 nm after preparative HPLC.
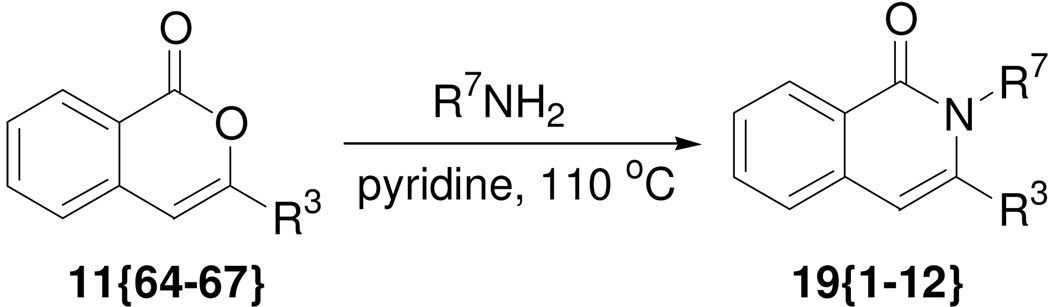
3-Aryl substituted isocoumarins 11{64–67}, prepared previously from 2-iodobenzoic acid (7), were used to generate a small set of isoquinolinones by reaction with various amines (Scheme 6).20 The isoquinolinone subunit is present in a large number of natural products (e.g. narciclasine, pancratistatin, lycoricidine, etc.) and its derivatives possess a broad spectrum of biological activities, including NK3 antagonists and inhibitors of potassium channels, Rho-kinase, NR5A1 and PARP-2.21 For the generation of the isoquinolinone library, commercially available amines (Figure 4) were chosen that contain polar functionality or heterocycles that would provide compounds with potentially attractive drug-like features. Cleaner reactions were achieved with amines containing hydroxyl functionality (i.e. for amines 20{1–4}). The resulting alcohols are attractive intermediates for expanding the isoquinolinone library via subsequent chemical modification. As summarized in Table 5, we have obtained the desired isoquinolinone products in high purity (>90%) in unoptimized yields in the range of 16–70%.
Scheme 6.
Conversion of isocoumarins to isoquinolin-1-ones.
Figure 4.
Diverse amines 20{1–8} used for isoquinolin-1-one library synthesis.
Table 5.
Library data for compounds 19{1–12}.
| compound | isocoumarin | amine | yielda (%) | purityc (%) |
|---|---|---|---|---|
| 19{1} | 11{64} | 20{1} | 46b | >99 |
| 19{2} | 11{64} | 20{2} | 50b | >99 |
| 19{3} | 11{64} | 20{5} | 57 | >99 |
| 19{4} | 11{64} | 20{6} | 54 | >99 |
| 19{5} | 11{64} | 20{7} | 70 | 95 |
| 19{6} | 11{64} | 20{8} | 29 | 97 |
| 19{7} | 11{65} | 20{1} | 63 | >99 |
| 19{8} | 11{65} | 20{2} | 64 | 96 |
| 19{9} | 11{66} | 20{1} | 16 | 92 |
| 19{10} | 11{66} | 20{3} | 24 | 96 |
| 19{11} | 11{67} | 20{3} | 25 | >99 |
| 19{12} | 11{67} | 20{4} | 26 | 90 |
Isolated yield after preparative HPLC.
Isolated yield after column chromatography.
UV purity determined at 214 nm after preparative HPLC.
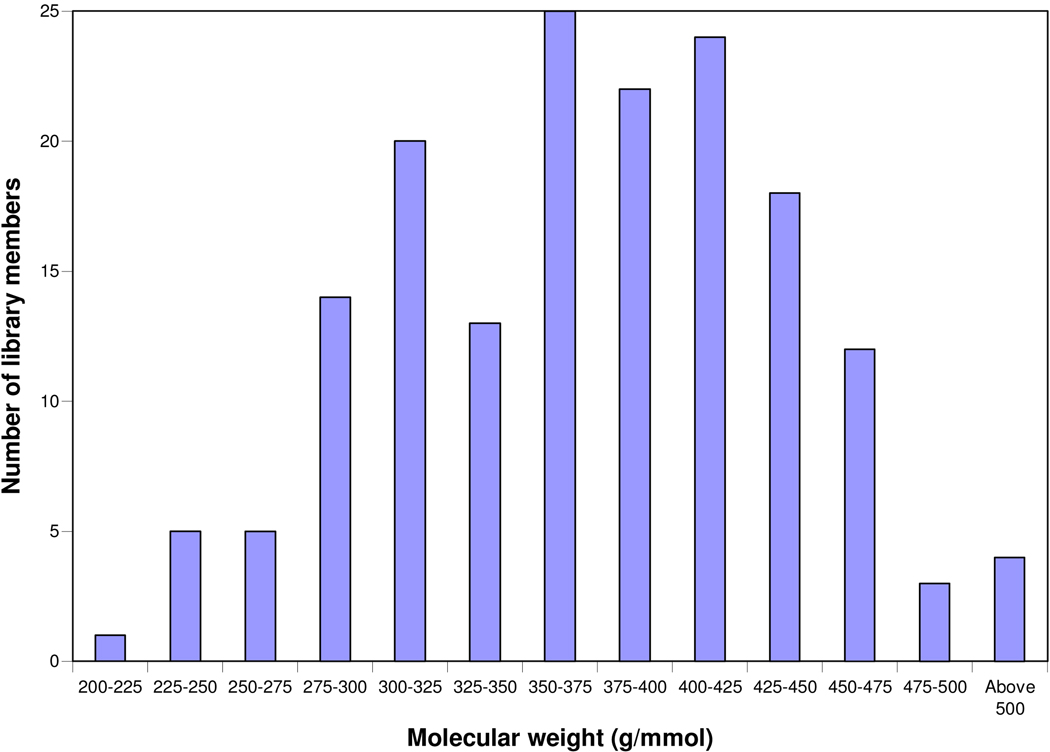
Most of the desired isocoumarin library members were highly Lipinski compliant.22 Overall, 80% of the library members are entirely compliant with Lipinski’s rules, 18% had one violation and 2% had two or more violations. The most common violation was clog P (calculated by EPI Suite)23 for which the average value for the entire library was around 4.0. The molecular weight distribution, shown in Figure 5, indicates that almost all of the members of the library reside in the desirable molecular weight range (<500).22
Figure 5.
Molecular weight distribution of library members.
In conclusion, we have efficiently constructed a 167 member library of isocoumarins with high purity (>90%). Our emphasis on high compound purity partially contributed to the lower yields in some instances. A couple of isocoumarins have also been converted to the corresponding isoquinolin-1-ones. These library members will be evaluated against various biological screens by the National Institutes of Health Molecular Library Screening Center Network.
Supplementary Material
Scheme 4.
Diversification of the 3-substituted isocoumarins.
Table 1.
Library data for compounds 11{1–63}.
| compound | isocoumarin | reactant | methoda | yieldb (%) | purityd (%) |
|---|---|---|---|---|---|
| 11{1} | 10{1} | 12{1} | A | 57c | >99 |
| 11{2} | 10{1} | 12{3} | A | 33 | 99 |
| 11{3} | 10{1} | 12{6} | A | 8 | >99 |
| 11{4} | 10{1} | 12{7} | A | 56 | 98 |
| 11{5} | 10{1} | 12{8} | A | 79 | 99 |
| 11{6} | 10{1} | 12{10} | A | 52 | 92 |
| 11{7} | 10{1} | 12{16} | A | 39 | 99 |
| 11{8} | 10{1} | 12{17} | A | 37 | 95 |
| 11{9} | 10{1} | 12{21} | A | 50 | 92 |
| 11{10} | 10{2} | 12{3} | A | 10 | 99 |
| 11{11} | 10{2} | 12{7} | A | 42 | >99 |
| 11{12} | 10{2} | 12{8} | A | 57 | >99 |
| 11{13} | 10{2} | 12{16} | A | 41 | 99 |
| 11{14} | 10{2} | 12{17} | A | 38 | 93 |
| 11{15} | 10{3} | 12{1} | A | 41 | 98 |
| 11{16} | 10{3} | 12{7} | A | 67 | 96 |
| 11{17} | 10{3} | 12{8} | A | 61 | 96 |
| 11{18} | 10{3} | 12{16} | A | 51 | >99 |
| 11{19} | 10{3} | 12{18} | A | 68 | 95 |
| 11{20} | 10{3} | 12{20} | A | 46 | 98 |
| 11{21} | 10{4} | 12{3} | A | 7 | 97 |
| 11{22} | 10{4} | 12{7} | A | 34 | 90 |
| 11{23} | 10{4} | 12{8} | A | 43 | 95 |
| 11{24} | 10{4} | 12{16} | A | 6 | >99 |
| 11{25} | 10{4} | 12{19} | B | 36 | 99 |
| 11{26} | 10{1} | 13{1} | B | 6 | 96 |
| 11{27} | 10{1} | 13{2} | B | 32 | 99 |
| 11{28} | 10{1} | 13{3} | B | 39 | >99 |
| 11{29} | 10{1} | 13{4} | B | 25 | 97 |
| 11{30} | 10{1} | 13{5} | B | 60 | 98 |
| 11{31} | 10{1} | 13{9} | B | 42 | 96 |
| 11{32} | 10{1} | 13{10} | B | 29 | 95 |
| 11{33} | 10{1} | 13{12} | B | 17 | 93 |
| 11{34} | 10{2} | 13{3} | A | 32 | 98 |
| 11{35} | 10{2} | 13{4} | B | 37 | >99 |
| 11{36} | 10{2} | 13{5} | B | 20 | 98 |
| 11{37} | 10{2} | 13{9} | B | 30 | 96 |
| 11{38} | 10{3} | 13{2} | B | 31 | 99 |
| 11{39} | 10{3} | 13{3} | B | 47 | 95 |
| 11{40} | 10{3} | 13{4} | B | 20 | 99 |
| 11{41} | 10{3} | 13{5} | B | 46 | 98 |
| 11{42} | 10{3} | 13{6} | B | 85 | >99 |
| 11{43} | 10{3} | 13{7} | B | 15 | 96 |
| 11{44} | 10{3} | 13{8} | B | 5 | >99 |
| 11{45} | 10{3} | 13{10} | B | 38 | 98 |
| 11{46} | 10{3} | 13{11} | B | 24 | 96 |
| 11{47} | 10{3} | 13{12} | B | 13c | 92 |
| 11{48} | 10{3} | 13{13} | B | 10 | 96 |
| 11{49} | 10{4} | 13{1} | B | 41 | 99 |
| 11{50} | 10{4} | 13{2} | B | 43 | 97 |
| 11{51} | 10{4} | 13{6} | B | 21 | 98 |
| 11{52} | 10{1} | 14{1} | C | 58 | 93 |
| 11{53} | 10{1} | 14{2} | C | 25 | 96 |
| 11{54} | 10{1} | 14{3} | C | 21 | 98 |
| 11{55} | 10{1} | 14{4} | C | 46c | >99 |
| 11{56} | 10{1} | 14{5} | C | 62 | 99 |
| 11{57} | 10{1} | 14{6} | C | 15 | >99 |
| 11{58} | 10{2} | 14{1} | C | 50 | >99 |
| 11{59} | 10{3} | 14{3} | C | 23 | 99 |
| 11{60} | 10{1} | - | D | 80c | 98 |
| 11{61} | 10{2} | - | D | 84c | 97 |
| 11{62} | 10{3} | - | D | 80c | >99 |
| 11{63} | 10{4} | - | D | 65c | 92 |
Method A: alkyne (1.5 equiv), PdCl2(PPh3)2 (3 mol %), CuI (2 mol %), 2:1 DMF/Et3N, 55 °C; Method B: boronic acid (1.5 equiv), Pd(PPh3)4 (5 mol %), Cs2CO3 (2 equiv), 1.5:0.3:0.2 DMF/EtOH/H2O, microwave irradiation, 120 °C; Method C: styrene (2 equiv), Pd(OAc)2 (15 mol %), Na2CO3 (2.5 equiv), TBAC (1.1 equiv), DMF, 80 °C; Method D: FSO2CF2CO2Me (5 equiv), CuI (1 equiv), DMF, 70 °C.
Isolated yield after preparative HPLC.
Isolated yield after column chromatography.
UV purity determined at 214 nm after preparative HPLC.
Acknowledgements
We would like to thank Dr. Jesse P. Waldo and Dr. Yu Chen of Iowa State University and Dr. Frank Schoenen of Kansas University for helpful discussions. We thank the National Institute of General Medical Sciences (GM070620 and GM079593) and the National Institutes of Health Kansas University Chemical Methodologies and Library Development Center of Excellence (GM069663) for support of this research and Johnson Matthey, Inc. and Kawaken Fine Chemicals Co., Ltd. for donating the palladium catalysts and Frontier Scientific and Synthonix for donating the boronic acids.
Footnotes
Supporting Information Available. Experimental details and characterization of a representative 20 library members, including full 1H and 13C NMR spectra and conditions for the high throughput liquid chromatography purification.
References
- 1.a) Hill RA. In: Progress in the Chemistry of Organic Natural Products. Herz W, editor. Vol. 49. New York: Springer-Verlag; 1986. pp. 1–78. [Google Scholar]; b) Mali RS, Babu KN. J. Org. Chem. 1998;63:2488–2492. doi: 10.1021/jo971622e. [DOI] [PubMed] [Google Scholar]; c) Barry RD. Chem. Rev. 1964;64:229–260. [Google Scholar]
- 2.a) Pochet L, Frederick R, Masereel B. Curr. Pharm. Des. 2004;10:3781–3796. doi: 10.2174/1381612043382684. [DOI] [PubMed] [Google Scholar]; b) Canedo-Hernandez L, Acebal-Sarabia C, Garcia-Gravalos D. PCT Int. Appl. WO 9627594, 1996. Chem. Abstr. 1996;125:273726. [Google Scholar]; c) Deck LM, Vander Jagt DL, Heynekamp JJ. U.S. Pat. Appl. Publ. US. 2006252823. Chem. Abstr. 2006;145:471397. 2006.; d) Bauta WE, Cantrell WR, Jr, Lovett DP. PCT Int. Appl. WO 2003004486, 2003. Chem. Abstr. 2003;138:106599. [Google Scholar]
- 3.a) Meepagala KM, Sturtz G, Wedge DE. J. Agric. Food Chem. 2002;50:6989–6992. doi: 10.1021/jf020466w. [DOI] [PubMed] [Google Scholar]; b) Engelmeier D, Hadacek F, Hofer O, Lutz-Kutschera G, Nagl M, Wurz G, Greger H. J. Nat. Prod. 2004;67:19–25. doi: 10.1021/np0301339. [DOI] [PubMed] [Google Scholar]; c) Whyte AC, Gloer JB, Scott JA, Malloch D. J. Nat. Prod. 1996;59:765–769. doi: 10.1021/np9603232. [DOI] [PubMed] [Google Scholar]
- 4.a) Yano K. J. Agric. Food Chem. 1987;35:889–891. [Google Scholar]; b) Yano K. Recent Res. Devel. Agri. Biol. Chem. 1998;2:293–305. [Google Scholar]
- 5.a) Nozawa K, Yamada M, Tsuda Y, Kawai K, Nakajima S. Chem. Pharm. Bull. 1981;29:2689–2691. doi: 10.1248/cpb.29.2689. [DOI] [PubMed] [Google Scholar]; b) Sonnenbichler J, Kovacs T. Eur. J. Biochem. 1997;246:45–49. doi: 10.1111/j.1432-1033.1997.t01-2-00045.x. [DOI] [PubMed] [Google Scholar]
- 6.a) Kurume A, Kamata Y, Yamashita M, Wang Q, Matsuda H, Yoshikawa M, Kawasaki I, Ohta S. Chem. Pharm. Bull. 2008;56:1264–1269. doi: 10.1248/cpb.56.1264. [DOI] [PubMed] [Google Scholar]; b) Matsuda H, Wang Q, Matsuhira K, Nakamura S, Yuan D, Yoshikawa M. Phytomedicine. 2008;15:177–184. doi: 10.1016/j.phymed.2007.09.010. [DOI] [PubMed] [Google Scholar]; c) Matsuda H, Shimoda H, Yamahara J, Yoshikawa M. Biol. Pharm. Bull. 1999;22:870–872. doi: 10.1248/bpb.22.870. [DOI] [PubMed] [Google Scholar]; d) Matsuda H, Shimoda H, Yamahara J, Yoshikawa M. Bioorg. Med. Chem. Lett. 1998;8:215–220. doi: 10.1016/s0960-894x(97)10221-9. [DOI] [PubMed] [Google Scholar]; e) Yoshikawa M, Harada E, Naitoh Y, Inoue K, Matsuda H, Shimoda H, Yamahara J, Murakami N. Chem. Pharm. Bull. 1994;42:2225–2230. doi: 10.1248/cpb.42.2225. [DOI] [PubMed] [Google Scholar]
- 7.a) Kumagai H, Wakazono K, Agata N, Isshiki K, Ishizuka M, Ikeda D. J. Antibiot. 2005;58:202–205. doi: 10.1038/ja.2005.24. [DOI] [PubMed] [Google Scholar]; b) Kumagai H, Masuda T, Ishizuka M, Takeuchi T. J. Antibiot. 1995;48:175–178. doi: 10.7164/antibiotics.48.175. [DOI] [PubMed] [Google Scholar]
- 8.Lim D-S, Kwak Y-S, Kim J-H, Ko S-H, Yoon W-H, Kim C-H. Chemotherapy. 2003;49:146–153. doi: 10.1159/000070621. [DOI] [PubMed] [Google Scholar]
- 9.a) Harris JP, Mantle PG. Phytochemistry. 2001;57:165–169. doi: 10.1016/s0031-9422(01)00004-8. [DOI] [PubMed] [Google Scholar]; b) Lee I-K, Seok S-J, Kim W-G, Yun B-S. Mycobiology. 2006;34:38–40. doi: 10.4489/MYCO.2006.34.1.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.a) Kawano T, Agata N, Kharbanda S, Avigan D, Kufe D. Cancer Chemother. Pharmacol. 2007;59:329–335. doi: 10.1007/s00280-006-0274-x. [DOI] [PubMed] [Google Scholar]; b) Agata N, Nogi H, Milhollen M, Kharbanda S, Kufe D. Cancer Res. 2004;64:8512–8516. doi: 10.1158/0008-5472.CAN-04-2626. [DOI] [PubMed] [Google Scholar]; c) Yin L, Ohno T, Weichselbaum R, Kharbanda S, Kufe D. Mol. Cancer Therapeutics. 2001;1:43–48. [PubMed] [Google Scholar]; d) Nakashima T, Hirano S-I, Agata N, Kumagai H, Isshiki K, Yoshioka T, Ishizuka M, Maeda K, Takeuchi T. J. Antibiot. 1999;52:426–428. doi: 10.7164/antibiotics.52.426. [DOI] [PubMed] [Google Scholar]; e) Reimer CL, Agata N, Takeuchi T, Kumagai H, Yoshioka T, Ishizuka M, Kufe DW, Weichselbaum RR. U.S. Pat. Appl. Publ. US. 2002019366. Chem. Abstr. 2002;136:161349. 2002.
- 11.a) Yao T, Larock RC. J. Org. Chem. 2003;68:5936–5942. doi: 10.1021/jo034308v. [DOI] [PubMed] [Google Scholar]; b) Yao T, Larock RC. Tetrahedron Lett. 2002;43:7401–7404. [Google Scholar]; c) Larock RC, Doty MJ, Han X. J. Org. Chem. 1999;64:8770–8779. doi: 10.1021/jo9821628. [DOI] [PubMed] [Google Scholar]; d) Larock R, Yang H. Synlett. 1994:748–750. [Google Scholar]; e) Larock RC, Varaprath S, Lau HH, Fellows CA. J. Am. Chem. Soc. 1984;106:5274–5284. [Google Scholar]; f) Larock RC, Yum EK, Doty MJ, Sham KKC. J. Org. Chem. 1995;60:3270–3271. [Google Scholar]
- 12.a) Liao H-Y, Cheng C-H. J. Org. Chem. 1995;60:3711–3716. [Google Scholar]; b) Subramanian V, Batchu VR, Barange D, Pal M. J. Org. Chem. 2005;70:4778–4783. doi: 10.1021/jo050440e. [DOI] [PubMed] [Google Scholar]; c) Inack-Ngi S, Rahmani R, Commeiras L, Chouraqui G, Thibonnet J, Duchene A, Abarbri M, Parrain J-L. Adv. Syn. Cat. 2009;351:779–788. [Google Scholar]; d) Lu Z-F, Yue J-J, Zhu Y, Hu H-W, Xu J-H. Photochem. Photobiol. Sci. 2009;8:217–223. [Google Scholar]; e) Chin L-Y, Lee C-Y, Lo Y-H, Wu M-JJ. Chinese Chem. Soc. 2008;55:643–648. [Google Scholar]; f) Le Bras G, Hamze A, Messaoudi S, Provot O, Le Calvez P-B, Brion J-D, Alami M. Synthesis. 2008:1607–1611. [Google Scholar]; g) Oezcan S, Balci M. Tetrahedron. 2008;64:5531–5540. [Google Scholar]; h) Marchal E, Uriac P, Legouin B, Toupet L, van de Weghe P. Tetrahedron. 2007;63:9979–9990. [Google Scholar]; i) Liang Y, Xie Y-X, Li J-H. Synthesis. 2007:400–406. [Google Scholar]; j) Uchiyama M, Ozawa H, Takuma K, Matsumoto Y, Yonehara M, Hiroya K, Sakamoto T. Org. Lett. 2006;8:5517–5520. doi: 10.1021/ol062190+. [DOI] [PubMed] [Google Scholar]; k) Chakravarty M, Swamy KCK. J. Org. Chem. 2006;71:9128–9138. doi: 10.1021/jo061525y. [DOI] [PubMed] [Google Scholar]; l) Langer J, Gaertner M, Goerls H, Walther D. Synthesis. 2006:2697–2706. [Google Scholar]; m) Cherry K, Parrain J-L, Thibonnet J, Duchene A, Abarbri M. J. Org. Chem. 2005;70:6669–6675. doi: 10.1021/jo050638z. [DOI] [PubMed] [Google Scholar]; n) Brasholz M, Luan X, Reissig H-U. Synthesis. 2005:3571–3580. [Google Scholar]; o) Yuan H, Junker B, Helquist P, Taylor RE. Curr. Org. Syn. 2004;1:1–9. [Google Scholar]; p) Rossi R, Carpita A, Bellina F, Stabile P, Mannina L. Tetrahedron. 2003;59:2067–2081. [Google Scholar]; q) Nakamura Y, Ukita T. Org. Lett. 2002;4:2317–2320. doi: 10.1021/ol025916k. [DOI] [PubMed] [Google Scholar]; r) Bellina F, Ciucci D, Vergamini P, Rossi R. Tetrahedron. 2000;56:2533–2545. [Google Scholar]; s) Mal D, Bandyopadhyay M, Ghorai SK, Datta K. Tetrahedron Lett. 2000;41:3677–3680. [Google Scholar]; t) Sashida H, Kawamukai A. Synthesis. 1999:1145–1148. [Google Scholar]; u) Minami T, Nishimoto A, Nakamura Y, Hanaoka M. Chem. Pharm. Bull. 1994;42:1700–1702. [Google Scholar]; v) Izumi T, Morishita N. J. Heterocycl. Chem. 1994;31:145–152. [Google Scholar]; w) Izumi T, Nishimoto Y, Kohei K, Kasahara A. J. Heterocycl. Chem. 1990;27:1419–1424. [Google Scholar]; x) Sakamoto T, Annaka M, Kondo Y, Yamanaka H. Chem. Pharm. Bull. 1986;34:2754–2759. [Google Scholar]; y) Batu G, Stevenson R. J. Org. Chem. 1980;45:1532–1534. [Google Scholar]; z) Korte DE, Hegedus LS, Wirth RK. J. Org. Chem. 1977;42:1329–1336. doi: 10.1021/jo00428a013. [DOI] [PubMed] [Google Scholar]
- 13.For a review of isocoumarin syntheses, see: Napolitano E. Org. Proc. Prep. Intl. 1997;29:631–664.
- 14.For the solid phase synthesis of isocoumarins, see: Peuchmaur M, Lisowski V, Gandreuil C, Maillard LT, Martinez J, Hernandez J-F. J. Org. Chem. 2009;74:4158–4165. doi: 10.1021/jo900281a.
- 15.a) Waldo JP, Mehta S, Neuenswander B, Lushington GH, Larock RC. J. Comb. Chem. 2008;10:658–663. doi: 10.1021/cc800055x. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Cho C-H, Neuenswander B, Lushington GH, Larock RC. J. Comb. Chem. 2008;10:941–947. doi: 10.1021/cc800120y. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Roy S, Roy S, Neuenswander B, Hill D, Larock RC. J. Comb. Chem. doi: 10.1021/cc9000949. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.a) Loughlin WA. Aust. J. Chem. 1998;51:875–893. [Google Scholar]; b) Nefzi Ac, Ostresh JM, Houghten RA. Chem. Rev. 1997;97:449–472. doi: 10.1021/cr960010b. [DOI] [PubMed] [Google Scholar]; c) Balkenhohl F, von dem Bussche-Huennefeld C, Lansky A, Zechel C. Angew. Chem., Int. Ed. Engl. 1996;35:2288–2337. [Google Scholar]
- 17.a) Hagmann WK. J. Med. Chem. 2008;51:4359–4369. doi: 10.1021/jm800219f. [DOI] [PubMed] [Google Scholar]; b) Hudlicky M, Pavlath AE. Chemistry of Organic Fluorine Compounds II: A Critical Review. 1995;187:888–912. ACS Monograph. [Google Scholar]; c) Hiyama T, editor. Organofluorine Compounds. Chemistry and Applications. New York: Springer Verlag; 2000. [Google Scholar]
- 18.a) Humphrey JM. Curr. Top. Med. Chem. 2003;3:1423–1435. doi: 10.2174/1568026033451925. [DOI] [PubMed] [Google Scholar]; b) Edmondson SD, Mastracchio A, Beconi M, Colwell LF, Habulihaz B, He H, Kumar S, Leiting B, Lyons KA, Mao A, Marsilio F, Patel RA, Wu JK, Zhu L, Thornberry NA, Weber AE, Parmee ER. Bioorg. Med. Chem. Lett. 2004;14:5151–5155. doi: 10.1016/j.bmcl.2004.07.056. [DOI] [PubMed] [Google Scholar]; c) Lin LS, Lanza TJ, Jewell JP, Liu P, Shah SK, Qi H, Tong X, Wang J, Xu S, Fong TM, Shen CP, Lao J, Chen J, Shearman LP, Stribling DS, Rosko K, Strack A, Marsh DJ, Feng Y, Kumar S, Samuel K, Yin W, Van der Ploeg L, Goulet MT, Hagmann WK. J. Med. Chem. 2006;49:7584–7587. doi: 10.1021/jm060996+. [DOI] [PubMed] [Google Scholar]
- 19.a) Chen Q-Y. J. Fluorine Chem. 1995;72:241–246. [Google Scholar]; b) De Angelis M, Stossi F, Waibel M, Katzenellenbogen BS, Katzenellenbogen JA. Bioorg. Med. Chem. 2005;13:6529–6542. doi: 10.1016/j.bmc.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 20.Kimura M, Waki I, Deguchi Y, Amemiya K, Maeda T. Chem. Pharm. Bull. 1983;31:1277–1282. doi: 10.1248/cpb.31.1277. [DOI] [PubMed] [Google Scholar]
- 21.a) Simonsen KB, Kehler J, Juhl K, Khanzhin N, Nielsen SM. U.S. Pat. Appl. Publ. US. 2009143402. Chem. Abstr. 2009;151:8316. 2009.; b) Zhou D, Gross JL, Robichaud AJ. U.S. Pat. Appl. Publ. US. 2009069370. Chem. Abstr. 2009;150:329630. 2009.; c) Hallam M, Martin B, Raubo P, Roberts B, St-Gallay S, Willis P. PCT Int. Appl. WO 2008122765, 2008. Chem. Abstr. 2008;149:471348. [Google Scholar]; d) Pellicciari R, Camaioni E, Costantino G, Formentini L, Sabbatini P, Venturoni F, Eren G, Bellocchi D, Chiarugi A, Moroni F. ChemMedChem. 2008;3:914–923. doi: 10.1002/cmdc.200800010. [DOI] [PubMed] [Google Scholar]; e) Roth J, Madoux F, Hodder P, Roush WR. Bioorg. Med. Chem. Lett. 2008;18:2628–2632. doi: 10.1016/j.bmcl.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Dinsmore CJ, Bergman JM, McIntyre CJ, Claremon DA. PCT Int. Appl. WO 2005046578, 2005. Chem. Abstr. 2005;143:7609. [Google Scholar]; g) Wang F, Liu H, Fu H, Jiang Y, Zhao Y. Org. Lett. 2009;11:2469–2472. doi: 10.1021/ol900847t. and references therein. [DOI] [PubMed] [Google Scholar]
- 22.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Adv. Drug Del. Rev. 1997;23:3–25. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 23.Estimation Programs Interface Suite™ for Microsoft® Windows, v[3.20] Washington, DC, USA: United States Environmental Protection Agency; cLog P values were calculated by EPI Suite: US EPA. [2007] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.